Abstract
A range of P2 receptor subtypes has been identified along the renal tubule, in both apical and basolateral membranes. Furthermore, it has been shown that nucleotides are released from renal tubular cells, and that ectonucleotidases are present in several nephron segments. These findings suggest an autocrine/paracrine role for nucleotides in regulating tubular function. The present review catalogues the known actions of extracellular nucleotides on tubular solute transport. In the proximal tubule, there is firm evidence that stimulation of apical P2Y1 receptors inhibits bicarbonate reabsorption, whilst basolaterally applied ATP has the opposite effect. Clearance studies suggest that systemic diadenosine polyphosphates profoundly reduce proximal tubular fluid transport, through as yet unidentified P2 receptors. To date, only circumstantial evidence is available for an action of nucleotides on transport in the loop of Henle; and no studies have been made on native distal tubules, though observations in cell lines suggest an inhibitory effect on sodium, calcium and magnesium transport. The nephron segment most studied is the collecting duct. Apically applied nucleotides inhibit the activity of small-conductance K+ channels in mouse collecting duct, apparently through stimulation of P2Y2 receptors. There is also evidence, from cell lines and native tissue, that apically (and in some cases basolaterally) applied nucleotides inhibit sodium reabsorption. In mice pharmacological profiling implicates P2Y2 receptors; but in rats, the receptor subtype(s) responsible is/are unclear. Recent patch-clamp studies in rat collecting ducts implicate apical P2Y and P2X subtypes, with evidence for both inhibitory and stimulatory effects. Despite considerable progress, clarification of the physiological role of the tubular P2 receptor system remains some way off.
Keywords: P2 receptors, Nucleotides, Solute transport, Proximal tubule, Loop of Henle, Distal tubule, Collecting duct
Introduction
The effects of stimulation of renal vascular P2 receptors (see article by Inscho in this Special Issue [1]), including those involved in tubuloglomerular feedback (see article by Bell et al. in this Special Issue [2]), clearly have implications for overall excretion rates. In addition, however, excretion rates can be altered directly by stimulation of tubular P2 receptors located along the whole length of the nephron. Here, we will examine nucleotide-P2 receptor interactions that affect tubular solute transport; water transport is dealt with elsewhere in this Special Issue (see article by Kishore et al. [3]).
In describing the distribution of P2 receptors along the nephron, we will focus on those present in native tissue (see Fig. 1). The various receptor subtypes have been identified by a combination of techniques: (1) Examination of the response of nephron segments to a range of agonists of varying receptor selectivity. (This approach has mainly utilised the ubiquitous coupling of P2 receptors to increases in intracellular Ca2+ concentration ([Ca2+]i), though it should be emphasised that such increases are not invariably linked to functional changes.) (2) Identification of mRNA using RT-PCR. (3) Identification of receptor protein using immunohistochemistry. Being a polarised epithelium, there is potential for different, or the same, receptor subtypes to be expressed in each membrane domain. Wherever possible, we will make the distinction.
Fig. 1.
Current information on P2 receptor subtypes along the rat nephron (native tissue only). P2 receptor subtypes were identified using immunohistochemistry and/or western blotting. Where possible, apical (a), basolateral (b) or intracellular (intra) location is indicated. Information is taken from references [4, 5, 9, 10, 36, 37]
The proximal tubule
The rat proximal tubule expresses mRNA for at least four P2Y receptor subtypes: P2Y1, 2, 4 and 6 [4, 5]. Expression at the mRNA level of other members of the P2Y family, or of any members of the P2X family, has not been investigated in native proximal tubule. Functional and immunological approaches have been used to localise receptor expression to a given membrane domain.
Basolateral membrane On the basis of nucleotide-induced increases in [Ca2+]i, the basolateral membrane of the rat proximal tubule appears to contain P2Y1 and P2Y6 receptors [4–6] and a pyrimidine receptor that has characteristics of P2Y2 and/or P2Y4 [4]. Agonist profiles cannot be used to distinguish between rat P2Y2 and P2Y4 receptors [7, 8], but the use of specific antibodies showed that the basolateral pyrimidine receptor is P2Y4, there being no discernible expression of P2Y2 in this segment [9]. The expression of P2Y6 receptors in the basolateral membrane was also confirmed immunologically [10]. Although this approach failed to detect basolateral P2Y1 receptor expression in the rat proximal convoluted tubule [9], P2Y1 receptors have been localised basolaterally in the proximal tubule of Necturus maculosus [11]. Immunological studies have also identified low-level expression of P2X4 and P2X6 receptors in the rat proximal tubule [9], but the membrane polarity of this expression was uncertain.
Apical membrane Apical expression of P2Y1-like receptors has been identified (on the basis of responses to “selective” agonists) in immortalised cell lines with proximal phenotype [12, 13]. Using an in vivo microperfusion approach, which permits the delivery of agonists/antagonists directly into the tubule lumen, strong functional evidence (see below) has also been provided for expression of P2Y1 receptors in the apical membrane of the rat proximal convoluted tubule (i.e., in native tissue) [14]. Although these functional data do not accord with the failure to demonstrate immunologically apical P2Y1 receptors in the S2 segment of the rat, expression being confined to the S3 segment [9], these conflicting observations have been reconciled by more sensitive Western blot analysis showing expression of P2Y1 receptors in rat S2 brush-border membrane vesicles (Fig. 2). Turner et al. additionally found apical expression of P2X5 receptors, again in the S3, rather than S1 or S2, segments [9].
Fig. 2.
Stimulation of apical P2Y1 receptors inhibits bicarbonate reabsorption in rat proximal convoluted tubule. a Expression of P2Y1 receptor protein in brush-border membrane vesicles harvested from rat proximal tubule. Total protein (30 μg/lane) from three animals (C1, C2, C3) was probed using an antibody raised against residues 242–258 of the human P2Y1 receptor protein (Alomone Labs, Israel); preabsorption of the antibody with this antigen prevented detection (right-hand side of the trace). Western blot is courtesy of Dr. Joanne Marks. b Effect of adenine nucleotides on bicarbonate reabsorption in the microperfused rat proximal tubule in vivo, expressed as percentage of the control flux measured in the same tubule. The inhibitory profile of naturally occurring adenine nucleotides is consistent with a P2Y1 receptor-mediated response. This was confirmed using the selective agonist 2MeSADP and specific antagonist MRS2179. c The effect of 2MeSADP was not additive to that of EIPA, suggesting an NHE-3-mediated response. Inhibition of bicarbonate flux by P2Y1 receptor activation was blocked by either U73122 or H89, indicating involvement of phospholipase C and protein kinase A, respectively. Data are taken from Bailey [14]. *P < 0.05, **P < 0.01 against paired control values
Effects of nucleotides on proximal tubular transport
Direct modulation of proximal tubule transport proteins was initially demonstrated in the amphibian proximal tubule, in which P2Y1 receptors activated basolateral chloride channels [11]. In the mammalian proximal tubule, in vivo perfusion of the peritubular capillaries with a solution containing ATP caused an increase in transepithelial bicarbonate reabsorption [15]. Proximal bicarbonate reabsorption was also stimulated by the addition of dextran to the peritubular perfusate, a manoeuvre that increased fluid viscosity by ∼30% yet altered neither osmolality nor charge. This stimulatory action of increased viscosity was abolished by non-selective purinoceptor antagonism or by blockade of nitric oxide synthesis.
In contrast to the above finding, perfusion of the apical membrane with adenine nucleotides in vivo caused a significant reduction in bicarbonate reabsorption [14]. The potency profile of adenine nucleotides/nucleosides implicated P2Y1 receptors in this response (Fig. 2). This was confirmed by two observations: first, the greatest degree of inhibition was observed using 2meSADP, a potent agonist of P2Y1 receptors; second, 2meSADP did not inhibit bicarbonate flux in the presence of the P2Y1 receptor-specific antagonist MRS2179. Bicarbonate reabsorption was reduced by ∼50% at a luminal 2meSADP concentration of ∼100 μmol/l, which is substantially greater than reported values for endogenous ATP concentrations in the proximal tubule [16] and suggests that submaximal inhibition is the physiological norm. The mechanism of inhibition appears to involve regulation of NHE3 by both phospholipase C and protein kinase A, since the inhibitory action of P2Y1 receptor activation was eliminated when this transporter, or either of these two pathways, was blocked (Fig. 2) [14]. Inhibition of NHE3 by a cyclic AMP/protein-kinase-A-dependent pathway has been observed in A6 cells and requires phosphorylation of the serines at positions 552 and 605 [17]. Phosphorylation of NHE3 by protein kinase A is facilitated by sodium-hydrogen exchange regulator factor (NHERF) and it appears that NHERF2 and P2Y1 receptors interact via a C-terminal PDZ domain [18] to facilitate regulation of the transporter.
A recent clearance study has reported profound renal effects of the naturally occurring diadenosine polyphosphate Ap4A [19]. When infused intravenously into rats, Ap4A increased the clearance of lithium—used as an index of end-proximal fluid delivery—almost twofold, despite a reduction in glomerular filtration rate. Fractional proximal tubular reabsorption was therefore reduced markedly. Although much of the extra sodium delivered to the distal nephron was reabsorbed therein, there was nevertheless a substantial natriuresis. It is difficult to interpret these data in terms of a specific receptor since Ap4A can activate multiple subtypes [20], including P2Y1 and P2Y4, which are both expressed in the proximal tubule. Moreover, the route of agonist delivery cannot separate apical from basolateral effects. However, perfusion of the proximal tubule lumen with UTP does not inhibit bicarbonate reabsorption [14], suggesting that if Ap4A inhibits proximal tubule sodium reabsorption via P2Y4 receptors, then it does so from the basolateral side.
The loop of Henle
P2 receptor expression in the thick descending limb of Henle (more generally known as the pars recta of the proximal tubule) has already been described. There is some evidence for pyrimidine receptors in rat descending and ascending thin limbs of Henle in that basolaterally applied ATP and UTP were equipotent in eliciting increases in [Ca2+]i [4]. The ascending thin limb expresses mRNA for both P2Y2 and P2Y4 receptors, but only P2Y2 has been identified at the protein level [9]. The identity of the UTP-sensitive receptor in the thin descending limb remains a mystery: P2Y4 is expressed at neither the mRNA nor the protein level [4, 9] and P2Y2 immunoreactivity is not evident [9], despite the expression of mRNA for this receptor [4]. In addition to that for P2Y2, mRNA for P2Y1 and P2Y6 subtypes has been found in the descending thin limb, although agonists for these receptors had no effect on [Ca2+]i when applied to the basolateral membrane; thus, these subtypes, if present, must be confined to the apical domain [4, 10]. To date, no P2Y1 or P2Y6 receptor protein has been identified in thin limbs.
In the rat thick ascending limb (TAL), basolaterally applied ATP has little effect on [Ca2+]i [4], although there is a species difference here: in the mouse TAL, basolateral ATP consistently evokes large calcium transients [21, 22]. The rat TAL expresses mRNA for P2Y1, 2, 4 and 6 receptors [4, 5], and immunohistochemical studies have identified P2Y2, P2X4 and P2X6 receptor protein [9], although their membrane polarities are not yet clear.
As well as the intracellular calcium transients seen in response to exogenously applied nucleotides, there is in vitro evidence for a flow-induced release of endogenous nucleotides in medullary thick ascending limb (TAL), leading to an autocrine/paracrine action to increase [Ca2+]i [23]. Two recent pieces of evidence imply a functional role for P2 receptors in the loop. Vallon’s group has demonstrated that in P2Y2 receptor ‘knockout’ mice there is increased expression of the apical Na+K+2Cl- co-transporter in the renal medulla, associated with an augmented natriuretic response to furosemide [24]. A separate study has shown that addition of ATP to suspensions of rat TAL in vitro stimulates intracellular nitric oxide production, an effect thought to be mediated by P2X receptors [25]. The fact that nitric oxide can inhibit transport processes in the TAL [26] makes this a particularly intriguing observation. However, it is notable that in contrast to the proximal tubule and collecting duct, no study has yet addressed directly the functional consequences of P2 receptor activation in the loop of Henle in terms of transepithelial electrolyte fluxes.
Distal tubule
Immunohistological techniques have so far identified only P2X4 and P2X6 receptors in the rat distal convoluted tubule (DCT), where they were confined to the basolateral membrane [9]. There are no corresponding investigations in other species. In the absence of functional studies on native distal tubule, our knowledge of the role of P2 receptors in these segments is restricted to findings from in vitro studies, either in primary cultures of native cells or, more commonly, in immortalised distal or “distal-like” cell lines.
There is evidence for a functional role (increased [Ca2+]i and chloride efflux) for basolateral P2Y1 and apical P2Y2 receptors in Xenopus A6 cells [27, 28], commonly used as a model for high-resistance distal epithelia. Another distal-like cell line, Madin–Darby canine kidney (MDCK) cells, expresses a number of P2Y subtypes and responds to ATP by increasing chloride secretion across monolayers [29]. Indeed, the Na+K+2Cl- co-transporter NKCC1, which is located in the basolateral membrane of MDCK cells, is regulated by nucleotides, being activated by apical P2Y2 and inhibited by basolateral P2Y1 receptors [30]. However, the significance of these findings in cell lines with respect to solute transport in the intact mammalian nephron is questionable. In the rat, at least, only P2X4 and P2X6 receptor proteins appear to be expressed in distal tubule (see above).
It has been demonstrated that changes in transepithelial pressure can induce transient increases in [Ca2+]i in MDCK cells, a phenomenon apparently mediated by apical and basolateral nucleotide release [31]. However, the pressures involved far exceeded those thought to exist in native tissue [32].
Experiments using an immortalised cell line derived from rabbit DCT have indicated that stimulation of apical P2 receptors, pharmacologically characterised as P2Y2, increases apical chloride conductance [33], while Quamme’s group, using their immortalised mouse DCT cell line, have provided evidence for P2 receptor-mediated increases in [Ca2+]i, coupled with inhibition of magnesium transport, a physiologically important function of the DCT [34]. In the latter instance, pharmacological profiling pointed towards P2X, rather than P2Y, mediation. Finally, cultured cells from rabbit connecting tubules respond to extracellular ATP with an increase in [Ca2+]i, together with inhibition of sodium and calcium absorption [35], probably via P2Y2 receptor stimulation; in this case the functional changes were found not to depend on the calcium transient.
The overall impression from these disparate findings in distal-like cell lines is that P2 receptors might have a role to play in distal tubular solute transport, but that the physiological significance of any such role awaits a comprehensive in vivo assessment.
Collecting duct
In native tissue (in the rat), mRNA has been identified for P2Y1, 2, 4 and 6 and P2X4 receptors in cortical and outer medullary collecting duct [4, 5, 36], and for P2Y2 receptors in inner medullary collecting duct [36, 37]. Intriguingly, mRNA for collecting duct P2X1 and P2X6 receptors is also detectable, but only during dietary sodium restriction [36]. Until recently, protein for only P2X4–6 receptors (expressed throughout the collecting duct) and P2Y2 receptors (in the medullary collecting duct) had been detected immunohistochemically in the rat [9, 37]. In the last year, however, further immunohistochemical studies have additionally indicated receptor protein for P2Y4 and P2Y6 subtypes in the apical membrane of principal cells (while P2Y2 [medulla only], P2X4 and P2X6 subtypes were confirmed in both apical and basolateral membranes) [36]. Moreover, a recent study has identified mRNA and protein for P2X1 and P2X4 receptors in native collecting duct of the mouse; immunohistochemistry revealed apical P2X1 and P2X4 receptors in principal cells throughout the medullary collecting duct, with some basolateral P2X4 expression in cortical collecting duct [38].
Despite continuing uncertainties over the precise receptor distribution, there is little doubt that stimulation of P2 receptors in the collecting duct can influence transport processes in this nephron segment—the final site of regulation of urinary output. Here, we will focus on potassium and sodium transport (and, where appropriate, chloride); water transport is reviewed elsewhere in this Special Issue (see article by Kishore et al. [3]).
Potassium
An early study in MDCK cells reported that nucleotides can activate K+ channels [39]. However, this is contradicted by more recent evidence in native collecting duct. An elegant patch-clamp investigation of split-open mouse native cortical collecting ducts (CCDs; allowing access to the apical membrane) has demonstrated that ATP reversibly inhibits the activity of the small-conductance K+ channels (Fig. 3), the major pathway through which potassium is secreted in the distal nephron [40]. On the basis of pharmacological profiling (i.e., equipotency of ATP and UTP and absence of effect of α, βmeATP and 2meSATP), it was concluded that apical P2Y2 receptors were responsible. The observation that P2Y2 receptor knockout mice are hypokalaemic is consistent with this view [24].
Fig. 3.
Effect of ATP on low-conductance K+ channels in the apical membrane of mouse cortical collecting duct. Patch-clamp techniques were applied to split-open tubules. a Channel recording showing that addition of ATP (100 µM) to the bathing solution (grey band) caused closure of the K+ channel, an effect that was reversible. b Dose–response curve for the effect of ATP on K+ channel open probability. The action of ATP was blocked by the P2 receptor antagonist suramin and was mimicked by UTP, whereas αβMeATP and 2MeSATP were without effect on K+ channel activity, suggesting the involvement of P2Y2 receptors. Taken, with permission, from Lu et al [40]
Indirect evidence for an inhibitory effect of the diadenosine polyphosphate Ap4A on potassium secretion in the distal nephron has come from the recent clearance study of Stiepanow-Trzeciak et al. [19]. As indicated earlier, this compound had a marked inhibitory effect on sodium reabsorption in the proximal tubule (of the rat), but much of the increased load of sodium was reabsorbed in the distal nephron. A compensatory increase in sodium reabsorption in the distal nephron would normally be associated with increased potassium secretion and consequent kaliuresis, yet, in this case, no such kaliuresis was seen [19]. The implication is reduced activity of the apical potassium channels.
Sodium
Studies using collecting duct cell lines generally indicate that extracellular nucleotides can reduce amiloride-sensitive short-circuit current (SCC; assumed to represent sodium transport) and stimulate chloride secretion. This has been demonstrated both in the mouse M-1 cell line [41, 42], where pharmacological profiling (of both apical and basolateral membranes) and RT-PCR pointed towards P2Y2 receptor mediation, and in the mouse IMCD-K2 cell line [43]. In the latter case, the effect was confined to the apical membrane and pharmacological profiling, in conjunction with RT-PCR, revealed involvement of both P2X and P2Y receptors. In the M-1 cell line, at least, the functional effects were associated with, but not dependent upon, increases in [Ca2+]i [41, 42]. In contrast to the above findings, a recent study, using the mouse IMCD-3 cell line, found that apical application of ATP and analogues induced an increase in SCC, thought to be mediated largely by P2X receptors [38]. The cause of this increase has not been established, but it is interesting to note that Wildman et al. [36] have recently shown that apical P2X receptor activation can, under certain conditions, stimulate collecting duct ENaC activity (see below).
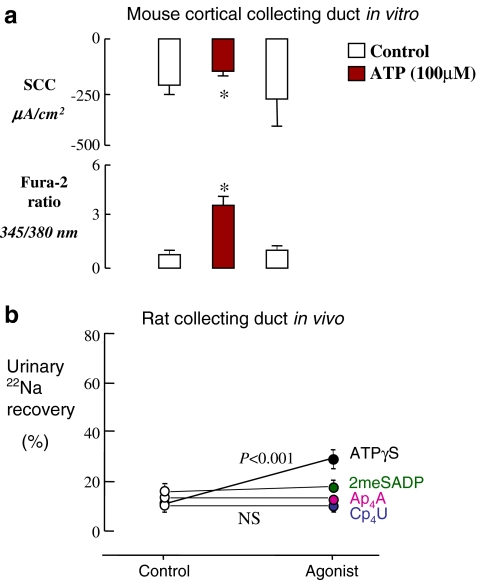
There is also considerable evidence for an effect of exogenous nucleotides in native tissue. In rabbit CCD, luminal perfusion in vitro with ATPγS (a broad-spectrum P2 agonist) or UTP was shown to increase [Ca2+]i in both principal and intercalated cells [44]. In contrast, in a different laboratory, only basolateral ATP was found to be effective [45]. Functional studies in mice showed that ATP and UTP, applied either luminally or basolaterally, caused an increase in [Ca2+]i and inhibition of amiloride-sensitive SCC [45, 46] (Fig. 4a), though the reduction in sodium transport did not depend on the calcium transient [46].
Fig. 4.
Inhibition of collecting duct sodium reabsorption by luminally applied nucleotides. a Effect of luminal application of ATP on short-circuit current (SCC) and [Ca2+]i (assessed as the fura-2 fluorescence emission ratio at 345/380 nm excitation) in mouse cortical collecting duct perfused in vitro. The mice had been kept on a low-sodium diet (to enhance ENaC activity). The SCC was shown to be amiloride-sensitive and was therefore taken to represent ENaC-mediated Na+ transport. The effect of ATP was mimicked by UTP. Taken, with permission, from Lehrmann et al. [46]. b Effect of intraluminal nucleotides on collecting duct 22Na absorption in sodium-restricted rats, in vivo. Late distal tubules were perfused with artificial tubular fluid containing 14C-inulin and 22Na, and urinary recoveries were monitored. Each distal tubule was perfused twice, first with a control perfusate, then with a P2 agonist. The broad-spectrum agonist ATPγS significantly increased urinary 22Na recovery, but the P2Y1 agonist 2MeSADP and the P2Y2/P2Y4 agonists Cp4U and Ap4A were without effect. Data are taken from Shirley et al. [47]
The effect of apical nucleotides on collecting duct sodium reabsorption in vivo has been investigated in our laboratory, using microperfusion of late distal tubules [47]. In rats fed a low-sodium diet in order to increase ENaC activity, addition of ATPγS to the luminal perfusate was found to increase urinary 22Na recovery (i.e., collecting duct 22Na reabsorption was reduced; Fig. 4b).
These studies, taken together, leave little room for doubt that exogenously applied nucleotides, albeit at relatively high doses, can inhibit sodium reabsorption in the collecting duct. Recently, circumstantial evidence for a paracrine/autocrine role for endogenous nucleotides in regulating collecting duct sodium transport has emerged from Schwiebert’s laboratory. ENaC-mediated sodium reabsorption has been shown to be up-regulated in CCD monolayers derived from Oak Ridge polycystic kidney [48]. These cells lack a well-formed apical central cilium, a deficiency that apparently diminishes their ability to release ATP in response to appropriate stimuli [49]. It takes only a small leap of imagination to link the reduced ATP release with the enhanced sodium reabsorption.
The P2 receptor subtype(s) responsible for the inhibitory effect on ENaC-mediated sodium reabsorption is a controversial topic, and species differences ensure that it will remain so for some time. Intriguingly, despite firm evidence from in vitro studies for P2Y2 mediation, “selective” P2Y2/P2Y4 and P2Y1 agonists were found to be ineffective in vivo in the rat (Fig. 4b), and a P2X heteromer-mediated effect was suggested [47]. In this connection, Wildman et al. have shown, using the Xenopus oocyte expression system, that co-expression of a number of P2X receptors (P2X2, P2X2/6, P2X4 or P2X4/6) with ENaC leads to its downregulation [50]. Recently, this whole topic has been opened up further by the discovery that when intraluminal sodium concentrations are low, activation of apical P2X4 and/or P2X4/6 receptors can stimulate ENaC activity [36]. However, we will not elaborate on this here, as the interaction between P2 receptors and ENaC is dealt with in depth elsewhere in this Special Issue (see article by Wildman et al. [51]).
Conclusion
As set-out in this article and elsewhere [52], there is now overwhelming evidence that the range of P2 receptors distributed along the nephron can influence the excretion of sodium, potassium and other ions. In some well-defined examples, stimulation of the apical P2 receptors is effective; in others, the polarity of receptor expression is less clear. In the majority of cases, the effect of P2 receptor stimulation on ion transport is inhibitory.
The tubular location of the P2 receptors, the observation that certain nucleotides are released from tubular cells and cell cultures [16, 53, 54], and the demonstration that a range of nucleotidase enzymes is available along the tubule to control nucleotide degradation (see article by Shirley et al. in this Special Issue [55]), all point towards a tubular nucleotide/P2 receptor system in the regulation of solute excretion. However, determination of the precise physiological/pathophysiological role of this system is fraught with difficulty and will clearly require an extensive and co-ordinated research effort.
Acknowledgements
Work in the authors’ laboratories was supported by The Wellcome Trust, Kidney Research UK, and St Peter’s Trust for Kidney, Bladder and Prostate Research.
References
- 1.Inscho E (2009) ATP, P2 receptors and the renal microcirculation. Purinergic Signalling in press [DOI] [PMC free article] [PubMed]
- 2.Bell, PD, Komlosi P, Zhang Z (2009) ATP as a mediator of macula densa cell signalling. Purinergic Signalling in press [DOI] [PMC free article] [PubMed]
- 3.Kishore, BK, RD Nelson, RL Miller et al (2009) P2Y2 receptors and water transport in the kidney. Purinergic Signalling in press [DOI] [PMC free article] [PubMed]
- 4.Bailey MA, Imbert-Teboul M, Turner C et al (2000) Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58:1893–1901 [DOI] [PubMed]
- 5.Bailey MA, Imbert-Teboul M, Turner C et al (2001) Evidence for basolateral P2Y6 receptors along the rat proximal tubule: functional and molecular characterization. J Am Soc Nephrol 12:1640–1647 [DOI] [PubMed]
- 6.Cha SH, Sekine T, Endou H (1998) P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol 274:F1006–F1014 [DOI] [PubMed]
- 7.Webb TE, Henderson DJ, Roberts JA et al (1998) Molecular cloning and characterization of the rat P2Y4 receptor. J Neurochem 71:1348–1357 [DOI] [PubMed]
- 8.Bogdanov Y, Rubino A, Burnstock G (1998) Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci 62:697–703 [DOI] [PubMed]
- 9.Turner CM, Vonend O, Chan C et al (2003) The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175:105–117 [DOI] [PubMed]
- 10.Bailey MA, Turner CM, Hus-Citharel A et al (2004) P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiol 96:79–90 [DOI] [PubMed]
- 11.Bouyer P, Paulais M, Cougnon M et al (1998) Extracellular ATP raises cytosolic calcium and activates basolateral chloride conductance in Necturus proximal tubule. J Physiol 510:535–548 [DOI] [PMC free article] [PubMed]
- 12.Jin W, Hopfer U (1997) Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: elevated cytosolic Ca2+ is not required. Am J Physiol 272:C1169–C1177 [DOI] [PubMed]
- 13.Anderson RJ, Breckon R, Dixon BS (1991) ATP receptor regulation of adenylate cyclase and protein kinase C activity in cultured renal LLC-PK1 cells. J Clin Invest 87:1732–1738 [DOI] [PMC free article] [PubMed]
- 14.Bailey MA (2004) Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287:F789–F796 [DOI] [PubMed]
- 15.Diaz-Sylvester P, Mac Laughlin M, Amorena C (2001) Peritubular fluid viscosity modulates H+ flux in proximal tubules through NO release. Am J Physiol Renal Physiol 280:F239–F243 [DOI] [PubMed]
- 16.Vekaria RM, Unwin RJ, Shirley DG (2006) Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17:1841–1847 [DOI] [PubMed]
- 17.Bagorda A, Guerra L, Di Sole F et al (2002) Extracellular adenine nucleotides regulate Na+/H+ exchanger NHE3 activity in A6-NHE3 transfectants by a cAMP/PKA-dependent mechanism. J Membr Biol 188:249–259 [DOI] [PubMed]
- 18.Fam SR, Paquet M, Castleberry AM et al (2005) P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA 102:8042–8047 [DOI] [PMC free article] [PubMed]
- 19.Stiepanow-Trzeciak A, Jankowski M, Angielski S et al (2007) P1, P4-diadenosine tetraphosphate (Ap4A) inhibits proximal tubular reabsorption of sodium in rats. Nephron Physiol 106:13–18 [DOI] [PubMed]
- 20.King BF, Townsend-Nicholson A (2003) Nucleotide and nucleoside receptors. Tocris Rev 23:1–11
- 21.Bailey MA, Hillman KA, Unwin RJ (2000) P2 receptors in the kidney. J Auton Nerv Syst 81:264–270 [DOI] [PubMed]
- 22.Paulais M, Bandouin-Legros M, Teulon J (1995) Extracellular ATP and UTP trigger calcium entry in mouse cortical thick ascending limb. Am J Physiol 268:F496–F502 [DOI] [PubMed]
- 23.Jensen ME, Odgaard E, Christensen MH et al (2007) Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18:2062–2070 [DOI] [PubMed]
- 24.Rieg T, Bundey RA, Chen Y et al (2007) Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21:3717–3726 [DOI] [PubMed]
- 25.Silva G, Beierwaltes WH, Garvin JL (2006) Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47:563–567 [DOI] [PubMed]
- 26.Ortiz PA, Hong NJ, Garvin JL (2001) NO decreases thick ascending limb chloride absorption by reducing Na+K+2Cl− cotransporter activity. Am J Physiol Renal Physiol 281:F819–F825 [DOI] [PubMed]
- 27.Leipziger J, Bailey MA, Unwin RJ (2003) Purinergic receptors in the kidney. In: Schwiebert EM (ed) Extracellular nucleotides and nucleosides. Release, receptors and physiological and pathophysiological effects. Academic, New York, pp 369–394
- 28.Guerra L, Favia M, Fanelli T et al (2004) Stimulation of Xenopus P2Y1 receptor activates CFTR in A6 cells. Pflügers Arch 449:66–75 [DOI] [PubMed]
- 29.Simmons NL (1981) Identification of a purine (P2) receptor linked to ion transport in a cultured renal (MDCK) epithelium. Br J Pharmacol 73:379–384 [DOI] [PMC free article] [PubMed]
- 30.Akimova OA, Grygorczyk A, Bundey RA et al (2006) Transient activation and delayed inhibition of Na+, K+, CI-cotransport in ATP-treated C11-MDCK cells involved distinct P2Y receptor subtype and signaling mechanisms. J Biol Chem 281:31317–31325 [DOI] [PubMed]
- 31.Praetorius HA, Frokiaer J, Leipziger J (2005) Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288:F133–F141 [DOI] [PubMed]
- 32.Burnett JC Jr, Haas JA, Larson MS (1985) Renal interstitial pressure in mineralocorticoid escape. Am J Physiol 249:F396–F399 [DOI] [PubMed]
- 33.Rubera I, Tauc M, Bidet M et al (2000) Extracellular ATP increases [Ca2+]i in distal tubule cells. II. Activation of a Ca2+-dependent Cl- conductance. Am J Physiol Renal Physiol 279:F102–F111 [DOI] [PubMed]
- 34.Dai LJ, Kang HS, Kerstan D et al (2001) ATP inhibits Mg2+ uptake in MDCT cells via P2X purinoceptors. Am J Physiol Renal Physiol 281:F833–F840 [DOI] [PubMed]
- 35.van Baal J, Hoenderop JG, Groenendijk M et al (1999) Hormone-stimulated Ca2+ transport in rabbit kidney: multiple sites of inhibition by exogenous ATP. Am J Physiol 277:F899–F906 [DOI] [PubMed]
- 36.Wildman SSP, Marks J, Turner CM et al (2008) Sodium-dependent regulation of amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19:731–742 [DOI] [PMC free article] [PubMed]
- 37.Kishore BK, Ginns SM, Krane CM et al (2000) Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278:F43–F51 [DOI] [PubMed]
- 38.Li L, Jeanette Lynch I, Zheng W et al (2007) Apical P2XR contribute to [Ca2+]i signaling and Isc in mouse renal MCD. Biochem Biophys Res Commun 359:438–444 [DOI] [PubMed]
- 39.Friedrich F, Weiss H, Paulmichl M et al (1991) Further analysis of ATP-mediated activation of K+ channels in renal epithelioid Madin Darby canine kidney (MDCK) cells. Pflügers Arch 418:551–555 [DOI] [PubMed]
- 40.Lu M, MacGregor GG, Wang W et al (2000) Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol 116:299–310 [DOI] [PMC free article] [PubMed]
- 41.Cuffe JE, Bielfeld-Ackermann A, Thomas J et al (2000) ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524:77–90 [DOI] [PMC free article] [PubMed]
- 42.Thomas J, Deetjen P, Ko WH et al (2001) P2Y2 receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. J Membr Biol 183:115–124 [DOI] [PubMed]
- 43.McCoy DE, Taylor AL, Kudlow BA et al (1999) Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol 277:F552–F559 [DOI] [PubMed]
- 44.Woda CB, Leite M Jr, Rohatgi R et al (2002) Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283:F437–F446 [DOI] [PubMed]
- 45.Deetjen P, Thomas J, Lehrmann H et al (2000) The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol 11:1798–1806 [DOI] [PubMed]
- 46.Lehrmann H, Thomas J, Kim SJ et al (2002) Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13:10–18 [DOI] [PubMed]
- 47.Shirley DG, Bailey MA, Unwin RJ (2005) In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288:F1243–F1248 [DOI] [PubMed]
- 48.Olteanu D, Yoder BK, Liu W et al (2006) Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Cell Physiol 290:C952–C963 [DOI] [PubMed]
- 49.Hovater MB, Olteanu D, Hanson EL et al (2008) Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Pur Sig 4:155–170 [DOI] [PMC free article] [PubMed]
- 50.Wildman SS, Marks J, Churchill LJ et al (2005) Regulatory interdependence of cloned epithelial Na+ channels and P2X receptors. J Am Soc Nephrol 16:2586–2597 [DOI] [PubMed]
- 51.Wildman SS, Kang ES-K, King BF (2009) ENaC, renal sodium excretion and extracellular ATP. Purinergic Signalling in press [DOI] [PMC free article] [PubMed]
- 52.Vallon V (2008) P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294:F10–F27 [DOI] [PubMed]
- 53.Schwiebert EM, Kishore BK (2001) Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280:F945–F963 [DOI] [PubMed]
- 54.Jankowski V, Karadogan S, Vanholder R et al (2007) Paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule epithelial cells. Kidney Int 71:994–1000 [DOI] [PubMed]
- 55.Shirley DG, Vekaria RM, Sévigny J (2009) Ectonucleotidases in the kidney. Purinergic Signalling in press [DOI] [PMC free article] [PubMed]