Abstract
Within each nephro-vascular unit, the tubule returns to the vicinity of its own glomerulus. At this site, there are specialised tubular cells, the macula densa cells, which sense changes in tubular fluid composition and transmit information to the glomerular arterioles resulting in alterations in glomerular filtration rate and blood flow. Work over the last few years has characterised the mechanisms that lead to the detection of changes in luminal sodium chloride and osmolality by the macula densa cells. These cells are true “sensor cells” since intracellular ion concentrations and membrane potential reflect the level of luminal sodium chloride concentration. An unresolved question has been the nature of the signalling molecule(s) released by the macula densa cells. Currently, there is evidence that macula densa cells produce nitric oxide via neuronal nitric oxide synthase (nNOS) and prostaglandin E2 (PGE2) through cyclooxygenase 2 (COX 2)-microsomal prostaglandin E synthase (mPGES). However, both of these signalling molecules play a role in modulating or regulating the macula-tubuloglomerular feedback system. Direct macula densa signalling appears to involve the release of ATP across the basolateral membrane through a maxi-anion channel in response to an increase in luminal sodium chloride concentration. ATP that is released by macula densa cells may directly activate P2 receptors on adjacent mesangial cells and afferent arteriolar smooth muscle cells, or the ATP may be converted to adenosine. However, the critical step in signalling would appear to be the regulated release of ATP across the basolateral membrane of macula densa cells.
Keywords: ATP, Macula densa, Cell signalling
Introduction
The kidney comprises repeating complex nephro-vascular units that are responsible for the filtration of blood and the processing of filtrate into final urine [1]. Once the afferent arteriole enters Bowman’s capsule it branches to form glomerular capillaries before coalescing and emerging from the glomerulus as the efferent arteriole. Filtrate processing occurs through distinct sequential tubular segments with differing cellular morphology and functions [2]. The initial tubular segment is the proximal tubule, the “work horse” of the nephron. The loop of Henle is the next tubular segment and it is a complex structure that is responsible for the concentration-dilution of tubular fluid. One important characteristic of the loop of Henle is that the thin and thick ascending limbs are impermeable to water [3]. Since active salt reabsorption continues in this segment, there is dilution of tubular fluid such that luminal sodium chloride concentration [NaCl] and osmolality approach values that are approximately one third of the values found in plasma [4].
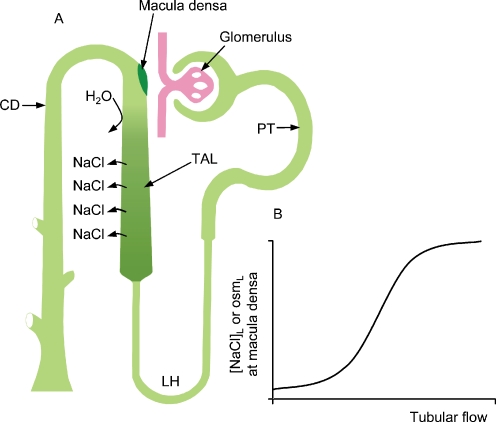
Shown in Fig. 1 is the fact that near the end of the loop of Henle, the tubule comes into contact with its own glomerulus. This appears to be an invariant feature of mammalian nephrons and this complex of tubule and parent glomerulus is termed the “juxtaglomerular apparatus” (JGA) [5, 6]. Early morphologists who identified this unique anatomical relationship also found that the cells that lie on the side of the tubule that faces the glomerulus are specialised and they called these cells macula densa cells, meaning “dense plaque of cells” [1]. The apical membrane of macula densa cells is exposed to luminal fluid while the basolateral membranes rest on mesangial cells. Electron micrographs have revealed that there are extensive interdigitations between basolateral membranes of macula densa cells and mesangial cells [7]. In spite of this specialised area of contact, however, there is no evidence for the presence of junctional complexes between these two types of cells [5]. This lack of specialised junctions was one reason to suspect chemical communication between these two cell types. Mesangial cells are modified smooth muscle cells that, in part, play a structural role supporting the glomerular capillary bed and are also in contact with the afferent and efferent arterioles [8–10]. These cells do exhibit gap junctions and connexins and there does appear to be communication between mesangial cells and also between mesangial cells and arteriolar segments and other elements within the JGA. Studies have demonstrated that electrical and calcium waves can be propagated throughout the mesangial cell field, arterioles, and even to the glomerular podocytes [8, 9, 11].
Fig. 1.
a Schematic representation of a nephro-vascular unit. The major nephron segments are identified; PT proximal tubule, LH loop of Henle, TAL thick ascending limb, and CD collecting duct. b Relationship between flow rate through the nephron and the sodium chloride concentration and osmolality of the tubular fluid at the macula densa
It has long been thought that the macula densa cells serve as sensor cells detecting changes in the tubular environment and transmitting signals to the mesangial cells/glomerular arterioles. One reason for this was simply based on the anatomical position of these cells located in the latter part of the nephron and in direct contact with the structure that is responsible for the filtration of fluid. It was also realised that glomerular filtration and nephron function appeared to be somewhat unrelated processes. Thus it made some sense that there would be a way of maintaining quality control, or, to put in another way, to synchronise filtration with the processes of reabsorption, secretion and excretion [12]. Another reason for suspecting that these cells were sensor cells was the fact that they are located very close to the end of the thick ascending limb (TAL). Before the “modern era” of macula densa research in the 1960s, work by Gottschalk and colleagues had found that the [NaCl] of the early distal tubule, that segment just beyond the macula densa, was very low, suggesting that there was salt transport without concomitant water abstraction in the TAL [13]. More importantly, the degree of dilution of tubular fluid was dependent upon the flow rate through this segment [14]. At low flow rates, luminal fluid [NaCl] and osmolality were very low, but as flow rate was elevated there were increases in [NaCl] and osmolality that approached plasma values. This relationship between flow, [NaCl] and osmolality at the macula densa provided an obvious signal that could be detected by macula densa cells.
Tubuloglomerular feedback (TGF)
Using micropuncture, it was possible to determine experimentally the existence of a macula densa feedback mechanism [14–22]. Termed tubuloglomerular feedback (TGF), it was demonstrated that artificial elevations in tubular fluid [NaCl] resulted in decreases in glomerular filtration rate and glomerular capillary pressure. TGF responses, therefore, involved transmission of a vasoconstrictive signal in response to an increase in luminal fluid [NaCl]. Careful analysis has indicated that the initiation of TGF responses occurs when luminal fluid [NaCl] is around 10–15 mM and that maximum responses are normally achieved when luminal fluid [NaCl] reaches 60 mM [14, 23]. Once the initial characterization of TGF signalling was accomplished, this led almost immediately to speculations concerning the nature of the signals that were transmitted from macula densa cells to the mesangial cell-glomerular arteriolar complex. This area remained mostly speculation until the last several years, during which substantial progress has been made in understanding the signalling transmission process of TGF.
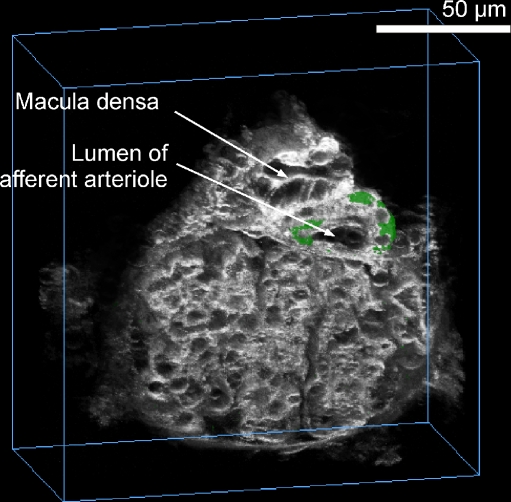
TGF signalling can be broken down into several arbitrary steps [14]. First is the mechanism that occurs at the apical membrane for the detection of tubular fluid and its constituents. Second is how the macula densa cell processes this initial signal. Third is signal or signals that are produced and elaborated by the macula densa. Fourth is the target or targets of macula densa signalling. Fifth is the change in glomerular filtration rate (GFR), which involves changes in glomerular arteriolar tone, mesangial cell contraction, and changes in the ultrafiltration coefficient of the glomerular capillaries. TGF signalling can also lead to the release of humoral factors such a renin [24–26] (Fig. 2).
Fig. 2.
A multiphoton confocal image of an isolated perfused JGA preparation in which both afferent arteriole and thick ascending limb are perfused. Note the close anatomical association between the macula densa plaque and the afferent arteriole. Also, the green fluorescence is quinacrine, which stains acidic vesicles and most likely represents renin-containing granules
The first step in the signal transmission process is the detection of some change in the tubular fluid environment. This process occurs at the apical membrane. Macula densa cells possess an apically located Na:2Cl:K cotransporter that is responsible for the majority (∼80%) of the entry of salt into macula densa cells [15, 27–30]. The apical membrane also possesses a Na:H exchanger, ROMK channel, and a Na,K(H)-ATPase [16, 31–37]. These cells are also permeable to water, at least to some degree, and possess a very long single cilium [30, 38]. As indicated, in response to an elevation in filtration rate or an increase in flow rate through the TAL, there are elevations in luminal [NaCl] and osmolality. With the rise in luminal salt concentration there is increased entry of Na, Cl and K into the macula densa cells. Potassium is recycled across the apical membrane via ROMK, as in the TAL [33]. Interestingly, macula densa intracellular [Na] and presumably [Cl] track or reflect luminal [NaCl] over a luminal concentration range from near zero to 60 mM [39]. This phenomenon does not occur in TAL cells; the fact that the intracellular salt concentration is a reflection of what is occurring in the lumen is certainly what would be expected of a sensor cell that is monitoring the luminal environment. The entry of chloride into macula densa cells has a secondary consequence, which is basolateral membrane depolarization via plasma membrane chloride channels [31]. As will be discussed subsequently, there are several types of basolateral chloride-permeable pathways, one of which conducts ATP [40]. However, control of membrane potential occurs through other chloride channels such as the ClCs [41, 42]. When luminal [NaCl] is low, macula densa cells are hyperpolarized; when luminal salt concentration is increased, there is concentration-dependent membrane depolarization. Membrane depolarization exactly “tracks” luminal [NaCl] from very low values up to 60 mM [31]. Macula densa basolateral membrane potential changes have been suggested to play an important role in transmission of TGF signalling. As shown by Garvin and colleagues [43], macula densa membrane depolarization with high potassium and valinomycin can lead to TGF responses. Other work by our laboratory has shown that in response to normal concomitant elevations in luminal [NaCl] and osmolality there is cell shrinkage that occurs because, unlike the cortical TAL cells, macula densa cells have a finite apical water permeability [30, 44]. There is also a modest elevation in cytosolic calcium concentration that occurs with elevated luminal salt concentration [45], and it has been suggested that changes in cystosolic calcium concentration may play an important role in TGF signalling [46, 47].
Thus, these cells exhibit the characteristics of an ideal sensor cell whose primary function is to monitor the luminal environment and to then transfer this information to the effector cells that control the rate of glomerular filtration. As stated, this process occurs over the range of very low luminal [NaCl] to around 60 mM; a range that most likely encompasses variations in luminal [NaCl] under normal conditions. The long unanswered question has been: how do macula densa cells transmit information to the underlying cells? This has been difficult to address experimentally because of the complex anatomical arrangement of the JGA and the issue of mediator(s) versus modulators. Over the years, a number of studies have been conducted to assess the possible role of vasoactive systems in TGF signalling. These have included thromboxanes, prostaglandins, angiotensin II, kinins, nitric oxide, etc. [14]. In a number of studies there was an effect, i.e., inhibition or stimulation of TGF, but there has always been uncertainty as to the meaning of the results on a least two levels. One is that these vasoactive systems may simply alter feedback signal sensitivity; the other is that they may directly affect smooth muscle cell contractility. In the 1960s it was proposed that macula densa signalling could be mediated by angiotensin II [48]. However, this was ruled out when it was established that increases in luminal fluid [NaCl] resulted in decreases in renin production and angiotensin II formation [49, 50]. This is exactly the opposite of what would be expected of a mediator of TGF, i.e., a decrease in the formation of a vasoconstrictive hormone, when the macula densa cells generate a vasoconstrictor signal; however, angiotensin is a modulator of TGF. A number of studies have shown that this hormone enhances TGF signalling and that this may occur by stimulating NaCl transport at the apical membrane of the macula densa cells and also sensitising afferent arteriole smooth muscle cells [51–58]. Thus, although this vasoactive hormone does not mediate feedback signal transduction, it is nevertheless involved in the overall process of TGF signalling.
Modulators of TGF produced by macula densa cells
There is a considerable amount of literature regarding the role of nitric oxide in the regulation of renal haemodynamics [59–72]. The involvement of macula densa cells in nitric oxide-mediated events came with the appreciation that these cells possess a high level of the neuronal form of nitric oxide synthase (nNOS) [59]. The presence of nNOS would presumably result in the macula-densa-mediated generation of NO, which then might lead to alterations in TGF signalling. There also appears to be short- and long-term regulation of the nitric oxide synthase-nitric oxide pathway, with acute TGF responses being an example of the former, and changes in dietary salt intake an example of the latter. We, and others, have taken advantage of fluorescent probes that have been developed to measure NO levels. In our studies we monitored extracellular levels of NO using the fluorescent probe DAF-FM and found that increases in luminal [NaCl] resulted in the generation of NO by macula densa cells [63, 73]. Further, we found that NO inhibited apical Na:2Cl:K cotransporter activity in macula densa cells. In addition, there was evidence that NO diffused into the mesangial cell field and that it could modify mesangial and arteriolar cell function. One interesting aspect of this study was the finding that macula densa stimulation of NO occurred also at higher luminal [NaCl] concentrations: between 60 and 150 mM, which suggests that NaCl transport through the cotransporter is not involved in the regulation of NO synthesis. It has been suggested that cell pH may play a role, and clearly macula densa Na:H exchanger-dependent alkalinization occurs from very low values of luminal [Na] up to 150 mM [37, 65]. However, the mechanism for nNOS stimulation and NO generation by macula densa cells is, at the present time, not entirely clear. We have proposed that NO serves as a brake or deterrent against excessive TGF-mediated vasoconstriction. It has been clearly shown that blockade of nNOS results in augmented feedback responses and that conditions in which NO is generated reduce TGF-mediated vasoconstriction [58, 70–72, 74].
Recent studies have also found that macula densa cells produce prostaglandin E2 in response to changes in the luminal environment [75, 76]. There is an extensive literature on the presence of cyclooxygenase 2 (COX-2) in macula densa cells [62, 77–79]. COX-2, along with microsomal-associated PGE synthase (mPGES), is expressed in macula densa cells, although the level of expression varies with salt intake [80]. Under conditions of normal to high salt diet, these two enzymes are minimally expressed, whereas on a low salt diet there is intense staining, measured by immunofluorescence, of both COX-2 and mPGES in macula densa cells. We used a biosensor assay (which will be described in more detail in the following section on ATP release) to determine if macula densa cells did in fact release PGE2. We were able to demonstrate that macula densa cells do in fact produce and release this prostanoid in a manner that is consistent with enzyme expression. In other words, little PGE2 was detected from macula densa cells obtained from animals on a normal or on a high salt diet, whereas in animals that had been maintained on a low salt diet there was marked PGE2 release at the basolateral membrane of macula densa cells. PGE2 release was stimulated by a reduction of luminal [NaCl] from ∼60 mM to zero. Macula densa PGE2 release was dependent on a functioning cotransporter since it was sensitive to furosemide: addition of furosemide to the lumen mimicked the effect of reducing luminal [NaCl] to zero. These studies and work by other laboratories suggest that macula densa PGE2 release, like NO, may play a role in protecting renal haemodynamics under conditions of a low salt diet or during volume depletion.
ATP as a mediator of TGF
The article by Dr. Inscho in this Special Issue [81] eloquently discusses the history of and evidence for a role of ATP in the regulation of renal haemodynamics. The work of this group, as well as studies from the Navar Laboratory at Tulane, set the stage for our contributions in this area [49, 82–88]. It should also be mentioned that around 1977, Osswald et al. proposed that adenosine played a role as a mediator of TGF. Thus, even early on, there was the suggestion that purines were involved in TGF signalling. Osswald’s proposal was based on the fact that salt reabsorption through the cotransporter, which does not directly use ATP, requires work and therefore energy metabolism [89]. It was proposed that elevations in luminal [NaCl] would result in increased salt reabsorption, which would then require ATP utilisation and thus formation of adenosine. The fact that adenosine receptors were known to exist made this a plausible hypothesis. As will be discussed, adenosine may be involved in TGF signalling, but with some modification in the initial hypothesis.
The suggestion that ATP may serve in TGF came from studies of autoregulation of GFR and renal blood flow. As discussed in more detail by Inscho, the kidney is a highly efficient autoregulatory organ and it is generally accepted that the TGF mechanism is responsible, at least in part, for this behaviour [90–93]. Thus, in response to an increase in blood pressure, there would be a transient elevation in GFR, leading to increased tubular flow into the proximal tubule. At the TAL, this increased flow rate would lead to less salt reabsorption per unit flow rate and a rise in luminal [NaCl] and in osmolality at the macula densa. This change in the luminal environment would be sensed by the macula densa and signals sent to the afferent arteriole, resulting in vasoconstriction and decreases in GFR and renal blood flow back to normal. There is certainly a component of autoregulation that is also due to myogenic behaviour of the smooth muscle cells; the current discussions in this area of research deal with the relative contributions of TGF and myogenic activity to autoregulation [94]. The contribution of both systems to autoregulation may not be fixed, but could vary due to changes in physiological conditions or disease processes.
There has been a convincing body of work that would suggest that ATP and purinergic receptors are involved in the process of GFR and blood flow autoregulation. Navar and colleagues [95–97] used microdialysis of the renal cortical interstitium to determine the concentration of nucleotides during autoregulation in response to changes in renal artery pressure. They found that interstitial ATP concentration correlated with changes in blood pressure, while interstitial adenosine concentration did not. Thus, when blood pressure was elevated renal cortical ATP levels also increased. A more direct means of assessing the role of ATP in autoregulatory responses is the in vitro blood-perfused juxtamedullary nephron preparation. Inscho and coworkers [49, 82, 87, 88, 91] found that pressure-induced afferent arteriolar vasoconstriction was attenuated during pharmacological blockade of P2 receptors with suramin, PPADS or the more selective blocker NF-279. Desensitisation of P2 receptors also inhibited pressure-induced autoregulatory adjustments in afferent arteriolar diameter. In addition, autoregulatory responses were diminished in a P2X1 receptor knockout mouse. Thus, there is convincing evidence for a role of ATP and purinergic receptors in the process of renal autoregulation (see article by Inscho [81] in this Special Issue).
These experimental approaches also suggested that TGF-mediated autoregulatory responses involve ATP. Nishiyama et al. [98] reported that renal cortical interstitial ATP concentration increased when distal volume delivery was elevated with the carbonic anhydrase inhibitor, acetazolamide, thereby inducing tubuloglomerular feedback responses, whereas interstitial ATP concentration was found to decrease when TGF responses were blocked with furosemide. In addition, the impaired autoregulatory response found in the P2X1 receptor knockout mice was not affected by manoeuvres that block TGF [88]. These results suggest that ATP may play a role in the TGF signalling process.
The ATP-signalling paradigm grew out of the work by Burnstock [99] through the identification of cell surface receptors that specifically bound ATP and the finding that cells release this nucleotide in a regulated or purposeful manner. In epithelial cells, which are polarised cells, purinergic receptors have been identified at both apical and basolateral surfaces [100–102]. In addition, ATP release by epithelial cells occurs across both membranes, although, in most confluent monolayers of epithelial cells, ATP release is greater across the apical membrane. Recent studies have demonstrated a role for cilia in the regulation of ATP release from the apical surface [103]. The mechanism for regulated release of ATP from epithelial cells has remained an unresolved issue. Certainly there is evidence for exocytosis of ATP; however, in terms of paracrine signalling there has been a search for an anion channel that would serve as an ATP conductive pathway. This has some appeal, since it would presumably favour a faster on/off transient and could provide a greater degree of precision regarding the amount of ATP that was released from the cell per unit time. Several ATP conductive candidate channels have been proposed, including CFTR, a nucleic acid channel, connexin hemichannels, and the maxi-anion channel [104–106]. It is also possible that more than one anion conductive pathway could be permeable to ATP.
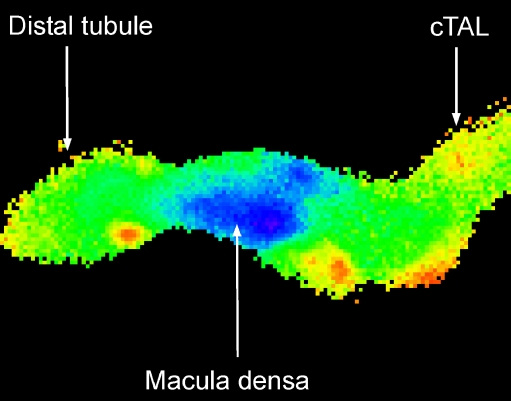
Macula densa cells have unique characteristics. The morphology of these cells is different from other epithelial cells and there is little resemblance to the surrounding TAL cells. They appear to have a small cytoplasm to nuclei ratio and to have an abundance of mitochondria [1, 7]. They have an interesting pattern of protein expression; one example, nNOS, has already been mentioned [59]. These cells are located near the end of the TAL at a site in which luminal [NaCl] is at a minimum. Thus, under normal physiological conditions, there may be minimal transport demands on these cells [29, 32, 33]. The finding that macula densa cells have a low abundance of Na+,K+-ATPase [107] is consistent with this notion. In fact we found that these cells use a Na+,K+(H+)-ATPase at the apical membrane to transport Na out of the cells [39]. What this suggests—though does not prove—is that these cells may have a readily available pool of ATP that could be used for cell-to-cell signalling. As stated previously, there are luminal [NaCl]-dependent changes in basolateral membrane potential such that increases in luminal salt concentration lead to cell depolarization. This is interesting since many channels are membrane voltage-dependent and this provides an attractive means of coupling an apical entry event to something that might regulate a basolateral channel. Also, as shown in Fig. 3, cytosolic calcium regulation in macula densa cells is unusual [45, 108]. Basal levels of calcium concentration are remarkably low compared with those of the surrounding TAL cells. One idea to explain this low calcium concentration in macula densa cells is based on the low cytoplasm to nuclei ratio and the large number of mitochondria. We have speculated that there is avid uptake of calcium into macula densa mitochondria and that this is one reason for the low cytoplasmic calcium concentration. Interestingly, mitochondrial ATP synthesis is enhanced by the uptake of calcium into mitochondria [109]. This further suggests that one of the primary functions of macula densa cells is to generate a pool of ATP that can be used for cell signalling. As will be discussed below, macula densa cells release ATP across the basolateral membrane. Interestingly, the basolateral membrane of macula densa cells expresses the P2Y2 receptor [110]. No purinergic receptor activity was detected at the apical membrane. One speculation for the existence of this receptor at the basolateral membrane is that upon release of ATP by macula densa cells, some of the ATP would bind to the P2Y2 receptors and trigger elevations in macula densa cells’ cystosolic calcium and thereby stimulate mitochondrial synthesis of ATP. In this manner these cells could couple ATP release with ATP synthesis.
Fig. 3.
Wide-field image of an isolated perfused thick ascending limb. The glomerulus is not shown because fura-2, the calcium-sensitive fluorophore, was loaded through the lumen. High levels of cytosolic calcium are red and yellow, while lower values are green and the lowest calcium concentrations are in blue. The macula densa cells have a much lower cytosolic calcium concentration compared to the surrounding epithelial cells
The use of patch-clamp techniques in macula densa cells is difficult. It requires dissection of the surrounding TAL, in order to expose the macula densa plaque, and an understanding the three dimensional orientation of macula densa-glomerulus to the patch pipette—which is complicated. Work on the maxi-anion channel in macula densa cells has involved our group, JY Lapointe (University of Montreal) and RZ Sabirov and Y Okada (National Institute of Physiological Sciences, Okakazi, Japan). It involved exposure of the basolateral surface and the successful patching of this membrane [111].
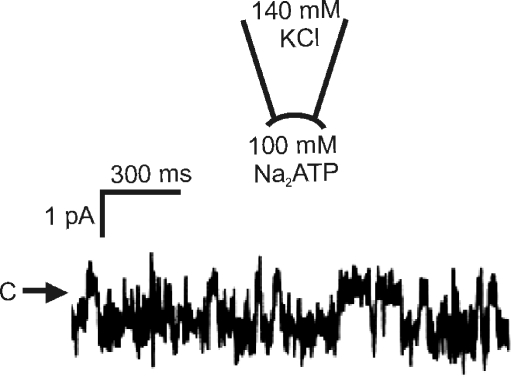
In a previous work from our laboratory, using microelectrodes to record basolateral membrane potentials, we determined that Cl− channels are the major charge carriers across the basolateral membrane [31]. Therefore, in our initial patch-clamp studies we wanted to address the nature of this Cl− channel conductance. We found evidence for a relatively small Cl− channel that may be responsible for the basolateral membrane depolarization seen when intracellular [Cl−] is elevated as a result of the enhanced entry of NaCl across the apical membrane. What was interesting to us was the presence of a large-conductance channel of ∼380 pS in cell-attached patches in the presence of 135 mM NaCl in the bathing solution. After excision and forming inside-out patches, channel activity was maintained, and the single-channel conductance was unchanged. There was a linear I–V relationship with a reversal potential close to zero. Interestingly, this channel exhibited voltage-dependent inactivation at large potentials (>50 mV) under symmetrical ionic conditions. We found that this was a Cl−-permeable pathway since the I–V relationship was unaltered when N-methyl-d-glucamine was substituted for Na+ and inward currents were reduced and the reversal potential was shifted to the left when bath [Cl−] was lowered by substituting with gluconate. The maxi-anion channel was permeable to large monovalent anions, and previous studies had found that this channel was permeable to ATP [112]. To assess whether the macula densa basolateral membrane channel was ATP-permeable, the intracellular (bath) solution was changed to 100 mM ATP solution (Fig. 4). At negative potentials, single-channel activity with inward current jumps was clearly detected at −50 mV. Since the only anion present at the intracellular surface of the patch (bathing solution) was ATP, then movement of ATP must be responsible for the inward currents. The permeability ratio was estimated to be PATP/PCl = 0.14. Macula densa maxi-anion channels were inhibited by 50 μM Gd3+, which is known to block ATP release from other cells and maxi-anion channels in C127 cells [113, 114]. In the presence of the Cl− channel blocker, 4-acetamido-4′-isothiocyanostilbene-2,2′-disulphonic acid (SITS), channel current was not suppressed, but became noisy, suggesting a flickery block. Channel activity was not sensitive to diphenylamine-2-carboxylate (DPC) or 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), nor was it sensitive to cytosolic calcium.
Fig. 4.
Representative trace recorded from an inside-out patch at the basolateral membrane of a macula densa cell. C and arrow indicate the closed current level. Current is mediated by a maxi-anion channel and the downward deflections reflect movement of ATP through the channel
Although the physical presence of a channel that conducts ATP has been firmly established using biophysical techniques, the molecular nature of this maxi-anion channel has remained elusive. Based on the biophysical properties of this channel, it has been suggested that it is a plasma membrane form of a mitochondrial porin (voltage-dependent anion channel, VDAC) [112]. This is especially appealing, since it is well known that this channel transports ATP across mitochondrial membranes. Also, the size and certain characteristics of the two channels, such as voltage inactivation at both positive and negative potentials, are similar. Unfortunately, as reported by Sabirov et al [115], deletion of the known VDAC genes in fibroblasts does not greatly diminish ATP release or the presence of a large conductive channel. However, it is possible that some VDAC-like protein, which has yet to be identified, could be involved in macula densa release of ATP. It is also possible that proteins such as connexin hemichannels or the nucleic acid channel [104, 116, 117] could be involved in ATP release. Thus, the unanswered question at this time is the molecular identity of this channel and what controls the opening and closure of this ATP pathway. Current evidence indicates that it is not a rise in intracellular calcium; rather this ion may function to promote mitochondrial ATP synthesis [111]. A likely candidate is membrane depolarization induced by a rise in intracellular chloride and this would make sense in terms of the overall TGF mechanism. Further work is needed to clarify the identity and regulation of this pathway in macula densa cells.
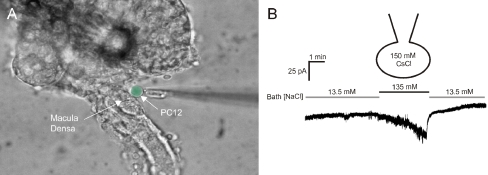
The presence of an ATP-permeable pathway does not, per se, establish that there is in fact ATP release and, importantly, that if there is release it occurs in a manner consistent with TGF signalling. To accomplish this goal, we used a “biosensor” approach to assess ATP release from macula densa cells [111]. This involved loading either a PC12 cell or a cultured mesangial cell with fura-2 and then placing these cells in a chamber mounted on a microscope, wherein the macula densa-glomerular preparation was being perfused. The dissection procedure was modified somewhat to expose the basolateral membrane of these cells, as shown in Fig. 5. A pipette was then used to place the biosensor cell next to the basolateral membrane of the macula densa cells and to measure changes in cytosolic calcium concentration of the biosensor cell. Remarkably, an increase in luminal [NaCl] elicited a rapid increase in cytosolic calcium concentration in either PC12 cells or mesangial cells. This increase in biosensor calcium concentration could be completely inhibited by the addition of the P2 receptor blocker suramin to the bath. In addition, the release of ATP by macula densa cells was blocked by the addition of furosemide, a blocker of Na:2Cl:K cotransport. Finally, we were able to show stepwise increases in ATP release as luminal [NaCl] increased incrementally to 60 mM [118]. This is direct evidence that macula densa cells release ATP in a manner that is consistent with TGF signalling.
Fig. 5.
a Photomicrograph showing the isolated perfused thick ascending limb-glomerular preparation. The glomerulus has been partially removed to allow access to the basolateral membrane. A biosensor (in this case a PC12 cell) is then placed at the basolateral membrane of these cells. ATP-P2X activity in the biosensor cell is measured with fura-2 or using whole-cell patch-clamp. Biosensor cell is pseudo-coloured green for identification. (b) Whole-cell patch-clamp of the biosensor cell showing the effects of increasing luminal sodium chloride concentration; these results are consistent with the activation of a P2X channel
Finally, is it ATP or some metabolite of this nucleotide that transmits information to the afferent arteriole? This has been controversial and the reader is referred to the review of Inscho in this issue [81] for a comprehensive discussion. There is evidence for a role of ATP conversion to adenosine, which then leads to arteriolar contraction via adenosine A1 receptors. There is also evidence that ATP directly leads to vasoconstriction. We and others have shown that cultured mesangial cells have P2Y2 receptors [40, 118, 119]. ATP can induce calcium responses at concentrations as low as 0.3 μM with a half-maximal concentration of 0.8 μM. Based on the biosensor assay, we calculated that macula densa cells release ATP across the basolateral membrane, giving rise to a local ATP concentration of as much as 10 μM. In addition, mesangial cells do not respond to adenosine. Thus, the mesangial cells are located directly adjacent to the macula densa cells, possess purinergic receptors, have gap junctions and are connected to other elements within the JGA, including the afferent arterioles. As shown by Peti-Peterdi [9], there is propagation of a TGF-mediated calcium wave that occurs throughout the JGA during TGF that is dependent upon ATP, and not on adenosine.
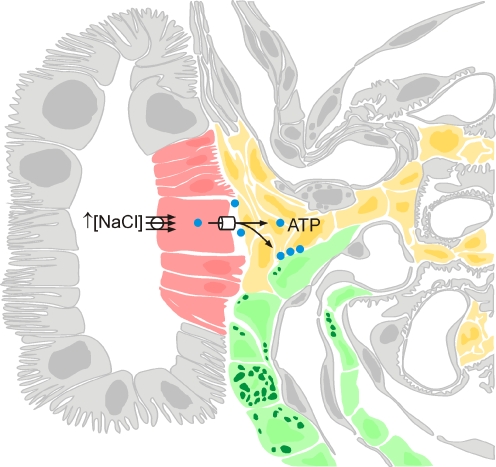
When released by macula densa cells, it is clear that ATP is rapidly degraded by several different forms of nucleotidases to adenosine and other metabolites. In fact, this is a very important point regarding this paracrine signalling process, i.e., it has a rapid onset and termination. However, to the extent that adenosine is involved in TGF, it is unlikely to be the step that is critical for the control of TGF signalling. There is little evidence that nucleotidases are rate-limiting in ATP metabolism [120]. The control point for macula densa cell signalling most likely resides at the point of ATP release across the basolateral membrane. For instance, it is known that TGF responsiveness varies with dietary salt. As shown by Komlosi et al [9, 118], the magnitude of ATP release by macula densa cells in response to luminal [NaCl] varied depending upon whether the animal had been on a normal or low salt diet. Thus, under conditions of a low salt diet where TGF responses are enhanced, macula densa cells release a greater quantity of ATP for any given level of luminal [NaCl]. This strongly suggests that the critical step or control point in macula densa TGF signalling is the release of ATP across the basolateral membrane of macula densa cells. Figure 6 shows a schematic summary of the TGF pathway.
Fig. 6.
Schematic representation of the macula densa cell signalling pathway in which ATP release by the macula densa cells plays a critical and essential role. Macula densa cells, extra/intraglomerular mesangial cells and vascular smooth muscle cells of the afferent arteriole are shown in red, yellow and green, respectively
Acknowledgements
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Disease (DK-32032) and by a Scientist Development Award from the American Heart Association and a VA Merit Grant. We thank Ms. Barbara Harris for administrative assistance.
References
- 1.Barajas L (1979) Anatomy of the juxtaglomerular apparatus. Am J Physiol 237:F333–F343 [DOI] [PubMed]
- 2.Schafer JA, Troutman SL, Andreoli TE (1974) Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol 64:582–607 [DOI] [PMC free article] [PubMed]
- 3.Burg MB, Green N (1973) Function of the thick ascending limb of Henle's loop. Am J Physiol 224:659–668 [DOI] [PubMed]
- 4.Rocha AS, Kudo LH (1982) Water, urea, sodium, chloride, and potassium transport in the in vitro isolated perfused papillary collecting duct. Kidney Int 22:485–491 [DOI] [PubMed]
- 5.Barajas L, Salido EC, Liu L, Powers KV (1995) The juxtaglomerular apparatus: a morphologic perspective. In: Laragh JH et al (eds) Hypertension: pathophysiology, diagnosis and management. Raven, New York, pp 1335–1348
- 6.Komlosi P, Fintha A, Bell PD (2005) Renal cell-to-cell communication via extracellular ATP. Physiology (Bethesda) 20:86–90 [DOI] [PubMed]
- 7.Bell PD, St John PL, Speyer M, Abrahamson DR (1992) Permeability of the macula densa basement membrane area to high molecular weight molecules. Ren Physiol Biochem 15:89–98 [DOI] [PubMed]
- 8.Iijima K, Moore LC, Goligorsky MS (1991) Syncytial organization of cultured rat mesangial cells. Am J Physiol 260:F848–F855 [DOI] [PubMed]
- 9.Peti-Peterdi J (2006) Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291:F473–F480 [DOI] [PubMed]
- 10.Pricam C, Humbert F, Perrelet A, Orci L (1974) Gap junctions in mesangial and lacis cells. J Cell Biol 63:349–354 [DOI] [PMC free article] [PubMed]
- 11.Goligorsky MS, Iijima K, Krivenko Y, Tsukahara H, Hu Y, Moore LC (1997) Role of mesangial cells in macula densa to afferent arteriole information transfer. Clin Exp Pharmacol Physiol 24:527–531 [DOI] [PubMed]
- 12.Navar LG and Bell PD (2004) Romancing the macula densa at UAB. Kidney Int Suppl :S34-S40 [DOI] [PubMed]
- 13.Ullrich KJ, Schmidt-Nielsen B, O'Dell R, Pehling G, Gottschalk CW, Lassiter WE, Mylle M (1963) Micropuncture study of composition of proximal and distal tubular fluid in rat kidney. Am J Physiol 204:527–531 [DOI] [PubMed]
- 14.Bell PD, Lapointe JY, Peti-Peterdi J (2003) Macula densa cell signaling. Annu Rev Physiol 65:481–500 [DOI] [PubMed]
- 15.Bell PD, Lapointe JY (1997) Characteristics of membrane transport processes of macula densa cells. Clin Exp Pharmacol Physiol 24:541–547 [DOI] [PubMed]
- 16.Bell PD, Navar LG (1982) Relationship between tubulo-glomerular feedback responses and perfusate hypotonicity. Kidney Int 22:234–239 [DOI] [PubMed]
- 17.Bell PD, Navar LG (1979) Stop-flow pressure feedback responses during reduced renal vascular resistance in the dog. Am J Physiol 237:F204–F209 [DOI] [PubMed]
- 18.Bell PD, Thomas C, Williams RH, Navar LG (1978) Filtration rate and stop-flow pressure feedback responses to nephron perfusion in the dog. Am J Physiol 234:F154–F165 [DOI] [PubMed]
- 19.Castrop H, Schweda F, Mizel D, Huang Y, Briggs J, Kurtz A, Schnermann J (2004) Permissive role of nitric oxide in macula densa control of renin secretion. Am J Physiol Renal Physiol 286:F848–F857 [DOI] [PubMed]
- 20.Schnermann J, Davis JM, Wunderlich P, Levine DZ, Horster M (1971) Technical problems in the micropuncture determination of nephron filtration rate and their functional implications. Pflugers Arch 329:307–320 [DOI] [PubMed]
- 21.Schnermann J, Ploth DW, Hermle M (1976) Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch 362:229–240 [DOI] [PubMed]
- 22.Schnermann J (1998) Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol 274:R263–R279 [DOI] [PubMed]
- 23.Schnermann J, Briggs JP (2000) Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: Seldin DW et al (eds) The Kidney Physiology & Pathophysiology. Lippincott Williams & Wilkins, Philadephia, pp 945–980
- 24.Barajas L, Powers K, Carretero O, Scicli AG, Inagami T (1986) Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int 29:965–970 [DOI] [PubMed]
- 25.Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH (2004) Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol 287:F329–F335 [DOI] [PubMed]
- 26.Schweda F, Wagner C, Kramer BK, Schnermann J, Kurtz A (2003) Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol 284:F770–F777 [DOI] [PubMed]
- 27.Bell PD, Lapointe JY, Cardinal J, Chang YS (1991) Transport pathways in macula densa cells. Kidney Int Suppl 32:S59–S64 [PubMed]
- 28.Laamarti MA, Lapointe JY (1997) Determination of NH4+/NH3 fluxes across apical membrane of macula densa cells: a quantitative analysis. Am J Physiol 273:F817–F824 [DOI] [PubMed]
- 29.Lapointe JY, Laamarti A, Bell PD (1998) Ionic transport in macula densa cells. Kidney Int Suppl 67:S58–S64 [DOI] [PubMed]
- 30.Komlosi P, Fintha A, Bell PD (2006) Unraveling the relationship between macula densa cell volume and luminal solute concentration/osmolality. Kidney Int 70:865–871 [DOI] [PubMed]
- 31.Bell PD, Lapointe JY, Cardinal J (1989) Direct measurement of basolateral membrane potentials from cells of the macula densa. Am J Physiol 257:463–468 [DOI] [PubMed]
- 32.Laamarti MA, Bell PD, Lapointe JY (1998) Transport and regulatory properties of the apical Na-K-2Cl cotransporter of macula densa cells. Am J Physiol 275:703–709 [DOI] [PubMed]
- 33.Lapointe JY, Laamarti A, Hurst AM, Fowler BC, Bell PD (1995) Activation of Na:2Cl:K cotransport by luminal chloride in macula densa cells. Kidney Int 47:752–757 [DOI] [PubMed]
- 34.Lapointe JY, Bell PD, Cardinal J (1990) Direct evidence for apical Na+:2Cl−:K+ cotransport in macula densa cells. Am J Physiol 258:F1466–F1469 [DOI] [PubMed]
- 35.Lapointe JY, Bell PD, Hurst AM, Cardinal J (1991) Basolateral ionic permeabilities of macula densa cells. Am J Physiol 260:F856–F860 [DOI] [PubMed]
- 36.Hurst AM, Lapointe JY, Laamarti A, Bell PD (1994) Basic properties and potential regulators of the apical K+ channel in macula densa cells. J Gen Physiol 103:1055–1070 [DOI] [PMC free article] [PubMed]
- 37.Fowler BC, Chang YS, Laamarti A, Higdon M, Lapointe JY, Bell PD (1995) Evidence for apical sodium proton exchange in macula densa cells. Kidney Int 47:746–751 [DOI] [PubMed]
- 38.Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L (1997) Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem 272:29672–29680 [DOI] [PubMed]
- 39.Peti-Peterdi J, Bebok Z, Lapointe JY, Bell PD (2002) Novel regulation of cell [Na(+)] in macula densa cells: apical Na(+) recycling by H-K-ATPase. Am J Physiol Renal Physiol 282:F324–F329 [DOI] [PubMed]
- 40.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y (2003) Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci U S A 100:4322–4327 [DOI] [PMC free article] [PubMed]
- 41.Uchida S (2000) Physiological role of CLC-K1 chloride channel in the kidney. Nephrol Dial Transplant 15(Suppl 6):14–15 [DOI] [PubMed]
- 42.Uchida S, Sasaki S (2005) Function of chloride channels in the kidney. Annu Rev Physiol 67:759–778 [DOI] [PubMed]
- 43.Ren Y, Yu H, Wang H, Carretero OA, Garvin JL (2001) Nystatin and valinomycin induce tubuloglomerular feedback. Am J Physiol Renal Physiol 281:F1102–F1108 [DOI] [PubMed]
- 44.Peti-Peterdi J, Morishima S, Bell PD, Okada Y (2002) Two-photon excitation fluorescence imaging of the living juxtaglomerular apparatus. Am J Physiol Renal Physiol 283:F197–F201 [DOI] [PubMed]
- 45.Peti-Peterdi J, Bell PD (1999) Cytosolic [Ca2+] signaling pathway in macula densa cells. Am J Physiol 277:F472–F476 [DOI] [PubMed]
- 46.Bell PD, Navar LG (1982) Cytoplasmic calcium in the mediation of macula densa tubulo-glomerular feedback responses. Science 215:670–673 [DOI] [PubMed]
- 47.Ren Y, Liu R, Carretero OA, Garvin JL (2003) Increased intracellular Ca++ in the macula densa regulates tubuloglomerular feedback. Kidney Int 64:1348–1355 [DOI] [PubMed]
- 48.Thurau K, Valtin H, Schnermann J (1968) Kidney. Annu Rev Physiol 30:441–524 [DOI] [PubMed]
- 49.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD (1996) Paracrine regulation of the renal microcirculation. Physiol Rev 76:425–536 [DOI] [PubMed]
- 50.Skott O, Briggs JP (1987) Direct demonstration of macula densa-mediated renin secretion. Science 237:1618–1620 [DOI] [PubMed]
- 51.Bell PD, Peti-Peterdi J (1999) Angiotensin II stimulates macula densa basolateral sodium/hydrogen exchange via type 1 angiotensin II receptors. J Am Soc Nephrol 10(Suppl 11):S225–S229 [PubMed]
- 52.Kovacs G, Peti-Peterdi J, Rosivall L, Bell PD (2002) Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol 282:F301–F306 [DOI] [PubMed]
- 53.Navar LG (1998) Integrating multiple paracrine regulators of renal microvascular dynamics. Am J Physiol 274:F433–F444 [DOI] [PubMed]
- 54.Navar LG, Imig JD, Zou L, Wang CT (1997) Intrarenal production of angiotensin II. Semin Nephrol 17:412–422 [PubMed]
- 55.Persson PB (2003) Renin: origin, secretion and synthesis. J Physiol 552:667–671 [DOI] [PMC free article] [PubMed]
- 56.Schnermann J, Briggs JP (1990) Restoration of tubuloglomerular feedback in volume-expanded rats by angiotensin II. Am J Physiol 259:F565–F572 [DOI] [PubMed]
- 57.Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, Briggs JP (1997) Absence of tubuloglomerular feedback responses in AT1A receptor- deficient mice. Am J Physiol 273:315–320 [DOI] [PubMed]
- 58.Vallon V (2003) Tubuloglomerular feedback in the kidney: insights from gene-targeted mice. Pflugers Arch 445:470–476 [DOI] [PubMed]
- 59.Bachmann S, Bosse HM, Mundel P (1995) Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol 268:F885–F898 [DOI] [PubMed]
- 60.Deng A, Wead LM, Blantz RC (2004) Temporal adaptation of tubuloglomerular feedback: Effects of COX-2. Kidney Int 66:2348–2353 [DOI] [PubMed]
- 61.Fischer E, Schnermann J, Briggs JP, Kriz W, Ronco PM, Bachmann S (1995) Ontogeny of NO synthase and renin in juxtaglomerular apparatus of rat kidneys. Am J Physiol 268:F1164–F1176 [DOI] [PubMed]
- 62.Harris RC (2002) Cyclooxygenase-2 and the kidney: functional and pathophysiological implications. J Hypertens Suppl 20:S3–S9 [PubMed]
- 63.Kovacs G, Komlosi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD (2003) Neuronal nitric oxide synthase: its role and regulation in macula densa cells. J Am Soc Nephrol 14:2475–2483 [DOI] [PubMed]
- 64.Liu R, Persson AE (2004) Angiotensin II stimulates calcium and nitric oxide release from Macula densa cells through AT1 receptors. Hypertension 43:649–653 [DOI] [PubMed]
- 65.Liu R, Carretero OA, Ren Y, Garvin JL (2005) Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67:1837–1843 [DOI] [PubMed]
- 66.Ollerstam A, Persson AE (2002) Macula densa neuronal nitric oxide synthase. Cardiovasc Res 56:189–196 [DOI] [PubMed]
- 67.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T (2004) Inhibition of nNOS expression in the macula densa by COX-2 derived prostaglandin E2. Am J Physiol Renal Physiol 287:F152–F159 [DOI] [PubMed]
- 68.Ren YL, Garvin JL, Carretero OA (2000) Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 58:2053–2060 [DOI] [PubMed]
- 69.Tojo A, Onozato ML, Fukuda S, Asaba K, Kimura K, Fujita T (2004) Nitric oxide generated by nNOS in the macula densa regulates the afferent arteriolar diameter in rat kidney. Med Electron Microsc 37:236–241 [DOI] [PubMed]
- 70.Wang H, Carretero OA, Garvin JL (2002) Nitric oxide produced by THAL nitric oxide synthase inhibits TGF. Hypertension 39:662–666 [DOI] [PubMed]
- 71.Wilcox CS, Deng X, Welch WJ (1998) NO generation and action during changes in salt intake: roles of nNOS and macula densa. Am J Physiol 274:R1588–R1593 [DOI] [PubMed]
- 72.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH (1992) Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89:11993–11997 [DOI] [PMC free article] [PubMed]
- 73.Liu R, Pittner J, Persson AE (2002) Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13:2688–2696 [DOI] [PubMed]
- 74.Wilcox CS, Welch WJ (1996) TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int Suppl 55:S9–S13 [PubMed]
- 75.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD (2003) Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112:76–82 [DOI] [PMC free article] [PubMed]
- 76.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J (2000) Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem 275:37922–37929 [DOI] [PubMed]
- 77.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC (2000) Nitric oxide regulates renal cortical cyclooxygenase-2 expression. Am J Physiol Renal Physiol 279:F122–F129 [DOI] [PubMed]
- 78.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD (1994) Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94:2504–2510 [DOI] [PMC free article] [PubMed]
- 79.Harris RC, Breyer MD (2001) Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol 281:F1–F11 [DOI] [PubMed]
- 80.Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J (2003) Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol 285:F558–F564 [DOI] [PubMed]
- 81.Inscho EW (2009) ATP, P2 receptors and the renal microcirculation. Pur. Sig., doi:10.1007/s11302-009-9147-1 [DOI] [PMC free article] [PubMed]
- 82.Inscho EW, Cook AK, Mui V, Miller J (1998) Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am J Physiol 274:718–727 [DOI] [PubMed]
- 83.Inscho EW, Carmines PK, Navar LG (1991) Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension 17:1033–1037 [DOI] [PubMed]
- 84.Inscho EW (2001) P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol 280:F927–F944 [DOI] [PubMed]
- 85.Inscho EW, Schroeder AC, Deichmann PC, Imig JD (1999) ATP-mediated Ca2+ signaling in preglomerular smooth muscle cells. Am J Physiol 276:F450–F456 [DOI] [PubMed]
- 86.Inscho EW, Cook AK, Navar LG (1996) Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol 271:F1077–F1085 [DOI] [PubMed]
- 87.Majid DS, Inscho EW, Navar LG (1999) P2 purinoceptor saturation by adenosine triphosphate impairs renal autoregulation in dogs. J Am Soc Nephrol 10:492–498 [DOI] [PubMed]
- 88.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ (2003) Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112:1895–1905 [DOI] [PMC free article] [PubMed]
- 89.Osswald H, Nabakowski G, Hermes H (1980) Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem 12:263–267 [DOI] [PubMed]
- 90.Casellas D, Moore LC (1990) Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol 258:F660–F669 [DOI] [PubMed]
- 91.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ (2004) Renal autoregulation in P2X knockout mice. Acta Physiol Scand 181:445–453 [DOI] [PubMed]
- 92.Navar LG, Burke TJ, Robinson RR, Clapp JR (1974) Distal tubular feedback in the autoregulation of single nephron glomerular filtration rate. J Clin Invest 53:516–525 [DOI] [PMC free article] [PubMed]
- 93.Navar LG, Bell PD, Burke TJ (1982) Role of a macula densa feedback mechanism as a mediator of renal autoregulation. Kidney Int Suppl 12:S157–S164 [PubMed]
- 94.Loutzenhiser R, Griffin K, Williamson G, Bidani A (2006) Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290:R1153–R1167 [DOI] [PMC free article] [PubMed]
- 95.Nishiyama A, Navar LG (2002) Response to J. Schnermann: adenosine mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283:Ra278–Ra280 [DOI] [PubMed]
- 96.Nishiyama A, Navar LG (2002) ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283:R273–R275 [DOI] [PubMed]
- 97.Nishiyama A, Majid DS, Walker MIII, Miyatake A, Navar LG (2001) Renal interstitial atp responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension 37:753–759 [DOI] [PubMed]
- 98.Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG (2000) Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86:656–662 [DOI] [PubMed]
- 99.Burnstock G (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36:1127–1139 [DOI] [PubMed]
- 100.Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, Rubini M, di Virgilio F (1996) An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 87:682–690 [PubMed]
- 101.Schwiebert EM, Zsembery A (2003) Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615:7–32 [DOI] [PubMed]
- 102.Schwiebert EM (2001) ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol 28:340–350 [DOI] [PubMed]
- 103.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM (2008) Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4:155–170 [DOI] [PMC free article] [PubMed]
- 104.Hanss B, Leal-Pinto E, Bruggeman LA, Copeland TD, Klotman PE (1998) Identification and characterization of a cell membrane nucleic acid channel. Proc Natl Acad Sci USA 95:1921–1926 [DOI] [PMC free article] [PubMed]
- 105.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J (2005) Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289:F1304–F1312 [DOI] [PubMed]
- 106.Egan ME (2002) CFTR-associated ATP transport and release. Methods Mol Med 70:395–406 [DOI] [PubMed]
- 107.Schnermann J, Marver D (1986) ATPase activity in macula densa cells of the rabbit kidney. Pflugers Arch 407:82–86 [DOI] [PubMed]
- 108.Komlosi P, Banizs B, Fintha A, Steele SL, Zhang ZR, Bell PD (2008) Oscillating cortical thick ascending limb cells at the juxtaglomerular apparatus. J Am Soc Nephrol 19:1940–1946 [DOI] [PMC free article] [PubMed]
- 109.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD (2004) Calcium and mitochondria. FEBS Lett 567:96–102 [DOI] [PubMed]
- 110.Liu R, Bell PD, Peti-Peterdi J, Kovacs G, Johansson A, Persson AE (2002) Purinergic receptor signaling at the basolateral membrane of macula densa cells. J Am Soc Nephrol 13:1145–1151 [DOI] [PubMed]
- 111.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y (2003) Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100:4322–4327 [DOI] [PMC free article] [PubMed]
- 112.Okada SF, O'neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC (2004) Voltage-dependent Anion Channel-1 (VDAC-1) Contributes to ATP Release and Cell Volume Regulation in Murine Cells. J Gen Physiol 124:513–526 [DOI] [PMC free article] [PubMed]
- 113.Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y (2000) Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl− conductances in murine C127 cells. J Physiol 523(Pt 1):1–11 [DOI] [PMC free article] [PubMed]
- 114.Sabirov RZ, Dutta AK, Okada Y (2001) Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol 118:251–266 [DOI] [PMC free article] [PubMed]
- 115.Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ (2006) Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem 281:1897–1904 [DOI] [PubMed]
- 116.Sabirov RZ, Prenen J, Tomita T, Droogmans G, Nilius B (2000) Reduction of ionic strength activates single volume-regulated anion channels (VRAC) in endothelial cells. Pflugers Arch 439:315–320 [DOI] [PubMed]
- 117.Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J (2008) Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294:R1769–R1776 [DOI] [PMC free article] [PubMed]
- 118.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD (2004) Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286:F1054–F1058 [DOI] [PubMed]
- 119.Gutierrez AM, Lou X, Erik A, Persson G, Ring A (1999) Ca2+ response of rat mesangial cells to ATP analogues. Eur J Pharmacol 369:107–112 [DOI] [PubMed]
- 120.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sevigny J, Robson SC (2005) Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288:F1032–F1043 [DOI] [PubMed]