Abstract
The CCA-adding enzyme synthesizes the CCA sequence at the 3′ end of tRNA without a nucleic acid template. The crystal structures of class II Thermotoga maritima CCA-adding enzyme and its complexes with CTP or ATP were determined. The structure-based replacement of both the catalytic heads and nucleobase-interacting neck domains of the phylogenetically closely related Aquifex aeolicus A-adding enzyme by the corresponding domains of the T. maritima CCA-adding enzyme allowed the A-adding enzyme to add CCA in vivo and in vitro. However, the replacement of only the catalytic head domain did not allow the A-adding enzyme to add CCA, and the enzyme exhibited (A, C)-adding activity. We identified the region in the neck domain that prevents (A, C)-adding activity and defines the number of nucleotide incorporations and the specificity for correct CCA addition. We also identified the region in the head domain that defines the terminal A addition after CC addition. The results collectively suggest that, in the class II CCA-adding enzyme, the head and neck domains collaboratively and dynamically define the number of nucleotide additions and the specificity of nucleotide selection.
Keywords: CCA, nucleotidyltransferase, template-independent, tRNA
Introduction
The CCA sequence is found at the 3′ end of every tRNA (at positions 74, 75 and 76), and is required for amino-acid attachment onto the 3′ end of tRNA (aminoacylation) by aminoacyl-tRNA synthetases (Sprinzl and Cramer, 1979). The CCA sequence is also required for interactions with the ribosome during protein synthesis (Green and Noller, 1997; Kim and Green, 1999; Nissen et al, 2000). The CCA is added and/or repaired by the CCA-adding enzyme (CTP:(ATP) tRNA nucleotidyltransferase)) (Deutscher, 1990; Weiner, 2004). The CCA-adding enzyme is a member of the nucleotidyltransferase super family (Holm and Sander, 1995; Martin and Keller, 1996, 2007) and is the only RNA polymerase that can synthesize the specific sequence onto the 3′ end of the specific tRNA primer, without any nucleic acid template. The CCA-adding enzyme has been identified in all three main kingdoms and has been classified into two classes (classes I and II) (Yue et al, 1996). Archaeal CCA-adding enzymes belong to class I, and eubacterial and eukaryotic CCA-adding enzymes belong to class II. Although both classes of CCA-adding enzymes catalyse the same reaction, there is no significant amino-acid similarity between the two classes of enzymes (Yue et al, 1996).
The recent crystallographic and biochemical studies on the class I Archaeoglobus fulgidus CCA-adding enzyme showed the detailed mechanism for the synthesis of the CCA sequence at the 3′ end of RNA, without a nucleic acid template (Okabe et al, 2003; Xiong et al, 2003; Xiong and Steitz, 2004; Tomita et al, 2006; Toh et al, 2008). In the class I CCA-adding enzyme, the nucleotide specificity is defined by the dynamic conformational changes of the enzyme and the RNA primer during polymerization. The bases of CTP and ATP interact with the phosphate backbones of the 3′ region of the RNA primers (Xiong and Steitz, 2004; Tomita et al, 2006). On CTP binding, the catalytic head domain relocates towards the neck domain, and the catalytic cleft of the enzyme shifts from an inactive open to an active closed form. The transition from the open to closed form of the enzyme is accompanied by the conformational change of the β-turn (Tomita et al, 2006). For the terminal AMP incorporation, the enzyme adopts an active closed form, and the reaction proceeds on ATP binding to the nucleotide pocket, composed of the enzyme and the 3′ end of the RNA. These findings indicate that the template for the CCA is the dynamic RNA–protein complex, rather than the protein itself (Shi et al, 1998; Yue et al, 1998; Schimmel and Yang, 2004; Weiner, 2004; Xiong and Steitz, 2004; Tomita et al, 2006). On the other hand, the crystal structures of the class II Bacillus stearothermophilus CCA-adding enzyme and its complex with CTP and ATP were reported (Li et al, 2002). The class II B. stearothermophilus CCA-adding enzyme adopts a seahorse-like structure consisting of four functional domains—catalytic head, neck, body and tail domains. In the structures, both CTP and ATP are recognized specifically through Watson–Crick-like base pairing between the bases and the amino-acid residues of the neck domain of the enzyme (Li et al, 2002). The NH2 groups of CTP and ATP hydrogen bond with Asp154, and the N3 atom of CTP and the N1 atom of ATP hydrogen bond with Arg157. The O2 atom of CTP also hydrogen bonds with Arg157. These two amino-acid residues in the neck domain are conserved among the class II CCA-adding enzymes, and even among the eubacterial polyA polymerases (Tomita and Weiner, 2002; Martin and Keller, 2004, 2007). It has been proposed that the template for the CCA addition by the class II enzymes is composed of only the protein, rather than the RNA–protein complex as in the class I CCA-adding enzyme. However, the detailed polymerization mechanism for CCA synthesis by the class II CCA-adding enzymes remains obscure.
Compounding the unsolved mechanistic questions about the class II CCA-adding enzymes, as described above, in some eubacteria, the CCA-adding activity is shared by two closely related, but distinct, class II enzymes—one adds C74C75 and the other adds A76 (Tomita and Weiner, 2001, 2002; Bralley et al, 2005; Neuenfeldt et al, 2008). In Aquifex aeolicus, which is located at the deepest root of the 16S rRNA-based phylogenetic tree and is a slowly evolving eubacterium (Pace, 1997; Woese, 2000), CCA addition is accomplished by a collaboration between CC-adding and A-adding enzymes (Tomita and Weiner, 2001). On the other hand, in Thermotoga maritima, which is also located at the deepest root of the phylogenetic tree and is close to A. aeolicus, a single enzyme, homologous to the A. aeolicus A-adding enzyme rather than the CC-adding enzyme, adds CCA (Tomita and Weiner, 2001, 2002). We previously determined the complex structure of A. aeolicus A-adding enzyme with a tRNA lacking the terminal A76 and an incoming ATP analog (AMPcpp) (Tomita et al, 2004). In the ternary complex structure, the binding pocket for the incoming AMPcpp is created by the 3′ end of the primer and the enzyme. The adenine of the incoming AMPcpp stacks with the C75 cytosine base of the primer tRNA, and the 6-NH2 group and the N1 atom of AMPcpp hydrogen bond with Asp149 and Arg152, respectively, as observed in the ATP recognition by the B. stearothermophilus CCA-adding enzyme (Li et al, 2002). However, the molecular basis for the different activities of the CCA-adding enzyme and the A-adding enzyme is still ambiguous, from the primary amino-acid sequence comparison (Tomita and Weiner, 2002; Martin and Keller, 2004).
Here, we determined the crystal structures of T. maritima CCA-adding enzyme and its complexes with CTP or ATP soaked in. The structure-guided swapping of the protein regions between T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme allowed us to generate, from the A-adding enzyme, an enzyme that can add CCA in vivo and in vitro. We identified the region in the neck domain that controls the number of nucleotide incorporations and the nucleotide specificity as well as the region in the head domain that defines the terminal A addition after CC addition. Our results suggest that, in the class II CCA-adding enzymes, the catalytic domain and the base-interacting neck domain define the register of the CCA sequence collaboratively and dynamically, and the specificity of nucleotide selection during the CCA-adding reaction. The possible mechanism of the CCA-adding reaction by the class II CCA-adding enzyme is also discussed.
Results and discussion
Structures of T. maritima CCA-adding enzyme (TmCCA)
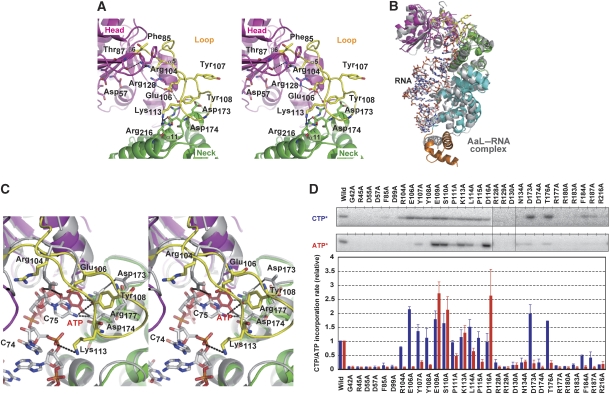
T. maritima CCA-adding enzyme (TmCCA) was crystallized under two different conditions (crystal forms I and II). The apo structures of forms I and II TmCCA and the complex structures of form I TmCCA with CTP or ATP soaked in were determined at 2.37–2.85 Å resolutions (Supplementary Table I). Both the crystal forms I and II belong to the same space group, C2, with different cell dimensions. TmCCA is composed of four domains: the head, neck, body and tail domains, and the structure is referred to as a seahorse (Figure 1A; Supplementary Figure S1) (Li et al, 2002). The head domain comprises antiparallel β-sheets containing three catalytic carboxylates (Asp55, Asp57 and Asp99), which are conserved in all known template-dependent DNA/RNA polymerases. The mutations of Asp55 to Ala, Asp57 to Ala and Asp99 to Ala reduced both the CMP and AMP incorporation rates into RNA primers ending in C74 and C74C75, respectively (Figure 2D). The neck domain contains the residues that interact with the incoming nucleobase (Asp174 and Arg177), as described below. The body and tail domains would recognize the top-half region of the tRNA primer in the same manner as those of the A. aeolicus A-adding enzyme (Tomita et al, 2004; Figure 2B).
Figure 1.
Crystal structures of T. maritima CCA-adding enzyme (TmCCA) and its complexes with CTP and ATP. (A) Overall structure of form I of apo TmCCA. The head, neck, body and tail domains of TmCCA are coloured magenta, green, cyan and orange, respectively. The loop between β6 and α5 in the head domain (amino acids 102–121) is disordered and is highlighted by a circle. (B) Superposition of the TmCCA forms I and II structures. The main chains of TmCCA forms I and II are coloured green and magenta, respectively. The loop region visible in the form II structure is circled. (C) CTP (upper panel) and ATP (lower panel) recognition by the TmCCA active site of the form I crystals. CTP and ATP are coloured blue and red, respectively. (D) Superposition of the active sites of complex structures with CTP and ATP. The CTP and ATP complex structures are coloured cyan and magenta, respectively. CTP and ATP are coloured blue and red, respectively, as in (C).
Figure 2.
Structure of the flexible loop in the catalytic head domain of T. maritima CCA-adding enzyme (TmCCA). (A) Structure of the catalytic head domain of crystal form II of TmCCA. The loop region (amino-acid residues 102–121) is coloured yellow. (B) The structure of crystal form II TmCCA (coloured as in Figure 1A) was superposed on the structure of Aquifex aeolicus A-adding enzyme complexed with tRNA lacking A76 and an incoming ATP analog (AaL–RNA complex, coloured gray, PDB code: 1VFG; Tomita et al, 2004). The head and neck domains of the two enzymes are superposed. (C) The detailed stereo view of the catalytic pocket of the superposition in (B). The loop region in the head domain is coloured yellow. ATP is coloured red. (D) In vitro relative CMP and AMP incorporation rates by mutant TmCCA variants. Asterisks indicate the α-32P-nucleotide used in the assays. The blue and red bars in the graph indicate the relative CMP and AMP incorporation rates, respectively. The CMP and AMP incorporation rates into mini-C74 and mini-C74C75 by the wild-type TmCCA are defined as 1.0. The bars in the graph are the standard deviations of more than three independent experiments.
In the TmCCA structure of the form I crystal solved at 2.85 Å resolution, the loop region (amino-acid residues 102–121) connecting β6 and α5 in the catalytic head domain is flexible and/or disordered (Figure 1A and B). The corresponding loop regions in the B. stearothermophilus CCA-adding enzyme, human CCA-adding enzyme and A. aeolicus A-adding enzyme (AaL) structures are also disordered and remained unresolved (Li et al, 2002; Augustin et al, 2003; Tomita et al, 2004). On the other hand, in the form II crystal structure of TmCCA solved at the higher resolution (2.37 Å), the electron density of the loop region is clearly visible (Figures 1B and 2A; Supplementary Figure S2). The root-mean-square deviation value between the forms I and II apo TmCCA structures was 1.26 Å, in the superposition of 386 Cα atoms. The orientations of the loop region are different between the forms I and II apo TmCCA structures (Figure 1B). In the complex structures of the form I TmCCA with CTP or ATP soaked in, the loop is disordered and flexible, as in the form I apo structure, and is oriented in the same direction as the form I apo TmCCA (Supplementary Figure S3). The flexible loop region might relocate towards the neck domain on RNA primer binding and subsequent nucleotide addition to the catalytic pockets. The possible function of the loop region in the CCA-adding reaction is described below.
In the complex structures of the form I TmCCA with CTP and ATP (2.80 and 2.85 Å resolutions, respectively), the bases of CTP and ATP form Watson–Crick-like hydrogen bonds with the amino-acid residues in the neck domain. The 4-NH2 of CTP and the 6-NH2 of ATP hydrogen bond with the Oδ atom of Asp174, whereas the N3 atom of CTP and the N1 atom of ATP hydrogen bond with the Nɛ atom of Arg177. The O2 atom of CTP hydrogen bonds with the Nη1 atom of Arg177 (Figure 1C; Supplementary Figure S4). The superposition of the two complex structures shows that only the conformations of the Arg177 side chain are different between the two complexes (Figure 1D). The interactions between the nucleobases and the side chains of Asp174 and Arg177 are the same as those observed in the structures of B. stearothermophilus CCA-adding enzyme, although the interaction of the CTP was not clearly visible at the mid-range resolution (3.5 Å) of the B. stearothermophilus CCA-adding enzyme structure (Li et al, 2002). The mutations of Asp174 to Ala and Arg177 to Ala reduced both the CMP and AMP incorporation rates into the RNA primers ending with C74 and C74C75, respectively (Figure 2D), confirming that the same amino acids are used for the specific recognition of both CTP and ATP by the class II CCA-adding enzymes. In our complex structures, the distances between the Nη2 atom of Arg177 and the Oδ atom of Asp173 in the CTP and ATP complexes are 4.8 Å and 4.5 Å, respectively. On the other hand, in the reported complex structures of the B. stearothermophilus CCA-adding enzyme with CTP and ATP, the corresponding Arg157 hydrogen bonds with Asp153 (Li et al, 2002).
The involvement of the flexible loop in the CCA-adding reaction by the T. maritima CCA-adding enzyme
In the TmCCA form II structure, the loop region between β6 and α5 (amino-acid residues 102–121) is clearly visible (Figures 1B and 2A; Supplementary Figure S2). The loop extends towards the neck domain, and the tip of the loop interacts with α11 in the neck domain. The loop region interacts with both the head and neck domains, thus bridging the two domains (Figure 2A). The Nη1 atom of Arg104 hydrogen bonds with the Oγ atom of Thr87. The Oɛ and Oɛ2 atoms of Glu106 hydrogen bond with the Nη of Arg128 and the OH group of Tyr108. Tyr108 interacts with the side chain of Asp173 through a hydrophobic interaction, and the Nζ atom of Lys113 hydrogen bonds with the Nɛ atom of Arg216.
The ternary structure of the A. aeolicus A-adding enzyme complexed with an RNA primer and an incoming ATP analog (Tomita et al, 2004) was superposed onto the form II TmCCA structure (Figure 2B). In the complex structure of A. aeolicus A-adding enzyme with tRNA and an ATP analog, the 3′-OH group of C75 is in the vicinity of the triphosphate moiety of the ATP analog. This geometry is similar to that of the 3′ end of a primer and an incoming nucleotide, as observed in the structure of DNA polymerase β (Pelletier et al, 1994). In the superposition, the incoming ATP analog in the A. aeolicus A-adding enzyme complex structure is located at the position where the 6-NH2 and N1 of ATP can hydrogen bond with the side chains of Asp174 and Arg177 in the T. maritima CCA-adding enzyme (Supplementary Figure S5). Therefore, the tRNA docking model, created by the superposition of the two structures, allows us to propose a possible mechanism for the recognition of the 3′-terminal region of RNA by the loop region of TmCCA, as described below. In the tRNA docking model, the loop region is in the vicinity of the 3′ end of the primer RNA and the incoming ATP analog (Figure 2C). The effects of mutations of the amino-acid residues in the loop region on the CMP and AMP incorporation were analysed. The point mutations of some of the amino-acid residues in the loop region of TmCCA reduced the AMP incorporation rate, without a significant reduction in the CMP incorporation rate (Figure 2D). Arg104Ala and Glu106Ala mutations reduced the AMP incorporation rate at position 76, without affecting the CMP incorporation rate at position 75. Tyr107Ala, Tyr108Ala and Pro115Ala mutations reduced only the AMP incorporation rate at position 76, to less than 30% of the wild-type TmCCA. Arg104, Glu106, Tyr108 and Pro115 are also conserved in the corresponding loop of the A. aeolicus A-adding enzyme, and the mutation of the amino-acid residues (Arg79, Glu81, Tyr83 and Pro90) of the A. aeolicus A-adding enzyme also reduced the AMP incorporation rate (Tomita et al, 2004). As mentioned (Neuenfeldt et al, 2008), Arg104 and Glu106 are a presumably conserved motif in the CCA-adding enzymes. In the complex structure of the A. aeolicus A-adding enzyme with tRNA and an incoming ATP analog, the Nη1 and Nη2 atoms of Arg79 hydrogen bond with the O2 atom of the C75 base and the 2′-OH of the C75 ribose, respectively (Tomita et al, 2004). It is plausible that the Nη1 of Arg104 of the T. maritima CCA-adding enzyme recognizes the O2 atom of C75 in the same manner as that of the A. aeolicus A-adding enzyme. In the RNA-binding model of TmCCA and tRNA (Figure 2C), Tyr108 is in the vicinity of the 4-NH2 of C75, and Lys113 is also in the vicinity of the phosphate backbone of C74. The OH group of Tyr108 and the Nζ atom of Lys113 would hydrogen bond with the 4-NH2 of C75 and the phosphate backbone of the RNA primer, respectively, in the AMP incorporation stage. As described, in the complex structures of the TmCCA with CTP or ATP, both CTP and ATP are specifically recognized by identical amino-acid residues (Asp174 and Arg177), and Arg177 is located proximally to Asp173 (Figure 1C and D). The side chain of Asp173 interacts with Tyr108. This hydrophobic interaction would affect the conformation of the Arg177 side chain. The mutation of Asp173 to Ala reduces only the AMP incorporation rate, without reducing the CMP incorporation rate (Figure 2D). These structural and biochemical studies as well as the RNA docking model collectively suggest that the loop region should be involved in the terminal AMP incorporation at position 76. In the AMP incorporation stage by TmCCA, the loop region would sense the 3′-terminal C74C75 and grab the 3′ end of the primer, and would fix the enzyme in the form in which the spatial size of the nucleotide-binding site and the conformation of the side chain of Asp174 and Arg177 in the neck domain would only be suitable for the accommodation of ATP. Other mutations, Glu109Ala, Ser110Ala and Asp116Ala, increased the AMP incorporation rate significantly and selectively. These residues might be involved in the maintaining the loop structure by intra-hydrogen bonding. The mutations of these residues might reduce the interaction between the head and neck domains, in which the loop serves as the bridge between the two domains. As a result, the release of the CCA end RNA product from the enzyme might be accelerated after AMP incorporation.
The compatibility of the loop regions between T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme
The T. maritima CCA-adding enzyme is closely related to the A. aeolicus A-adding enzyme, rather than the A. aeolicus CC-adding enzyme, based on the phylogenetic tree (Tomita and Weiner, 2001, 2002). It was recently proposed that the CC-adding enzyme might have arisen from the CCA-adding enzyme by a short deletion of the amino acids in the flexible loop regions, and that the flexible region might be required as a hinge for reorganization of the NTP-binding pocket to switch the nucleotide specificity from CTP to ATP during CCA synthesis (Neuenfeldt et al, 2008). The compatibility of the loop regions in the catalytic head domains between the T. maritima CCA-adding enzyme and the A. aeolicus A-adding enzyme was examined. First, the conditional amber suppression system was used to analyse the CCA-adding activity in vivo (Tomita and Weiner, 2001). The TmCCA variants with an altered amino-acid sequence in the loop region between β6 and α5 were expressed in the Escherichia coli strain CA244cca− (Reuven and Deutscher, 1993) along with the conditional suppressor tRNASup3+ (CGGOH), with the CGG end at positions 74–76. The lacZ amber mutation is only suppressed by the suppressor tRNA when the 3′-G75G76 sequence is removed and the C75A76 is added, and the colonies are coloured blue on plates containing X-gal. The expression of the TmCCA bearing the corresponding loop of the A. aeolicus A-adding enzyme (AaL, amino-acid residues 76–96) exhibited amber suppression activity in vivo to the same extent as that of the wild-type TmCCA (Tm-AaL-loop in Figure 3A and B). On the other hand, the expression of the TmCCA carrying the shorter putative loop of the A. aeolicus CC-adding enzyme (AaS, amino-acid residues 114–124; Tomita and Weiner, 2001, 2002; Neuenfeldt et al, 2008) showed slight suppressor activity, to the same extent as that of the A. aeolicus CC-adding enzyme (Tm-AaS-loop and AaS in Figure 3A and B). The slight suppressor activity observed by the expression of AaS is due to the activities of the endogenous polyA polymerase or related enzymes after the C74C75 addition (Reuven et al, 1997; Tomita and Weiner, 2001). Furthermore, the replacement of the loop region of the A. aeolicus A-adding enzyme with that of either TmCCA or A. aeolicus CC-adding enzyme (AaL-Tm-loop, AaL-AaS-loop in Figure 3A and B) did not result in any suppressor activity. The recombinant chimaeric enzymes were purified, and their enzymatic activities were analysed in vitro. Tm-AaL-loop retained both the C75-adding and A76-adding activities, whereas Tm-AaS-loop retained the C75-adding activity but lost the A76-adding activity (Figure 3C). AaL-Tm-loop retained the A76-adding activity, whereas AaL-AaS-loop lost the A76-adding activity. Neither AaL-Tm-loop nor AaL-AaS-loop had C75-adding activity. These in vitro results are consistent with the in vivo amber suppression results (Figure 3B). tRNASup3+ can only suppress the amber mutation when the enzyme adds C75 and A76. The in vivo (Figure 3B) and in vitro (Figures 2D and 3C) results collectively suggest that the loop regions of TmCCA and A. aeolicus A-adding enzyme, not A. aeolicus CC-adding enzyme, have equivalent functions in the terminal A-adding reaction, and highlight the exclusive involvement of the loop region in only the terminal A addition, and not in the C addition. The results also explain the recent report that the deletion of the flexible loop in the B. subtilus CCA-adding enzyme generates the CC-adding enzyme (Neuenfeldt et al, 2008). As the short loop of the CC-adding enzyme could not recognize the terminal C75 of the primer and could not fix the 3′ end of the primer, the CC-adding enzyme would not be able to add the terminal A.
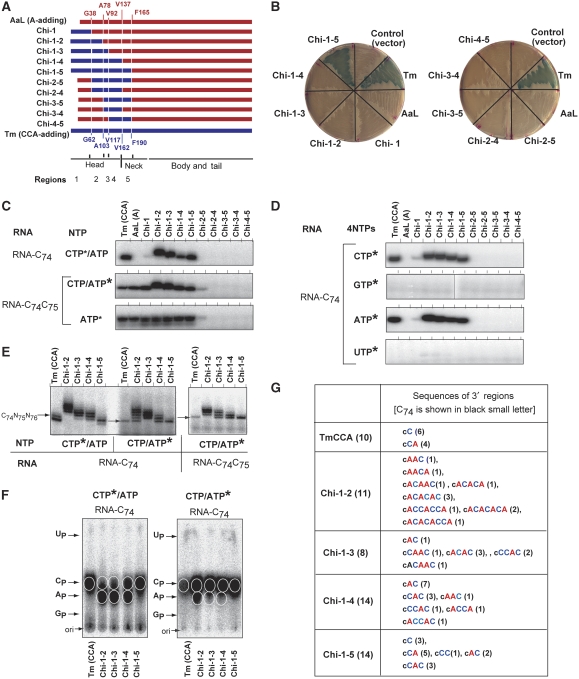
Figure 3.
Compatibility of the loop regions between T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme. (A) Representation of the chimaeric proteins of T. maritima CCA-adding enzyme (Tm; blue), A. aeolicus A-adding enzyme (AaL; red) and A. aeolicus CC-adding enzyme (AaS, green) (upper). The amino-acid sequences of the loop regions of TmCCA (amino-acid residues 101–121, coloured blue), Aquifex aeolicus A-adding enzyme (amino-acid residues 76–96, coloured red) and CC-adding enzyme (amino-acid residues 114–124, coloured green) (lower). The catalytic residues are underlined. (B) In vivo conditional suppression assays by the chimaeric enzymes. E. coli strain CA224cca−, carrying the plasmid encoding an amber suppressor tRNA gene [pSup(CGGOH)], was transformed by the pMW118 plasmid either with no insert (control), the Tm, Tm-AaL-loop and Tm-AaS-loop genes (upper left plate), the AaL, AaL-Tm-loop and AaL-AaS-loop genes (upper right plate), or the AaS, AaS-Tm-loop and AaS-AaL-loop genes (lower plate). The transformants were inoculated on LB plates containing ampicillin, chloramphenicol, tetracycline, IPTG and X-gal. (C) In vitro CCA-adding assays by the recombinant chimaeric proteins in (A). CMP incorporation into mini-C74 in the presence of 32P-CTP and unlabelled ATP (upper panel), and AMP incorporation into mini-C74C75 in the presence of 32P-ATP (lower panel). Asterisks indicate the α-32P-nucleotide used in the assays.
We also replaced the loop region of the A. aeolicus CC-adding enzyme (AaS) with that of either the A. aeolicus A-adding enzyme or T. maritima CCA-adding enzyme (AaS-AaL-loop and AaS-Tm-loop in Figure 3A), and the CCA-adding activities were analysed by the amber suppression system (Figure 3B). The expression of the AaS variants did not significantly enhance the suppressor activities as compared with the expression of wild-type AaS, suggesting that the AaS with the loop of either the CCA-adding enzyme or A-adding enzyme did not act as the CCA-adding enzyme in vivo (Figure 3B). The recombinant AaS variants retained the C75-adding activity, but did not show the A76-adding activity in vitro (Figure 3C), consistent with the results obtained by the suppression assay. It was recently reported that, when the putative loop of the B. subtilis CCA-adding enzyme was transplanted into the corresponding region of the B. halodurans CC-adding enzyme lacking the loop (Bralley et al, 2005), the enzyme exhibited the CCA-adding activity (Neuenfeldt et al, 2008). However, the loop replacement of A. aeolicus CC-adding enzyme with the corresponding loop of either the A. aeolicus A-adding enzyme or T. maritima CCA-adding enzyme did not convert the CC-adding enzyme into the CCA-adding enzyme in vivo and in vitro (Figure 3). Among the slowly evolving eubacteria, such as A. aeolicus and T. maritima, the simple replacement or deletion of the loop would not be sufficient to generate a CCA-adding enzyme from a CC-adding enzyme.
Generation of the CCA-adding enzyme from the A-adding enzyme by region exchanges between T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme
To explore the mechanism for the CCA addition by the CCA-adding enzyme, chimaeras of T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme were constructed, based on a comparison of the amino-acid sequences and the two available crystal structures (Figure 1; Tomita et al, 2004). Several regions of the A. aeolicus A-adding enzyme were replaced with the corresponding regions of the T. maritima CCA-adding enzyme (Figure 4A; Supplementary Figure S6). The head domain was divided into four regions (regions 1–4). The four regions in the head domain and the N-terminal half of the neck domain (region 5) were considered for the construction of the chimaeric enzymes. First, the CCA-adding activity was analysed by using the conditional amber suppression system (Figure 4B). The expression of the chimaeric enzyme, in which the entire catalytic head domain (regions 1–4) of the A. aeolicus A-adding enzyme was replaced with that of the T. maritima CCA-adding enzyme, did not show significant suppressor activity (Figure 4B, Chi-1-4). However, the expression of the chimaeric enzyme, in which the entire catalytic head domain along with part of the neck domain (region 5) was replaced, showed suppression activity to the same extent as the wild-type TmCCA (Figure 4B, Chi-1-5). The expression of other chimaeric enzymes, except for that of Chi-1-5, did not show significant suppression activity. These results suggest that, for the CCA-adding activity, both the entire head domain and part of the neck domain are indispensable in vivo.
Figure 4.
Generation of the CCA-adding enzyme from the A-adding enzyme in vivo and in vitro. (A) Representations of the chimaeric TmCCA–AaL enzymes. The protein regions of Aquifex aeolicus A-adding enzyme (AaL) and T. maritima CCA-adding enzyme (TmCCA) are coloured red and blue, respectively. The amino-acid sequence numbering of AaL and TmCCA is coloured red and blue, respectively. (B) In vivo amber suppression assays by chimaeric TmCCA–AaL enzymes in (A). The transformants were inoculated on LB plates as in Figure 3. (C) In vitro CCA-adding assays using the recombinant chimaeric TmCCA–AaL variants in (A). CMP incorporation into mini-C74 in the presence of 32P-CTP and unlabelled ATP (upper panel). AMP incorporation into mini-C74C75 in the presence of 32P-ATP and unlabelled CTP (middle panel) and in the presence of 32P-ATP (lower panel). (D) CMP or AMP incorporation by chimaeric enzymes into mini-C74 RNA in the presence of all four nucleotides. Neither GMP nor UMP is incorporated by the chimaeric enzymes. (E) Separation of reaction products at one nucleotide resolution. CMP or AMP incorporation into RNA ending with C74 (left) and into RNA ending with C74C75 (right) by TmCCA and chimaeric enzymes (Chi-1-2, Chi-1-3, Chi-1-4 and Chi-1-5). The arrows in the gel margins indicate the product with the expected CCA end. (F) Neighbouring nucleotide analysis of 32P-labelled products in (E) by thin-layer chromatography. Tm(CCA), Chi-1-2, Chi-1-3, Chi-1-4 and Chi-1-5 indicate RNase T2 hydrolysates of 32P-labelled products in the gels in the left part of (E) (left, CTP-labelled product) and middle part of (E) (right, ATP-labelled product). (G) Nucleotide sequences of 3′-terminal regions of reaction products generated by chimaeric enzymes. The total number of the clones analysed (left columns) and the number of the clones with the respective sequences (right columns) are shown in parentheses. Asterisks in (C), (D), (E) and (F) indicate the α-32P-nucleotide used in the assays.
These chimaeric recombinant enzymes were tested for their CCA-adding activity in vitro (Figure 4C). All of the chimaeric enzymes, except for Chi-2-5, were expressed well in E. coli. The expression level of Chi-2-5 was approximately 10 times lower than those of the others (data not shown). The chimaeric enzymes, Chi-1, Chi-1-2, Chi-1-3, Chi-1-4 and Chi-1-5, incorporated AMP into RNA ending with C74C75 in the presence of only ATP (lower panel in Figure 4C). The incorporation of AMP by Chi-2-5 was drastically lower than that of the wild-type TmCCA, and the other chimaeric enzymes lacked A76-adding activity. Unexpectedly, Chi-1-2, Chi-1-3 and Chi-1-4, which lacked in vivo sufficient suppressor activities (Figure 4B), could incorporate CMP into the RNA ending with C74 in the presence of both CTP and ATP (upper panel in Figure 4C). However, the lengths of 32P-labelled products generated by Chi-1-2, Chi-1-3, and Chi-1-4 varied and the products were longer than 32P-labelled products by TmCCA and Chi-1-5. The longer 32P-labelled products generated by Chi-1-2, Chi-1-3 and Chi-1-4 were also observed in the assays using RNA ending with C74C75 in the presence of both CTP and ATP (middle panel in Figure 4C). It should be noted that the nucleotide additions by these chimaeric enzymes were specific for CMP and AMP, and neither UMP nor GMP was incorporated in the presence of all four nucleotides (Figure 4D). The 32P-labelled products generated by TmCCA, Chi-1-2, 1-3, 1-4, and 1-5 were separated at one nucleotide resolution (Figure 4E). The lengths of the respective 32P-labelled products were not homogeneous. The Chi-1-2, Chi-1-3 and Chi-1-4 enzymes added more than one to five nucleotides beyond position 76, depending on the chimaeric enzyme. On the other hand, the length of the reaction product generated by Chi-1-5 was the same as that of the reaction product from TmCCA.
The E. coli class II CCA-adding enzyme exhibits polyC polymerase activity in the absence of ATP (Hou, 2000; Tomari et al, 2000), and the polyC polymerization is inhibited in the presence of an ATP concentration as low as 25 μM (Hou, 2000). However, for the chimaeric enzymes, Chi-1-2, 1-3 and 1-4, the nucleotide incorporation beyond position 76 was not inhibited in the presence of a higher concentration of ATP (1 mM) (data not shown).The neighbouring nucleotide of the 32P-labelled products generated by the chimaeric enzymes was analysed (Figure 4F). Chi-1-2 and Chi-1-3 predominantly incorporated CMP to the 3′-end A of RNA and less efficiently to the 3′-end C of RNA, and Chi-1-4 incorporated CMP to both the 3′ ends of A and C of RNAs (Figure 4F, left panel). Chi-1-2, Chi-1-3 and Chi-1-4 can incorporate AMP onto the 3′-end C of RNA, and less efficiently to the 3′-end A of RNA (Figure 4F, right panel). On the other hand, Chi-1-5 predominantly incorporated CMP onto the 3′-end C of RNA and incorporated AMP onto the 3′ end of C of RNAs, similar to wild-type TmCCA. These analyses suggested that the reaction products generated by Chi-1-2, Chi-1-3, and Chi-1-4 have heterogeneous sequences, and the sequences would not be the stretches of C residues ending with a terminal A. The reaction products generated by these chimaeric enzymes were amplified by ligation-mediated RT–PCR, cloned and sequenced. The 3′ sequences of the reaction products of Chi-1-2, Chi-1-3 and Chi-1-4 were heterogeneous (Figure 4G), and no clones with C74C75A76 were isolated. These in vitro results well explain the absence of sufficient suppression activities by the expression of these chimaeric enzymes in vivo (Figure 4B). On the other hand, among the sequences of the reaction products generated by Chi-1-5, 5 of the 14 clones analysed had C74C75A76, and the sequences of the remaining clones were C74C75 (three clones), C74C75C76 (one clone), C74A75C76 (two clones) and C74C75A76C77 (three clones). Although Chi-1-5 synthesized sequences other than CCA in vitro, the fraction of the tRNA with the CCA end would be sufficient to function in vivo, and thus Chi-1-5 can function as the CCA-adding enzyme in vivo. The in vivo and in vitro results collectively suggest that the entire head domain and the part of neck domain are indispensable for the correct CCA addition to tRNAs. The possible mechanisms for the variation of the length and nucleotide compositions of the reaction products by the chimaeric enzymes are described below.
It is noteworthy that the other chimaeric enzymes (Chi-2-4, Chi-3-5, Chi-3-4 and Chi-4-5) did not exhibit significant CMP or AMP incorporation into RNAs in vitro (Figure 4C). Although the inability of Chi-2-5 to incorporate CMP (and AMP) in vivo and in vitro might be a consequence of the instability of the chimaeric enzyme, these results imply that the entire head domain and the neck domain are necessary for the CCA-adding activity, and that regions 1 and 2 might be essential for the CMP incorporation activity by the CCA-adding enzyme. These results also suggest that the protein elements in regions 1 and 2 required for the incorporation of CMP and AMP overlap. Although the loop region in the head domain was found to be required for the terminal A addition (Figures 2 and 3), the present results (Figure 4) suggest that other regions or amino-acid residues are also involved in the A-adding reaction, as described below.
The involvement of the neck domain in CCA addition by the CCA-adding enzyme
The results obtained from biochemical and genetic analyses using the chimaeric enzymes of T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme (Figure 4) suggest that the correct CCA addition to the 3′ end of RNA is defined by the entire head domain and the neck domain, in a collaborative manner. The catalytic head domain of the CCA-adding enzyme alone does not define the correct CCA synthesis. The nucleobase-interacting amino acids, Asp174 and Arg177, reside in the neck domain (Figure 1), and these two residues are conserved among the class II CCA-, A- and CC-adding enzymes (Tomita and Weiner, 2002; Martin and Keller, 2004). To identify the amino-acid residues in the neck domain (region 5 in Figure 4A) required for the regulation of the number of nucleotide incorporations and the nucleotide specificity by the CCA-adding enzyme, the amino-acid residues in region 5 of the Chi-1-4 (Figure 5A) were partially randomized. In the randomization, only the different amino acids in the regions 5 between the T. maritima CCA-adding enzyme and the A. aeolicus A-adding enzyme were degenerated to the amino-acid sequences of either the CCA-adding or A-adding enzyme. The library of the Chi-1-4 gene with the randomized region 5 was used for the transformation of CA244cca-carrying pSup3+(CGGOH). The plasmids were purified from the blue-coloured colonies, and the sequences of region 5 were analysed (Supplementary Table 2). Most of the Chi-1-4 variants with the suppressor activity selected from the library had either Glu185Gln186 or Glu185Glu186 (residue numbers are those of TmCCA) in region 5 of the neck domain (54 out of 62 clones). In addition to these variants, other variants with Glu185Arg186 (four clones), Ala185Glu186 (two clones), Ala185Gln186 (one clone) and Ala185Arg186 (one clone) were isolated. No variants with the original AlaGly (amino-acid residues 160 and 161 in the A. aeolicus A-adding enzyme) sequence in region 5 of Chi-1-4 were isolated. The expression of the Chi-1-4 enzyme with Glu185Gln186 (residue numbers are those of TmCCA) in region 5 indeed exhibited suppressor activity in vivo, to the same extent as Chi-1-5 (Figure 5B, left, Chi-1-4 EQ). The recombinant Chi-1-4 with the Glu185Gln186 sequence in region 5 (Chi-1-4 EQ) added a single CMP at position 75 of the RNA primer ending with C74, and added a single AMP at position 76 in vitro, consistent with the in vivo suppression results (Figure 5B, right).
Figure 5.
Hydrogen bonds in the neck domain involved in the correct CCA addition. (A) Detailed representation of chimaeras Chi-1-4 and Chi-1-5 in Figure 4A. The protein regions of Aquifex aeolicus A-adding enzyme (AaL) and T. maritima CCA-adding enzyme (TmCCA) are coloured red and blue, respectively. The amino-acid sequences of region-5 of AaL and TmCCA are shown. The different amino-acid residues are underlined. (B) In vivo amber suppression assays by Chi-1-4 bearing the Glu185Gln186 sequence in region 5 (left plate). The transformants were inoculated on LB plates, as in Figure 3. In vitro CCA-adding assays by the recombinant protein Chi-1-4 EQ (right). The CMP incorporation into mini-C74 (upper panel) and the AMP incorporation into mini-C74C75 (lower panel). Asterisks indicate the α-32P-nucleotide used in the assays. Arrows at the gel margin indicate the RNA products with the CCA end. (C) Structures of the backside of the neck domains of TmCCA (left) and AaL (middle). The hydrogen bonds between α9 and α13 of TmCCA are depicted by dashed lines. A model structure of AaL with the mutations of Ala160Gly161 to Glu160Gln161 (AaL-EQ model: right).
In the TmCCA structure, the Oɛ1 and Oɛ2 atoms of Glu185 hydrogen bond with the Nη1 of Arg236 in α13 of the neck domain and the Nɛ atom of Gln186, respectively. The Oɛ atom of Gln186 hydrogen bonds with the Nζ atom of Lys232 in α13 of the neck domain (Figure 5C). On the other hand, in the A. aeolicus A-adding enzyme (AaL) structure, the corresponding Ala160 and Gly161 do not interact with the amino-acid residues Glu207 and Leu211, which are located at the positions corresponding to Lys232 and Arg236 in α13 of TmCCA. The Ala160Gly161 of AaL was manually mutated to Glu160Gln161 and the putative hydrogen-bond interactions between the helices in the neck domain could be modelled (Figure 5C). In the model structure of mutated AaL (AaL-EQ in Figure 5C), the Nɛ atom of Gln161 could hydrogen bond with the Oɛ1 atom of Glu210 and the Oɛ1 and Oɛ2 atoms of Glu161 could hydrogen bond with the Nζ atom of Lys214 and the OH-group of Tyr215, respectively (residue numbering is that of AaL). Therefore, the hydrogen-bond interaction between α9 and α13 in Chi-1-4 with Glu160Gln161 (residue numbering is that of AaL) would be equivalent to that between α9 and α13 in TmCCA for the CCA synthesis. Interestingly, the Glu185Gln186 sequence found in TmCCA is not conserved among the class II CCA-adding enzymes. The other hydrogen-bond interaction(s) between the helices in the neck domain in the other CCA-adding enzymes might have equivalent roles to those observed in TmCCA. In fact, other types of clones with suppressor activity were isolated from the region 5 randomized library (Supplementary Table S2). The suppression activities of these clones varied, as judged from the intensity of the blue colour of the colonies (Supplementary Figure S7). The expression of Chi-1-4 with Glu185Arg186 or with Ala185Arg186 exhibited sufficient suppressor activity. On the other hand, the suppression by the expression of Chi-1-4 with Ala185Glu186 was slightly weaker, and the suppression by the expression of Chi-1-4 with Ala185Gln186 was apparently weaker than the others. The mutation modelling of these residues in the helices in the neck domain showed that other hydrogen bonds between the two helices in the neck could be formed, and that the number of hydrogen bonds between the helices varied (Supplementary Figure S8). The number and the strength of the hydrogen bonds between helices and the location of the hydrogen bonds might define the efficiency of the correct CCA-synthesis in vivo.
During the CCA-adding reaction, the TΨC loop of the tRNA primer is recognized by the tail domain, which acts as an anchor to prevent the tRNA primer from dislodging from the enzyme surface (Shi et al, 1998; Yue et al, 1998). As the CMP is incorporated at the 3′ end of the tRNA primer by an induced-fit mechanism, as described below, the hydrogen bond between α9 and α13 would function as a springy hinge that regulates the processibility and specificity of nucleotide incorporation. When the 3′ end of C74C75 fills the catalytic pocket, the hydrogen-bond network between α9 and α13 would no longer be able to function as the springy hinge, thus preventing the additional CMP incorporation and defining the number of CMP incorporations to the 3′ end of the tRNA. After the CC addition, the loop in the head domain would grab the 3′ end of the RNA, and the terminal A would be added to complete the CCA sequence, and thus both the head and neck domains collaboratively define the correct CCA addition.
The variation in the number of nucleotide incorporations and the specificity by Chi-1-2, Chi-1-3 and Chi-1-4 (Figure 4C) could be explained by the absence of the hydrogen bonds, the springy hinge, between the helices in the neck domains, and by the non-uniformity of the head domain structure in the context of the enzyme. The absence of the springy hinge in the neck domain, as in the Chi-1-4 protein, would prevent the proper communication between the catalytic head domain and the base-interacting neck domain for the dynamic polymerization reaction. As a result, the number and the specificity of nucleotide incorporation would not be defined properly. Moreover, in the cases of the reactions by Chi-1-2 and Chi-1-3, the dynamic, non-uniform structure of the head domain in the context of the chimaeric enzyme would also influence unusual nucleotide incorporation. This dynamic, non-uniform structure might increase the enzymatic processibility—the movement of the head domain relative to the neck domain—which in turn would reduce the specificity of the enzymes.
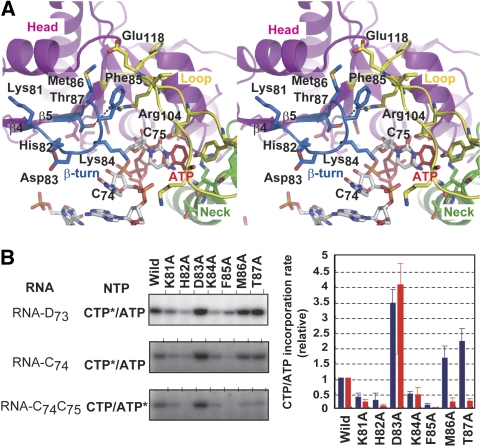
The involvement of the β-turn in the head domain in the CCA-adding reaction by CCA-adding enzyme
As described above, the chimaeric enzymes, Chi-1-2, Chi-1-3 and Chi-1-4, exhibited CMP incorporation activity in vitro (Figure 4C), although these chimaeric enzymes could not synthesize the correct CCA sequence (Figure 4F and G). The other chimaeric enzymes, Chi-2-4, Chi-2-5, Chi-3-5, Chi-3-4 and Chi-4-5, lacked the CMP incorporation activity. Therefore, it is plausible that at least regions 1 and 2 in the head domain, along with the neck domain, are required, but not sufficient, for the CMP incorporation activity. The β-turn between β4 and β5 (amino-acid residues 81–87) in region 2 is in the vicinity of the 3′ end of the tRNA primer, as shown by the tRNA docking model structure, using the structure of the A. aeolicus A-adding enzyme complexed with tRNA (Figure 6A). Biochemical and structural studies of class I Ar. fulgidus CCA-adding enzyme suggested that the β-turn in the catalytic head domain direct the consecutive three nucleotide additions (Xiong and Steitz, 2004; Cho et al, 2005, 2006; Tomita et al, 2006; Martin and Keller, 2007). Therefore, the effects of mutations of the amino-acid residues in the β-turn were analysed (Figure 6B). The Met86Ala and Thr87Ala mutations reduced only the AMP incorporation rate, without reducing the CMP incorporation rates into RNAs ending with discriminator nucleotide (D73) and C74. The side chain of Met86 interacts with Phe85 through a hydrophobic interaction, and both Met86 and Phe85 are located proximally to Glu118 in the loop region. The Oγ atom of Thr87 hydrogen bonds with the Nη2 atom of Arg104 in the loop region. As the loop region of the catalytic domain is involved in the terminal A76 addition (Figures 2 and 3), the interactions of Met86 and Thr87 with the loop might indirectly contribute to the recognition of the 3′-terminal C75, only at the terminal A-adding stage. On the other hand, the Lys81Ala, His82Ala, Lys84Ala and Phe85Ala mutations reduced all three nucleotide additions (Figure 6B). According to the spatial positions of these amino-acid residues (Figure 6A), these residues in the β-turn might recognize the phosphate backbone of the 3′ end of RNA primers at the CMP incorporation stages, and also contribute to the proper positioning of the C74C75 at the AMP incorporation stage along with the C75 recognition by the loop region. As described above, the CTP and ATP bases are recognized by identical amino-acid residues (Asp174 and Arg177) in the neck domain (Figure 1C). During the C74C75- and A76-adding reactions, the tRNA primer does not translocate on the enzyme surface (Shi et al, 1998). To use a single nucleotide pocket and a single catalytic site for the CCA addition, consecutive dynamic conformational changes of the enzyme and the 3′ end of RNA primer should be required. During the CCA-adding reaction, the consecutive conformation changes of the β-turn in the catalytic domain might be accompanied by the CMP and AMP incorporation reactions. The mutation of Asp83 to Ala reduced neither the CMP nor AMP incorporation rate, instead it increased the incorporation rates significantly (Figure 6B). The Asp83 in the β-turn might hydrogen bond with the side chain of Lys113 in the loop region, and the side chain of Asp83 might stack with the bases at the 3′ end of the primer in the primer–protein binary state, before the nucleotide accommodation state in the catalytic pocket. In the class I enzyme, the β-turn is stacked with the base of the nucleoside at the 3′ end of the primer in the primer–enzyme binary complex state, and the enzyme adopts the catalytically inactive open conformation. On CTP binding, the head domain relocates towards the neck domain, and the catalytic cleft of the enzyme shifts from an inactive open to an active closed form. The transition from the open to closed form of the enzyme is accompanied by the conformational change of the β-turn, which allows the 3′-end nucleotide to flip, where the base of C74 stacks with that of the incoming CTP (Tomita et al, 2006). This scheme might also be applicable to the class II CCA-adding enzymes. Without the stacking interaction between the side chain of Asp83 and the bases of the RNA primer, the conformation of the 3′ end of the RNA primer might be able to readily transit from an open inactive form to a closed active one for the nucleotide incorporation to proceed, as in the class I CCA-adding enzyme (Tomita et al, 2006).
Figure 6.
Involvement of the β-turn in the head domain in the CCA addition by T. maritima CCA-adding enzyme. (A) Stereo views of the superposition of the form II TmCCA structure on the structure of A. aeolicus A-adding enzyme complexes with the RNA primer and ATP, as in Figure 2C. The protein structure of A. aeolicus A-adding enzyme is not shown. Only the catalytic domain of TmCCA is shown. The β-turn (amino-acid residues 81–87) of TmCCA is coloured blue. (B) In vitro relative CMP and AMP incorporation rates by mutant TmCCA variants. The CMP and AMP incorporation into mini-D73, mini-C74 and mini-C74C75 by the wild-type TmCCA (left gel panels). The blue and red bars in the graph indicate the relative CMP and AMP incorporation rates into mini-C74 and mini-C74C75, respectively. The bars in the graph are the standard deviations of more than three independent experiments. As the incorporation of CMP into mini-D73 represents the CMP incorporation at positions 74 and 75, the quantification of the CMP incorporation rate was performed only for the CMP incorporation into mini-C74.
A possible mechanism for the CCA-adding reaction by the class II CCA-adding enzyme
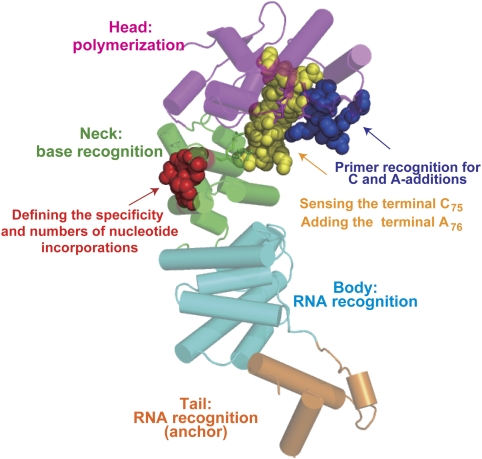
Extensive biochemical and structural studies on the class I archaeal CCA-adding enzymes showed the molecular basis for the CCA-adding reaction by the class I CCA-adding enzymes (Shi et al, 1998; Yue et al, 1998; Okabe et al, 2003; Xiong et al, 2003; Xiong and Steitz, 2004; Cho et al, 2005; Tomita et al, 2006). However, the mechanism for the CCA-adding reaction by the class II eubacterial and eukaryotic CCA-adding enzymes remains obscure. A comparison of the amino-acid sequence and the structure of the T. maritima CCA-adding enzyme with those of the closely related A. aeolicus A-adding enzyme (Tomita and Weiner, 2001, 2002; Tomita et al, 2004) allowed us to generate the CCA-adding enzyme from the A-adding enzyme in vivo and in vitro. By using biochemical and genetic approaches based on the structures, as well as an RNA docking model, we identified a flexible loop in the catalytic domain that defines the terminal A addition (Figures 2, 3 and 7). We discovered hydrogen bonds between the two α-helices in the base-interacting neck that defines the number and the specificity of nucleotide incorporations (Figures 4, 5 and 7). We also identified a β-turn in the catalytic domain, which would be involved in the recognition of the 3′ end of the RNA during the CCA-adding reaction (Figures 6 and 7).
Figure 7.
Functional elements for the CCA-adding reaction. The overall structure of form II of TmCCA. The head, neck, body and tail domains of TmCCA are coloured magenta, green, cyan and orange, respectively. The loop in the head domain (presented as yellow spheres) is involved in the recognition of the terminal C75 of the primer and in A76 incorporation. The hydrogen bond between the two helices in the neck domain defines the specificity and the number of nucleotide incorporations into the RNA. Amino-acid residues forming hydrogen bonds are depicted as red spheres. The β-turn in the catalytic domain (represented as blue spheres) is involved in the recognition of 3′ end of the primer RNA for CMP and AMP incorporations.
These results have allowed us to propose a possible mechanism for the CCA-adding reaction by the class II CCA-adding enzyme. On binding to tRNA primers, the enzyme holds the top-half region of the tRNA (the acceptor and TΨC helix of tRNA). The tail domain of the enzyme interacts with the TΨC loop of the tRNA (Tomita et al, 2004), thus preventing the tRNA from dislodging from the surface of the enzyme. As a result, the 3′ end of the primer is located in the vicinity of the active pocket of the enzyme. The enzyme starts to incorporate CMP onto the 3′ end of the RNA primer. Although the mechanism for the CMP incorporation at positions 74 and 75 is still obscure, at least, the entire catalytic domain itself defines the C-adding reaction along with the neck domain (Figure 3). CTP might be incorporated by an induced-fit mechanism similar to that observed in the class I CCA-adding enzyme (Tomita et al, 2006), although the specificity for the CTP is determined by the interaction between the side chains of Asp174 and Arg177 and the CTP base (Figure 1C). Only when the correct nucleotide, CTP, binds to the catalytic pocket, the head domain of the enzyme might relocate towards the neck domain, thus creating the catalytically active form for the nucleotide incorporation. The consecutive conformation change of the β-turn in the catalytic domain would monitor the 3′ end of RNA synthesis for the correct positioning of the 3′ end of the RNA at the catalytic active site during the polymerization reaction. The hydrogen bonds between the two helices in the neck domain, which act as a springy hinge, could prevent the incorporation of incorrect and/or excess nucleotides. After two CMP incorporations at positions 74 and 75, the loop in the catalytic domain would sense the terminal C75, and fix the 3′ end of the RNA. Finally, the base-interacting residues, Asp174 and Arg177, are suitable only for ATP accommodation, and the CCA is synthesized. The template for the AMP incorporation by the class II enzyme is not the protein itself. As described above, the selection of ATP would be achieved through the recognition of the growing 3′ end of the RNA primer by the loop and the β-turn in the catalytic domain. Moreover, the hydrogen bonds between the two helices in the neck domain regulate the specificity and the number of the nucleotide incorporations. In these respects, as discussed earlier (Shi et al, 1998; Schimmel and Yang, 2004; Yue et al, 1998; Tomita et al, 2004; Weiner, 2004; Lizano et al, 2008; Toh et al, 2008), the template is actually the dynamic RNA–protein complex, as in the class I CCA-adding enzyme, although the bases of the CTP and ATP hydrogen bond with specific amino acids. The assessment of the class II CCA-adding model presented here awaits the determination of the sequential structures of the CCA-adding reaction.
Materials and methods
Preparation of T. maritima CCA-adding enzyme, its variants and RNAs
The C-terminal half of T. maritima CCA-adding enzyme was over-expressed in E. coli (Tomita and Weiner, 2001). The hexahistidine-tagged C-terminal half of the TmCCA was first purified on a Ni-chelating column and then further purified on HiTrap-Q and HiTrap-heparin columns. Finally, the TmCCA was purified by chromatography on a Superdex 200 column, in a buffer containing 50 mM Tris–Cl, pH 7.0, 200 mM KCl and 10 mM β-mercaptoethanol, and was concentrated (final concentration was 10 mg/ml). All of the columns used for the enzyme purification were purchased from GE Healthcare, Japan. The protein was stored at −70°C until use. The mutations were introduced by site-directed mutagenesis. The chimaeric genes of TmCCA and A. aeolicus A-adding enzyme (AaL) were constructed by PCR and cloned into the pET22(+) plasmid. The mutant TmCCA variants, the A. aeolicus A-adding enzyme (AaL), the CC-adding enzyme (AaS) and the chimaeric enzymes were purified on a Ni-chelating column followed by Nap-5 chromatography. tRNA mini-helices, derived from T. maritima tRNAPhe, ending in C74 (mini-C74) and C74C75 (mini-C74C75), were prepared as described (Tomita et al, 2006).
Crystallization and data collection
For the crystallization of T. maritima CCA-adding enzyme (TmCCA), 1 μl of protein solution (10 mg/ml) was mixed with 1 μl of reservoir solution, containing 50 mM Tris–Cl, pH 8.4, and 20% (v/v) ethylene glycol, and the drop solution was equilibrated against the reservoir solution at 20°C by the hanging drop vapour diffusion method (form I crystal). TmCCA was also crystallized under different conditions, in which the protein solution was mixed with a reservoir solution containing 50 mM HEPES, pH 8.1, 5 mM MgCl2, 0.2 M KCl, and 10% (v/v) polyethylene glycol 4000, and the drop solution was equilibrated against the reservoir solution. The crystals were cryo-protected with 20% (v/v) ethylene glycol and were flash-cooled in a 100-K nitrogen stream, and the data were collected at the beam-lines NW-12A, BL-5A and BL-17A of KEK (Tsukuba, Japan). All of the data were processed using the program HKL2000 (Otwinowski and Minor, 1997).
Structure determination of T. maritima CCA-adding enzyme
For structure determination, three data sets for the multiple wavelength anomalous dispersion (MAD) method with the selenomethionine derivative were collected for crystal form I. The crystal form I belongs to the space group C2, with a=190.6 Å, b=62.7 Å, c=152.4 Å, α=90.00°, β=103.96°, γ=90.00°, and contains two molecules in the asymmetric unit. The crystal form II belongs to the space group C2, with a=173.3 Å, b=50.5 Å, c=69.9 Å, α=90.00°, β=91.0°, γ=90.00°, and contains one molecule in the asymmetric unit. The data set at the peak wavelength up to 2.85 Å resolution was used for locating the selenium atoms with the program SnB (Weeks and Miller, 1999), and 18 peaks were picked out of 20 atoms in the asymmetric unit. An initial phase set was calculated by the MAD method with the program SHARP (de La Fortelle and Bricogne, 1997), and density modification with solvent flattening was performed (Cowtan, 1994). A model with two TmCCA molecules in the asymmetric unit was built using the program O (Jones et al, 1991), and was refined by the program CNS (Brunger et al, 1998) and the program PHENIX (Adams et al, 2002). The crystal form II structure was determined by AMoRe (Navaza, 1994), using the form I structure as a search model. For the preparation of the complex with CTP or ATP, a native form I crystal was soaked in the reservoir solution containing 2 mM CTP or ATP at 20°C for 2 h. The refinement statistics are shown in Supplementary Table 1.
In vitro CMP and AMP incorporation assays
In vitro CCA adding assays were performed as follows. For AMP incorporation into mini-C74C75, reaction mixtures containing 50 mM glycine-KOH, pH 8.5, 150 mM KCl, 10 mM MgCl2, 10 mM β-mercaptoethanol, 100 μM ATP, 100 nM α-32P ATP (3000Ci/mmole, GE Healthcare), 2 μM mini-helix, and 50 nM TmCCA were incubated at 45°C for 7 min. The assay procedures for CMP incorporation into mini-C74 were the same as those described above, except that 100 μM CTP, 100 μM ATP, and 100 nM α-32P CTP (3000Ci/mmol, GE Healthcare) were used instead of the 100 μM ATP and 100 nM α-32P ATP. The reaction was stopped by adding an equal volume of stop buffer (9 M urea, 0.02% BPB, 0.02% XC). Under these conditions, the reaction proceeds in a linear range. The products were separated by 12% (w/v) polyacrylamide gel electrophoresis under denaturing conditions, and the intensity of the 32P-labelled RNAs was quantified by a BAS-5000 imager (Fuji Film, Japan). For the assays in Figures 3, 4 and 5, 200 nM of enzyme (for TmCCA, AaL and chimaeric enzymes) was used and the incubation time was extended to 1 h, to detect the residual activity. To analyse the nucleotide specificities of the chimaeric enzymes in Figure 4, the assays were performed in the presence of 100 μM of all four nucleotides and 100 nM of each α-32P nucleotide.
In vivo amber suppression assays
An amber suppressor tRNASup3+ gene, with the CGG sequence at positions 74–76 between the llp promoter and rrn terminator, was synthesized and cloned into the PstI and HindIII sites of the pBR322 plasmid, to create pSup3+(CGGOH). The genes encoding T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme within the pET22 (+) plasmids (Tomita and Weiner, 2001) were digested by XbaI and SalI. The XbaI/SalI-digested DNA fragments were each cloned into the same sites of the pMW118 plasmid (Nippon Gene, Japan). The chimaeric genes of T. maritima CCA-adding enzyme and A. aeolicus A-adding enzyme were generated by PCR and cloned into the pMW118 plasmid in the same manner. E. coli strain CA244cca− (Reuven and Deutscher, 1993) was transformed by the enzyme-expressing plasmids along with pSup3+(CGGOH) and was cultured on an LB plate containing 50 μg/ml ampicillin, 34 μg/ml chloramphenicol, 10 μg/ml tetracycline, 1 mM IPTG (isopropyl-β-D(−)-thiogalactopyranoside) and 20 μg/ml X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) at 37°C.
Sequence analysis of reaction products
The reaction product RNAs generated by the chimaeric enzymes (Chi-1-2, Chi-1-3, Chi-1-4, and Chi-1-5) were extracted by phenol–chloroform and ethanol precipitated. The product RNAs were further purified by chromatography on a Sepharose-Q column (GE Healthcare) to remove the nucleotides. A linker oligonucleotide, pUATACTCATGGTCATAGCTGTT (Sigma Aldrich, Japan), was ligated to the product RNAs in a buffer containing 50 mM Tris–Cl, pH7.4, 15 mM MgCl2, 3.5 mM DTT, 15 μg/ml BSA, 5% (v/v) PEG6000, 0.5 mM ATP, 2 μM linker oligonucleotide and 1000 units/ml T4 RNA ligase (NEB Biolabs, Japan) at 16°C for 12 h. The RNAs were phenol extracted and ethanol precipitated. The cDNAs were synthesized by Primer Script Reverse Transcriptase (Takara, Japan) according to the manufacturer's instructions, using RT-primer (5′-AACAGGTATGACCATGAGT-3′), and were amplified by PCR using RT-primer and For-primer (5′-GGCCCGGGGCGGTTCGATTC-3′). The PCR products were ligated to the pCR 2.1-TOPO vector (Invitrogen, Japan) and the sequences were analysed. About 30% of the clones sequenced were found to have the correct PCR products. The remaining clones had no PCR-products inserted or PCR products derived from truncated reaction products with a linker ligated to the 3′ end. The low percentage of the clones obtained partly resulted from the low efficiency of the linker ligation to the reaction products.
Neighbouring nucleotide analysis by thin-layer chromatography
The 32P-labelled reaction product RNAs were extracted with phenol-chloroform and ethanol-precipitated. The product RNAs were further purified by chromatography on a Sepharose-Q column (GE Healthcare) to remove the nucleotides and were ethanol-precipitated. The 32P-labelled products were digested in a 10 μl solution containing 50 mM Tris-Cl, pH 7.0, and 1 unit of RNase T2 (Invitrogen), at 37°C for 12 h. The hydrolysed products were separated by thin-layer chromatography (Kodak) using a developing solution [2-propanol/HCl/water (70:15:15 v/v/v)] (Kuchino et al, 1987), and the 32P-labelled nucleotides were visualized by a BAS-5000 imager (Fuji Film).
Construction of the region 5 library
The amino-acid residues in region 5 of Chi-4 (Figure 5A) on the pMW118 plasmid were partially randomized by PCR, using a pair of degenerate DNA primers (TmLib5 and AaLib5). The DNA nucleotide sequences of the primers, TmLib5 and AaLib5, are 5′-AATCCTTRYAGGGTCSTCTAYAAAACTATMAGKGTGAAGAACCCTTATTAC-3′ and 5′-TTACGTGCCMTAAGATTTGMGSRAAGAYTCRATTTTAAACTCTCAAGA-3′, respectively (R=A or G, Y=C or T, S=C or G, M=A or C and K=G or T). The expected amino-acid sequence of region 5, generated by PCR using the primer pair, would be V-I-R-V-L-H-(T/P)-(L/V)-S-F-(V/I)-(D/E)-D-P-(T/V/I/A)-R-I-L-R-A-(I/L)-R-F-(E/A)-(Q/E/R/G)-R-(F/L)-(D/N)-F (residues 162–190, residue numbers are those of TmCCA). The PCR product was gel purified and self-ligated by T4 DNA ligase (Toyobo, Japan). E. coli JM109 was transformed by the ligation product. Approximately 4000 colonies were combined and inoculated into LB medium, and the plasmids were isolated. The plasmid library was used for the transformation of E. coli strain CA244cca−-carrying pSup3+(CGGOH). About 1000 colonies were obtained, and approximately 40% were coloured blue on the LB plates containing X-gal.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank Murray Deutscher, of the University of Miami School of Medicine, for E. coli strains and Azusa Hamada of AIST for technical assistance. We thank the beam-line staffs of BL-5A, NW12A and BL-17A (KEK, Tsukuba) for technical assistance during data collection. This work was supported by grants from the PRESTO program of JST and Toray Science and Technology Foundation to KT. The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 3H37, 3H38, 3H39, and 3H3A).
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new soft warefor automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954 [DOI] [PubMed] [Google Scholar]
- Augustin MA, Reichert AS, Betat H, Huber R, Mörl M, Steegborn C (2003) Crystal structure of the human CCA-adding enzyme: insights into template-independent polymerization. J Mol Biol 328: 985–994 [DOI] [PubMed] [Google Scholar]
- Bralley P, Chang SA, Jones GH (2005) A phylogeny of bacterial RNA nucleotidyltransferases: Bacillus halodurans contains two tRNA nucleotidyltransferases. J Bacteriol 187: 5927–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D 54: 905–921 [DOI] [PubMed] [Google Scholar]
- Cho HD, Verlinde CL, Weiner AM (2005) Archaeal CCA-adding enzymes: central role of a highly conserved beta-turn motif in RNA polymerization without translocation. J Biol Chem 280: 9555–9566 [DOI] [PubMed] [Google Scholar]
- Cho HD, Chen Y, Varani G, Weiner AM (2006) A model for C74 addition by CCA-adding enzymes: C74 addition, like C75 and A76 addition, does not involve tRNA translocation. J Biol Chem 281: 9801–9811 [DOI] [PubMed] [Google Scholar]
- Cowtan K (1994) An automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EACBM. Newsl Protein Crystallogr 31: 34–38 [Google Scholar]
- de La Fortelle E, Bricogne G (1997) Maximum-likelihood heavy-atom parameter refinement for the multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol 276: 472–494 [DOI] [PubMed] [Google Scholar]
- Deutscher MP (1990) Transfer RNA nucleotidyltransferase. Methods Enzymol 181: 434–439 [DOI] [PubMed] [Google Scholar]
- Green R, Noller HF (1997) Ribosomes and translation. Annu Rev Biochem 66: 679–716 [DOI] [PubMed] [Google Scholar]
- Holm L, Sander C (1995) DNA polymerase beta belongs to an ancient nucleotidyltransferase superfamily. Trends Biochem Sci 20: 345–347 [DOI] [PubMed] [Google Scholar]
- Hou YM (2000) Unusual synthesis by the Escherichia coli CCA-adding enzyme. RNA 6: 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou YY, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Kim DF, Green R (1999) Base-pairing between 23S rRNA and tRNA in the ribosomal A site. Mol Cell 4: 859–864 [DOI] [PubMed] [Google Scholar]
- Kuchino Y, Hanyu N, Nishimura S (1987) Analysis of modified nucleosides and nucleotide sequence of tRNA. Methods Enzymol 155: 379–396 [DOI] [PubMed] [Google Scholar]
- Li F, Xiong Y, Wang J, Cho HD, Tomita K, Weiner AM, Steitz TA (2002) Crystal structures of the Bacillus stearothermophilus CCA-adding enzyme and its complexes with ATP or CTP. Cell 111: 815–824 [DOI] [PubMed] [Google Scholar]
- Lizano E, Scheibe M, Rammelt C, Betat H, Mörl M (2008) A comparative analysis of CCA-adding enzymes from human and E. coli: differences in CCA addition and tRNA 3'-end repair. Biochimie 90: 762–772 [DOI] [PubMed] [Google Scholar]
- Martin G, Keller W (1996) Mutational analysis of mammalian poly(A) polymerase identifies a region for primer binding and catalytic domain, homologous to the family X polymerases, and to other nucleotidyltransferases. EMBO J 15: 2593–2603 [PMC free article] [PubMed] [Google Scholar]
- Martin G, Keller W (2004) Sequence motifs that distinguish ATP(CTP):tRNA nucleotidyl transferases from eubacterial poly(A) polymerases. RNA 10: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Keller W (2007) RNA-specific ribonucleotidyl transferases. RNA 13: 1834–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaza J (1994) AMoRe: an automated package for molecular replacement. Acta Crystallogr A 50: 157–163 [Google Scholar]
- Nissen P, Hansen J, Ban N, Moore PB, Steitz TA (2000) The structural basis of ribosome activity in peptide bond synthesis. Science 289: 920–930 [DOI] [PubMed] [Google Scholar]
- Neuenfeldt A, Just A, Betat H, Mörl M (2008) Evolution of tRNA nucleotidyltransferases: a small deletion generated CC-adding enzymes. Proc Natl Acad Sci USA 105: 7953–7958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Tomita K, Ishitani R, Ishii R, Takeuchi N, Arisaka F, Nureki O, Yokoyama S (2003) Divergent evolutions of trinucleotide polymerization revealed by an archaeal CCA-adding enzyme structure. EMBO J 22: 5918–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol 276: 307–326 [DOI] [PubMed] [Google Scholar]
- Pace NR (1997) A molecular view of microbial diversity and the biosphere. Science 276: 734–740 [DOI] [PubMed] [Google Scholar]
- Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J (1994) Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science 264: 1891–1903 [PubMed] [Google Scholar]
- Reuven NB, Deutscher MP (1993) Substitution of the 3′ terminal adenosine residue of transfer RNA in vivo. Proc Natl Acad Sci USA 90: 4350–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuven NB, Zhou Z, Deutscher MP (1997) Functional overlap of tRNA nucleotidyltransferase, poly(A) polymerase I, and polynucleotide phosphorylase. J Biol Chem 272: 33255–33259 [DOI] [PubMed] [Google Scholar]
- Schimmel P, Yang XL (2004) Two classes give lessons about CCA. Nat Struct Mol Biol 11: 807–808 [DOI] [PubMed] [Google Scholar]
- Shi PY, Maizels N, Weiner AM (1998) CCA addition by tRNA nucleotidyltransferase: polymerization without translocation? EMBO J 17: 3169–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M, Cramer F (1979) The -C-C-A end of tRNA and its role in protein biosynthesis. Prog Nucleic Acid Res Mol Biol 22: 1–69 [DOI] [PubMed] [Google Scholar]
- Toh Y, Numata T, Watanabe K, Takeshita D, Nureki O, Tomita K (2008) Molecular basis for maintenance of fidelity during the CCA-adding reaction by a class I CCA-adding enzyme. EMBO J 27: 1944–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomari Y, Suzuki T, Watanabe K, Ueda T (2000) The role of tightly bound ATP in Escherichia coli tRNA nucleotidyltransferase. Genes Cells 5: 689–698 [DOI] [PubMed] [Google Scholar]
- Tomita K, Weiner AM (2001) Collaboration between CC- and A-adding enzymes to build and repair the 3′-terminal CCA of tRNA in Aquifex aeolicus. Science 294: 1334–1336 [DOI] [PubMed] [Google Scholar]
- Tomita K, Weiner AM (2002) Closely related CC- and A-adding enzymes collaborate to construct and repair the 3′-terminal CCA of tRNA in Synechocystis sp. and Deinococcus radiodurans. J Biol Chem 277: 48192–48198 [DOI] [PubMed] [Google Scholar]
- Tomita K, Fukai S, Ishitani R, Ueda T, Takeuchi N, Vassylyev DG, Nureki O (2004) Structural basis for template-independent RNA polymerization. Nature 430: 700–704 [DOI] [PubMed] [Google Scholar]
- Tomita K, Ishitani R, Fukai S, Nureki O (2006) Complete crystallographic analysis of the dynamics of CCA sequence addition. Nature 443: 956–960 [DOI] [PubMed] [Google Scholar]
- Weeks CM, Miller R (1999) The design and implementation of SnB version 2.0. J Appl Crystallogr 32: 120–124 [Google Scholar]
- Weiner AM (2004) tRNA maturation: RNA polymerization without a nucleic acid template. Curr Biol 14: 883–885 [DOI] [PubMed] [Google Scholar]
- Woese CR (2000) Interpreting the universal phylogenetic tree. Proc Natl Acad Sci USA 97: 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Steitz TA (2004) Mechanism of transfer RNA maturation by CCA-adding enzyme without using an oligonucleotide template. Nature 430: 640–645 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Li F, Wang J, Weiner AM, Steitz TA (2003) Crystal structures of an archaeal class I CCA-adding enzyme and its nucleotide complexes. Mol Cell 12: 1165–1172 [DOI] [PubMed] [Google Scholar]
- Yue D, Maizels N, Weiner AM (1996) CCA-adding enzymes and poly(A) polymerases are all members of the same nucleotidyltransferase superfamily: characterization of the CCA-adding enzyme from the archaeal hyperthermophile Sulfolobus shibatae. RNA 2: 895–908 [PMC free article] [PubMed] [Google Scholar]
- Yue D, Weiner AM, Maizels N (1998) The CCA-adding enzyme has a single active site. J Biol Chem 273: 29693–29700 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File