Abstract
The kidneys play a critical role in the maintenance of water homeostasis. This is achieved by the inherent architecture of the nephron along with the expression of various membrane transporters and channels that are responsible for the vectorial transport of salt and water. The collecting duct has become a focus of attention by virtue of its ability to transport water independent of solutes (free-water transport), and its apparent involvement in various water balance disorders. It was originally believed that the water transport capability of the collecting duct was solely under the influence of the circulating hormone, arginine vasopressin (AVP). However, during the past decade, locally produced autocrine and/or paracrine factors have emerged as potent modulators of transport of water by the collecting duct. Recently, much attention has been focused on the purinergic regulation of renal water transport. This review focuses on the role of the P2Y2 receptor, the predominant purinergic receptor expressed in the collecting duct, in the modulation of water transport in physiological and pathophysiological conditions, and its therapeutic potential as a drug target to treat water balance disorders in the clinic. Studies carried out by us and other investigators are unravelling potent interactions among AVP, prostanoid and purinergic systems in the medullary collecting duct, and the perturbations of these interactions in water balance disorders such as acquired nephrogenic diabetes insipidus. Future studies should address the potential therapeutic benefits of modulators of P2Y2 receptor signalling in water balance disorders, which are extremely prevalent in hospitalised patients irrespective of the underlying pathology.
Keywords: Collecting duct, Water transport, Prostanoid signalling, Vasopressin, Purinergic receptors, Diabetes insipidus, Aquaporins
Molecular physiology of water handling by the mammalian kidney
Sixty percent of the human body by weight is water. The movement of water among the intracellular, extracellular and intravascular compartments and visceral cavities is very important for the proper function of the various organs. In order to maintain water homeostasis, the human kidneys are equipped with the capacity to filter and reabsorb a large amount (~180 l) of water in a day. The reabsorption is achieved by aquaporin (AQP) water channels, which allow rapid movement of water across biological membranes, as well as by membrane transporters that are responsible for vectorial transport of solutes generating osmotic gradients. In addition, the inherent architecture of the nephrons, the functional units of the kidney, is critically important (Fig. 1). The ability of the mammalian kidney to absorb the bulk of the large amount of filtered water is due to coupling to the absorption of sodium and other solutes in the proximal nephron, which is very rich in AQP1 protein on both apical (brush border) and basolateral aspects of the cells. In this part of the nephron, 60–70% of the filtered solutes and water are reabsorbed into the blood. The part of the nephron from the tip of the loop of Henle to the collecting duct is impermeable to water, but transports sodium out of the lumen (diluting segment). This creates the necessary osmotic gradients in the interstitium which are crucial for the osmotic reabsorption of water in the adjacent collecting duct.
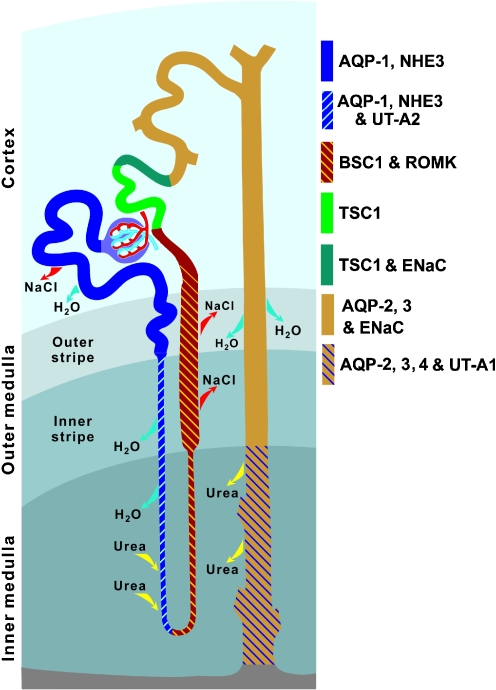
Fig. 1.
Architecture of rat nephron and collecting duct showing segmental localization of major transporters and channels that play critical roles in water and solute reabsorption (modified from [55]). AQP1, AQP2, AQP3 and AQP4 Aquaporin water channel isoforms 1 through 4, NHE3 sodium–hydrogen exchanger isoform 3, UT-A1 and UT-A2 urea transporter isoforms A1 and A2, BSC1 bumetanide-sensitive cotransporter-1, ROMK renal outer medullary potassium channel, TSC1 thiazide-sensitive cotransporter-1, ENaC epithelial sodium channel
Although the collecting duct system accounts for the reabsorption of only a small fraction of filtered water (~15%), this is very important for the maintenance of water homeostasis. First, unlike the proximal nephron, the reabsorption of water in the collecting duct is regulated independently of sodium reabsorption, by hormones and autocrine/ paracrine agents. Second, if the collecting duct system fails, there are no other tubular segments downstream which can compensate. Third, since the collecting duct system starts beyond the juxtaglomerular apparatus and macula densa, unlike failures in the more proximal part of the nephron, failure in the collecting duct system is exempt from sensing by the tubuloglomerular feedback mechanism. In addition, most forms of nephrogenic diabetes insipidus (NDI), whether acquired or hereditary, are due to defect(s) in the collecting duct system. In view of these facts, the collecting duct system has become the focus in recent years for intense research by renal physiologists and pathophysiologists, including our group.
Cell biology of vasopressin action on collecting duct
The collecting duct system determines the concentration of the final voided urine. Arginine vasopressin (AVP), acting through the V2 receptor, a G-protein-coupled receptor, in the collecting duct principal cell, activates membrane-bound adenylyl cyclase to produce cyclic AMP (cAMP) as a second messenger. The cellular effects of cAMP are connected to the activation of protein kinase A, which phosphorylates various key proteins and thus increases the water permeability of the principal cells of the collecting duct (Fig. 2) [1, 2].
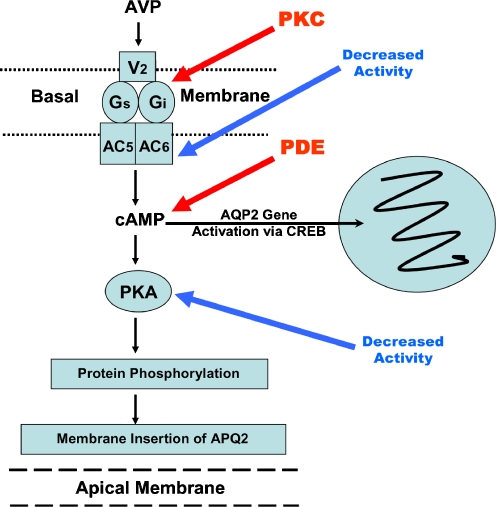
Fig. 2.
Schematic representation of the major G protein-coupled receptors and the channels involved in regulated reabsorption of water and sodium in the principal cell of the collecting duct. The scheme also depicts the major points of interaction between mutually opposing cyclic AMP and phosphoinositide signalling pathways through which these receptors act (modified from [16] and used with permission). For details refer to the text. AVP Arginine vasopressin; ET endothelin, PGE2 prostaglandin E2, V2-R vasopressin V2 receptor, ET-R endothelin receptor, EP3-R prostanoid receptor type 3, AC adenylyl cyclase, PLC phospholipase C, Gs stimulatory G protein, Gi inhibitory G protein, cAMP cyclic AMP, PKA protein kinase A, PKC protein kinase C, PDE phosphodiesterases, IP3 inositol triphosphate, ER endoplasmic reticulum, CaM calcium calmodulin, DAG diacyl glycerol, cPLA2 cytosolic phospholipase A2, AQP2, AQP3 and AQP4 aquaporin water channel isoforms 2, 3 and 4, ENaC epithelial sodium channel, α, β, γ subunits, Aldo aldosterone, PIP2 phosphatidyl-inositol 4,5-bisphosphate, PI3-K phosphoinositide 3-kinase
AVP has both short- and long-term effects on collecting duct water permeability. The short-term effect involves the translocation of AQP2 water channels from a pool of subapical vesicles to the apical plasma membrane [3]. The apical plasma membrane is the rate-limiting barrier for the transepithelial water movement, as AQP3 and AQP4 water channels are constitutively expressed on the basolateral domain of the collecting duct principal cells under normal conditions (Fig. 2). The long-term effect involves an increase in the absolute amount of AQP2 mRNA and protein that result from increased transcription and translation of the AQP2 gene. Increased cyclic AMP generated by AVP action leads to protein-kinase-A-mediated phosphorylation and activation of CREB, which binds to CRE (cAMP response element) in the AQP2 promoter. AQP2 gene transcription is ultimately activated via both CRE and AP1 (Activator Protein-1) sites in the AQP2 promoter [4–6].
Non-AVP regulators of collecting duct function
Apart from AVP, a variety of autocrine and paracrine agents, such as prostaglandin E2 (PGE2), endothelin and extracellular nucleotides (ATP/UTP), can regulate collecting duct water transport [7–14]. Acting through their respective receptors, and the associated phosphoinositide signalling pathway, these agents decrease AVP-stimulated osmotic water permeability in the collecting duct (Fig. 2). In the collecting duct, cAMP and phosphoinositide systems are mutually opposing signalling pathways (Fig. 2). There are specific points of interaction between these two pathways. Figure 3 illustrates the potential sites in the AVP signalling pathway that can be regulated. In general, AVP exerts a tonic effect on the collecting duct, while the non-AVP regulators, including PGE2, endothelin or ATP, exert an opposing action, largely through a reduction of cAMP levels. In summary, cAMP levels and water permeability are tightly regulated by a balance between AVP and non-AVP regulators.
Fig. 3.
Sequence of intracellular events leading from the binding of AVP to its V2 receptor (top) to the insertion of AQP2 water channels into the apical membrane (bottom). The potential pre-cAMP formation sites that can be disrupted are (a) activation of inhibitory G protein (Gi) by the increased activity of PKC brought about by diacylglycerol (DAG) formed as a result of stimulation of the PI signalling pathway by agents such as PGE2 and ATP, and (b) decreased activity of adenylyl cyclase (AC) isoforms 5 and 6 expressed in the medullary collecting duct. The potential post-cAMP formation sites which can be disrupted are (a) rapid hydrolysis of cAMP by phosphodiesterases (PDE; isoforms III and IV) by calcium-calmodulins activated through the PI signalling pathway by agents such as PGE2 and ATP and (b) decreased activity of PKA, resulting in decreased protein phosphorylation and membrane insertion of AQP2
Physiology of the P2Y2 receptor in the collecting duct
The intracellular concentrations of ATP are between 3 and 5 mM. Being a highly polar molecule, ATP cannot exit cells by diffusing passively through the lipid bilayer of the cell membrane. However, virtually all cells, including renal cells, release nucleotides into the extracellular milieu through specific membrane transporters in a regulated fashion [15, 16; and see article by Praetorius and Leipziger [17] in this Special Issue]. When extracellular ATP and other nucleotides reach even low micromolar concentrations locally, they stimulate a variety of P2 receptors, eliciting short- and long-term biological effects. In recent years, the potential roles of extracellular nucleotides in the regulation of renal tubular transport, especially salt and water, through P2 receptors are increasingly being recognised [15–21].
P2 receptor signal transduction is tightly controlled by a narrow range of extracellular concentrations of nucleotides, the latter being regulated by control of exocytosis or specific transporters, as well as by rapid enzymatic hydrolysis of released nucleotides [22]. Studies conducted by us and by others have documented that the collecting duct system is capable of releasing nucleotides [15, 16], and the enzymes that hydrolyze the released nucleotides, such as NTPDases (nucleoside triphosphate diphosphohydrolases) and NPPs (nucleotide pyrophosphatases) are expressed in the kidney and/or in the vicinity of the collecting duct in the medulla [23, 24]. The expression and functional roles of ectonucleotidases in the kidney are dealt with elsewhere in this Special Issue (see article by Shirley et al. [25]); hence, we will not elaborate on ectonucleotidases here.
The P2Y2 receptor (originally designated as P2u purinoceptor) is a G protein-coupled extracellular nucleotide receptor with an agonist potency order of UTP ≥ ATP > ATPγS >> 2-MeS-ATP. Pharmacological, functional and molecular methodologies localised the P2Y2 receptor to the medullary collecting duct [26, 27]. Currently, the P2Y2 subtype is the predominant P2Y receptor whose expression in native collecting duct of rat or mouse has been established by both molecular and functional approaches. However, the expression of other P2Y receptors has been reported in cultured cell lines of renal origin, using molecular and/or functional methodologies [16, 28]. Recently, Unwin’s group showed that collecting ducts of sodium-restricted rats have significant amounts of P2Y4 and P2X4 receptor mRNA, whereas the mRNA expression of these receptors in sodium-replete rats is insignificant. [29]. Hence, it appears that other subtypes of P2Y receptors, which may not be detectable in the collecting duct under normal conditions, may express significantly in pathophysiological states. In contrast to the murine collecting duct, functional studies in rabbit cortical collecting tubule (CCT) revealed the apparent expression of both P2Y2 and P2Y1 receptors [10]. Since pharmacological and/or functional characterization of the expression of multiple forms of P2Y receptors is a complex issue, additional molecular studies need to be performed to support these findings.
It is important to note that the activation of P2Y2 receptors induces at least two signalling systems that affect collecting duct water transport. First, activation of the P2Y2 receptor per se down regulates AVP-stimulated water transport in a protein kinase C-dependent and inhibitory G protein (Gi)-mediated manner [10, 11]. Secondly, activation of the P2Y2 receptor causes the production and release of PGE2 by the medullary collecting duct [30]. We have demonstrated that P2Y2 receptor-mediated PGE2 release is markedly enhanced in physiological polyuria induced by water loading, and blunted in oliguria caused by water deprivation or chronic dDAVP (V2 receptor-specific analogue of AVP) infusion in rats [31, 32].
PGE2 antagonises AVP-stimulated water permeability in the medullary collecting duct [7, 9], an effect that is partly due to activation of EP (E-prostanoid)3 receptor-mediated retrieval of AQP2 from the apical membrane [13]. In addition, PGE2 can chronically elevate phoshoinositides and thus potentially reduce the cAMP levels (Fig. 2).
Kishore, Knepper and Nielsen were the first group of investigators to generate a peptide-derived polyclonal antibody to the P2Y2 receptor and to immunolocalise the receptor protein in rat kidney and lung [27]. The receptor protein is expressed on both apical and basolateral domains of rat medullary collecting duct [27]. While Kishore and Knepper, as well as others, have demonstrated that the basolateral P2Y2 receptor is involved in the inhibition of AVP-induced water transport in medullary collecting duct [10, 11, 14], recent studies have demonstrated a role for the apical P2Y2 receptor in the inhibition of amiloride-sensitive sodium transport via the epithelial sodium channel (ENaC) in the collecting duct [19, 33–35]. P2-receptor-mediated regulation of sodium transport in the collecting duct is dealt with elsewhere in this Special Issue (see articles by Bailey and Shirley [36] and Wildman et al. [37]), and so we will not elaborate this aspect here; instead we will focus on regulation of water transport by the P2Y2 receptor. Thus, by acting on different domains—basolateral or apical—of the principal cell of the collecting duct, P2Y2 receptors can antagonise the effect of AVP or aldosterone, respectively (Fig. 2). Thus, independent modulation of these transport processes by extracellular nucleotides, released locally either at the basolateral or apical domains, can potentially exert profound effects in disorders of water or sodium, including hypertension. Therefore, modulators of purinergic signalling in the collecting duct have the potential for the development of innovative therapies for the treatment of water and/or sodium balance disorders.
P2Y2 receptor deletion results in manifestation of renal phenotype
The availability of P2Y2 receptor null mice (courtesy Dr. Beverly Koller, Univ. of North Carolina at Chapel Hill [38, 39]) has allowed us and others to establish further the potential role of the P2Y2 receptor in renal or collecting duct water transport. Our studies show that when subjected to metabolic balance using a specially formulated gel diet containing fixed amounts of nutrients and normal water content, the P2Y2 receptor gene knockout mice had significantly lower urine output with higher urine concentration as compared with wild-type mice. Western analysis of renal medullary tissue revealed that P2Y2 receptor knockouts had 1.8-fold higher protein abundance of AQP2 than wild-type mice.
Surprisingly, there were no significant differences between the P2Y2 receptor knockout and wild-type mice with respect to plasma osmolalities, AVP levels and total osmolar excretion [40]. These data indicate that genetic deletion of P2Y2 receptors increases the sensitivity of the medullary collecting duct to circulating AVP levels. This in turn leads to increased protein abundance of the AQP2 water channel, and increased urinary concentrating ability. These findings also confirm that P2Y2 receptor activation inhibits the tonic stimulatory effect of AVP on the collecting duct, since genetic deletion of the P2Y2 receptor apparently increases AVP’s tonic stimulatory effect. Similar results have been reported by the group of Vallon and Insel [41].
Interactions among AVP, prostanoid and purinergic systems in the collecting duct
The interactions among AVP, prostanoid and purinergic systems in the collecting duct have been partially unravelled. The left panel in Fig. 4 schematically illustrates these interactions in normal conditions, and the right panel shows the potential perturbations of these interactions in acquired NDI. The following description briefly summarises these findings. The letters A, B, C etc at the beginning of each paragraph correspond to the letters in the schemes.
Acute interaction of purinergic system with AVP: in collecting ducts from normal animals, microperfused in vitro, acute activation of P2Y2 receptors down regulates AVP-stimulated water flow [11]. This interaction may remain intact or accentuated in acquired NDI.
Chronic interaction of AVP with purinergic system: chronically elevated circulating AVP levels, due to water deprivation or to dDAVP infusion in normal rats, down regulates the expression of P2Y2 receptors [32, 42]. This chronic effect of AVP is blunted in acquired NDI where the collecting duct is resistant to AVP action. This is supported by our data showing that the expression of the P2Y2 receptor did not change significantly in acquired NDI models [43].
Purinergic-mediated prostanoid production: activation of P2Y2 receptors in ex vivo preparations of medullary collecting ducts from normal rats stimulates the production and release of PGE2 [30]. This response is markedly accentuated in rats with non-pathological polyuria induced by sucrose-water drinking [31]. This interaction is also apparently accentuated in acquired NDI, as demonstrated in ex vivo preparations of medullary collecting ducts (see below).
Interactions between PGE2 and AVP system: published studies have documented the inhibitory effect of PGE2 on AVP-induced water flow in the collecting duct, and on the AVP-induced trafficking of AQP2 water channel to the apical plasma membrane [7, 9, 12, 13]. Increased production of PGE2 in NDI results in potent inhibition of AVP’s action, leading to an AVP-resistant state. Blocking the synthesis of PGE2 by indomethacin reduces the polyuria of NDI [44, 45].
Chronic effect of elevated circulating AVP levels on purinergic-mediated PGE2 production: in normal rats chronically infused with high doses of dDAVP and in water-deprived rats (with raised circulating AVP levels), purinergic-mediated ex vivo PGE2 production by medullary collecting ducts is significantly decreased [31, 32]. This effect is blunted in NDI, since purinergic-mediated ex vivo prostanoid production by medullary collecting ducts is actually increased, despite the fact that circulating AVP levels are either normal or elevated [43].
Fig. 4.
Proposed models for the interaction among AVP, purinergic (ATP) and prostanoid (PGE2) systems in medullary collecting duct principal cell under normal condition (left), and how they are deranged in acquired NDI (right). (−) and (+) signs denote inhibition and stimulation, respectively. X marks indicate blocked pathways. Thicker arrows indicate accentuation of pathways. The letters A, B, C, D and E are keyed to the explanations in the text
Potential role of the P2Y2 receptor in acquired NDI
Diabetes insipidus (DI) causes considerable morbidity and even mortality, in addition to significant social inconvenience. Patients with DI have an elevated risk of dehydration, hypernatraemia, alterations in consciousness, and haemodynamic instability from hypovolaemia [44, 45]. Central diabetes insipidus is due to defects in AVP synthesis or release, whereas NDI is due to defects in the kidney collecting duct, or to reduced medullary osmolality, making the collecting duct non-responsive to the action of circulating AVP. Familial NDI, usually due to mutations in the genes coding for the vasopressin V2 receptor or the AQP2 water channel, is relatively rare. It presents in children, with failure to thrive and hypernatraemic dehydration, and persists in adults resulting in lifelong disability due to only partially effective treatments. Acquired NDI, the more common form, can occur at any age. Patients with acquired NDI present with polyuria, polydipsia and a reduced ability to concentrate urine, and are unresponsive to AVP treatment [44, 45]. The most common cause of acquired NDI is chronic administration of lithium for the treatment of bipolar disorders [46]. In addition to its long-standing and proven beneficial effect in bipolar disorders, lithium is recently emerging as a potential treatment for acute brain injury as well as chronic neurodegenerative diseases [47–49]. Other drugs that are capable of inducing NDI are colchicine, methoxyflurane, amphotericin B, gentamicin, loop diuretics and demeclocycline. In addition to drugs, acquired NDI occurs as a result of certain diseases. These include, but are not limited to, chronic kidney diseases, hypokalaemia, hypercalcaemia, sickle cell disease, post-obstructive uropathy (due to benign prostatic hyperplasia or cancer), reflux nephropathy, renal dysplasia, cystic kidney disease and low protein diets. Despite these different aetiologies, the hallmark of all forms of acquired NDI is a marked decrease in the protein abundance of AQP2 even in the setting of normal or elevated circulating AVP levels [50]. Thus, in acquired NDI, the collecting duct is resistant to AVP action.
In view of the clinical importance of lithium as a potent drug for the treatment of bipolar disorders and chronic neurodegenerative diseases such as Alzheimer’s, Parkinson and Huntington diseases, we have been studying the molecular physiology of the medullary collecting duct in lithium-induced NDI. Previous studies have attributed the AVP resistance of the collecting duct in lithium-induced NDI to increased production of renal PGE2 [51, 52]. As described above, PGE2 is a potent antagonist of AVP action on the collecting duct. Accordingly, administration of inhibitors of cyclooxygenases (COX) that decrease PGE2 biosynthesis, such as indomethacin, ameliorates the polyuric condition of lithium-induced NDI (Fig. 5). Since the P2Y2 receptor is known to antagonise the effect of AVP on the collecting duct, and can mediate increased production of PGE2 by the collecting duct, we hypothesised that the P2Y2 receptor may be involved in the genesis of polyuria in lithium-induced NDI. We found that in rats subjected to lithium-induced NDI, stimulation of medullary collecting duct by ATPγS results in markedly enhanced production of PGE2, as compared with that seen in normal rats. Since previous studies in our laboratory showed that only ATPγS and UTP, but not ADP, are capable of inducing the production of PGE2 by medullary collecting duct [30], it is logical to presume that the observed effect is due to the P2Y receptor family, most probably the P2Y2 subtype, although involvement of other P2Y receptors, such as the P2Y4 subtype, in acquired NDI cannot be ruled out at this stage. This apparent increase in the sensitivity of P2Y2 receptors in lithium-induced NDI is associated with upregulation of PGE2 synthesizing machinery, exemplified by increased expression of mRNA for cytosolic phospholipase A2, COX-1 and COX-2 [43]. At present, our data suggest a potential role for the P2Y2 receptor in the genesis of polyuria in lithium-induced NDI. This notion has been substantiated by studies using P2Y2 receptor knockout mice. When subjected to lithium feeding for 2 weeks, the P2Y2 receptor null mice were markedly resistant to the development of lithium-induced polyuria, with about 50% lower urine volume and significantly higher urine osmolalities as compared with wild-type mice subjected to a similar regimen. Terminal serum osmolalities were the same in both groups, indicating that none of the animals were dehydrated under the experimental conditions [53]. If confirmed by more extensive and long-term studies, these novel findings provide a rationale to develop a new class of drugs for the treatment of lithium-induced NDI which specifically target the P2Y2 receptor and desensitise it, thereby counteracting the AVP-resistant state in NDI. This approach may prove safer as compared to the current approach of directly inhibiting the activities of cyclooxygenases in lithium-induced NDI (Fig. 5).
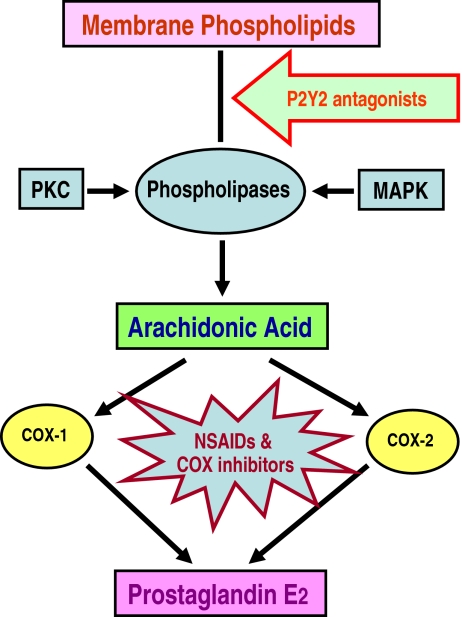
Fig. 5.
Potential utility of P2Y2 receptor antagonists to block the production of PGE2 in acquired NDI. The availability of free arachidonic acid is the rate-limiting step in the biosynthesis of PGE2. Phospholipases, which release arachidonic acid from membrane phospholipids, are activated by calcium or protein kinase C (PKC) or mitogen-activated protein kinases (MAPK). Once arachidonic acid is released, either cyclooxygenase (COX)-1 or COX-2, or both, may be involved in the synthesis of prostaglandin E2 (PGE2)
Another commonly encountered acquired NDI condition among ageing men is post-obstructive uropathy due to benign prostatic hyperplasia (BPH) or cancer. About 300,000 surgical procedures, mostly transurethral resection of prostate, are performed each year in the USA for BPH. Release of the obstruction is often associated with diuresis, despite persistent decreases in renal cortical perfusion and glomerular filtration rate [54]. Although post-obstructive diuresis is a self-limiting condition, the transient loss of fluid can result in debilitating conditions in elderly patients, who often exhibit an impaired thirst mechanism [55, 56]. Increased production of renal PGE2 is also implicated in the genesis of the polyuria of post-obstructive uropathy, which responds to treatment with inhibitors of PGE2 biosynthesis [57]. In view of this, in parallel with our experiments with lithium-induced NDI, we also examined the potential involvement of P2Y2 receptors in the genesis of polyuria in a rat model of post-obstructive uropathy. Our preliminary results indicate that in rats with post-obstructive uropathy, agonist-stimulation of P2Y2 receptors in the medullary collecting duct results in markedly enhanced production of PGE2 when compared with sham-operated control rats [43].
Based on the preliminary results from these two NDI models, as well as the published data, we have depicted in Fig. 4, right panel, the changes in the interactions among AVP, prostanoid and purinergic systems in acquired NDI.
Future perspectives
The potential involvement of P2Y2 receptors in water transport in the medullary collecting duct opens the possibility of exploring the utility of P2Y2 receptor agonists and antagonists for the treatment of a variety of water balance disorders. These include water-losing disorders such as acquired NDI, as well as water-retaining conditions or disorders, such as pregnancy, hepatic cirrhosis, nephrosis and hypertension. For example, specific antagonists of P2Y2 receptors could be used to overcome the AVP resistance in acquired NDI, as opposed to the current usage of a combination of a thiazide with a potassium-sparing diuretic or prostaglandin synthesis inhibitor (indomethacin or other COX-2 inhibitors). Thiazide diuretics should be used with caution in lithium-induced NDI, as they reduce the renal excretion of lithium, potentially resulting in lithium intoxication; while indomethacin, a non-steroidal anti-inflammatory drug (NSAID), increases the risk of gastrointestinal disorders and bleeding. In addition, recent reports indicate that the use of NSAIDs, especially COX-2-specific inhibitors (celecoxib and rofecoxib), in patients on lithium therapy is associated with increased serum lithium concentrations [58, 59]. Currently, the safety of long-term use of COX-2 inhibitors with their cardiovascular complications is in question and their future is uncertain. Replacement of the current side-effect-prone drugs with new ones based on improved understanding of molecular pathophysiology in NDI might result in improved efficacy and fewer side effects. In this context, targeting the P2Y2 receptor with specific antagonists may prove to be a better and safer alternative (Fig. 5).
Specific agonists of P2Y2 receptors may act on the collecting duct as diuretics or aquaretics in water-retaining conditions such as pregnancy, cirrhosis, nephrosis and cardiac failure, conditions which can potentially lead to hyponatraemia if not corrected promptly. Conditions such as pregnancy, cirrhosis and cardiac failure apparently cause water retention and dilutional hyponatraemia by a unified mechanism that involves arterial vasodilatation due to increased activity of nitric oxide (NO), haemeoxygenases or prostaglandins, leading to arterial underfilling. The latter stimulates the non-osmotic release of AVP, resulting in increased expression and apical membrane targeting of AQP2 in the renal collecting duct, leading to water retention. To disrupt this pathway, recently introduced specific antagonists of vasopressin V2 receptors, the so called “aquaretics”, are proving to be promising agents to increase “free-water” excretion in hyponatraemic states [60, 61]. In this context, the potential utility of P2Y2 receptor agonists in water retention states that may result in hyponatraemia is worth exploring. Furthermore, because of the potential diuretic and natriuretic effects of these agents, the utility of P2Y2 receptor agonists should also be explored in hypertension.
Acknowledgements
The authors thank Drs. Mark Knepper, Simon Robson and Erik Schwiebert for critical reading of the manuscript and helpful suggestions. Authors’ work cited in this review has been supported by grants from the National Institutes of Health, Department of Veterans Affairs, the National Kidney Foundation of Utah and Idaho, Catalyst Grant from the University of Utah, and the resources and facilities at the VA Salt Lake City Health Care System. Parts of our work presented here have been performed in collaboration with Drs. Mark Knepper, Søren Nielsen, Chung-Lin Chou, Carissa Krane, Leslie Myatt, Simon Robson, Herbert Zimmermann, Mathieu Bollen, Jean Sévigny, and Jorge Isaac. Thanks are due to post-doctoral fellows, graduate and undergraduate students, and technicians who assisted us in our studies over the past several years. The proprietary information presented in this review article has been protected by an international patent under Patent Cooperation Treaty (PCT/US2005/038231) and published by the World Intellectual Property Organization (WO/2006/066679).
References
- 1.Nielsen S, Frokiær J, Marples D et al (2002) Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82:205–244 [DOI] [PubMed]
- 2.Valenti G, Giuseppe P, Tamma G et al (2005) Minireview : aquaporin 2 trafficking. Endocrinology 146:5063–5070 [DOI] [PubMed]
- 3.Nielsen S, Chou C-L, Marples D et al (1995) Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92:1013–1017 [DOI] [PMC free article] [PubMed]
- 4.Hozawa S, Holtzman EJ, Ausiello D (1996) cAMP motifs regulating transcription in the aquaporin-2 gene. Am J Physiol 270:C1695–C1702 [DOI] [PubMed]
- 5.Matsumura Y, Uchida S, Rai T et al (1997) Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8:861–867 [DOI] [PubMed]
- 6.Yasui M, Zelenina SM, Celsi G et al (1997) Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol 272:F442–F450 [DOI] [PubMed]
- 7.Nadler SP, Zimplemann JA, Hebert RL (1992) PGE2 inhibits water permeability at a post-cAMP site in rat terminal inner medullary collecting duct. Am J Physiol 262:F229–F235 [DOI] [PubMed]
- 8.Kohan DE, Hughes AK (1993) Autocrine role of endothelin in rat IMCD: inhibition of AVP-induced cAMP accumulation. Am J Physiol 265:F126–F129 [DOI] [PubMed]
- 9.Han JS, Maeda Y, Ecelbarger C et al (1994) Vasopressin-independent regulation of collecting duct water permeability. Am J Physiol 266:F139–F146 [DOI] [PubMed]
- 10.Rouse D, Leite M, Suki WN (1994) ATP inhibits the hydrosmotic effect of AVP in rabbit CCT: evidence for a nucleotide P2u receptor. Am J Physiol 267:F289–F295 [DOI] [PubMed]
- 11.Kishore BK, Chou C-L, Knepper MA (1995) Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol 269:F863–F869 [DOI] [PubMed]
- 12.Rouch AJ, Kudo LH (2000) Role of PGE2 in α2-induced inhibition of AVP- and cAMP-stimulated H2O, Na+, and urea transport in rat IMCD. Am J Physiol 279:F294–F301 [DOI] [PubMed]
- 13.Zelenina M, Christensen BM, Palmer J et al (2000) Prostaglandin E(2) interaction with AVP: effect on AQP2 phosphorylation and distribution. Am J Physiol 278:F388–F394 [DOI] [PubMed]
- 14.Edwards RM (2002) Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol 438:179–181 [DOI] [PubMed]
- 15.Schwiebert EM (2001) ATP release mechanisms, ATP receptors and purinergic signaling along the nephron. Clin Exp Pharmacol Physiol 28:340–350 [DOI] [PubMed]
- 16.Schwiebert EM, Kishore BK (2001) Extracellular nucleotide signaling along the renal epithelium. Am J Physiol 280:F945–F963 [DOI] [PubMed]
- 17.Praetorius HA, Leipziger J (2009) ATP release from non-excitable cells. Pur Sig doi:10.1007/s11302-009-9146-2 [DOI] [PMC free article] [PubMed]
- 18.Chan CM, Unwin RJ, Burnstock G (1998) Potential functional roles of extracellular ATP in kidney and urinary tract. Exp Nephrol 6:200–2007 [DOI] [PubMed]
- 19.Leipziger J (2003) Control of epithelial transport via luminal P2 receptors. Am J Physiol 284:F419–F432 [DOI] [PubMed]
- 20.Unwin RJ, Bailey MA, Burnstock G (2003) Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci 18:237–241 [DOI] [PubMed]
- 21.Bucheimer RE, Linden J (2003) Purinergic regulation of epithelial transport. J Physiol 555(2):311–321 [DOI] [PMC free article] [PubMed]
- 22.Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure, function relationships and pathophyiological significance. Purinergic Signalling 2:409–430 [DOI] [PMC free article] [PubMed]
- 23.Kishore BK, Isaac J, Tripp SR et al. Cellular localization of nucleotide pyrophosphatases / phosphodiesterases (NPPs) in rat kidney. Proceedings of the Experimental Biology 2004 Meeting, Washington DC. FASEB J 18:A733
- 24.Kishore BK, Isaac J, Fausther M et al (2005) Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol 288:F1032–F1043 [DOI] [PubMed]
- 25.Shirley DG, Vekaria RM, Sévigny J (2009) Ectonucleotidases in the kidney. Purinergic Signalling doi:10.1007/s11302-009-9152-4 [DOI] [PMC free article] [PubMed]
- 26.Ecelbarger CA, Maeda Y, Gibson CC et al (1994) Extracellular ATP increases intracellular calcium in rat terminal collecting duct via nucleotide receptor. Am J Physiol 267:F998–F1006 [DOI] [PubMed]
- 27.Kishore BK, Ginns SM, Krane CM et al (2000) Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol 278:F43–F51 [DOI] [PubMed]
- 28.Insel PA, Ostrom RS, Zambon AC et al (2001) P2Y receptors of MDCK cells: epithelial cell regulation by extracellular nucleotides. Clin Exp Pharmacol Physiol 28:351–354 [DOI] [PubMed]
- 29.Wildman SSP, Marks J, Turner CM et al (2008) Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 recptors. J Am Soc Nephrol 19:731–742 [DOI] [PMC free article] [PubMed]
- 30.Welch BD, Carlson NG, Shi H et al (2003) P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol 285:F711–F721 [DOI] [PubMed]
- 31.Sun R, Carlson NG, Hemmert AC et al (2005) P2Y2 receptor-mediated release of prostaglandin E2 by IMCD is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol 289:F585–F592 [DOI] [PubMed]
- 32.Sun R, Miller RL, Hemmert AC et al (2005) Chronic dDAVP infusion in rats decreases the expression of P2Y2 receptor in inner medulla and P2Y2 receptor-mediated PGE2 by IMCD. Am J Physiol 289:F768–F776 [DOI] [PubMed]
- 33.Kunzelman K, Bachhuber T, Regeer R et al (2005) Purinergic inhibitionof the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19:142–143 [DOI] [PubMed]
- 34.Shirley DG, Bailey MA, Unwin RJ (2005) In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol 288:F1243–F1248 [DOI] [PubMed]
- 35.Ma HP, Eaton DC (2005) Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16:182–187 [DOI] [PubMed]
- 36.Bailey MA, Shirley DG (2009) Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signalling doi:10.1007/s11302-009-9149-z [DOI] [PMC free article] [PubMed]
- 37.Wildman SSP, Kang ES-K, King BF (2009) ENaC, renal sodium excretion and extracellular ATP. Purinergic Signalling doi:10.1007/s11302-009-9150-6 [DOI] [PMC free article] [PubMed]
- 38.Cressman VL, Lazarowsko E, Homolya L et al (1999) Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl- transport. J Biol Chem 274:26461–26468 [DOI] [PubMed]
- 39.Homolya L, Watt WC, Lazarowski ER et al (1999) Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor (−/−) mice. J Biol Chem 274:26454–26460 [DOI] [PubMed]
- 40.Kishore BK, Judge JP, Sun R et al (2006) Manifestation of renal phenotype in mice with genetic deletion of P2Y2 receptor. Proceedings of the 39th Annual Meeting of the American Society of Nephrology, Washington DC. J Am Soc Nephrol 17:30A [DOI]
- 41.Rieg T, Bundey R, Chen Y et al (2006) Absence of P2Y2 receptor facilitates free water excretion. Proceedings of the 39th Annual Meeting of the American Society of Nephrology, Washington DC. J Am Soc Nephrol 17:31A 16338965
- 42.Kishore BK, Krane CM, Miller RL et al (2005) P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rat: relevance to AVP-independent regulation of IMCD function. Am J Physiol 288:F1164–F1172 [DOI] [PubMed]
- 43.Sun R, Nelson RD, Carlsons NG et al (2005) Enhanced purinergic-mediated PGE2 release from the medullary collecting duct of rats with acquired nephrogenic diabetes insipidus: pathophysiological and therapeutic implications. Proceedings of the Experimental Biology 2005 Meeting, San Diego. FASEB J 19:A58 [DOI]
- 44.Bell TN (1994) Diabetes insipidus. Crit Care Nurs Clin North Am 6:675–685 [PubMed]
- 45.Sands JM, Bichet DG (2006) Nephrogenic diabetes insipidus. Ann Intern Med 144:186–194 [DOI] [PubMed]
- 46.Lenox RH, McNamara RK, Papke RL et al (1998) Neurobiology of lithium. J Clin Psychiatry 58:37–47 [PubMed]
- 47.Chuang DM (2004) Neuroprotective and neurotropic actions of the mood stablilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol 16:83–90 [DOI] [PubMed]
- 48.Rowe MK, Chuang DM (2004) Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med 18:1–18 [DOI] [PubMed]
- 49.Wada A, Yokoo H, Yanagita T et al (2005) Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci 99:307–321 [DOI] [PubMed]
- 50.Nielsen S, Kwon TH, Christensen BM et al (1999) Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 10:647–663 [DOI] [PubMed]
- 51.Allen HM, Jackson RL, Winchester MD et al (1989) Indomethacin in the treatment of lithium-induced nephrogenic diabetes insipidus. Arch Intern Med 149:1123–1126 [DOI] [PubMed]
- 52.Sugawara M, Hashimot K, Ota Z (1988) Involvement of prostaglandin E2, cAMP, and vasopressin in lithium-induced polyuria. Am J Physiol 254:R863–R869 [DOI] [PubMed]
- 53.Judge JP, Kohan DE, Carlson NG et al (2006) Mice with genetic deletion of P2Y2 receptor are markedly resistant to lithium-induced poluria. Proceedings of the 39th Annual Meeting of the American Society of Nephrology, Washington DC. J Am Soc Nephrol 17:31A 16338965
- 54.Kramer HJ (1985) Mechanisms of postobstructive polyuria. Klin Wochenschr 63:934–943 [DOI] [PubMed]
- 55.Kishore BK, Krane CM, Reif M et al (2001) Molecular physiology of urinary concentration defect in elderly population. Int Urol Nephrol 33:235–248 [DOI] [PubMed]
- 56.Luckey AE, Parsa CJ (2003) Fluid and electrolytes in the aged. Arch Surg 138:1055–1060 [DOI] [PubMed]
- 57.Fradet Y, Simard J, Grose JH et al (1980) Enhanced urinary prostaglandin E2 in postobstructive diuresis in humans. Prostaglandins Med 5:29–30 [DOI] [PubMed]
- 58.Ratz Bravo AE, Egger SS, Crespo S et al (2004) Lithium intoxication as a result of an interaction with refecoxib. Ann Pharmacother 38:1189–1193 [DOI] [PubMed]
- 59.Phelan KM, Mosholder AD, Lu S (2003) Lithium interaction with the cyclooxygenases 2 inhibitors refecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs. J Clin Psychiatry 64:1328–1334 [DOI] [PubMed]
- 60.Wang F, Feng X-C, Li Y-M et al (2006) Aquaporins as potential drug targets. Acta Pharmacol Sin 27:395–401 [DOI] [PubMed]
- 61.Verbalis JG (2006) AVP receptor antagonists as aquaretics: review and assessment of clinical data. Cleve Clin J Med 73:S24–S33 [DOI] [PubMed]