Abstract
BAR (Bin/Amphiphysin/Rvs) domains and amphipathic α-helices (AHs) are believed to be sensors of membrane curvature thus facilitating the assembly of protein complexes on curved membranes. Here, we used quantitative fluorescence microscopy to compare the binding of both motifs on single nanosized liposomes of different diameters and therefore membrane curvature. Characterization of members of the three BAR domain families showed surprisingly that the crescent-shaped BAR dimer with its positively charged concave face is not able to sense membrane curvature. Mutagenesis on BAR domains showed that membrane curvature sensing critically depends on the N-terminal AH and furthermore that BAR domains sense membrane curvature through hydrophobic insertion in lipid packing defects and not through electrostatics. Consequently, amphipathic motifs, such as AHs, that are often associated with BAR domains emerge as an important means for a protein to sense membrane curvature. Measurements on single liposomes allowed us to document heterogeneous binding behaviour within the ensemble and quantify the influence of liposome polydispersity on bulk membrane curvature sensing experiments. The latter results suggest that bulk liposome-binding experiments should be interpreted with great caution.
Keywords: amphipathic α-helix, BAR domain, membrane curvature sensing, membrane insertion, single liposomes
Introduction
BAR (Bin/Amphiphysin/Rvs) domains are found in a number of membrane-associated proteins involved in endocytosis, actin regulation and cell signalling, and are thought to sense curvature of lipid bilayers (Habermann, 2004; Peter et al, 2004; McMahon and Gallop, 2005)—a characteristic also attributed to amphipathic helices (AHs) (Drin et al, 2007). Membrane curvature sensing is defined as the ratio of protein density in high membrane curvature areas over flat or low curvature areas. Membrane curvature sensing facilitates assembly of protein complexes in a spatio/temporal context dictated by the membrane shape during, for example, endocytosis and sorting in the endocytic pathway, thus showing an additional layer of membrane compartmentalization (McMahon and Gallop, 2005; Parthasarathy and Groves, 2007; Manneville et al, 2008; Trajkovic et al, 2008). At higher protein concentrations, several BAR domain proteins can also deform membranes and hence induce and/or stabilize membrane curvature (Zimmerberg and Kozlov, 2006; Lemmon, 2008), thus they actively participate in vesicle budding and tubulation, basic remodelling of the membrane, which is a fundamental need of any growing cell (Takei et al, 1999; Farsad et al, 2001). Recently, much attention has been focused at the mechanism by which BAR domains induce and stabilize membrane curvature (Takei et al, 1999; Farsad et al, 2001; Shimada et al, 2007; Frost et al, 2008; Saarikangas et al, 2009); however, little effort has been directed at the mechanism by which BAR domains sense membrane curvature.
The currently accepted curvature sensing mechanism of BAR domains was deduced from the crystal structure of the Amphiphysin BAR domain showing an elongated crescent-shaped homodimer (Peter et al, 2004). On the basis of its overall curved quaternary structure the BAR domain was proposed to act as a membrane curvature sensor of negatively charged membranes using two distinct features: (i) the overall curved structure of the dimer and (ii) the net positive charge along its inner/concave surface (Peter et al, 2004). Hence, BAR domains are a rare example of biological recognition mediated by the overall structure of a protein domain and the only example of proteins that, unlike AHs, are not using hydrophobic insertions to sense membrane curvature. Curvature sensing of BAR domains was confirmed by a centrifugation assay that showed higher densities of bound protein on smaller liposomes. The proposed sensing mechanism was validated by charge reversions on the concave surface that knocked out the ability of BAR domains to induce curvature (Carlton et al, 2004; Peter et al, 2004; Pylypenko et al, 2007); these mutants, however, were never tested for sensing, mainly because sensing and induction of membrane curvature are currently assumed to be mediated by the same physical mechanism.
Structural similarity to the arfaptin coiled-coil domain led to identification of a family of BAR domain proteins, now comprising close to 40 proteins (Habermann, 2004). The intuitive mechanism of curvature sensing through the crescent-shaped dimer structure was generalized to other families—the F-BAR domains (formerly FCH) (Henne et al, 2007; Shimada et al, 2007) and I-BAR domains (formerly IMD) (Millard et al, 2005; Lee et al, 2007). Here, we use measurements on Single Liposomes of different diameters and therefore Curvature (SLiC assay) to investigate up to what extent sensing of membrane curvature by BAR domains depends on the curved BAR scaffold or to insertions of amphipathic residues in the membrane.
Results
Membrane curvature sensing by BAR domains on single liposomes
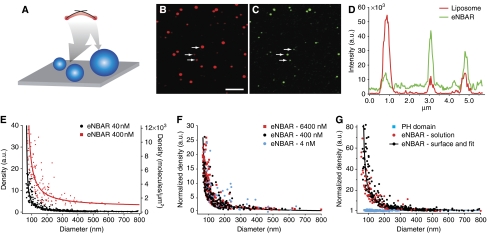
Using a method we developed recently (Hatzakis et al, 2009) we evaluated the binding of membrane curvature sensing proteins using quantitative fluorescence imaging of single liposomes with different diameters (50–800 nm) and therefore membrane curvature. Similarly to other groups we isolated single liposomes (Folch fraction) by tethering them on surfaces at low densities (Figure 1A) (Stamou et al, 2003; Kuyper et al, 2006; Yoon et al, 2006; Chan et al, 2007; Christensen and Stamou, 2007). Briefly, we used tethering conditions that are known to keep liposomes intact to water soluble fluorescent molecules (Lohse et al, 2008) and ionic gradients (Stamou et al, 2003). As we showed in a recent publication (Bendix et al, 2009) under appropriate conditions, immobilization does not modify the spherical shape of liposomes. Fluorescent labelling of the membrane allowed us to image them with confocal microscopy (Stamou et al, 2003; Bagatolli, 2006) (Figure 1B). We imaged the bound protein in a second fluorescence channel (Figure 1C). A line trace through liposomes of different size qualitatively illustrated that smaller liposomes bound more protein (Figure 1D). The diameter of these nanoscopic objects was calculated with high accuracy (±5 nm) by integrating the fluorescence intensity of each liposome according to the procedure of Kunding et al (see Supplementary Figure 1; Kunding et al, 2008). In the same way, we calculated the total amount of labelled protein bound on each liposome. The ratio of the two signals gave us the density of protein bound on single liposomes of different diameters.
Figure 1.
BAR domain binding on single liposomes of different diameters and therefore membrane curvature. (A) Sketch of the SLiC assay. Single liposomes are tethered on surfaces using streptavidin–biotin linkage and imaged with confocal microscopy. BAR domains sense membrane curvature by binding with higher density on liposomes of smaller diameter, and this is schematically indicated by the large arrow towards the smaller liposomes. (B, C) Confocal microscopy images of brain liposomes (exc. 633 nm) and BAR protein (eNBAR) (exc. 488 nm), respectively. Scale bar is 3 μm and applies to both images. (D) Intensity profile from cross section of three liposomes indicated by arrows in (B) (274, 95 and 115 nm in diameter calculated by their total intensity), which shows qualitatively membrane curvature sensing by eNBAR. Protein signal (green) is higher for smaller liposomes, notice this effect will be greatly amplified when signals are converted into density. (E) eNBAR density versus liposome diameter for two different concentrations 40 and 400 nM. We typically record a relative eNBAR density increase of ∼20–100-fold. A higher concentration increases the density, i.e. number of proteins pr. unit area. (F) eNBAR normalized density versus liposome diameter for three different concentrations—4, 400 and 6400 nM. We recorded identical membrane curvature sensing graphs for different concentrations. Density is normalized to the baseline (density of largest liposomes was set to 1). (G) A control with the lipid-binding PH domain of Centaurin β2 shows no curvature selectivity as expected. A second control excludes any immobilization artefacts by demonstrating matching membrane curvature sensing curves for liposomes incubated with eNBAR directly in solution (red dots) or after their immobilization on a surface (black dots). Protein concentration was 40 nM.
One of the first BAR domain proteins we tested for membrane curvature sensing was rat endophilin A1 1–247 (eNBAR). eNBAR is a cytoplasmic protein enriched at synapses and implicated in synaptic vesicle endocytosis (Ringstad et al, 1999). It has an N-terminal NBAR domain and a C-terminal SH3-domain, which binds the GTPase dynamin and the lipid phosphatase synaptojanin (Ringstad et al, 1999; Farsad et al, 2001). Recent work identified how the N-terminal AH and the BAR crescent dimer structure collectively drive membrane deformation (Gallop et al, 2006; Masuda et al, 2006). The BAR domain is believed to be inherently capable of sensing membrane curvature.
Figure 1E shows eNBAR density versus liposome diameter (membrane curvature sensing graph) for liposomes that were immobilized and then incubated with two different concentrations of eNBAR, 40 and 400 nM. To compare proteins, we work at non-deforming conditions (Supplementary Figure 2). If the protein is deforming the liposomes, the protein density is identical for all sizes (Peter et al, 2004). Each point on the graph is a single liposome of a given diameter. The assay documents a remarkable curvature sensing efficiency (ratio of density of small over large liposomes) of up to ∼100, as compared with efficiencies of ∼1.5 that are commonly reported for BAR domains by bulk assays (Carlton et al, 2004; Peter et al, 2004; Gallop et al, 2005; Pylypenko et al, 2007). Higher concentrations increased the density as expected (Figure 1E; Supplementary Figure 3). We calibrated the intensity signals (see Materials and methods and Lohse et al, 2008) to determine the absolute protein density and found that at 40 nM on flat membranes (800 nm) ∼0.3% of the surface area is covered assuming a BAR dimer covers 3 × 12 nm2. The highest densities we recorded for the smallest liposomes at the highest concentration (6.4 μM) were ∼70% of the available surface area.
To test the contribution of the dimer in sensing we recorded sensing curves at concentrations in which we have predominantly monomers (4 nM) or dimers (6.4 μM). The sensing curves of eNBAR appear nearly identical at different concentrations, suggesting the dimer has a minor contribution to sensing, if any (Figure 1F; Supplementary Figure 3).
As a negative control we incubated the liposomes with the lipid-binding Pleckstrin Homology domain of Centaurin β2 (PH) that should not exhibit curvature-dependent binding (Peter et al, 2004). Indeed, we observed that the PH domain efficiently bound liposomes of all sizes but at constant densities, i.e. it showed no size selectivity as expected (Figure 1G). The incubation with the PH domain also serves as a control for multilamellar liposomes that would appear in the SLiC assay as one large liposome with relatively low protein density. The constant PH to lipid ratio shows that the small number of oligolamellar liposomes (∼10%, see Supplementary Figure 4) does not bias the measurements in any significant manner, though it probably contributes to the overall noise of the data. To exclude any influence of the immobilization procedure on the shape/curvature of the liposomes and their protein-binding properties, we pre-incubated liposomes with eNBAR in solution and then immobilized them. The membrane curvature sensing graphs for the two different incubation protocols were identical, showing that immobilized and freely soluble liposomes bind eNBAR in an identical manner (Figure 1G).
Testing current model for membrane curvature sensing by BAR domains
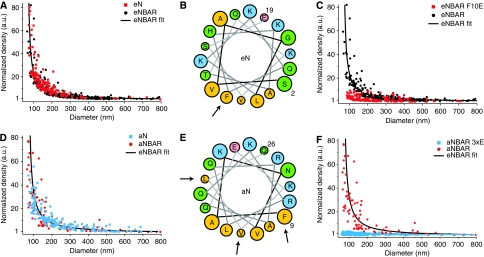
In a next step we used the SLiC assay to investigate the molecular origin of membrane curvature sensing by BAR domains, measuring on members of the BAR superfamily including NBARs, F-BARs and I-BARs. Both the overall charge and dimer structure vary significantly within the BAR superfamily (Figure 2A) suggesting that these may not be the ubiquitous features used to sense membrane curvature. To test the hypothesis that curvature sensing is mediated by positive charges on the concave surface of an NBAR dimer, we incubated single liposomes with the mutant of endophilin that lacks three positively charged amino acids on its concave surface (residues 171–173 3KE, hereafter eNBAR-3KE). This type of mutation has been inhibiting tubulation in a very efficient manner in vivo and in vitro (Carlton et al, 2004; Peter et al, 2004; Gallop et al, 2006; Tsujita et al, 2006; Pylypenko et al, 2007; Shimada et al, 2007), a result that was interpreted as a proof that charge distribution is critical for sensing curvature. Comparison of the mutant with the wild type showed that the 3KE mutation at both high and low concentration does not affect sensing in any way (Figure 2B). Thus, although charge mutations are critical for the induction of membrane curvature they seem not to have a function in sensing membrane curvature.
Figure 2.
Comparison of membrane curvature sensing graphs for various BAR domains. Testing the current hypothesis of the molecular mechanism of membrane curvature sensing by BAR domains. All samples are incubated with protein at 40 nM for 1 h unless otherwise stated. (A) Representative members of three BAR domain super families. Surface representation coloured according to electrostatic potential and mesh equipotential surfaces at −5 kT/e to 5 kT/e (blue is +, red is −). Arrows indicate the position of AHs. Neither dimer shape nor electrostatic potential is conserved for the different proteins. (B) Comparison of eNBAR and eNBAR-3KE shows that charge mutations do not alter the ability of this BAR domain to sense membrane curvature. Sensing is identical for eNBAR 3KE at high concentration (5.2 μM). (C) Comparison of eNBAR and oFBAR shows that the intrinsic radius of curvature of the dimer (11 nm versus 65 nm) does not influence membrane curvature sensing. (D) eNBAR shows identical membrane curvature sensing as an IBAR (mIBAR), which has an negative radius of curvature according to the crystal structure. Sensing is identical for mIBAR at high concentration (0.9 μM). Thus, neither charges nor BAR dimer structure is essential for curvature sensing.
To test the hypothesis that membrane curvature sensing is mediated by the crescent-shaped quaternary structure of the dimer, we compared eNBAR with two BAR domains of significantly different quaternary structure. The BAR domain of FCHo2 (residues 1–272, here termed oFBAR) (Henne et al, 2007) has ∼6 times the internal radius of curvature of eNBAR (∼65 nm versus ∼11 nm) (Masuda et al, 2006; Henne et al, 2007). The BAR domain of MIM (residues 1–254, here termed mIBAR), member of the related I-BAR family, has a radius of curvature inverse to NBARs (Lee et al, 2007; Mattila et al, 2007) and is therefore speculated to bind preferentially to liposomes of larger diameter. oFBAR has been shown to convert liposomes to tubes with larger diameter than eNBAR, whereas mIBAR has been shown to produce inverse tubes (invaginations) in liposomes (Henne et al, 2007; Mattila et al, 2007; Shimada et al, 2007). Contrary to expectation, however, we observed similar membrane curvature sensing graphs for an N-BAR, an F-BAR and an I-BAR domain, indicating that though the shape of the dimer may be important for the induction of membrane curvature, it is not critical for the sensing of membrane curvature (Figure 2C and D).
Determination of NBAR–membrane interaction
To better understand the nature of the binding of eNBAR on membranes of different curvature we performed quantitative membrane curvature sensing experiments at different protein concentrations (1 nM–6.4 μM, see Supplementary Figure 3). This allowed us to measure for the first time binding curves for a BAR domain as a function of membrane curvature. In Figure 3A, we show three representative binding curves. Fitting the curves with equation (1),
Figure 3.
Binding curves of eNBAR on membranes of different curvature. (A) Quantitative determination of the concentration-dependent binding of eNBAR as a function of liposome diameter. Depicted are representative binding curves for three different liposome diameters fitted with Langmuir isotherms (100, 200 and 650 nm). Strikingly, the eNBAR shows a strong curvature selectivity even at saturation. (B) From the fits in (A) we extract the size dependency of the apparent equilibrium disassociation constant (KD) and the apparent free energy of binding. The KD varies from ∼400 to 140 nM illustrating a marginally higher affinity of eNBAR for curved membranes. (C) The saturation density (Bmax) extracted from (A) as a function of liposome diameter. The size dependence of the Bmax changes to a much greater extent than KD. The inset depicts the Bmax in a log–log plot. Membrane curvature sensing is not mediated by higher affinity but relies predominantly on a curvature-dependent Bmax.
 |
where BC is the density of bound protein at a given concentration (c), allowed us to extract the apparent disassociation constant (KD) and the saturation density (Bmax) for binding of eNBAR on liposomes of different diameters and hence curvature.
All curvatures can be fitted with a classical Langmuir isotherm indicating the existence of one binding mode for a range of concentrations from far below up to the dimerization dissociation constant of eNBAR. The value of the KD depends on the size of the liposomes (Figure 3B). For binding at flat membranes the KD converges to a value of 397±8 nM. Surprisingly, however the maximal gain in ΔG for eNBAR binding at curved membranes is only ∼1.1 kBT that is at the order of thermal fluctuations. In other words, eNBAR does not recognize curved membranes so efficiently because it has a higher affinity for them. This observation is at odds with the current hypothesis that BAR domains sense curvature by having higher affinities for membranes that match their shape and therefore maximize the electrostatic-binding energy.
Surprisingly the Bmax depends on liposome diameter, thus even at concentrations much higher than the KD, eNBAR is able to sense and upconcentrate to highly curved membranes (Figure 3C). Again this observation cannot be explained by an affinity-based model of BARs adsorbing and packing on the membrane, but is identical to the characteristic mechanism that AHs (including the N-terminal AH of eNBAR) use to sense membrane curvature (Hatzakis et al, 2009). Recently, it was shown that membrane curvature sensing by AHs is not because of higher affinity for smaller liposomes. Sensing is rather dominated by an intrinsic property of curved membranes to accommodate larger densities of amphipathic molecules inside hydrophobic defects generated by membrane curvature (Hatzakis et al, 2009). These results complement the conclusions reached by mutagenesis that membrane curvature sensing by BAR domains does not depend on electrostatic attraction between surfaces of different curvature, furthermore they suggest that membrane curvature sensing by eNBAR may primarily be due to its N-terminal AH (eNBAR 1–33, hereafter eN).
The N-terminal AH dominates membrane curvature sensing by NBARs
Many AHs are known to be sensors of membrane curvature (Drin et al, 2007; Ramamurthi et al, 2009). In an attempt to identify the contribution of eN to the sensing of eNBAR we isolated the peptide and compared its sensing ability to the full-length eNBAR. Figure 4A shows eN is able to sense membrane curvature equally well as eNBAR, by being within the uncertainty of the eNBAR fit. To further probe the role of the peptide, we used a known point mutation to disrupt its amphipathic nature (F10E, here termed eNBAR-F10E) (Figure 4B; Supplementary Figure 5) (Farsad et al, 2001). The mutant still bound to the liposomes possibly because of the electrostatic contribution from the BAR domain itself; however, it was essentially unable to sense membrane curvature (Figure 4C). Earlier studies, apart from point mutations (Farsad et al, 2001), have used deletions of the first 33 amino acids to isolate the BAR domain (Gallop et al, 2005, 2006; Masuda et al, 2006). In the SLiC assay, consistent with the behaviour of the eNBAR-F10E mutant, the eNBAR-Δ33 seemed not to be a sensor of membrane curvature (Supplementary Figure 6A). However, we caution against the use of the latter constructs as they show limited stability (own experience and literature; Nie et al, 2006). Surprisingly, we do not see the appendage loop of eNBAR (a second potential AH motif, residues 63–79) contributing significantly to membrane curvature sensing (Figure 4C; Supplementary Figure 6A). This may be due to its role in dimerization or to the relatively limited degree of orientational freedom that may restrict optimal interaction with the lipid membrane. The N-terminal helix therefore emerges as a minimal motif able to fully replicate the sensing ability of wild-type eNBAR.
Figure 4.
Membrane curvature sensing of BAR domains is dominated by the N-terminal amphipathic helix. All graphs are measured at 40 nM protein unless otherwise stated. (A) Membrane curvature sensing by eN fully replicates the sensing ability of eNBAR. (B) Helical wheel representation of eN, arrow indicates the point mutation F10E. Yellow, indicates hydrophobic residues; green, polar; blue, basic; pink, acidic. (C) Membrane curvature sensing graph for eNBAR-F10E measured at 740 nM. The point mutation disrupting the formation of the N-terminal AH impaired severe membrane curvature sensing. The concentration was increased to get sufficient binding. (D) The data for aN and aNBAR overlay completely with the fit for eNBAR showing the three molecules have identical membrane curvature sensing ability. (E) Helical wheel representation of aN, arrows indicate point mutations to glutamate (3xE). (F) Membrane curvature sensing of aNBAR was also severely impaired by mutations in the N-terminal peptide (aNBAR 3xE), binding measured at 4 μM. Thus, membrane curvature sensing by BAR domains seems to originate from the insertion of the N-terminal AHs in the bilayer.
To validate whether our conclusion could be extended to other BAR domains we tested the founding member of the BAR family, Amphiphysin 1–377 (aNBAR) (Peter et al, 2004). A known mutant with two charges converted on the concave interface (aNBAR 159/161 2KE) did not revert aNBARs ability to sense membrane curvature (Supplementary Figure 6B). More importantly, the N-terminal peptide (aNBAR 1–38, aN) of aNBAR was able to replicate the sensing ability of the wild-type NBAR (Figure 4D). In addition, disrupting the N-terminal helix of aNBAR (F9, V26, L27 converted to E, aNBAR-3xE) eliminated sensing, (see Figure 4E and F; Supplementary Figure 6C). Additional membrane curvature sensing experiments with different BARs (Sortin Nexin 1 and Centaurin β2), whose structure is not yet resolved, showed that they had similar sensing curves as eNBAR, eN, aNBAR, aN, oFBAR and mIBAR (Supplementary Figure 6D) corroborating further our conclusion that the curvature of the BAR dimer and its charge distribution are not responsible for membrane curvature sensing.
The combined results of Figures 2, 3 and 4 strongly suggest that the structural motif endophilin and amphiphysin are using to sense membrane curvature, is the insertion of the N-terminal AH peptide and not the BAR dimer. We searched the BAR domain phylogeny by Habermann (2004) to identify whether the presence of BAR domains correlates tightly with the presence of helices. Indeed, our search resulted in the identification of seven putative AHs N-terminals to BAR domains that were thought not to have any (Arfaptin, Sortin Nexin 1, 2 and 4, Centaurin β2, Oligophrenin 1 and protein interacting with C-kinase 1; see Supplementary Figure 7). This was further supported by the recent identification of AHs in SNX9 and several IBARs (Pylypenko et al, 2007; Saarikangas et al, 2009). Nevertheless, recent reports show that direct insertion of different hydrophobic motifs (amino acids in non-helical conformation; Martens et al, 2007; Ramachandran and Schmid, 2008) can also occur in a membrane curvature-dependent manner. Though our data indicate this is not the case for endophilin and amphiphysin, we cannot exclude that other BAR domains may use such non-N-terminal hydrophobic insertions for sensing, either in conjunction or in the place of the AHs. We therefore decided to devise a generic negative control experiment, able to discriminate whether membrane curvature sensing is mediated by hydrophobic insertion or electrostatic attraction without the use of mutations.
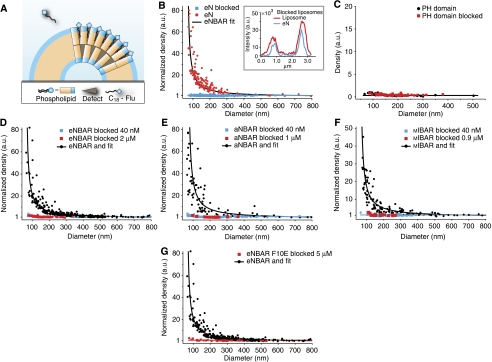
Membrane curvature sensing can be impaired by blocking lipid packing defects
Amphipathic and hydrophobic motifs are known to sense membrane curvature by inserting in lipid packing defects created in membranes because of curvature stress (Drin et al, 2007; Ramachandran and Schmid, 2008; Hatzakis et al, 2009). Lysophospholipids have been used previously to block curvature-induced defects (Chen et al, 2006), accordingly we incubated liposomes after their formation with a small amphiphilic molecule (C18-fluorescein) that, similar to a lysophospholipid, would insert in the membrane, block defects, prevent the insertion of hydrophobic residues and may be consequently inhibit membrane curvature sensing (Figure 5A). We tested and validated our hypothesis first on the N-terminal AH of eNBAR. Indeed, eN was able to bind to ‘blocked' liposomes but with the same density on all curvatures that is it was not able to sense membrane curvature anymore (Figure 5B). To test that the general lipid-binding properties were not affected by blocking lipid packing defects, we performed a control in which the PH domain was incubated with normal and blocked liposomes. The fact that the PH domain bound with the same absolute density to the blocked liposomes indicated that neither lipid packing defects nor C18-fluorescein in any way affected binding of the protein and further validated the assay (Figure 5C).
Figure 5.
Membrane curvature sensing by BAR domains is inhibited by blocking the curvature-induced hydrophobic defects of a bilayer. Graphs are measured at 40 nM protein unless otherwise stated. (A) Sketch showing blockage of membrane curvature-induced defects in the bilayer by pre-incubation of liposomes with a small amphiphilic molecule (C18-fluorescein). (B) Blocking of defects impaired membrane curvature sensing mediated by hydrophobic insertion for an AH (eN). Inset: typical line scan showing qualitatively that eN binds to blocked liposomes but without discriminating different diameters. (C) Control with PH domain (1 μM) on normal and blocked liposomes. Both conditions show identical absolute density assuring that blocking with amphiphiles does not cause any general lipid-binding effects. (D–F) Blocking of defects impaired membrane curvature sensing for eNBAR, aNBAR and mIBAR, respectively at both high (2, 1 and 0.9 μM, respectively) and low concentration, further supporting that membrane curvature sensing by BAR domains depends on curvature-induced defects in the bilayer and not charge-mediated interactions. (G) Control with eNBAR F10E at 5 μM also show that the residual binding from a BAR domain is not capable of sensing through charge-mediated interactions.
We then incubated the blocked liposomes with a series of BAR domains (NBARs and IBARs, Figure 5D–F). In agreement with our previous conclusions, wild-type BAR domains were not able to sense membrane curvature at both high and low concentration if bilayer surfaces did not expose hydrophobic defects. Finally, we performed a control designed to block sensing because of the N-terminal amphipathic helix and show any residual sensing because of the BAR dimer. The control recorded at 5 μM protein showed that the BAR dimer indeed binds through electrostatic interactions between the charged dimer scaffold and the membrane, however it was not able to sense curvature (Figure 5G), thus supporting the results achieved with high concentration of wt BAR domains (Figure 5D–F). The combined experiments of Figure 5 demonstrate further that membrane curvature sensing of BAR domain proteins is mediated by, and depends on, hydrophobic insertions.
BAR domains do not bind equally to all liposomes
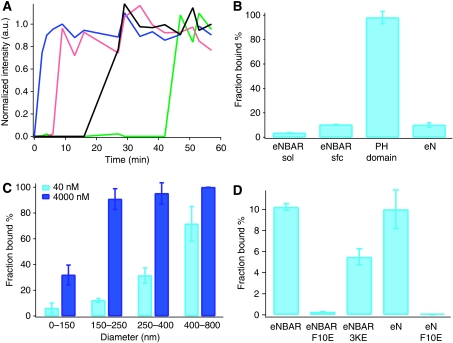
The SLiC assay, by measuring on single liposomes, avoided ensemble averaging and allowed us to document accurately how liposomes of different diameter interact with BAR domains. The ability to record binding on single particles also allowed us to check for heterogeneous binding behaviour for liposomes of the same diameter. The data of Figures 1, 2, 3, 4 and 5 include all liposomes in our samples that showed binding (signal was above the background) after incubation of typically few hours. Indeed all data points collapse in the same curves suggesting that liposomes of the same diameter exhibit identical binding properties as expected. However, time-resolved measurements of BAR domain binding with the SLiC assay showed strong heterogeneities in the behaviour of apparently identical single liposomes. This is illustrated by the binding kinetics of eNBAR in Figure 6A. Instead of synchronized kinetics starting at time zero, different liposomes exhibited a variable lag phase of several tens of minutes. The effect of asynchronous binding kinetics was so prominent that even for incubation times of hours at physiological protein concentrations (∼10–1000 nM) a fraction of liposomes did not have a protein signal above the background (Supplementary Figure 8).
Figure 6.
Heterogeneous binding of eNBAR wild type and mutants showed in single liposome measurements. All graphs are measured at 40 nM protein unless otherwise stated. (A) Typical binding kinetics for eNBAR to four single liposomes (blue, red, black and green traces). A variable lag phase is identified lasting up to several tens of minutes. (B) Heterogeneous binding kinetics results in a variable fraction of liposomes showing binding (Bfrac). Bfrac is documented for different binding conditions (protein incubation (1 h) in solution or on the surface) and several different binding partners (eNBAR, PH domain (1 μM) and eN). (C) Measured eNBAR Bfrac as a function of liposome diameter for two different concentrations within a fixed incubation time (1 h). Error bars represent the s.d. for three different samples of approximately 800–1000 liposomes each. (D) Bfrac for different eNBAR and eN mutants. Bfrac is primarily affected by a point mutation in the AH (F10E) and to a lesser degree by mutations that modify the electrostatic potential on the concave side of eNBAR (3KE). Incubation time and error bars as in (C). A full-colour version of this figure is available at The EMBO Journal Online.
In line with a recent report (Ramamurthi et al, 2009) we also used the fraction of liposomes that showed binding above background to describe the protein-binding behaviour. We hereafter refer to the fraction of liposomes that showed binding above background at a given incubation condition as fraction bound % (Bfrac). All BAR domains that we measured exhibited different degrees of Bfrac. Bfrac is not an artefact originating from the immobilization of liposomes, as a control sample incubated in solution showed a similar behaviour (Figure 6B). Studies with giant unilamellar vesicles further supported the conclusion that Bfrac is an inherent property of the system (Supplementary Figure 8).
In contrast, liposomes incubated with the PH domain showed essentially full Bfrac (98±2%), suggesting that Bfrac may be a property of the eNBAR domain. To test this hypothesis we incubated liposomes with eN. The observation that both eNBAR and eN showed a low degree of Bfrac suggested that Bfrac is a feature associated to hydrophobic insertion (and not electrostatic binding) in lipid bilayers. Further analysis of Bfrac data showed a strong dependence both on liposome radius (r) and protein concentration (c) (Figure 6C). A Bayesian analysis of these data (see Supplementary data) showed that Bfrac scaled with the square of the radius (∝ rα, where α=2±0.1) suggesting that it increases with the surface area available for binding. The analysis also indicated with good confidence that Bfrac scaled with the square root of concentration (∝[c]β, where β=0.5±0.02).
All liposome-binding experiments that are carried out in bulk make the implicit assumption that all liposomes in the ensemble have identical or similar properties. Our data, however, indicate that for certain systems (BAR domains and AHs) this assumption is not valid as the presence of Bfrac may have a pronounced effect on membrane binding. Though at this stage we do not fully understand the molecular mechanism behind Bfrac we feel it is a quantity that is important to consider when evaluating the binding of proteins on lipid membranes. Therefore, below in line with literature (Ramamurthi et al, 2009), we used the fraction of liposomes (Bfrac) that show binding above background to compare different BAR domain mutants and also discuss important consequences for measuring membrane curvature sensing with liposomes in bulk experiments.
Bulk experiments have earlier shown that mutations that alter the charge distribution of BARs (Carlton et al, 2004; Peter et al, 2004; Gallop et al, 2006; Tsujita et al, 2006; Pylypenko et al, 2007; Shimada et al, 2007) had a pronounced effect on protein binding to membranes. Our sensing curves however did not indicate such clear differences; we therefore screened different mutants for their Bfrac parameter under identical incubation conditions. The first striking observation was that the Bfrac of eNBAR is dramatically reduced (a factor of ∼30) by the point mutation that disrupts the amphipathic nature of the N-terminal AH; these results, in addition to the fact that eN shows similar Bfrac to eNBAR, indicate that the AH is a motif that critically affects Bfrac of BAR domains. Interestingly, the eNBAR-3KE mutant also showed lower (∼50%) Bfrac than the wild type, an effect in line with previous bulk experiments, although once the mutant binds it has identical densities and sensing ability (Figure 2B). Thus, the probability of nucleating binding on a liposome though mainly driven by the hydrophobic AH–bilayer interaction, seems also to be regulated by the local electrostatic potential (McLaughlin and Murray, 2005) around the AH that may inhibit its insertion by negative (3KE mutant) long-range electrostatic forces (Supplementary Figure 9). If Bfrac is present in vivo (e.g. on endocytotic buds or cargo vesicles), local charge modifications in a protein (e.g. through phosphorylation) (McLaughlin and Murray, 2005) may modulate Bfrac and function as an additional on/off switch triggering the association of AH-anchored proteins.
Bulk ensemble measurements may average out membrane curvature sensing
Fractional binding is a quantity inherently different from the density of bound protein on a single liposome; however, in a bulk experiment these two parameters cannot be distinguished as they would both contribute equally to the total amount of protein bound to an ensemble of liposomes. To illustrate the influence of ensemble averaging in the results produced by bulk experiments, we reconstructed in Figure 7 a bulk experiment using single liposome data at different protein concentrations and in the presence or absence of Bfrac.
Figure 7.
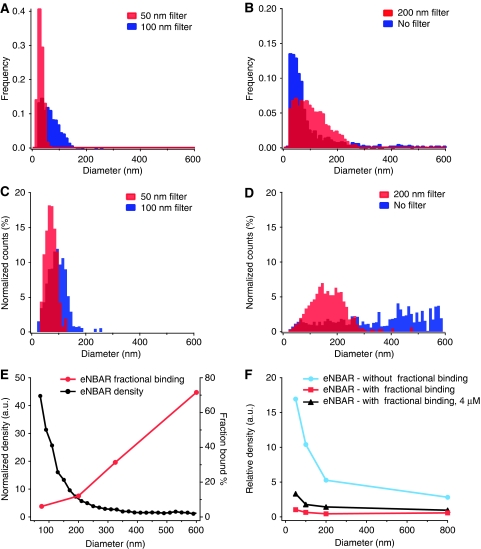
Reconstruction of bulk membrane curvature measurements from single liposome data. (A, B) Distribution of liposome diameters for populations extruded with filters of different pore size (50, 100 and 200 nm) or not extruded at all. (C, D) Distribution of the lipid mass at given diameter range. Conditions as in (A, B). (E) eNBAR density (black) and fractional binding (red) measured at 40 nM. (F) Simulation of bulk ensemble averaging without fractional binding (Bfrac) (blue), with Bfrac at 40 nM (red) or 4000 nM (black). Bulk ensemble assays may potentially provide false-negative results because of a convolution of density and fractional binding. A full-colour version of this figure is available at The EMBO Journal Online.
Membrane curvature sensing experiments in bulk typically use ∼4 populations of liposomes extruded with filters of different pore size (Carlton et al, 2004; Peter et al, 2004; Bigay et al, 2005; Gallop et al, 2005; Drin et al, 2007; Pylypenko et al, 2007). As the bound protein density ρ(d) depends on liposome diameter (d), to reconstruct the total amount of protein bound in each of these populations it is necessary to know the actual distribution of liposome sizes for each extrusion. In Figure 7A and B we plot the full size distributions of liposome samples not extruded at all and extruded at 50, 100 and 200 nm. The size distribution can be converted to the distribution of membrane surface area, A(d) (Figure 7C and D). Notice that the extensive overlap (∼70% for 50 and 100 nm) in A(d) between the different populations is ‘masking' to some extent size depended observations in bulk experiments. In a bulk experiment, the total amount of protein (P(d)) bound on each population is given by equation (2),
 |
The first reconstruction (Figure 7F, blue curve) assumes Bfrac=100% and when compared with the SLiC data it shows the influence of liposome polydispersity on the membrane curvature sensing assay. SLiC showed that for eNBAR the membrane curvature sensing efficiency (i.e. the ratio of protein density of small over large liposomes) is × 50– × 100 (Figures 1G and 7E), however, because of ensemble averaging in a bulk experiment, the apparent increase is calculated to be only × 6.
More significant however is the observation that the Bfrac has a radius dependence that is inverse to the one of density (Figure 7E), hence it will decrease the bulk sensing efficiency (see equation (2)). Indeed, if we use the values of Bfrac for different concentrations we see that the sensing efficiency reported by a bulk experiment would be reduced from × 6 to × 3.5 and × 1.8 for 40 nM and 4 μM, respectively (Figure 7F). Actually if the true sensing efficiency of eNBAR was not so high, Bfrac could inverse completely the tendency and indicate an apparent preference for larger diameters.
These results highlight that inherent properties of liposome ensembles such as polydispersity and heterogeneous binding may critically influence the outcome of bulk experiments. For example, mutations of hydrophobic (Bigay et al, 2005; Gallop et al, 2006; Henne et al, 2007) or charged residues (Carlton et al, 2004; Peter et al, 2004; Gallop et al, 2006; Tsujita et al, 2006; Pylypenko et al, 2007; Shimada et al, 2007) that will change the Bfrac but not necessarily sensing (e.g. eNBAR-3KE), when tested in a bulk curvature sensing assay will appear to modulate the sensing ability of the particular mutant, leading to the false conclusion that they are regulating membrane curvature sensing of the wild-type protein.
Discussion
Sensing of membrane curvature by a protein with a curved quaternary structure is an attractive hypothesis; however, we have presented evidence that neither the radius of curvature nor the charge distribution of the concave side of BAR domains is involved in sensing. As strongly suggested by mutations of hydrophobic residues and by specifically blocking the defects of curved membranes, membrane curvature sensing is rather because of the insertion of hydrophobic motifs such as AHs in the membrane. This conclusion was corroborated further by quantitative binding curves of eNBAR as a function of membrane curvature, which were dominated by the characteristic property of hydrophobic motifs to have a Bmax that increases with curvature (Hatzakis et al, 2009). A potential reason why BAR dimers do not sense membrane curvature could be that they bind with their long axis perpendicular to the membrane. However, though in this study we do not investigate the orientation of the dimer, a plethora of published evidence (experiments and simulations) (Carlton et al, 2004; Peter et al, 2004; Gallop et al, 2006; Mattila et al, 2007; Pylypenko et al, 2007; Shimada et al, 2007; Blood et al, 2008; Frost et al, 2008; Saarikangas et al, 2009; Yin et al, 2009) shows dimers bind flat on the membrane. Thus, at this stage the reason why BAR dimers do not sense membrane curvature remains unclear. Measurements on single liposomes allowed us to document heterogeneous binding behaviour within the ensemble (Bfrac) and quantify the influence of liposome polydispersity on bulk membrane curvature sensing experiments. Both results suggest that bulk liposome-binding experiments should be interpreted with great caution.
Our data suggest that although proven earlier to induce membrane curvature, BAR dimers do not detect it; hence, sensing and induction of curvature are not necessarily a low/high-affinity version of a single process but rather two distinct mechanisms that are most likely partially assigned to disparate motifs. The presence of a membrane curvature sensing motif that does not depend on dimerization would enable a closed amplification loop that would first detect and subsequently stabilize membrane curvature. BAR domains, whether in monomeric or dimeric form (Figure 1F), would be able to target preferentially membranes that are already curved (e.g. endocytotic buds) using amphipathic motifs and would thus create locally the concentrations necessary for dimerization and multimerization. As extensively described before both by experiment and simulations, the organized arrangement of BAR domains, similarly to other proteins coats, will then collectively amplify and stabilize membrane curvature (Takei et al, 1999; Farsad et al, 2001; Blood and Voth, 2006; Frost et al, 2008; Saarikangas et al, 2009) using both the attractive interaction of the positively charged dimer scaffold with the membrane and the helix-induced membrane deformation (Gallop et al, 2006; Blood et al, 2008). Sensing pre-curved areas and thus focusing the action of BAR domains would minimize the indiscriminate non-physiological deformation of flat membranes. At the same time it would amplify the localized effect of BAR domains thus allowing them to operate from low, physiologically relevant, concentrations in solution. The conclusion that BAR domains are not sensors of membrane curvature underlines the importance of hydrophobic insertions (in particular AH peptides) as motifs that may endow proteins with the ability to sense the curvature of biological membranes (Hatzakis et al, 2009).
Materials and methods
Protein expression, purification and labelling
All constructs were expressed as GST fusion proteins from the pGEX 6P1, 4T2 or 2T vector in BL21 DE3 pLysS cells. Bacteria were grown at 37°C until OD600 of ∼0.8, induced with 100 μM IPTG and grown overnight at 30°C. Cells were collected and resuspended in buffer A (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM DTT, 10 μg/ml DNaseI, bacterial protease inhibitor (Roche)) and lysed by freeze thawing. The lysate was centrifuged for 30 min at 48 000 g, 4°C and the supernatant was incubated with 0.5 ml of glutathione beads (GE healthcare) per 1 l of culture for 1.5 h. Beads were washed three times with 25 ml buffer B (50 mM Tris pH 7.4, 150 mM NaCl). An endogenous cysteine on the protein was fluorescently labelled with 100 μM Alexa 488 maleimide (Invitrogen) at 4°C for 1.5 h and washed three times with 10 ml (buffer B+1 mM DTT). The PH domain from hCentaurin β2 was labelled with Alexa 488 succinimidyl ester because maleimide labelling was not possible because of absence of surface accessible cysteins. All endophilin and amphiphysin proteins are rat versions. GST eN 1–33, GST eN 1–33 F10E, GST aN 1–38 were eluted in (buffer B+1 mM DTT, 20 mM glutathione). hCentaurin β2 PH, eNBAR 1–247, eNBAR 3KE (171–173 3KE), eNBAR F10E, eNBAR-Δ33, eN 1–33, eN 1–33 F10E, aNBAR 1–377, aNBAR 2KE (159/161 2KE), aNBAR F9E and aNBAR 3xE (F9, V26, L27 mutated to E) were cleaved from GST at 4°C overnight with 0.3 U thrombin protease or Prescission protease. hFCHo2 BAR 1–272 (oFBAR) (Henne et al, 2007) were received purified and labelled with Alexa 488 from McMahon lab, Cambridge. mIBAR 1–254 (Mattila et al, 2007) was received purified from Lappalainen lab, Helsinki and labelled subsequently with Alexa 488.
Purity and integrity of the protein were inspected by SDS–PAGE, and labelling efficiency was determined by spectroscopy. Labelling efficiency was always below 1.
Single liposome curvature assay
Liposomes were prepared using Folch bovine brain extracts (fraction 1, Sigma) adding 0.5% 1,2 dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Cap Biotinyl) (DOPE–biotin), (Avanti) and 0.5% 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indodicarbocyanine perchlorate (DiD) (Molecular probes) dye. The lipids were mixed, dried slowly under nitrogen and placed under vacuum for 1 h to remove remaining solvent. A rehydration solution of 200 mM D-Sorbitol (Fluka) was added to a final concentration of 1 mg/ml, and was followed by overnight incubation at 37°C. The unilamellarity of the liposomes was strongly favoured by the absence of charged ions/molecules in the rehydration solution and the presence of charged lipids in the membrane. Freeze–thaw cycles further promoted unilamellarity and a polydisperse population of liposomes with a well-defined upper limit was achieved by extruding once (Northern Lipids extruder) through isopore polycarbonate membrane (Millipore) with a pore size of 0.8 μm. Before surface immobilization the liposomes were diluted in equiosmolar buffer—4 mM phosphate pH 7.4, 94 mM NaCl (hereafter PBS buffer) to a concentration of 20 μg/ml.
Assay surfaces with immobilized liposomes were prepared according to earlier work (Stamou et al, 2003) by consecutive incubation of clean glass slides with 0.2 mg/ml BSA-biotin (Sigma), 1 mg/ml BSA (Sigma) and streptavidin (0.1 mg/ml) for 15 min at room temperature. Before a new protein was incubated at the surface, the surface was rinsed thoroughly with PBS buffer. A final volume of 500 μl buffer was left above the surface in a custom made microscope chamber and to this 10 μl of reconstituted brain liposomes solution was added making a final concentration of 0.4 μg/ml. For assaying binding at high protein concentrations (μM range) a chamber with sample volume of 50 μl and corresponding 1 μl of liposomes was used. Under these conditions, as described earlier (Stamou et al, 2003; Bendix et al, 2009), the liposomes were immobilized without deforming on the surface through the streptavidin–biotin interactions. The absence of streptavidin leads to practically zero binding of liposomes. Surface immobilization of liposomes was allowed for 15 min before the unbound ones were removed by gently diluting with PBS buffer. BAR domain proteins were kept on ice prior assay and post addition to assay surface incubated at room temperature for ∼1 h before imaging.
Giant liposome binding
We were unable to generate giant liposomes with Folch lipids and therefore an artificial lipid mixture mimicking a synapse membrane was prepared using 39% 1,2 dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Avanti), 39% 1,2 dioleoyl-sn-glycero-3-phosphocholine (DOPC) (Avanti), 10% phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) (Avanti), 10% cholesterol (Avanti), 1% DOPE–biotin and 0.5% DiD. Liposomes were prepared as for the SLiC assay, with 2 days of rehydration at 37°C and no extrusion. The giant liposomes were immobilized on an assay surface and incubated with protein for 30 min at room temperature.
Confocal laser scanning microscopy
All samples were examined with a Leica TCS SP5 inverted confocal microscope. All images were acquired using an oil immersion objective HCX PL APO CS x100 (NA 1.4), detecting BAR domain protein at 495–600 nm (exc. 488 nm) and liposomes at 640–750 nm (exc. 633 nm). Sequential scanning insured the absence of crosstalk between the two channels. Images had a resolution of 2048 × 2048 pixel, with a pixel size of 38 nm and a bit depth of 16. Image acquisition settings for the concentration series were continuously optimized to take advantage of the full dynamic range of the detection system. Temperature was monitored in the room and remained constant at 22±1°C. Binding kinetics was monitored with external Avalanche Photodiode Detector at low excitation power to allow consecutive images to be acquired in the same area with minimal bleaching.
Circular dichroism
The folding of the N-terminal amphipathic helix on interaction with liposomes was monitored by CD spectroscopy. The 33 amino-acids peptide (eN and eN F10E) was cleaved from GST at 4°C overnight using a 0.3 U thrombin protease. Measurements were carried out on a Jasco J-710 at room temperature with a quartz cell of 0.1 cm path length, with typically 0.4 g/l of sonicated brain liposomes mixed with 35 μM of peptide. Each spectrum is the average of three scans monitoring 190–260 nm at 1 nm step resolution and a speed of 30 nm/min. Control spectra of buffer with and without liposomes were subtracted from the protein spectra.
Image analysis and size calibration
The images were analysed using ImageJ 1.37v for particle tracking, getting out individual integrated fluorescence intensities for the liposome and protein channel, respectively. Thresholding, minimum particle area and ellipticity were used as tools to faithfully track particles. Custom made software routine in Igor Pro 5.03v found colocalized particles in the two channels and gave out liposome diameter and protein density.
Calibration of liposome diameter was accomplished by a combination of confocal fluorescence microscopy and dynamic light scattering measurements as has been described earlier (Kunding et al, 2008). Briefly, the integrated intensity (I) is proportional to the number of dye molecules in the membrane and hence the surface area (A). Thus, the liposome diameter is equal to the square root of I multiplied by a constant (Ccal),
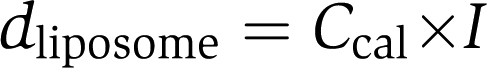 |
The Ccal was determined by size calibration to reference liposomes.
Protein density was calculated on the basis that protein intensity is directly proportional to number of molecules and again that liposome intensity is proportional to surface area. Density is the ratio of integrated protein intensity over integrated liposome intensity. Calibration of protein intensity was adapted from Lohse et al (2008), using a surface model instead of volume. Briefly, we record an image with the same microscope settings in a solution with a known concentration (ccalib) of the same dye. Knowledge of the illumination volume (Vill) of the microscope allows us to relate the total recorded intensity to the total number of fluorophores Ntot (Ntot=ccalib × Vill) and thus extract the average intensity per molecule.
Typical signal to noise ratios are 10–66 (i.e. the background is 10–2% of the signal) for the liposome channel and 7–13 for the protein channel and provide therefore high quality data points. In the membrane curvature sensing graphs in Figure 1 the average s.d. for a point is ±7.5%.
Reconstruction of bulk ensemble measurements based on single liposome data
Liposome populations extruded with 50, 100 and 200 nm filter and without one were prepared and imaged according to previous procedures. For each population, the effective protein density was calculated as the ratio f between total protein signal and total lipid mass. As the protein signal is directly proportional to adsorbed protein mass, f is directly comparable with earlier published density measurements extracted from bulk ensemble experiments. We calculated f as
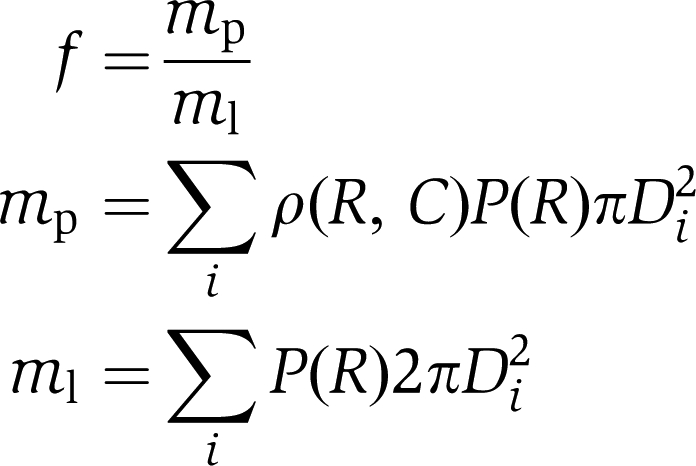 |
Here, mp is non-normalized protein mass, ml is lipid mass, D is the binned diameter, C is concentration of protein, ρ(R, C) is the recorded non-normalized protein density profile and P(R) is the radius distribution histogram. The expression above does not take heterogeneous protein adsorption into account, and hence overestimates the density. We included this contribution by introducing a weighting function C(R), which provides the fraction of vesicles, with a certain radius, having a detectable amount of protein bound:
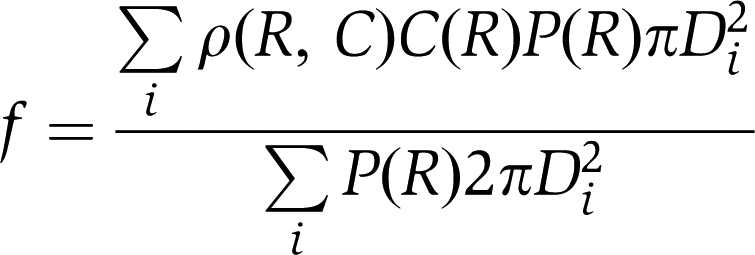 |
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank B Peter and HT McMahon for critically reading the paper, HT McMahon for generously providing NBAR plasmids as well as purified oFBAR and P Lappalainen for contributing purified mIBAR. This work was supported by the Danish Council for Scientific and Strategic Research.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bagatolli LA (2006) To see or not to see: lateral organization of biological membranes and fluorescence microscopy. Biochim Biophys Acta Biomembr 1758: 1541–1556 [DOI] [PubMed] [Google Scholar]
- Bendix PM, Pedersen MS, Stamou D (2009) Quantification of nano-scale intermembrane contact areas by using fluorescence resonance energy transfer. Proc Natl Acad Sci USA 106: 12341–12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Casella JF, Drin G, Mesmin B, Antonny B (2005) ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J 24: 2244–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood PD, Swenson RD, Voth GA (2008) Factors influencing local membrane curvature induction by N-BAR domains as revealed by molecular dynamics simulations. Biophys J 95: 1866–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood PD, Voth GA (2006) Direct observation of Bin/amphiphysin/Rvs (BAR) domain-induced membrane curvature by means of molecular dynamics simulations. Proc Natl Acad Sci USA 103: 15068–15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Peter BJ, Oorschot VMJ, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ (2004) Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol 14: 1791–1800 [DOI] [PubMed] [Google Scholar]
- Chan YHM, Lenz P, Boxer SG (2007) Kinetics of DNA-mediated docking reactions between vesicles tethered to supported lipid bilayers. Proc Natl Acad Sci USA 104: 18913–18918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Arac D, Wang TM, Gilpin CJ, Zimmerberg J, Rizo J (2006) SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys J 90: 2062–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SM, Stamou D (2007) Surface-based lipid vesicle reactor systems: fabrication and applications. Soft Matter 3: 828–836 [DOI] [PubMed] [Google Scholar]
- Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B (2007) A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol 14: 138–146 [DOI] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P (2001) Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol 155: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman E, De Camilli P, Unger V (2008) Structural basis of membrane invagination by F-BAR domains. Cell 132: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop JL, Butler PJG, McMahon HT (2005) Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature 438: 675–678 [DOI] [PubMed] [Google Scholar]
- Gallop JL, Jao CC, Kent HM, Butler PJG, Evans PR, Langen R, McMahon HT (2006) Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J 25: 2898–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann B (2004) The BAR-domain family of proteins: a case of bending and binding? The membrane bending and GTPase-binding functions of proteins from the BAR-domain family. EMBO Rep 5: 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzakis N, Bhatia VK, Larsen J, Madsen K, Bolinger P, Kunding A, Castillo J, Hedegard P, Gether U, Stamou D (2009) How curved membranes recruits amphipathic helices and protein anchoring motifs. Nat Chem Biol (doi:10.1038/nchembio.213) [DOI] [PubMed] [Google Scholar]
- Henne WM, Kent HM, Ford MGJ, Hegde BG, Daumke O, Butler PJG, Mittal R, Langen R, Evans PR, McMahon HT (2007) Structure and analysis of FCHo2F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure 15: 839–852 [DOI] [PubMed] [Google Scholar]
- Kunding A, Mortensen MW, Christensen SM, Stamou D (2008) A fluorescence-based technique to construct size distributions from single object measurements: application to the extrusion of lipid vesicles. Biophys J 95: 1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyper CL, Fujimoto BS, Zhao Y, Schiro PG, Chiu DT (2006) Accurate sizing of nanoparticles using confocal correlation spectroscopy. J Phys Chem B 110: 24433–24441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kerff F, Chereau D, Ferron F, Klug A, Dominguez R (2007) Structural basis for the actin-binding function of missing-in-metastasis. Structure 15: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA (2008) Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9: 99–111 [DOI] [PubMed] [Google Scholar]
- Lohse B, Bolinger PY, Stamou D (2008) Encapsulation efficiency measured on single small unilamellar vesicles. JACS 130: 14372–14373 [DOI] [PubMed] [Google Scholar]
- Manneville JP, Casella JF, Ambroggio E, Gounon P, Bertherat J, Bassereau P, Cartaud J, Antonny B, Goud B (2008) COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc Natl Acad Sci USA 105: 16946–16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT (2007) How synaptotagmin promotes membrane fusion. Science 316: 1205–1208 [DOI] [PubMed] [Google Scholar]
- Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N (2006) Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J 25: 2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P (2007) Missing-in-metastasis and IRSp53 deform PI(4,5)P-2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol 176: 953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Murray D (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438: 605–611 [DOI] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL (2005) Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438: 590–596 [DOI] [PubMed] [Google Scholar]
- Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, Futterer K (2005) Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J 24: 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie ZZ, Hirsch DS, Luo RB, Jian XY, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA (2006) A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol 16: 130–139 [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Groves JT (2007) Curvature and spatial organization in biological membranes. Soft Matter 3: 24–33 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Pylypenko O, Lundmark R, Rasmuson E, Carlsson SR, Rak A (2007) The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J 26: 4788–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Schmid SL (2008) Real-time detection reveals that effectors couple dynamin's GTP-dependent conformational changes to the membrane. EMBO J 27: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Lecuyer S, Stone HA, Losick R (2009) Geometric cue for protein localization in a bacterium. Science 323: 1354–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, De Camilli P (1999) Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron 24: 143–154 [DOI] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Pykalainen A, Laurinmaki P, Mattila PK, Kinnunen PKJ, Butcher SJ, Lappalainen P (2009) Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol 19: 95–107 [DOI] [PubMed] [Google Scholar]
- Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen LR, Liu ZJ, Wang BC, Yamamoto M, Terada T, Miyazawa A, Tanaka A, Sugano S, Shirouzu M, Nagayama K, Takenawa T et al. (2007) Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 129: 761–772 [DOI] [PubMed] [Google Scholar]
- Stamou D, Duschl C, Delamarche E, Vogel H (2003) Self-assembled microarrays of attoliter molecular vessels. Angew Chem Int Ed Engl 42: 5580–5583 [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P (1999) Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol 1: 33–39 [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247 [DOI] [PubMed] [Google Scholar]
- Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T (2006) Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol 172: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Arkhipov A, Schulten K (2009) Simulations of membrane tubulation by lattices of amphiphysin N-BAR domains. Structure 17: 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon TY, Okumus B, Zhang F, Shin YK, Ha T (2006) Multiple intermediates in SNARE-induced membrane fusion. Proc Natl Acad Sci USA 103: 19731–19736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J, Kozlov MM (2006) How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol 7: 9–19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File