SUMMARY
Multiple myeloma, and the associated osteolytic bone disease, is highly dependent upon cellular interactions within the bone marrow microenvironment. A major limitation of existing myeloma models is the requirement for a specific host strain of mouse, preventing molecular examination of the bone marrow microenvironment. The aim of the current study was to develop a model of myeloma in which the host microenvironment could be modified genetically. The Radl 5T murine model of myeloma is well characterized and closely mimics human myeloma. In the current study, we demonstrate 5T myeloma establishment in recombination activating gene 2 (RAG-2)-deficient mice, which have improper B- and T-cell development. Importantly, these mice can be easily bred with genetically modified mice to generate double knockout mice, allowing manipulation of the host microenvironment at a molecular level. Inoculation of 5TGM1 myeloma cells into RAG-2−/− mice resulted in myeloma development, which was associated with tumor growth within bone and an osteolytic bone disease, as assessed by microcomputed tomography (microCT), histology and histomorphometry. Myeloma-bearing RAG-2−/− mice displayed many features that were similar to both human myeloma and the original Radl 5T model. To demonstrate the use of this model, we have examined the effect of host-derived matrix metalloproteinase 9 (MMP-9) in the development of myeloma in vivo. Inoculation of 5TGM1 myeloma cells into mice that are deficient in RAG-2 and MMP-9 resulted in a reduction in both tumor burden and osteolytic bone disease when compared with RAG-2-deficient wild-type myeloma-bearing mice. The establishment of myeloma in RAG-2−/− mice permits molecular examination of the host contribution to myeloma pathogenesis in vivo.
INTRODUCTION
Multiple myeloma is one of the most common hematological malignancies in the USA (Jemal et al., 2004). Myeloma is characterized by the clonal expansion of malignant plasma cells within the bone marrow, which is associated with the development of a destructive osteolytic bone disease, anemia and immune suppression. The mechanisms involved in the development of myeloma are not well understood; therefore, despite many advances in the treatment of multiple myeloma, it remains an incurable and fatal malignancy. Myeloma progression and the development of osteolytic bone disease are inextricably linked and are dependent upon cellular interactions within the bone marrow microenvironment. Therefore, the study of the bone marrow microenvironment in myeloma is crucial for both our understanding of mechanisms involved in disease progression, and the identification of novel therapeutic targets.
The advances in the treatment of myeloma are limited owing to the number of clinically relevant animal models that allow for the in vivo study of myeloma development in the context of a bone marrow microenvironment. The current animal models for myeloma include the severe combined immunodeficiency (SCID)-hu/rab xenograft model, a conditional mouse model that is dependent upon Myc activation in germinal center B cells, and the Radl 5T model. The SCID-hu/rab xenograft model provides a system where primary human myeloma cells can be injected into either a fetal human bone or rabbit bone that is implanted subcutaneously into an immunocompromised mouse (Yaccoby et al., 1998; Yaccoby et al., 2007). The Radl model uses 5T myeloma cells that arose spontaneously in aged, inbred C57BL/KaLwRijHsd mice and is propagated by the inoculation of these myeloma cells into syngeneic mice (Radl et al., 1979; Radl et al., 1988; Garrett et al., 1997). Both of these models allow the study of tumor growth and myeloma bone disease, and have proven to be effective preclinical models to test novel therapeutic approaches for the treatment of myeloma bone disease (Dallas et al., 1999; Croucher et al., 2001; Croucher et al., 2003; Oyajobi et al., 2003; Yaccoby et al., 2004; Edwards et al., 2007; Yaccoby et al., 2007; Edwards et al., 2008). Activation of Myc under the control of the kappa light chain regulatory elements results in the development of myeloma with features that are similar to human multiple myeloma (Chesi et al., 2008). A major limitation of all existing models is that manipulation of the bone marrow microenvironment, independent of the tumor, is limited to systemic pharmacological reagents, rendering it impossible to elucidate specific cellular and molecular mechanisms of myeloma bone disease within the bone marrow microenvironment. Current research has demonstrated the crucial role that the tumor microenvironment plays in disease progression, but the existing animal models for the study of the tumor microenvironment in myeloma severely impair both clinical and basic research in this field.
The aim of the current study was to develop a murine model of myeloma in which the host microenvironment could subsequently be modified genetically, thus enabling molecular studies of the host contribution to multiple myeloma progression to be conducted in vivo. The Radl 5T murine model of myeloma was originally identified to occur spontaneously in aging mice of the C57BL/KaLwRij strain. Several 5T cell lines have been developed from this model, including 5T2 and 5TGM1, which result in tumor growth within bone and osteolytic bone disease when cells are inoculated into either syngeneic C57BL/KaLwRij mice or bg/nu/Xid mice (Garrett et al., 1997; Asosingh et al., 2000). By contrast, myeloma does not develop when cells are inoculated into C57BL/6 mice. The genetic mutation that defines C57BL/KaLwRij mice is unknown, and the deleterious effects of the bg/nu/Xid mutation on breeding and life span mean that neither of these strains can be crossed with genetically modified mice in order to modify the host microenvironment in mice which are permissive to myeloma growth. In the current study, we investigated the establishment of 5TGM1 myeloma cells in immunocompromised recombination activating gene 2 (RAG-2)-deficient mice on a C57BL/6 background. These mice have a targeted disruption of the Rag2 gene, which results in the absence of functional recombinases, leading to improper B- and T-cell development (Shinkai et al., 1992). Importantly, these mice can be easily bred with genetically modified mice to generate double knockout mice, therefore greatly improving our ability to genetically manipulate the host microenvironment.
RESULTS
RAG-2−/− mice develop a characteristic myeloma tumor burden
RAG-2-deficient mice on a C57BL/6 background were inoculated with 106 green fluorescent protein (GFP)-tagged 5TGM1 myeloma cells by intravenous tail vein injection. Tumor burden was measured by serum IgG2bκ ELISA, histomorphometric analysis of tumor burden in bone, and flow cytometric analysis of tumor burden in the bone marrow and spleen. Myeloma development in RAG-2-deficient mice was compared with the rate of development in syngeneic C57BL/KaLwRij mice, C57BL/6 mice, bg/nu/Xid mice, and T-cell-deficient athymic nude mice.
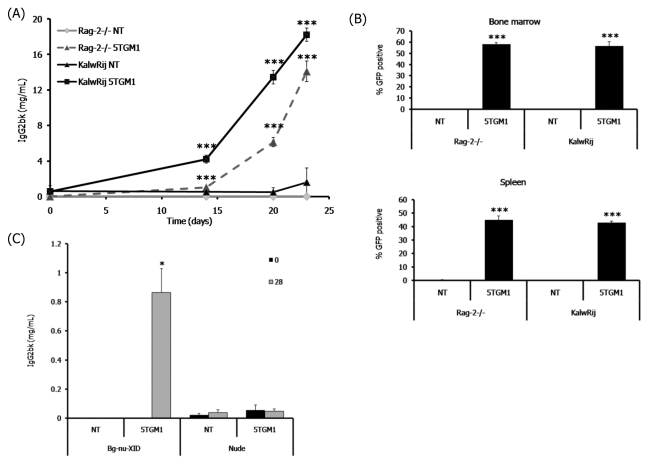
Following intravenous inoculation of 5TGM1 myeloma cells, the RAG-2-deficient mice developed myeloma at the same rate as that observed with the syngeneic C57BL/KaLwRij mice from the 5T model. The tumor burden of the RAG-2-deficient mice increased over time, as determined by measuring the serum levels of the myeloma-specific immunoglobulin IgG2b (Fig. 1A). The increase of IgG2b levels in the RAG-2-deficient mice was comparable to the tumor burden found in the myeloma-bearing C57BL/KaLwRij mice (Fig. 1A). Inoculation of 5TGM1 cells into immune-competent C57BL/6 mice did not result in myeloma development. Tumor burden was also assessed by measuring the percentage of GFP-positive myeloma cells present in the bone marrow and spleen. The myeloma-bearing RAG-2-deficient mice showed a significant accumulation of GFP-positive myeloma cells in both the bone marrow and spleen (Fig. 1B), and this burden was comparable to that observed in the C57BL/KaLwRij mice. Therefore, the development of multiple myeloma in RAG-2-deficient mice occurs in an identical manner to C57BL/KaLwRij mice, both with respect to the time for tumor development and the extent of tumor burden.
Fig. 1.
RAG-2−/− mice develop a characteristic myeloma tumor burden. Intravenous inoculation of 5TGM1 myeloma cells into RAG-2−/− mice, which have improper B- and T-cell development, or C57BL/KaLwRij (KaLwRij) mice. (A) Tumor burden represented by the levels of the myeloma-specific immunoglobulin IgG2bκ in the serum. (B) Tumor burden represented by the percentage of GFP-positive 5TGM1 myeloma cells within the bone marrow and spleen, measured by flow cytometry. (C) Serum IgG2b levels in non-tumor (NT)-bearing and 5TGM1 myeloma-bearing bg/nu/Xid (deficient in B cells, T cells and natural killer cells) and nude (deficient in T cells) mice at day 0 and day 28 following tumor inoculation. Data are expressed as mean±S.E. *P<0.05, **P<0.01, ***P<0.001 compared with non-tumor (NT)-bearing mice.
In contrast to the accumulation of myeloma cells that was observed in RAG-2-deficient mice and bg/nu/Xid mice, when 5TGM1 myeloma cells were inoculated into T-cell-deficient athymic nude mice, the measurements of myeloma-specific immunoglobulin levels in the serum demonstrated that there was no increase in IgG2b levels in 5TGM1-bearing athymic nude mice (Fig. 1C). This demonstrates that a lack of T cells is not sufficient to permit myeloma development in vivo.
RAG-2−/− mice develop myeloma-associated bone disease
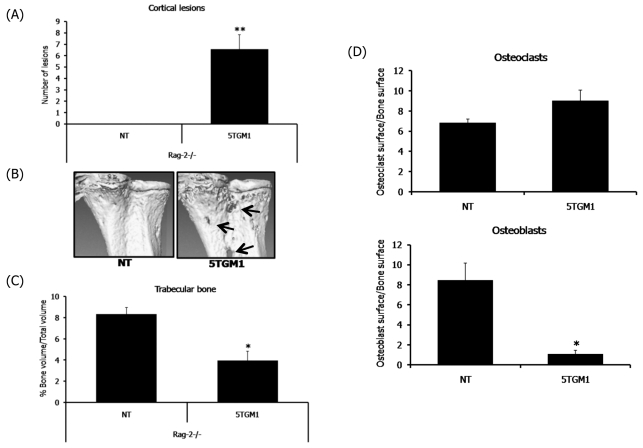
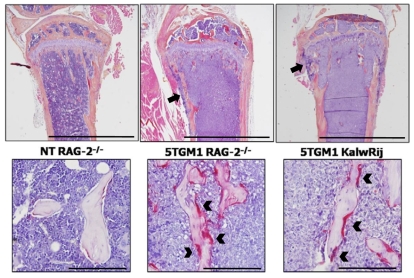
In addition to indices of tumor burden, we also evaluated the myeloma-associated osteolytic bone disease in RAG-2-deficient mice in comparison to the well-characterized bone disease of the C57BL/KaLwRij mice. Trabecular bone volume and osteolytic lesions were analyzed by microcomputed tomography (microCT), and osteoclast and osteoblast numbers were determined by bone histomorphometry. Myeloma-bearing RAG-2-deficient mice were found to have characteristic features of myeloma bone disease, which were identical to those seen in C57BL/KaLwRij mice and strikingly similar to human multiple myeloma. The myeloma-bearing RAG-2-deficient mice had a significant number of osteolytic lesions within the cortical bone, whereas the non-tumor mice had no lesions (Fig. 2A,B). Histological analysis confirmed areas where the cortical bone had been destroyed, with tumor cells expanding through the cortices, leading to the development of discrete osteolytic lesions (Fig. 3). We found that the myeloma-bearing RAG-2-deficient mice had a significant decrease in the overall trabecular bone volume when compared with the non-tumor control mice (Fig. 2C; Fig. 3). Histomorphometric analysis of the RAG-2-deficient myeloma-bearing mice demonstrated other features that are characteristic of myeloma-associated bone disease, such as an increase in bone-resorbing osteoclasts and a decrease in bone-forming osteoblasts (Fig. 2D; Fig. 3). Histological analysis demonstrated a striking similarity between 5TGM1 myeloma-bearing RAG-2−/− mice and myeloma-bearing syngeneic KaLwRij mice in terms of both tumor expansion within the bone marrow cavity and development of myeloma bone disease (Fig. 3).
Fig. 2.
RAG-2−/− mice develop myeloma-associated bone disease. Myeloma-associated bone disease assessed by microCT analysis, histomorphometry and histology. (A) MicroCT analysis of osteolytic bone lesions through the cortical bone. (B) Representative microCT images of cortical bone lesions. (C) MicroCT analysis of trabecular bone volume. (D) Histomorphometric analysis of the osteoclast and osteoblast surface area (mm2) to trabecular bone surface area (mm3) in RAG-2−/− mice. Data are expressed as mean±S.E. *P<0.05, **P<0.01, ***P<0.001 compared with non-tumor (NT)-bearing mice.
Fig. 3.
RAG-2−/− mice develop characteristic pathology that is typical of clinical myeloma. The pathology present in myeloma-bearing RAG-2-deficient mice is similar to that seen in the well-established Radl 5T murine model of myeloma in C57BL/KaLwRij mice. (Top row) Myeloma cell growth within the bone marrow cavity, and osteolytic lesions through the cortical bone (black arrows). Bars, 2 mm. (Bottom row) Myeloma-bearing mice display a characteristic increase in TRAP-positive osteoclasts (black arrowheads). NT=non-tumor bearing. Bars, 200 μm.
Deficiency in matrix metalloproteinase 9 (MMP-9) decreases both tumor burden and the severity of the associated osteolytic bone disease
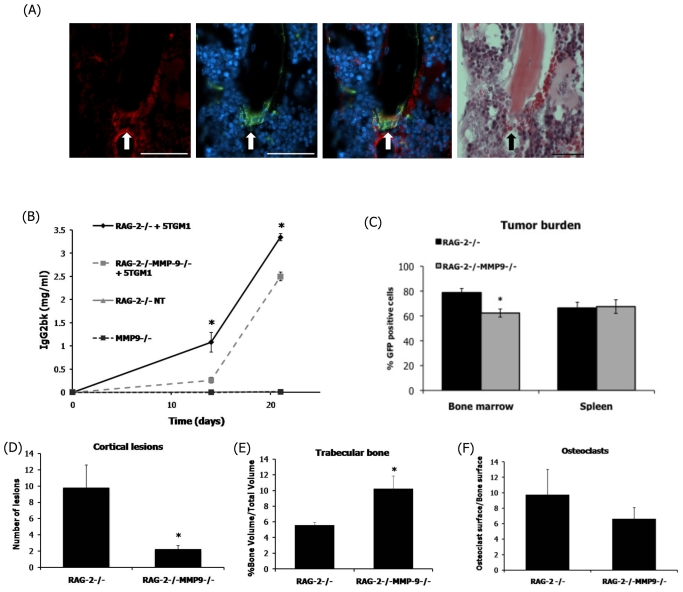
The MMP family of proteolytic enzymes has been studied extensively for their role in extracellular matrix degradation, which can result in cancer progression in various tumor cell types including myeloma (Barille et al., 1997; Vacca et al., 1998; Barille et al., 1999; Vacca et al., 1999). Previous studies have demonstrated a role for tumor-derived MMP-9 in myeloma progression, but also revealed the presence of host-derived MMP-9 within the bone marrow microenvironment (Van Valckenborgh et al., 2005). In order to demonstrate the use of this RAG-2−/− model of myeloma, we chose to investigate myeloma development in mice that were deficient in MMP-9. MMP-9 expression in the bone marrow of C57BL/KaLwRij mice was demonstrated by immunohistochemistry in tartrate-resistant acid phosphatase (TRAP)-positive multi-nucleated osteoclasts on the surface of trabecular bone (Fig. 4A). A similar level of expression was observed in RAG-2-deficient mice (data not shown). Mice that were deficient in both RAG-2 and MMP-9, in addition to mice that were deficient in RAG-2 alone, were inoculated intravenously with 5TGM1 myeloma cells to determine how MMP-9 deficiency would affect tumor burden and the associated bone disease. When compared with myeloma-bearing mice that were deficient in only RAG-2, the tumor burden, as indicated by IgG2b serum levels, in mice that were deficient in both RAG-2 and MMP-9 was decreased significantly at 14 and 21 days following tumor inoculation (Fig. 4B). Mice that were deficient in both RAG-2 and MMP-9 showed a significant decrease in the proportion of GFP-positive 5TGM1 myeloma cells that were present in the bone marrow when compared with myeloma-bearing RAG-2-deficient mice; however, there was no significant difference in the proportion of GFP-positive myeloma cells in the spleens of these mice (Fig. 4C). The contribution of host-derived MMP-9 from the osteoclasts within the bone marrow microenvironment also had significant effects on myeloma bone disease. The number of lesions present through the cortical bone of myeloma-bearing mice that were deficient in both RAG-2 and MMP-9 was significantly decreased when compared with myeloma-bearing RAG-2-deficient mice (Fig. 4D). Additionally, the overall bone loss in myeloma-bearing double-deficient mice was significantly less when compared with the control RAG-2-deficient mice, as indicated by microCT analysis of trabecular bone volume (Fig. 4E). Histomorphometric analysis demonstrated a trend towards a reduction in osteoclasts in myeloma-bearing double-deficient mice when compared with myeloma-bearing RAG-2-deficient mice (Fig. 4F). No significant difference in osteoblast number was observed (data not shown).
Fig. 4.
A lack of host-derived MMP-9 significantly reduces tumor burden and myeloma bone disease in vivo. Intravenous inoculation of 5TGM1 cells into either RAG-2−/− mice or mice that were deficient in both RAG-2 and MMP-9 was followed by an assessment of tumor burden. (A) MMP-9 localization in KaLwRij bone marrow. Fluorescent TRAP staining (green) was used to localize osteoclasts (arrows), whereas immunofluorescence was used to localize MMP-9 (red). Non-specific staining was observed in red blood cells. DAPI (blue) was used as a nuclear stain. Murine IgG was used as a negative control. Bars, 50 μM. (B) Tumor burden represented by the IgG2bκ levels present in the serum. (C) Tumor burden represented by the percentage of GFP-positive 5TGM1 myeloma cells within the bone marrow and spleen, measured by flow cytometry. Myeloma bone disease was assessed by microCT analysis, histomorphometry and histology. (D) MicroCT analysis of osteolytic bone lesions through the cortical bone. (E) MicroCT analysis of trabecular bone volume. (F) Histomorphometric analysis of osteoclast number. Data are expressed as mean±S.E. *P<0.05 compared with tumor-bearing RAG-2−/− mice.
DISCUSSION
The present study demonstrates a new in vivo system for the examination of the host tumor microenvironment and the contributions of this specialized niche to myeloma development. Despite many therapeutic advancements in the treatment of myeloma using existing mouse models, the field of myeloma research has long been limited by the inability of these models to permit specific investigation of the tumor microenvironment. The results from the current study demonstrate that myeloma development in RAG-2-deficient mice shares many of the clinical and histological features of human myeloma and the associated osteolytic bone disease that is also demonstrated in the established Radl 5T model. Myeloma-bearing RAG-2-deficient mice displayed extensive tumor burden within the bone marrow, an increase in osteoclasts, a decrease in osteoblasts, and the development of destructive lytic lesions and overall bone loss. In the 5TGM1 model of myeloma, inoculation of myeloma cells results in them homing to both the bone marrow and spleen, with homing to the spleen being a result of the hematopoietic nature of this organ in mice. The growth of myeloma cells in bone and non-bone sites is a useful tool for elucidating the role of the bone marrow microenvironment; this important feature was also observed in myeloma-bearing RAG-2-deficient mice, with an accumulation of myeloma cells within the bone marrow and spleen. The use of RAG-2-deficient mice in a myeloma model is an extremely important advancement for myeloma research, as gene expression in the host compartment of the tumor microenvironment can be more specifically manipulated.
The results from this study also provide compelling evidence that the bone marrow microenvironment is crucial for myeloma development. We are able to demonstrate crucial differences between 5TGM1 myeloma-permissive and non-permissive strains of mice. We found significant differences in myeloma establishment and progression in various strains of mice despite their similar genetic backgrounds. The most interesting example is the difference between tumor establishment in the C57BL/KaLwRij mice that are used in the Radl model and the lack of tumor take and growth in the C57BL/6 mice of the same genetic background. Of additional interest is the difference in tumor establishment between two immunocompromised strains of mice: athymic nude mice do not develop characteristics of myeloma, whereas RAG-2-deficient mice develop a pathology that is identical to the Radl C57BL/KaLwRij mice. Although the use of RAG-2-deficient mice will not allow for the investigation of the immune system, specifically B and T cells, in myeloma, our results demonstrates that a lack of T cells is not sufficient to permit myeloma development in vivo. Since the major difference between RAG-2-deficient mice and nude mice is the absence of mature B cells, it raises the intriguing possibility that the development of 5T myeloma in RAG-2-deficient mice may not simply be the result of immunodeficiency, but may in part be dependent on specific B-cell regulation. Nude mice are also known to have increased natural killer cell and macrophage activity, and it is possible that theses differences may also contribute to their inability to permit myeloma development (Budzynski and Radzikowski, 1994).
The use of the RAG-2-deficient mice in a model of multiple myeloma creates many opportunities to improve current therapies by increasing our understanding of specific mechanisms within the host tumor microenvironment. The use of this animal model will allow specific manipulation of the host tumor microenvironment through genetic mutation; for example, this model system allowed for the specific examination of host-derived MMP-9 and its contribution to myeloma progression. MMPs are known to have important roles in tumor progression; however, it is impossible to discern their specific contributions owing to the lack of specificity of MMP inhibitors. The ability to inhibit specific MMP expression in the host microenvironment using MMP-deficient mice permits the investigation of the specific roles of individual MMPs in myeloma pathogenesis. In a previous study by Van Valckenborgh et al., in which an MMP-9 pro-drug was used to specifically target tumor cells within the bone marrow microenvironment, MMP-9 activity was higher in myeloma-bearing mice compared with non-tumor-bearing mice (Van Valckenborgh et al., 2005). However, cells in the bone marrow of non-tumor mice still showed elevated levels of MMP-9 expression, suggesting that MMP-9 was present in the bone marrow of C57BL/KaLwRij mice. Our studies confirmed this by using immunofluorescence to demonstrate MMP-9 expression in osteoclasts within both C57BL/KaLwRij and RAG-2−/− bone marrow. By investigating the development of 5TGM1 myeloma in mice that were deficient in both RAG-2 and MMP-9, we were able to demonstrate a significant reduction in both tumor burden and the associated osteolytic bone disease in MMP-9-deficient mice. This both identifies a role for host-derived MMP-9 in myeloma pathogenesis, and illustrates the potential for this model in studies of the host microenvironment in myeloma.
There are many important questions in myeloma research regarding the relative contribution of host-derived factors versus tumor-derived factors, such as receptor activator of nuclear factor-κB ligand (RANKL) and Dickkopf homolog 1 (DKK1), which are known to be expressed by both tumor cells and other cells within the bone marrow microenvironment, including stromal cells. The study of myeloma growth in vivo combined with genetic modification of the host microenvironment offers a novel molecular approach to elucidate the specific host-tumor interactions. Overall, the establishment of multiple myeloma in RAG-2-deficient mice, and the resulting ability to study myeloma growth and the associated bone disease in a genetically modified host microenvironment, is a major advancement in myeloma research and a highly important tool for the myeloma research community.
METHODS
Cell culture
The 5TGM1-GFP myeloma cell line was cultured as described previously (Dallas et al., 1999; Oyajobi et al., 2007).
In vivo 5TGM1 myeloma studies
Studies were performed using 8–10-week-old female RAG-2−/−, C57BL/6 (Harlan U.S., Indianapolis, IN), C57BL/KaLwRijHsd (Harlan Netherlands, Horst, The Netherlands), or RAG-2−/−/MMP-9−/− mice. Studies were approved by the Institution of Animal Care and Use Committee at Vanderbilt University and were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Myeloma was propagated in these animals by the intravenous inoculation of 106 5TGM1-GFP-tagged myeloma cells in 100 μl of phosphate-buffered saline (PBS). Tumor burden was assessed by serum analysis of the myeloma-specific immunoglobulin IgG2bκ, as described previously (Dallas et al., 1999).
Bone histomorphometric analysis
Histomorphometric analysis was performed to quantify bone volume; osteoclast and osteoblast surface area to bone surface area; trabecular number; and trabecular spacing. Tibias and femurs were formalin-fixed, decalcified in 14% EDTA, paraffin-embedded, and sectioned along the mid-sagittal plane in 4 μm-thick sections. To visualize osteoclasts, sections were stained with hematoxylin and eosin, and for TRAP activity. Three non-consecutive sections were evaluated using Osteomeasure histomorphometry software, as described previously (Edwards et al., 2008).
Immunohistochemistry
For MMP-9 and TRAP localization, the following technique was employed: sections were rehydrated through a series of ethanol solutions and then rinsed in Tris-buffered saline (TBS; 10 mM Tris at pH 7.4, 150 mM NaCl) with Tween 20 (0.05%). For antigen retrieval, slides were immersed in a 20 μg/ml solution of proteinase K, according to the manufacturer’s instructions, for 10 minutes at room temperature. Following washing in TBS, tissue sections were blocked using standard blocking criteria for 1 hour at room temperature. MMP-9 antibodies (Oncogene) were added as part of a blocking solution overnight at 4°C at a dilution of 1:100. Slides were washed extensively in TBS with Tween 20 before the addition of a species-specific fluorescently labeled secondary antibody (Alexa Fluor 568 nm, Invitrogen), diluted 1:1000 in blocking solution, for 1 hour at room temperature. Slides were washed in TBS and then equilibrated in an acetate buffer, as described (Filgueira, 2004). The ELF97 TRAP stain (Invitrogen) was diluted 1:1000 in acetate buffer, and slides were incubated for 15 minutes at room temperature. Following washing, slides were aqueously mounted in media (Biomeda Corp.) containing 2 μM DAPI (4′ ,6-diamidino-2-phenylindole) for nuclear localization.
MicroCT analysis
Cortical bone lesions were measured using microCT analysis on the proximal tibia. Bones were fixed in formalin and scanned at an isotropic voxel size of 12 μm using a microCT40 (SCANCO Medical, Bassersdorf, Switzerland). For analysis of cortical bone lesions, cross-sectional images of the entire metaphysis including the cortices and extending 0.25 mm from the growth plate were imported into Amira 3D graphics software (Mercury Computer Systems, Chelmsford, MA). The Amira software generated a 3D reconstruction of the metaphyses using a consistent threshold. The number of osteolytic lesions that completely penetrated the cortical bone, as seen in the virtual reconstruction, were counted. MicroCT analysis was also performed on the trabecular bone to assess the overall volume and structural characteristics of the trabeculae. Contours were drawn within the cortices of the proximal tibia using the microCT40. The analysis provided a ratio measurement of bone volume to total tissue volume within the cortical bone.
Flow cytometry
Bone marrow was flushed from the tibia and femur of 5TGM1 myeloma-bearing mice. Splenic cells from myeloma-bearing mice were obtained by homogenization in tissue culture media. Cell suspensions from both organs were filtered through a 70 μm filter followed by analysis for GFP fluorescence using a 3-laser BD LSRII (Becton Dickinson, San Jose, CA).
Acknowledgments
We thank Kathy Carter from the Department of Cancer Biology for help with the intravenous injections. This work was supported by NCI through P01 CA-40035 (G.R.M.). C.M.E. is supported by the International Myeloma Foundation and the Elsa U. Pardee Foundation. We gratefully acknowledge support from the Vanderbilt University Institute of Imaging Science. We are grateful to Kevin P. Weller and David K. Flaherty for their assistance with flow cytometry. Flow cytometry experiments were performed in the Vanderbilt Medical Center (VMC) Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.A.F., G.R.M., C.C.L. and C.M.E. conceived and designed the experiments; J.A.F., C.C.L. and S.T.L. performed the experiments; J.A.F. analyzed the data; C.C.L. contributed reagents/animals; J.A.F. and C.M.E. wrote the paper.
REFERENCES
- Asosingh K, Radl J, Van Riet I, Van Camp B, Vanderkerken K. (2000). The 5TMM seies: a useful in vivo mouse model of human multiple myeloma. Hematol J. 1, 351–356 [DOI] [PubMed] [Google Scholar]
- Barille S, Akhoundi C, Collette M, Mellerin MP, Rapp MJ, Harousseau JL, Bataille R, Amiot M. (1997). Metalloproteinases in multiple myeloma: production of matrix metalloproteinase-9 (MMP-9), activation of proMMP-2, and induction of MMP-1 by myeloma cells. Blood 90, 1649–1655 [PubMed] [Google Scholar]
- Barille S, Bataille R, Rapp MJ, Harousseau JL, Amiot M. (1999). Production of metalloproteinase-7 (matrilysin) by human myeloma cells and its potential involvement in metalloproteinase-2 activation. J Immunol. 163, 5723–5728 [PubMed] [Google Scholar]
- Budzynski W, Radzikowski C. (1994). Cytotoxic cells in immunodeficient athymic mice. Immunopharmacol Immunotoxicol. 16, 319–346 [DOI] [PubMed] [Google Scholar]
- Chesi M, Robbiani DF, Sebag M, Chng WJ, Affer M, Tiedemann R, Valdez R, Palmer SE, Haas SS, Stewart AK, et al. (2008). AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell 13, 167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, Brabbs AC, van Beek EJ, Holen I, Skerry TM, et al. (2001). Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood 98, 3534–3540 [DOI] [PubMed] [Google Scholar]
- Croucher PI, De Hendrik R, Perry MJ, Hijzen A, Shipman CM, Lippitt J, Green J, Van Marck E, Van Camp B, Vanderkerken K. (2003). Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 18, 482–492 [DOI] [PubMed] [Google Scholar]
- Dallas SL, Garrett IR, Oyayobi BO, Dallas MR, Boyce BF, Bauss F, Radl J, Mundy GR. (1999). Ibandronate reduces osteolytic lesions but not tumour burden in a murine model of myeloma bone disease. Blood 93, 1697–1706 [PubMed] [Google Scholar]
- Edwards CM, Mueller G, Roelofs AJ, Chantry A, Perry M, Russell RG, Van Camp B, Guyon-Gellin Y, Niesor EJ, Bentzen CL, et al. (2007). Apomine, an inhibitor of HMG-CoA-reductase, promotes apoptosis of myeloma cells in vitro and is associated with a modulation of myeloma in vivo. Int J Cancer 120, 1657–1663 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR. (2008). Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood 111, 2833–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueira L. (2004). Fluorescence-based staining for tartrate-resistant acidic phosphatase (TRAP) in osteoclasts combined with other fluorescent dyes and protocols. J Histochem Cytochem. 52, 411–414 [DOI] [PubMed] [Google Scholar]
- Garrett IR, Dallas S, Radl J, Mundy GR. (1997). A murine model of human myeloma bone disease. Bone 20, 515–520 [DOI] [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer Thun MJ. (2004). Cancer Statistics, 2004. CA Cancer J Clin. 54, 8–29 [DOI] [PubMed] [Google Scholar]
- Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, et al. (2003). Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 102, 311–319 [DOI] [PubMed] [Google Scholar]
- Oyajobi BO, Munoz S, Kakonen R, Williams PJ, Gupta A, Wideman CL, Story B, Grubbs B, Armstrong A, Dougall WC, et al. (2007). Detection of myeloma in skeleton of mice by whole-body optical fluorescence imaging. Mol Cancer Ther. 6, 1701–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radl J, de Glopper E, Schuit HER, Zurcher C. (1979). Idiopathic paraprotienemia II. Transplantation of the paraprotein-producing clone from old to young C587Bl/KaLwRij mice. J Immunol. 122, 609–613 [PubMed] [Google Scholar]
- Radl J, Croese JW, Zurcher C, Van Den Enden-Vieveen MHM, Margreet de Leeuw A. (1988). Animal model of human disease; multiple myeloma. Am J Pathol. 132, 593–597 [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. (1992). RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Iurlaro M, Albini A, Minischetti M, Bussolino F, Pellegrino A, Ria R, Rusnati M, Presta M, et al. (1998). Human lymphoblastoid cells produce extracellular matrix-degrading enzymes and induce endothelial cell proliferation, migration, morphogenesis, and angiogenesis. Int J Clin Lab Res. 28, 55–68 [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. (1999). Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 93, 3064–3073 [PubMed] [Google Scholar]
- Van Valckenborgh E, Mincher D, Di Salvo A, Van Riet I, Young L, Van Camp B, Vanderkerken K. (2005). Targeting an MMP-9-activated prodrug to multiple myeloma-diseased bone marrow: a proof of principle in the 5T33MM mouse model. Leukemia 19, 1628–1633 [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Barlogie B, Epstein J. (1998). Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood 92, 2908–2913 [PubMed] [Google Scholar]
- Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J. (2004). Cancer and the microenvironment:Myeloma-osteoclast interactions as a model. Cancer Res. 64, 2016–2023 [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr (2007). Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109, 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]