Abstract
Regulation of the CLN1 and CLN2 G1 cyclin genes controls cell cycle progression. The SBF activator binds to these promoters but is kept inactive by the Whi5 and Stb1 inhibitors. The Cdc28 cyclin-dependent kinase phosphorylates Whi5, ending the inhibition. Our chromatin immunoprecipitation (ChIP) experiments show that SBF, Whi5 and Stb1 recruit both Cdc28 and the Rpd3(L) histone deacetylase to CLN promoters, extending the analogy with mammalian G1 cyclin promoters in which Rb recruits histone deacetylases. Finally, we show that the SBF subunit Swi6 recruits the FACT chromatin reorganizer to SBF- and MBF-regulated genes. Mutations affecting FACT reduce the transient nucleosome eviction seen at these promoters during a normal cell cycle and also reduce expression. Temperature-sensitive mutations affecting FACT and Cdc28 can be suppressed by disruption of STB1 and WHI5, suggesting that one critical function of FACT and Cdc28 is overcoming chromatin repression at G1 cyclin promoters. Thus, SBF recruits complexes to promoters that either enhance (FACT) or repress (Rpd3L) accessibility to chromatin, and also recruits the kinase that activates START.
Keywords: cell cycle, chromatin, cyclin genes, transcriptional regulation, yeast
Introduction
Cell cycle progression is tightly regulated, particularly at critical points such as the transition from G1 to S phase. In metazoans, the E2F family of heterodimeric transcription factors bind to the promoters of genes that drive progression of the cell cycle (Iaquinta and Lees, 2007; van den Heuvel and Dyson, 2008). E2F binds to promoters throughout the cell cycle, but during most of the cell cycle it is kept in an inactive form due to the binding of the Rb repressor protein. Rb represses transcription in part by masking activity of the E2F activation domain, and in part by recruiting histone deacetylases (HDAC) that modify histones at the promoters. The rise in cyclin-dependent kinase (CDK) activity during late G1 results in phosphorylated Rb, which no longer binds to E2F. This ends the recruitment of the inhibitory HDAC and exposes the E2F activation domain, allowing expression of genes such as cyclins that drive cell cycle progression.
In Saccharomyces cerevisiae, expression of the CLN1 and CLN2 G1 cyclin genes drives the transition from G1 to S known as START (Wittenberg and Reed, 2005; Bloom and Cross, 2007). CLN1 and CLN2 expression is activated by the heterodimeric SBF DNA-binding factor composed of Swi4 and Swi6, and SBF participates in a positive feedback loop that is important for producing the burst of cyclins that accompanies START (Skotheim et al, 2008). Swi6 is also present, with Mbp1, in the heterodimeric MBF factor that promotes expression of other G1/S genes. Swi6 is present in the nucleus only during the G1 phase of the cell cycle (Sidorova et al, 1995; Harrington and Andrews, 1996; Koch et al, 1996).
There are strong analogies between metazoans and yeast in terms of regulation of the G1 cyclin genes. In both cases, there are heterodimeric DNA-binding activators, E2F or SBF, which are kept inactive by an inhibitor, either Rb or Whi5 in yeast (Schaefer and Breeden, 2004). Interestingly, E2F and SBF recognize very similar sequences, GCGCGAAA (Thalmeier et al, 1989) and CNCGAAA (Badis et al, 2008), even though they are unrelated by sequence or structural motifs. In both cases, gene activation requires a CDK (Cdc28 in yeast) that phosphorylates and inactivates the inhibitor (Costanzo et al, 2004; de Bruin et al, 2004). Phosphorylation of the Whi5 inhibitor in yeast results in export from the nucleus, and this is an important part of the positive feedback loop that drives the cell cycle (Skotheim et al, 2008).
The Stb1 protein also contributes to the regulation of SBF and MBF target genes. Stb1 interacts with SBF and MBF, and Stb1 binds to SBF- and MBF-specific promoters during G1 phase (Ho et al, 1999; Costanzo et al, 2003; de Bruin et al, 2008). Stb1 has a function in both transcriptional repression and activation, as stb1 mutants reduce both the repression in early G1 and also the induced RNA level of late G1 (de Bruin et al, 2008). Stb1 has been shown to interact with Sin3, a component of Rpd3(L) (Kasten and Stillman, 1997), and also with Swi6, present in both SBF and MBF (Ho et al, 1999).
Activation of the yeast HO gene requires a number of activators, including SBF (Breeden and Nasmyth, 1987; Nasmyth, 1993). We have recently described alterations in chromatin structure that occur at the HO promoter during the cell cycle, and showed that changes at the URS2 region of the promoter require SBF (Takahata et al, 2009). SBF is required both for eviction of nucleosomes and for the recruitment of three coactivator complexes, Swi/Snf, SAGA and Mediator, to HO URS2. Additionally, SBF recruits the FACT chromatin reorganizing complex to the promoter, and FACT and Swi6 co-immunoprecipitate (Takahata et al, 2009). The FACT chromatin reorganizing complex changes the accessibility of DNA within a nucleosome, but unlike Swi/Snf remodellers, FACT activity is ATP independent (Formosa, 2008). Genetic and biochemical studies suggest FACT functions in DNA replication, transcription initiation, and transcription elongation. We recently showed that FACT mutations lower HO expression by reducing nucleosome eviction from the region of the HO promoter bound by SBF (Takahata et al, 2009). FACT mutants are defective in expressing G1 cyclin genes and show impaired G1/S transition (Rowley et al, 1991; Lycan et al, 1994; Schlesinger and Formosa, 2000; Biswas et al, 2008b).
Here, we investigate events at the promoters of the CLN1 and CLN2 genes. We show that SBF binds transiently during the cell cycle to these promoters, and that SBF recruits Cdc28, Rpd3(L) and FACT to these promoters. FACT binds transiently to the promoters of the G1/S regulon genes, and FACT mutations reduce both nucleosome eviction and gene transcription. We extend the analogy between yeast and metazoan cyclin genes by showing that the Rpd3(L) HDAC is recruited to the promoters of the yeast G1 cyclin genes by SBF. Rpd3(L) recruitment requires the Whi5 inhibitor and also the Stb1 protein. Stb1 has been shown to interact with Sin3, a component of Rpd3(L) (Kasten and Stillman, 1997), and also with Swi6 (Ho et al, 1999). Finally, mutations in STB1 and WHI5 permit growth at normally nonpermissive temperatures of strains with conditional mutations affecting FACT and Cdc28, suggesting that transcriptional activation of G1/S target genes by overcoming Stb1/Whi5 inhibition is a critical function of FACT and Cdc28.
Results
SBF binds to CLN2 transiently during the cell cycle
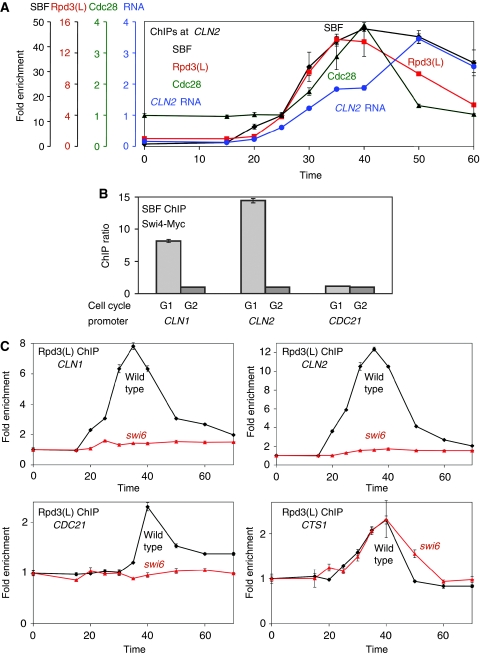
We used ChIP assays to measure Swi4-Myc binding to the CLN2 promoter during the cell cycle (Figure 1A). Cells were synchronized by GALp∷CDC20 arrest and release, and samples were taken at various times to determine SBF binding. SBF binding begins at ∼20 min after release and peaks at 50 min. Late G1 in these synchronized cells is about 30 min, when CLN2 mRNA levels start to rise; CLN2 RNA levels peak at about 50 min. Using synchronized cells, we see SBF binding to the CLN1 and CLN2 promoters during G1, but binding is not seen in G2-arrested cells (Figure 1B). SBF does not bind to CDC21, an MBF-activated gene. SBF association with the cyclin promoters is therefore transient and correlates with transcription of the genes.
Figure 1.
SBF recruits Rpd3(L) to promoters. (A) DY12794 (GALp∷CDC20 SWI4-Myc), DY12247 (GALp∷CDC20 SDS3-Myc) and DY6669-Cdc28-HA (GALp∷CDC20 YCp:KanMX:CDC28-HA) cells were synchronized in mitosis by removing galactose, followed by release by addition of galactose (0 min). The DY6669-Cdc28-HA cells were grown in medium containing G418. The CDC20 arrest is at the G2/M transition, and CLN2 expression begins at 30 min after release corresponds to late G1 phase. Binding of the Swi4-Myc subunit of SBF and the Sds3-Myc subunit of Rpd3(L) were measured ChIP using samples taken at various times after the release. CLN2 RNA measurements were from synchronized DY12752 (GALp∷CDC20 GCN5-Myc). (B) Swi4-Myc binding at CLN1 (SBF regulated), CLN2 (SBF regulated) and CDC21 (MBF regulated) was assessed in DY12794 cells (GALp∷CDC20 SWI4-Myc) either in G1 (35 min after CDC20 arrest and release) or arrested in G2 (0 min). (C) Strains DY12247 (GALp∷CDC20 SDS3-Myc) and DY12828 (GALp∷CDC20 SDS3-Myc swi6) were synchronized and ChIP experiments preformed to measure Rpd3(L) binding at CLN1, CLN2, CDC21 and CTS1. A full-colour version of this figure is available at The EMBO Journal Online.
Rpd3(L) is recruited to SBF- and MBF-dependent promoters by Swi6
SBF is functionally analogous to mammalian E2F, and E2F recruits HDACs to promoters (Iaquinta and Lees, 2007; van den Heuvel and Dyson, 2008). On the basis of this analogy, we asked whether the Rpd3(L) HDAC was recruited to the CLN2 promoter. We performed ChIP assays with synchronized cells to detect Sds3-Myc at promoters. Sds3 is a subunit of Rpd3(L), but absent from the Rpd3(S) complex (Carrozza et al, 2005). Rpd3(L) and Rpd3(S) appear to have different functions, with Rpd3(L) localized primarily to promoter regions and Rpd3(S) at transcribed regions (Joshi and Struhl, 2005). Rpd3(L) binds to CLN2 with kinetics very similar to that observed for SBF (Figure 1A). However, Rpd3(L) binding decays more rapidly than does SBF. The similar kinetics of binding for SBF and Rpd3(L) suggest that SBF recruits Rpd3(L). To test this idea we examined Rpd3(L) binding to promoters in swi6 mutants (Figure 1C). Binding of the Sds3-Myc subunit of Rpd3(L) to CLN1 and CLN2 is lost in the swi6 mutant. Rpd3(L) also binds to the promoter of the MBF-dependent CDC21 gene, although later in the cell cycle. Swi6 is a subunit of both SBF and MBF, and Rpd3(L) binding to CDC21 is also lost in the swi6 mutant. Rpd3(L) also binds to the CTS1 promoter, recruited by the Fkh1 and Fkh2 factors (Voth et al, 2007). No defect in Rpd3(L) binding to CTS1 is seen in the swi6 mutant, and thus the swi6 mutation does not simply affect the integrity of the HDAC complex.
Whi5 and Stb1 are required for Rpd3(L) binding to SBF-dependent genes
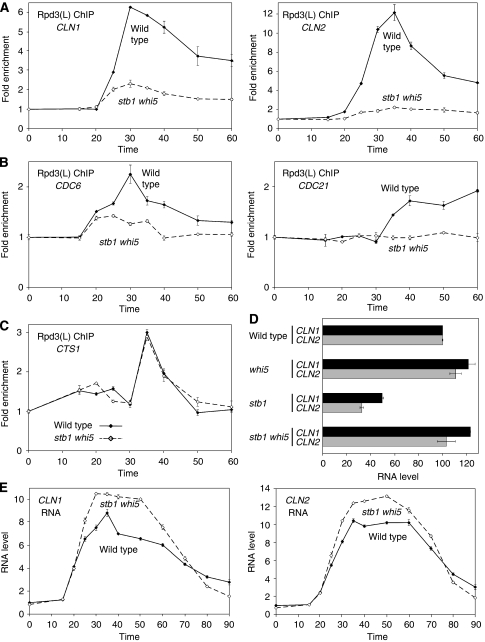
Stb1 and Whi5 interact with SBF and are described as inhibitors of SBF (Costanzo et al, 2004; de Bruin et al, 2004, 2008). The analogy between these inhibitors and mammalian Rb protein suggested that they could be the intermediaries that allow SBF to recruit the Rpd3(L) HDAC. We examined Rpd3(L) binding to the CLN1 and CLN2 promoters in strains with either a stb1 or a whi5 mutation (Supplementary Figure S1). A stb1 mutation modestly reduces Rpd3(L) binding to CLN1 or CLN2, and a whi5 mutation has little effect on Rpd3(L) binding. We next examined Rpd3(L) binding to SBF-dependent promoters in a strain lacking both Stb1 and Whi5 (Figure 2A). Rpd3(L) binding to the SBF-dependent CLN1 and CLN2 promoters is essentially eliminated in the stb1 whi5 mutant. Importantly, Rpd3(L) binding to CTS1 is not affected in the stb1 whi5 double mutant (Figure 2C). Additionally, SBF binding to CLN1 and CLN2 is not affected by either a stb1 or a whi5 mutation (Supplementary Figure S2). We conclude that Whi5 and Stb1 both link Rpd3(L) to SBF.
Figure 2.
Rpd3(L) binding to SBF and MBF promoters requires Stb1 and Whi5. (A–C) Strains DY12248 (GALp∷CDC20 SDS3-Myc) and DY13502 (GALp∷CDC20 SDS3-Myc stb1 whi5) were synchronized and ChIP experiments preformed to measure Rpd3(L) binding at (A) SBF-dependent CLN1 and CLN2, (B) MBF-dependent CDC6 and CDC21 and (C) SBF- and MBF-independent CTS1. (D) CLN1 and CLN2 expression is reduced in stb1 mutants but not in stb1 whi5 double mutants. RNA was isolated from log phase wild-type (DY150), whi5 (DY10474), stb1 (DY13454) and stb1 whi5 (DY13494), strains and mRNA levels measured by RT–qPCR. (E) CLN1 and CLN2 expression is modestly increased in synchronized stb1 whi5 double mutants. Strains DY6669 (GALp∷CDC20) and DY13496 (GALp∷CDC20 stb1 whi5) were synchronized and mRNA levels measured by RT–qPCR.
Whi5 has been described as a negative regulator of SBF-dependent genes (Costanzo et al, 2004; de Bruin et al, 2004), consistent with its ability to recruit Rpd3(L). This predicts that a whi5 mutation should result in increased expression of target genes. However, RNA measurements show nearly normal expression of CLN1 and CLN2 in whi5 mutants (Figure 2D). Stb1 has been described as promoting transcriptional activation (de Bruin et al, 2008), and consistent with this, we find decreased expression of SBF-dependent genes in a stb1 mutant (Figure 2D). Interestingly, the stb1 defect in transcriptional activation is suppressed by a whi5 mutation. As a control, expression of the CYC1 gene is unaffected by whi5 or stb1 mutations (Supplementary Figure S3). These results support the idea that Stb1 is an activator, whereas Whi5 is a transcriptional inhibitor, but additional mechanisms appear to limit the level of CLN1 and CLN2 transcription to near the WT level in cells lacking only Whi5.
We expected that CLN1 and CLN2 RNA levels would be significantly higher in the stb1 whi5 double mutant, as Rpd3(L) binding to these promoters is lost in this mutant. However, in the asynchronous stb1 whi5 cells CLN2 RNA was at wild-type levels and CLN1 RNA was only slightly increased (Figure 2D). We therefore synchronized wild-type and stb1 whi5 cells and measured CLN1 and CLN2 RNA during the cell cycle (Figure 2E). There is no significant effect on the kinetics or RNA accumulation, and only a modest increase in the levels of CLN1 and CLN2 RNA.
Only Stb1 is required for Rpd3(L) binding to MBF-dependent genes
Rpd3(L) binds to the promoters of MBF-dependent genes, and this binding requires the Swi6 protein common to both SBF and MBF (Figure 1C). Similarly, Rpd3(L) binding to CDC6 and CDC21, which are MBF dependent, is lost in the stb1 whi5 double mutant (Figure 2B), like the SBF-dependent CLN1 and CLN2 promoters. Rpd3(L) binding to MBF target genes is not affected by a whi5 mutation (Supplementary Figure S1C), similar to SBF genes (Supplementary Figure S1A). However, a major difference in factor dependence was seen for Rpd3(L) binding to MBF-dependent and SBF-dependent promoters. A stb1 mutation essentially eliminates Rpd3(L) binding to CDC6 and CDC21 (Supplementary Figure S1D), whereas the stb1 mutation results in only a modest reduction in Rpd3(L) binding to the SBF-dependent CLN1 and CLN2 promoters (Supplementary Figure S1B). Expression of MBF-dependent genes is reduced in a stb1 mutant (Supplementary Figure S3), as was seen for SBF-dependent genes (Figure 2D). However, although a whi5 mutation strongly suppresses the stb1 defect in the activation of MBF-dependent genes, there is less suppression at MBF-dependent genes by whi5.
Although Stb1 interacts with the Swi6 subunit present in both SBF and MBF (de Bruin et al, 2008), there is discrepancy in the literature as to whether Whi5 interacts with both SBF and MBF or with SBF alone SBF (Costanzo et al, 2004; de Bruin et al, 2004). To address this question in our strain background we performed co-immunoprecipitation experiments with tagged alleles. The experiment performed with extracts prepared from a Whi5-Flag and Swi4-Myc strain show that Whi5 interacts with the SBF-specific Swi4 protein, irrespective of which antibody is used for the immunoprecipitation (Supplementary Figure S4A). In contrast, we saw no interaction between Whi5-Flag and the Mbp1-Myc subunit of MBF (Supplementary Figure S4B). We conclude that Whi5 does not interact with MBF, and this is consistent with our result that only Stb1 is required for recruiting Rpd3(L) to MBF-dependent genes.
Cdc28 is recruited to SBF- and MBF-dependent promoters
The Cdc28 CDK has long been implicated in transcriptional activation by SBF and MBF (Wittenberg and Reed, 2005). Cdc28 has been shown to be directly recruited to the CDC20 promoter when it is expressed in late G2 (Morris et al, 2003), so we performed ChIP experiments and found that Cdc28 is also recruited directly to both SBF- and MBF-dependent promoters (Supplementary Figure S5). Disruption of either SWI6 or WHI5 eliminates Cdc28 binding, whereas a stb1 mutation only modestly reduces Cdc28 recruitment. Whi5 does not bind to MBF (Supplementary Figure S4; de Bruin et al, 2004), and thus it is surprising that Cdc28 binding is reduced in the whi5 mutant. A whi5 mutation affects cell cycle progression (Jorgensen et al, 2002), and thus it could indirectly affect Cdc28 recruitment in these asynchronous cells. CDC20 expression is independent of SBF or MBF (Simon et al, 2001), and Cdc28 recruitment to CDC20 is not affected by swi6, whi5 or stb1 mutations. We also examined Cdc28 binding to CLN2 in synchronized cells (Figure 1A). The kinetics of Cdc28 binding is similar to that seen for SBF, although Cdc28 binding decreases more rapidly after peak binding. This rapid decline in Cdc28 binding is consistent with this kinase phosphorylating Whi5 resulting in nuclear export of Whi5 (Costanzo et al, 2004; de Bruin et al, 2004), and the loss of Whi5 would end Cdc28 binding. We conclude that Cdc28 recruitment to SBF- and MBF-dependent promoters requires Swi6 and Whi5.
FACT and Swi6 are required for nucleosome eviction at CLN2
Chromatin disassembly at promoters often accompanies gene activation (Workman, 2006; Williams and Tyler, 2007). We determined earlier that nucleosome eviction at the SBF-dependent HO promoter requires both FACT and Asf1 histone chaperones (Takahata et al, 2009). FACT co-immunoprecipitates with Swi6 (Takahata et al, 2009), and we wanted to determine whether this interaction was direct. We expressed a GST-Swi6 fusion protein in Escherichia coli, and loaded the purified GST-Swi6 protein onto glutathione-agarose beads. Purified FACT was loaded onto the beads, washed and eluted. The Spt16 and Pob3 subunits of FACT bind to GST-Swi6, but not to the GST-only or the GST-Swi5 controls (Figure 3). We conclude that FACT and Swi6 interact directly.
Figure 3.
FACT interacts directly with Swi6. Glutathione-agarose beads were loaded with either GST only, GST-Swi6 or GST-Swi5 and purified FACT was applied to the beads, washed and eluted. The eluted material is analysed on western blots with antibodies to GST, Spt16 and Pob3. There are significant degradation products, particularly for GST-Swi6. Lane 1 contains the input extract (corresponding to 50% of the amount of material applied to the beads.
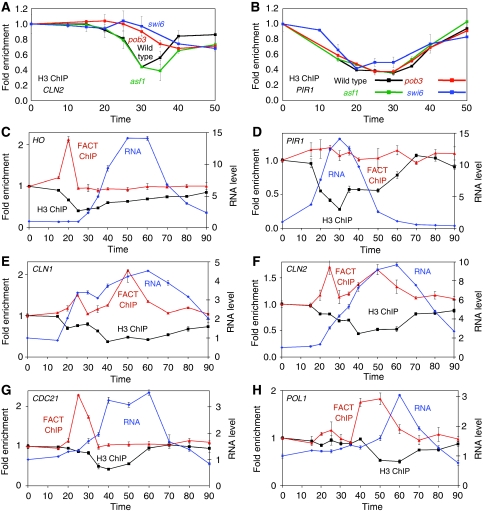
We next examined nucleosome eviction from cyclin gene promoters during their activation, and any function for FACT and Asf1 in this process. We used synchronized cells for a ChIP experiment to measure histone H3 occupancy at CLN2 during the cell cycle in wild-type cells and in cells with mutations in ASF1, SWI6 and POB3 (Figure 4A). The Spt16 and Pob3 subunits of FACT are encoded by essential genes (Formosa, 2008), and in these experiments we used a pob3(L78R) mutation that strongly decreases the level of Pob3 protein even at the permissive temperature (VanDemark et al, 2008). In wild-type cells, nucleosomes are transiently evicted from the CLN2 promoter at 30 min after release from GALp∷CDC20 arrest. Nucleosome eviction at CLN2 is sharply reduced in pob3 or swi6 mutants, but the asf1 mutation has no effect. Thus, CLN2 differs from PHO5 and HO in which Asf1 is required for chromatin disassembly (Adkins et al, 2004; Takahata et al, 2009). PIR1 is a cell cycle-regulated gene expressed in M/early G1, and nucleosomes are evicted from the PIR1 promoter transiently during the cell cycle (Figure 4B). The pob3 and swi6 mutations have no effect on nucleosome loss at PIR1. Our data suggest that chromatin disassembly occurs at many cell cycle genes coincident with transcriptional activation, but the mechanism of eviction varies widely. Importantly, FACT and Swi6 are required for the changes in chromatin structure at the CLN2 promoter.
Figure 4.
FACT binding at SBF and MBF promoters precedes nucleosome eviction and gene expression. (A) Histone H3 ChIP was performed to measure nucleosome occupancy at the CLN2 promoter using synchronized strains DY6669 (GALp∷CDC20), DY12914 (GALp∷CDC20 asf1), DY11246 (GALp∷CDC20 pob3) and DY13373 (GALp∷CDC20 swi6). (B) Histone H3 ChIP measuring nucleosome occupancy at the PIR1 promoter, as in panel A. (C–H) Strain DY6669 (GALp∷CDC20) was synchronized and samples taken for FACT (Spt16) ChIP, histone ChIP and mRNA measurements for (C) HO URS2, (D) PIR1, (E) CLN1, (F) CLN2, (G) CDC21 and (H) POL1. Supplementary Figure S8 shows the same experiment conducted with a swi6 mutant.
Nucleosome eviction at CLN2 is reduced, or possibly delayed, in strains with mutations affecting FACT or SBF. On the basis of this result, we examined CLN2 mRNA levels during the cell cycle in synchronized wild-type, pob3 or swi6 strains. There is a marked delay in the appearance of CLN2 mRNA in the pob3 and swi6 mutants (Supplementary Figure S6A), but transcription eventually occurs. Earlier work has suggested a function for Rme1 in activating CLN2 (Toone et al, 1995). Overexpression of Rme1 increases CLN2 expression and also permits growth of normally inviable cells lacking both SBF subunits. The Rme1 DNA-binding protein binds to the CLN2 promoter in vitro and activates through promoter sequences distinct from the SBF-binding sites. To address the function of RME1 in CLN2 activation, we constructed rme1 mutant strains and also made double mutants with swi6 and pob3. RNA measurements with asynchronous cells show reduced CLN2 expression in rme1 mutants, and strong additive defects in swi6 rme1 and pob3 rme1 double-mutant strains (Supplementary Figure S7). Although the swi6 and pob3 mutations reduce HO expression, little additive effect was seen in the swi6 rme1 and pob3 rme1 double-mutant strains. Finally, PIR1 expression was unaffected by rme1, swi6 or pob3 mutations. These results are consistent with the idea that Rme1 promotes the residual CLN2 activation in strains with SBF or FACT mutations.
In contrast to the situation at CLN2 in which there is residual expression in the swi6 and pob3 mutants (Supplementary Figure S6A), expression of the SBF- and FACT-dependent HO gene is largely eliminated in both pob3 and swi6 cells (Supplementary Figure S6B). HO gene transcription requires recruitment of coactivator complexes to the URS2 region of the promoter, and mutations affecting FACT or SBF eliminate this coactivator recruitment (Takahata et al, 2009). We therefore examined binding of the Gcn5 subunit of the SAGA coactivator to CLN2 using ChIP assays to see whether this mechanism is also used at this promoter (Supplementary Figure S6C). In wild-type cells Gcn5 binds transiently to CLN2 during the cell cycle, at about the same time as SBF that binds. The pob3 and swi6 mutations do not eliminate Gcn5 binding to CLN2, but rather cause a delay similar to the delay in mRNA levels. We conclude that FACT and Swi6 are required to express the CLN2 promoter at the appropriate time because they allow eviction of nucleosomes and coactivator recruitment to occur rapidly. This is unlike the situation at the HO promoter in which the gene is completely transcriptionally inactive in FACT or SBF mutants. In contrast, nucleosome eviction, coactivator recruitment and gene expression eventually occur at CLN2 in these mutants, and thus FACT and Swi6 are not absolutely required here.
Swi6 is required for FACT binding and nucleosome eviction at SBF- and MBF-regulated genes
On the basis of the delay in CLN2 activation in swi6 mutants during the cell cycle, we decided to repeat the histone H3 ChIP experiment but with a longer time course, so that we could discriminate between events that are required for CLN2 activation and those that affect its rate. FACT binds to HO URS2 in a SWI6-dependent manner (Takahata et al, 2009), and so we measured FACT binding in wild-type cells (Figure 4C–H), and in swi6 mutants (Supplementary Figure S8). Both wild-type and swi6 cells were synchronized by GALp∷CDC20 arrest and release, and samples were taken at time points for H3 ChIP, FACT ChIP and RNA measurements; each measurement is therefore from the same culture of synchronized cells.
FACT binds to HO URS2 at 20 min, just before nucleosomes are evicted, with strong gene expression seen starting at 40 min (Figure 4C), consistent with our earlier experiments (Takahata et al, 2009). The PIR1 gene, whose cell cycle expression is independent of Swi6, serves as a control (Figure 4D). PIR1 expression is not affected by a FACT mutation (Takahata et al, 2009), and FACT does not bind to this promoter. Nucleosomes are transiently evicted during the cell cycle as the gene is expressed, and these events are not affected in a swi6 mutant (Supplementary Figure S8). Thus, the swi6 mutation does not disrupt events at all cell cycle-regulated genes.
Expression of the SBF-dependent genes CLN1 and CLN2 is reduced in FACT mutants (Rowley et al, 1991; Lycan et al, 1994; Takahata et al, 2009), and this is also seen in our experiments analysing RNA levels in synchronized cells (Figure 4; Supplementary Figure S8). There are two peaks of FACT binding at these promoters, one at 25 min after release and one at 50 min. (See also the independent ChIP experiments in Figure 5 showing this result is reproducible.) Nucleosome occupancy decreases first at 20 min, and then further at 40 min; return of nucleosomes to the promoters begins at 50 min. There is no FACT binding to these promoters in the swi6 mutant (Supplementary Figure S8C and D). However, the swi6 mutation causes only a modest change in nucleosome disassembly, despite the markedly reduced and delayed gene expression. Although no FACT binds to the promoters in the swi6 mutant, nucleosome eviction and gene transcription still occur, albeit more slowly. The delayed and reduced residual chromatin disassembly and transcription may be induced by Rme1 (Toone et al, 1995). We conclude that although FACT recruitment is correlated with timely gene induction, it is not absolutely required for nucleosome disassembly at these promoters.
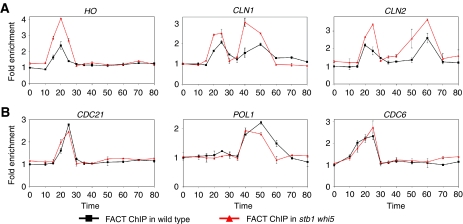
Figure 5.
FACT binding to SBF-regulated genes increases in stb1 whi5 mutant. (A) FACT binding to SBF promoters increases in a stb1 whi5 mutant. Strains DY6669 (GALp∷CDC20) and DY13496 (GALp∷CDC20 swi6) were synchronized and FACT binding to SBF promoters HO URS2, CLN1 and CLN2 was measured. (B) FACT binding to MBF promoters does not change in a stb1 whi5 mutant. The ChIP material used in panel A was analysed for FACT binding to MBF promoters CDC21, POL1 and CDC6. A full-colour version of this figure is available at The EMBO Journal Online.
We also examined promoter events at two MBF-dependent promoters, CDC21 and POL1. At CDC21 there is a sharp peak of FACT binding at 20 min, followed by nucleosome loss and gene transcription (Figure 4G). All of these events are eliminated in a swi6 mutant (Supplementary Figure S8E). The kinetics at CDC21 resemble those seen at HO (Figure 4C), except that FACT binding and chromatin disassembly occur slightly sooner at HO. Events at POL1 occur later in the cell cycle (Figure 4H). Major FACT binding is seen at 40 min after release, although there may be weak transient binding at 20–25 min. Nucleosome eviction and gene transcription also occur later, after the peak in FACT binding. FACT binding, chromatin disassembly, and gene activation are all lost in a swi6 mutant (Supplementary Figure S8F).
These studies allow us to make a number of conclusions regarding the common features of promoters that are expressed at different times during the cell cycle. FACT binds transiently during the cell cycle to promoters of SBF- and MBF-activated genes. This FACT binding is followed first by changes in chromatin structure at the promoter and subsequently by gene transcription. Swi6 is required for recruitment of FACT to these promoters and for chromatin disassembly, although there is significant nucleosome loss at CLN1 and CLN2 in a swi6 mutant so FACT is not required for all nucleosome loss, and nucleosome loss is not sufficient to cause increased transcription.
Whi5 and Stb1 inhibit FACT binding to SBF-dependent promoters
SBF recruits both Stb1 and Whi5 to promoters, and SBF also recruits FACT. We wondered whether Stb1 or Whi5 were required for recruiting FACT. We measured FACT binding in synchronized wild-type cells and a stb1 whi5 double mutant. To our surprise, in stb1 whi5 mutants we observed markedly increased FACT binding to three SBF-dependent genes, HO, CLN1 and CLN2 (Figure 5A). This suggests that Stb1 or Whi5, or both, inhibit FACT binding to SBF-dependent promoters. In contrast, FACT binding to three MBF-dependent promoters was not affected in the stb1 whi5 double mutants (Figure 5B). Whi5 binds to SBF but not to MBF (Supplementary Figure S4; de Bruin et al, 2004). We suggest that Whi5 antagonizes FACT binding to SBF-dependent promoters, consistent with Whi5's function as a transcriptional inhibitor.
stb1 and whi5 suppress FACT and cdc28 mutations
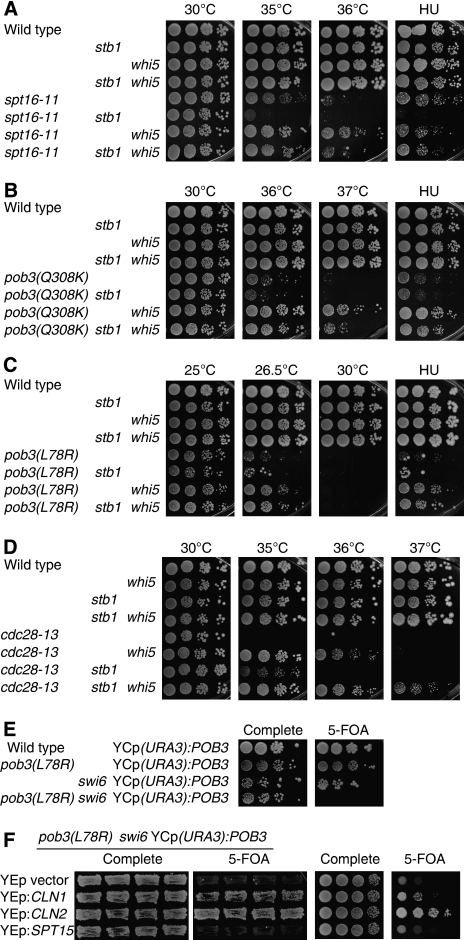
Consistent with an antagonistic relationship between FACT and Whi5, we identified genetic interactions between FACT, WHI5 and STB1. An spt16-11 mutation in FACT shows growth defects at higher temperatures, but this defect is suppressed by whi5 (Figure 6A). In contrast, the spt16-11 stb1 double mutant shows an additive defect compared with either single mutant at 35°C or on media containing hydroxyurea (HU). This defect is suppressed in the spt16-11 stb1 whi5 triple mutant, and thus whi5 is epistatic to stb1. A whi5 mutation also suppresses both the temperature- and HU-sensitive phenotypes caused by two pob3 alleles (Figure 6B and C); a synthetic defect is not seen in the pob3 stb1 double mutants.
Figure 6.
stb1 and whi5 suppress cdc28 and FACT mutations. (A) Serial dilutions of strains DY150 (wild type), DY13454 (stb1), DY9558 (whi5), DY13640 (stb1 whi5), DY7815 (spt16-11), DY13714 (spt16-11 stb1), DY13716 (spt16-11 whi5) and DY13718 (spt16-11 stb1 whi5) were incubated on medium at 30°C for 2 days, at 35°C for 2 days, at 36°C for 3 days, or on medium containing 50 mM HU at 25°C for 5 days. (B) Serial dilutions of strains DY150 (wild type), DY13454 (stb1), DY9558 (whi5), DY13640 (stb1 whi5), DY12236 (pob3(Q308K)), DY13696 (pob3(Q308K) stb1), DY13698 (pob3(Q308K) whi5) and DY13700 (pob3(Q308K) stb1 whi5) were incubated on medium at 30°C for 2 days, at 36°C for 3 days, at 37°C for 3 days, or on medium containing 25 mM HU at 25°C for 3 days. (C) Serial dilutions of strains DY150 (wild type), DY13454 (stb1), DY9558 (whi5), DY13640 (stb1 whi5), DY7379 (pob3(L78R)), DY13704 (pob3(L78R) stb1), DY13702 (pob3(L78R) whi5) and DY13706 (pob3(L78R) stb1 whi5) were incubated on medium at 25°C for 3 days, at 26.5°C for 4 days, at 30°C for 2 days, or on medium containing 100 mM HU at 25°C for 5 days. (D) Serial dilutions of strains DY150 (wild type), DY10474 (whi5), DY13454 (stb1), DY13494 (stb1 whi5), DY12836 (cdc28-13), DY11982 (cdc28-13 whi5), DY13541 cdc28-13 stb1) and DY13543 (cdc28-13 stb1 whi5) were incubated on medium at 30°C for 3 days, at 35°C for 3 days, at 36°C for 3 days or at 37°C for 5 days. (E) Serial dilutions of strains DY150 (wild type), DY7379 (pob3(L78R)), DY13357 (swi6) and DY14024 (pob3(L78R) swi6), each transformed with the YCp(URA3):POB3 plasmid, were incubated on either complete medium or 5-FOA plates at room temperature for 4 days. (F) Strain DY14024 (pob3(L78R) swi6) with the YCp(URA3):POB3 plasmid was transformed with one of four multicopy plasmids, the empty YEp-LEU2 vector, YEp:CLN1, YEp:CLN2, or YEp:SPT15, and the transformants tested for the ability to lose the YCp(URA3):POB3 plasmid and grow on 5-FOA plates. In the left two panels, four transformants were patched on medium lacking leucine and uracil, and replica plated and grown at room temperature for 4 days. In the right two panels, serial dilutions of a single transformant were incubated at room temperature for 5 days.
At the nonpermissive temperature, cdc28-13 mutants arrests in G1, before START (Mendenhall et al, 1988). We next examined whether stb1 or whi5 mutations could suppress the cdc28-13 defects. Although cdc28-13 cells do not form colonies at temperatures of 35°C or above, growth is restored by either a stb1 or whi5 mutation (Figure 6D). Suppression by whi5 is more robust, and only the cdc28-13 whi5 strain is able to grow at 36°C, whereas cdc28-13 stb1 cells cannot. Finally, suppression by stb1 and whi5 is additive, as only the cdc28-13 stb1 whi5 triple mutant can grow at 37°C. This stb1 whi5 double mutant has a growth defect at elevated temperatures (Supplementary Figure S9), making this suppression of cdc28-13 all the more impressive. These genetic results confirm that Whi5 antagonizes both FACT and CDK functions, but Stb1's function is more complex as it appears to promote FACT function but to antagonize CDK.
CLN2 overexpression suppresses pob3 swi6 synthetic lethality
CLN2 transcription is reduced in both pob3 and swi6 mutants, and we wanted to see whether CLN2 expression is further reduced in a double-mutant strain. A doubly heterozygous +/pob3 +/swi6 diploid strain was transformed with a YCp(URA3) plasmid with a wild-type POB3 gene. After sporulation, a haploid pob3 swi6 double-mutant strain with the YCp(URA3):POB3 was isolated. We tested whether this strain could grow on medium containing 5-FOA, as only strains lacking the URA3 plasmid can grow on these plates. The pob3 swi6 YCp(URA3):POB3 cells are unable to grow on 5-FOA (Figure 6E), showing that pob3 and swi6 are synthetically lethal. We next transformed these pob3 swi6 YCp(URA3):POB3 cells with multicopy YEp(LEU2) plasmids, and tested these transformants for the ability to grow on 5-FOA medium. As shown in Figure 6F, a multicopy plasmid with CLN2 allows cells to lose the YCp(URA3):POB3 plasmid and grow on 5-FOA; multicopy CLN1 also suppresses the pob3 swi6 synthetic lethality but to a lesser extent. This genetic experiment suggests that the pob3 and swi6 mutations are synthetically lethal because of insufficient G1 cyclin gene expression.
Discussion
High level expression of the CLN1 and CLN2 G1 cyclin genes are required for a yeast cell to pass through START, the commitment point of the cell cycle (Wittenberg and Reed, 2005; Bloom and Cross, 2007). Here, we provide new mechanistic insights into the control of these tightly regulated genes, with implications for control of metazoan cell cycle genes. (1) Nucleosomes are transiently evicted from the CLN1 and CLN2 promoters during the cell cycle when the genes are activated, and the FACT chromatin reorganizing complex is required for both nucleosome eviction and transcriptional activation. (2) The Swi6 subunit of the SBF and MBF G1-specific transcription factors recruits FACT to G1-specific promoters. (3) The Rpd3(L) HDAC is also recruited to SBF-dependent promoters through two intermediary proteins, Stb1 and Whi5, that interact with SBF and thus inhibit transcription by recruiting the HDAC. (4) Only Stb1 is required to recruit Rpd3(L) to MBF-dependent promoters, as Whi5 does not interact with MBF. (5) The Cdc28 CDK that drives cell cycle progression is also recruited to SBF-dependent genes by Swi6 and Whi5. (6) FACT and the Cdc28 CDK are both required for viability, but normally temperature-sensitive mutations can be suppressed by mutations in STB1 and WHI5, suggesting that FACT and Cdc28 have important functions in the activation of G1 targets genes. (7) Finally, combining mutations in FACT and SBF results in lethality, but the suppression of this synthetic lethality by a multicopy CLN2 plasmid, originally isolated as a multicopy suppressor of a cdc28 temperature-sensitive mutant (Hadwiger et al, 1989), shows a critical function for FACT and SBF in activating expression of G1 cyclin genes to regulate the G1/S transition.
The Whi5 inhibitor of G1-specific transcription has been described as analogous to the mammalian Rb transcriptional inhibitor (Schaefer and Breeden, 2004). In mammalian cells, G1-specific promoters are bound by the heterodimeric E2F activator, which is inhibited by Rb. Rb phosphorylation by cyclin D-CDK4/6 at G1/S ends the Rb–E2F interaction and thus allows transcriptional activation (Iaquinta and Lees, 2007; van den Heuvel and Dyson, 2008). In yeast, Whi5 binds to and inhibits the heterodimeric SBF activator, and Whi5 phosphorylation by the Cdc28 CDK results in nuclear export (Costanzo et al, 2004; de Bruin et al, 2004). Rb inhibits transcriptional activation by recruiting HDACs and we show that the Rpd3(L) HDAC is recruited to the promoters of yeast G1-specific genes. Nuclear entry by Swi6 is regulated within the cell cycle (Sidorova et al, 1995), and ChIP experiments show that SBF binds to promoters transiently in the cell cycle. Comparing the binding kinetics at CLN2 supports the hypothesis that SBF recruits Rpd3(L) (Figure 1A). However, Rpd3(L) binding decays more rapidly, consistent with the idea that Cdc28 phosphorylation of Whi5 results in loss of both Whi5 and Rpd3(L) from the promoter even while SBF remains bound (Figure 7). The kinetics of Cdc28 recruitment to the CLN2 promoter (Figure 1A) are consistent with this model.
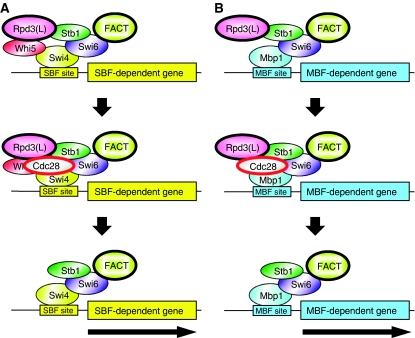
Figure 7.
SBF recruits multiple components to promoters. (A) Swi6 enters the nucleus in late M/early G1, interacts with Swi4 to form SBF, and binds to promoters. Whi5 is recruited through interaction with Swi4, and Stb1 and FACT are recruited through interaction with Swi6. Whi5 and Stb1 recruit the Rpd3(L) HDAC. As cells pass START in the cell cycle, the Cdc28 kinase is active and phosphorylates Whi5 and Stb1. Phosphorylated Whi5 leaves the nucleus. We suggest phosphorylated Stb1 remains at the promoter and stimulates gene activation. There appears to be two pulses of FACT binding to CLN1 and CLN2 promoters. (B) Mbp1 and Swi6 form the MBF factor, which binds to MBF-dependent promoters. Whi5 is not recruited. Stb1 and FACT are recruited through interaction with Swi6, and Stb1 recruits Rpd3. We suggest that Stb1 phosphorylation by Cdc28 results in disassociation of Rpd3(L). The kinetics of FACT binding is different at distinct MBF-dependent promoters. A full-colour version of this figure is available at The EMBO Journal Online.
stb1 and whi5 mutations have different effects on transcriptional activation of cyclin genes (Figure 2D). RNA levels are decreased in a stb1 mutant, consistent with Stb1 functioning as a transcriptional activator (de Bruin et al, 2008). Interestingly, a whi5 mutation suppresses the defect in SBF-dependent gene expression caused by stb1. One might expect to see increased expression of G1 cyclin genes in the whi5 single mutant, as WHI5 is a negative regulator of SBF-dependent genes (Costanzo et al, 2004; de Bruin et al, 2004). However, the major effect of a whi5 mutation is seen in assays measuring the size of cells when they pass START (Di Talia et al, 2007). Although SBF-dependent genes are activated prematurely in a whi5 mutant, this would not be seen in our experiments in which the synchrony method allows growth of the bud before cell division. The growth defects caused by FACT mutations were suppressed by whi5, whereas a stb1 mutation showed additive growth defect in combination with a FACT mutation (Figure 6). Additionally, the growth defects of the FACT stb1 double mutants were suppressed by whi5. These growth results are consistent with FACT and Stb1 both stimulating CLN gene expression, and Whi5 acting as an inhibitor. We also find that FACT binding to SBF-dependent promoters increases in a stb1 whi5 mutant, but no change is seen at MBF promoters (Figure 5). Whi5 interacts with the Swi4 subunit of SBF, but not with Mbp1 of MBF (Supplementary Figure S4), and we therefore suggest that Whi5 antagonizes FACT binding. Thus, the defect in CLN gene expression caused by the lack of Stb1 could be counteracted at least in part by increased FACT binding, restoring normal expression levels in the stb1 whi5 double mutant.
FACT binds to the promoters of SBF- and MBF-activated genes transiently during the cell cycle (Figure 4). Interestingly, FACT binding slightly precedes both chromatin disassembly and gene transcription. FACT binding at POL1 is later in the cell cycle, and chromatin disassembly and POL1 expression also occur later. A swi6 mutation eliminating both SBF and MBF prevents FACT recruitment, chromatin disassembly, and gene expression at CDC21, and POL1, both MBF genes. In contrast, we see a number of differences at HO and the two CLN genes, although all are activated by SBF. Synchrony experiments show that HO expression is largely eliminated by mutations affecting SBF or FACT, but expression of CLN2 is delayed in these mutants (Supplementary Figure S6). Similarly, although these mutations completely eliminate coactivator binding to HO URS2 (Takahata et al, 2009), they merely cause a delay in binding of the SAGA coactivator to CLN2. How is CLN2 activated in the absence of SBF? Overexpression of Rme1 increases expression of CLN2 and also permits growth of normally inviable cells lacking both SBF subunits (Toone et al, 1995). The Rme1 DNA-binding protein binds to the CLN2 promoter in vitro and activates through promoter sequences distinct from the SBF-binding sites (Toone et al, 1995). We suggest that Rme1 is responsible for the residual CLN2 activation in strains with SBF or FACT mutations, and that the HO promoter lacks Rme1-binding sites. Supporting this, there is an additive decrease in CLN2 expression in swi6 rme1 and pob3 rme1 double-mutant strains, but HO expression is not affected by a rme1 mutation (Supplementary Figure S7). Finally, we see two peaks of FACT binding at CLN1 and CLN2, one at 25 min after release and one at 50 min. The two waves of FACT binding could contribute to nucleosome disassembly and reassembly, respectively, but further studies are needed to verify this speculation. It is also surprising that the kinetics of FACT binding to HO most closely resemble that of CDC21, an MBF-activated gene, instead of CLN1 and CLN2. Although HO, CLN1 and CLN2 are all SBF-dependent genes, there are important differences, including stringent chromatin repression uniquely seen at the HO promoter (ST, YY and DJS, paper in preparation). Overall, these results underscore the way that the same factors can have fundamentally different functions at different promoters.
It is critically important for a cell to tightly regulate expression of genes such as CLN1 and CLN2 that control the G1/S transition. These genes are transcriptionally silent, and then rapid expression is stimulated by a positive feedback loop (Skotheim et al, 2008). SBF recruits a number of regulatory components to these promoters: Stb1, Whi5, Rpd3(L), FACT and Cdc28 (Figure 7). Chromatin structure at the promoters is probably affected in opposing manner by Rpd3(L) and FACT. We suggest that the Rpd3(L) HDAC produces a repressed state to help keep the gene inactive. Meanwhile, FACT is working to disassemble nucleosomes from the promoter to activate the gene. SBF also recruits the Cdc28 CDK that triggers START through positive feedback, causing nuclear export of Whi5 by phosphorylation. We suggest that Cdc28 also acts on Stb1 at this time, as the whi5 mutation causes only a mild reduction in Rpd3(L) binding (Supplementary Figure S1A), whereas Rpd3(L) binding is eliminated in the stb1 whi5 double mutant (Figure 2A). Stb1 is a target for Cdc28 phosphorylation, and phosphorylated Stb1 no longer interacts with Swi6 (Ho et al, 1999; Costanzo et al, 2003). ChIP experiments show Stb1 bound at SBF- and MBF-dependent promoters during G1 phase, and the loss of Stb1 binding when transcriptional stops is consistent with phosphorylation of Stb1 eliminating binding to Swi6 (de Bruin et al, 2008). Thus, Stb1 and Whi5 both contribute to Rpd3(L) recruitment during early G1, before Cdc28 is activated.
There are significant differences in the regulation of SBF- and MBF-dependent genes. Rpd3(L) is recruited to SBF genes by both Stb1 and Whi5, and Rpd3(L) binding is only lost in the stb1 whi5 double mutant. Whi5 does not bind to MBF, and only Stb1 is required for Rpd3(L) recruitment to MBF genes. This raises questions as to how Stb1 regulates gene activity. ChIP experiments show Stb1 bound to MBF promoters throughout G1, until transcription terminates (de Bruin et al, 2008); this correlates with the time Swi6 is nuclear (Sidorova et al, 1995; Geymonat et al, 2004). Stb1 is phosphorylated by Cdc28 at START (Ho et al, 1999), and this Stb1 modification could release Rpd3(L) from the promoter. We suggest that phosphorylated Stb1 remains bound at the promoter when the gene is transcribed, as a stb1 mutation reduces activation of SBF and MBF target genes. However, an in vitro experiment shows that Stb1 phosphorylation reduces its interaction with Swi6 (Costanzo et al, 2003), and thus further work is needed to understand how Stb1 regulates target genes.
How does loss of Rpd3(L) by the positive feedback loop trigger gene activation? One possibility is that FACT is much less effective at evicting deacetylated nucleosomes from a promoter. Although there is no biochemical data to support this idea, genetic experiments show that FACT defects can be suppressed by an rpd3 mutation (Formosa et al, 2001), and FACT histone acetyltransferase double mutants show synthetic growth defects (Formosa et al, 2002). An alternative and nonexclusive regulatory mechanism has Rpd3(L) deacetylating transcription factors at the promoter and thus blocking transcription initiation. In support of this idea, Swi6 interacts both genetically (Macpherson et al, 2000) and physically (Sanders et al, 2002) with subunits of TFIID, and TAF mutants are defective in the activation of G1/S cyclin genes (Walker et al, 1997). Further work is needed to decipher the regulatory mechanisms at these promoters.
Materials and methods
All yeast strains used are listed in Supplementary Table I and are isogenic in the W303 background (Thomas and Rothstein, 1989). Standard genetic methods were used for strain construction (Rothstein, 1991; Sherman, 1991). Plasmid M5056 (YCp:KanMX:CDC28-HA) was constructed by inserting the CDC28-HA allele from plasmid YCp:TRP1:CDC28-HA (Morris et al, 2003), kindly provided by Steve Reed, into plasmid pLEJ009 (Jauert et al, 2005), provided by David Kirkpatrick. The YCp(URA3):POB3 plasmid pJW4 (Schlesinger and Formosa, 2000) was provided by Tim Formosa. Multicopy plasmids were the YEp(LEU2):CLN1 (pJH1-38) and YEp(LEU2):CLN2 (pJH3-45) (Hadwiger et al, 1989), provided by Steve Reed, and YEp(LEU2):SPT15 (pSH223) provided by Steve Hahn, and YEp13 (Broach et al, 1979) as the empty vector control. Cell cycle synchronization was performed by galactose withdrawal and re-addition with a GALp∷CDC20 strain grown at 25°C in YEP medium containing 2% galactose and 2% raffinose (Bhoite et al, 2001). A high degree of synchrony was shown by flow cytometry analysis, budding indices and analysis of cycle-regulated mRNAs (data not shown). In other experiments, cells were grown in YEPD medium (Sherman, 1991).
ChIPs were performed as described (Bhoite et al, 2001; Voth et al, 2007) using 9E11 (Abcam) or 4A6 (Upstate) monoclonal antibody to the Myc epitope, monoclonal antibody to the HA epitope (12CA5, University of Utah Bioprocessing Resource), anti-histone H3 (07-690, Upstate), rabbit anti-Spt16 (provided by Tim Formosa), and antibody-coated magnetic beads (Rabbit and Pan Mouse IgG beads, Dynal Biotech). ChIP assays were analysed by real time PCR as described (Eriksson et al, 2004). PCR primers are listed in Supplementary Table II. Each ChIP sample was first normalized to an input DNA sample and then to the ChIP signal for a control region on chromosome I. Error bars in ChIP assays reflect the standard deviation of three replicate PCRs. For cell synchrony experiments, the ChIP values were normalized so that the zero time point was 1.0. RT–qPCR was used to measure HO mRNA levels as described (Voth et al, 2007) using primers listed in Supplementary Table II, except that RDN25 RNA was used as the internal control. Error bars in RT–qPCR assays reflect the standard deviation of three replicate PCRs. The GST-Swi6 expression plasmid M5435 contains a PCR fragment with the entire SWI6 open reading frame cloned into pGEX4-T1 (Amersham) cleaved with BamHI and XhoI. GST-Swi6 and GST-Swi5 were expressed and purified from E. coli as described (Takahata et al, 2009). Immunoprecipitations were performed as described earlier (Biswas et al, 2008a) using and anti-Myc (4A6, Upstate) and anti-Flag (M2, Sigma) antibodies, and blots probed with anti-Myc, anti-Flag, anti-Spt16 and anti-Pob3 (provided by Tim Formosa) antibodies and scanned using a Odyssey Infrared Imaging System (Li-Cor Biosciences).
Supplementary Material
Supplemental Information
Review Process File
Acknowledgments
We thank Tim Formosa and Dean Tantin for many helpful discussions and for comments on the manuscript. We thank Tim Formosa, Steve Hahn, David Kirkpatrick and Steve Reed for antibodies, plasmids and purified FACT. This work was supported by a grant from the National Institutes of Health.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adkins MW, Howar SR, Tyler JK (2004) Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Mol Cell 14: 657–666 [DOI] [PubMed] [Google Scholar]
- Badis G, Chan ET, van Bakel H, Pena-Castillo L, Tillo D, Tsui K, Carlson CD, Gossett AJ, Hasinoff MJ, Warren CL, Gebbia M, Talukder S, Yang A, Mnaimneh S, Terterov D, Coburn D, Li Yeo A, Yeo ZX, Clarke ND, Lieb JD et al. (2008) A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol Cell 32: 878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoite LT, Yu Y, Stillman DJ (2001) The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev 15: 2457–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D, Takahata S, Stillman DJ (2008a) Different genetic functions for the Rpd3(L) and Rpd3(S) complexes suggest competition between NuA4 and Rpd3(S). Mol Cell Biol 28: 4445–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D, Takahata S, Xin H, Dutta-Biswas R, Yu Y, Formosa T, Stillman DJ (2008b) A role for Chd1 and Set2 in negatively regulating DNA replication in Saccharomyces cerevisiae. Genetics 178: 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J, Cross FR (2007) Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol 8: 149–160 [DOI] [PubMed] [Google Scholar]
- Breeden L, Nasmyth K (1987) Cell cycle control of the yeast HO gene: cis- and trans-acting regulators. Cell 48: 389–397 [DOI] [PubMed] [Google Scholar]
- Broach JR, Strathern JN, Hicks JB (1979) Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene 8: 121–133 [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, Shia WJ, Anderson S, Yates J, Washburn MP, Workman JL (2005) Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123: 581–592 [DOI] [PubMed] [Google Scholar]
- Costanzo M, Nishikawa JL, Tang X, Millman JS, Schub O, Breitkreuz K, Dewar D, Rupes I, Andrews B, Tyers M (2004) CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117: 899–913 [DOI] [PubMed] [Google Scholar]
- Costanzo M, Schub O, Andrews B (2003) G1 transcription factors are differentially regulated in Saccharomyces cerevisiae by the Swi6-binding protein Stb1. Mol Cell Biol 23: 5064–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin RA, Kalashnikova TI, Wittenberg C (2008) Stb1 collaborates with other regulators to modulate the G1-specific transcriptional circuit. Mol Cell Biol 28: 6919–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin RA, McDonald WH, Kalashnikova TI, Yates J III, Wittenberg C (2004) Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117: 887–898 [DOI] [PubMed] [Google Scholar]
- Di Talia S, Skotheim JM, Bean JM, Siggia ED, Cross FR (2007) The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature 448: 947–951 [DOI] [PubMed] [Google Scholar]
- Eriksson P, Biswas D, Yu Y, Stewart JM, Stillman DJ (2004) TATA-binding protein mutants that are lethal in the absence of the Nhp6 high-mobility-group protein. Mol Cell Biol 24: 6419–6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T (2008) FACT and the reorganized nucleosome. Mol Biosyst 4: 1085–1093 [DOI] [PubMed] [Google Scholar]
- Formosa T, Eriksson P, Wittmeyer J, Ginn J, Yu Y, Stillman DJ (2001) Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J 20: 3506–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T, Ruone S, Adams MD, Olsen AE, Eriksson P, Yu Y, Rhoades AR, Kaufman PD, Stillman DJ (2002) Defects in SPT16 or POB3 (yFACT) in Saccharomyces cerevisiae cause dependence on the Hir/Hpc pathway. Polymerase passage may degrade chromatin structure. Genetics 162: 1557–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geymonat M, Spanos A, Wells GP, Smerdon SJ, Sedgwick SG (2004) Clb6/Cdc28 and Cdc14 regulate phosphorylation status and cellular localization of Swi6. Mol Cell Biol 24: 2277–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger JA, Wittenberg C, Richardson HE, de Barros Lopes M, Reed SI (1989) A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci USA 86: 6255–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LA, Andrews BJ (1996) Binding to the yeast SwI4,6-dependent cell cycle box, CACGAAA, is cell cycle regulated in vivo. Nucleic Acids Res 24: 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Costanzo M, Moore L, Kobayashi R, Andrews BJ (1999) Regulation of transcription at the Saccharomyces cerevisiae start transition by Stb1, a Swi6-binding protein. Mol Cell Biol 19: 5267–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaquinta PJ, Lees JA (2007) Life and death decisions by the E2F transcription factors. Curr Opin Cell Biol 19: 649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauert PA, Jensen LE, Kirkpatrick DT (2005) A novel yeast genomic DNA library on a geneticin-resistance vector. Yeast 22: 653–657 [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M (2002) Systematic identification of pathways that couple cell growth and division in yeast. Science 297: 395–400 [DOI] [PubMed] [Google Scholar]
- Joshi AA, Struhl K (2005) Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Mol Cell 20: 971–978 [DOI] [PubMed] [Google Scholar]
- Kasten MM, Stillman DJ (1997) Identification of the Saccharomyces cerevisiae STB1–STB5 genes encoding Sin3p binding proteins. Mol Gen Genet 256: 376–386 [DOI] [PubMed] [Google Scholar]
- Koch C, Schleiffer A, Ammerer G, Nasmyth K (1996) Switching transcription on and off during the yeast cell cycle: Cln/Cdc28 kinases activate bound transcription factor SBF (Swi4/Swi6) at start, whereas Clb/Cdc28 kinases displace it from the promoter in G2. Genes Dev 10: 129–141 [DOI] [PubMed] [Google Scholar]
- Lycan D, Mikesell G, Bunger M, Breeden L (1994) Differential effects of Cdc68 on cell cycle-regulated promoters in Saccharomyces cerevisiae. Mol Cell Biol 14: 7455–7465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson N, Measday V, Moore L, Andrews B (2000) A yeast taf17 mutant requires the Swi6 transcriptional activator for viability and shows defects in cell cycle-regulated transcription. Genetics 154: 1561–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall MD, Richardson HE, Reed SI (1988) Dominant negative protein kinase mutations that confer a G1 arrest phenotype. Proc Natl Acad Sci USA 85: 4426–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Kaiser P, Rudyak S, Baskerville C, Watson MH, Reed SI (2003) Cks1-dependent proteasome recruitment and activation of CDC20 transcription in budding yeast. Nature 423: 1009–1013 [DOI] [PubMed] [Google Scholar]
- Nasmyth K (1993) Regulating the HO endonuclease in yeast. Curr Opin Genet Dev 3: 286–294 [DOI] [PubMed] [Google Scholar]
- Rothstein R (1991) Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Meth Enzymol 194: 281–302 [DOI] [PubMed] [Google Scholar]
- Rowley A, Singer RA, Johnston JC (1991) CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol Cell Biol 11: 5718–5726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Jennings J, Canutescu A, Link AJ, Weil PA (2002) Proteomics of the eukaryotic transcription machinery: identification of proteins associated with components of yeast TFIID by multidimensional mass spectrometry. Mol Cell Biol 22: 4723–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer JB, Breeden LL (2004) RB from a bud's eye view. Cell 117: 849–850 [DOI] [PubMed] [Google Scholar]
- Schlesinger MB, Formosa T (2000) POB3 is required for both transcription and replication in the yeast Saccharomyces cerevisiae. Genetics 155: 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F (1991) Getting started with yeast. Meth Enzymol 194: 1–21 [DOI] [PubMed] [Google Scholar]
- Sidorova JM, Mikesell GE, Breeden LL (1995) Cell cycle-regulated phosphorylation of Swi6 controls its nuclear localization. Mol Biol Cell 6: 1641–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon I, Barnett J, Hannett N, Harbison CT, Rinaldi NJ, Volkert TL, Wyrick JJ, Zeitlinger J, Gifford DK, Jaakkola TS, Young RA (2001) Serial regulation of transcriptional regulators in the yeast cell cycle. Cell 106: 697–708 [DOI] [PubMed] [Google Scholar]
- Skotheim JM, Di Talia S, Siggia ED, Cross FR (2008) Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature 454: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata S, Yu Y, Stillman DJ (2009) FACT and Asf1 regulate nucleosome dynamics and coactivator binding at the HO promoter. Mol Cell 34: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmeier K, Synovzik H, Mertz R, Winnacker EL, Lipp M (1989) Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev 3: 527–536 [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Rothstein R (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56: 619–630 [DOI] [PubMed] [Google Scholar]
- Toone WM, Johnson AL, Banks GR, Toyn JH, Stuart D, Wittenberg C, Johnston LH (1995) Rme1, a negative regulator of meiosis, is also a positive activator of G1 cyclin gene expression. EMBO J 14: 5824–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S, Dyson NJ (2008) Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9: 713–724 [DOI] [PubMed] [Google Scholar]
- VanDemark AP, Xin H, McCullough L, Rawlins R, Bentley S, Heroux A, Stillman DJ, Hill CP, Formosa T (2008) Structural and functional analysis of the Spt16p N-terminal domain reveals overlapping roles of yFACT subunits. J Biol Chem 283: 5058–5068 [DOI] [PubMed] [Google Scholar]
- Voth WP, Yu Y, Takahata S, Kretschmann KL, Lieb JD, Parker RL, Milash B, Stillman DJ (2007) Forkhead proteins control the outcome of transcription factor binding by antiactivation. EMBO J 26: 4324–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SS, Shen WC, Reese JC, Apone LM, Green MR (1997) Yeast TAF(II)145 required for transcription of G1/S cyclin genes and regulated by the cellular growth state. Cell 90: 607–614 [DOI] [PubMed] [Google Scholar]
- Williams SK, Tyler JK (2007) Transcriptional regulation by chromatin disassembly and reassembly. Curr Opin Genet Dev 17: 88–93 [DOI] [PubMed] [Google Scholar]
- Wittenberg C, Reed SI (2005) Cell cycle-dependent transcription in yeast: promoters, transcription factors, and transcriptomes. Oncogene 24: 2746–2755 [DOI] [PubMed] [Google Scholar]
- Workman JL (2006) Nucleosome displacement in transcription. Genes Dev 20: 2009–2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information
Review Process File