Abstract
Members of all four families of ectonucleotidases, namely ectonucleoside triphosphate diphosphohydrolases (NTPDases), ectonucleotide pyrophosphatase/phosphodiesterases (NPPs), ecto-5′-nucleotidase and alkaline phosphatases, have been identified in the renal vasculature and/or tubular structures. In rats and mice, NTPDase1, which hydrolyses ATP through to AMP, is prominent throughout most of the renal vasculature and is also present in the thin ascending limb of Henle and medullary collecting duct. NTPDase2 and NTPDase3, which both prefer ATP over ADP as a substrate, are found in most nephron segments beyond the proximal tubule. NPPs catalyse not only the hydrolysis of ATP and ADP, but also of diadenosine polyphosphates. NPP1 has been identified in proximal and distal tubules of the mouse, while NPP3 is expressed in the rat glomerulus and pars recta, but not in more distal segments. Ecto-5′-nucleotidase, which catalyses the conversion of AMP to adenosine, is found in apical membranes of rat proximal convoluted tubule and intercalated cells of the distal nephron, as well as in the peritubular space. Finally, an alkaline phosphatase, which can theoretically catalyse the entire hydrolysis chain from nucleoside triphosphate to nucleoside, has been identified in apical membranes of rat proximal tubules; however, this enzyme exhibits relatively high Km values for adenine nucleotides. Although information on renal ectonucleotidases is still incomplete, the enzymes’ varied distribution in the vasculature and along the nephron suggests that they can profoundly influence purinoceptor activity through the hydrolysis, and generation, of agonists of the various purinoceptor subtypes. This review provides an update on renal ectonucleotidases and speculates on the functional significance of these enzymes in terms of glomerular and tubular physiology and pathophysiology.
Keywords: Renal tubule, Ectonucleotidases, Ectonucleoside triphosphate diphosphohydrolases, Ectonucleotide pyrophosphatase/phosphodiesterases, Ecto-5′-nucleotidase, Alkaline phosphatases
Introduction
Adenosine 5′-triphosphate (ATP) can be released from renal epithelial cells across the apical membrane into the tubular lumen and also, to a lesser extent, across the basolateral membrane [1, 2]. As described elsewhere in this Special Issue, these nucleotides have the potential to activate renal P2 purinoceptors located along the nephron and thereby elicit a variety of autocrine/paracrine effects on tubular transport processes. Aside from their cellular distribution and expression levels, the extent of activation of the various renal P2 receptor subtypes by extracellular nucleotides will depend on the rate of nucleotide release, on the rate of generation of nucleotides by phosphorylating enzymes, and on how rapidly the nucleotides are hydrolyzed by nucleotidases.
The phosphorylating enzymes that can synthesise nucleotides include nucleoside diphosphate kinases (EC 2.7.4.6) and adenylate kinases (EC 2.7.4.3) [3, 4]. The former catalyse the transfer of the terminal phosphate of nucleoside 5′-triphosphates to nucleoside 5′-diphosphates. For example, ATP can donate a phosphate to GDP to produce GTP; i.e., ATP + GDP ⇆ ADP + GTP. Although initially believed to be restricted to the cell cytosol, there is evidence that nucleoside diphosphate kinases are also present in the cell membrane, where they could potentially generate extracellular nucleoside triphosphates. Both mRNA and enzyme protein for nucleoside diphosphate kinases have been identified in rat kidney [5], but their distribution along the nephron is unknown. The other phosphorylating enzymes, adenylate kinases, will either catalyse the production of ADP from ATP and AMP or vice versa, depending on the concentrations of the respective nucleotides; thus: ATP + AMP ⇆ 2ADP. Adenylate kinase activity has been documented in the glomerulus, proximal convoluted tubule, thick ascending limb of Henle and distal tubule [6], though it was not possible to distinguish between intracellular and extracellular activity.
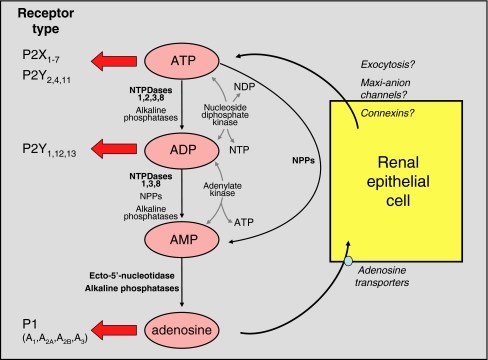
Ectonucleotidases have been detected in virtually all tissues, including kidney. They are a heterogeneous group of enzymes with differing, though partly overlapping, catalytic properties (Table 1). Four families exist: the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family, the ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP) family, ecto-5′-nucleotidase and alkaline phosphatases. Their activities are dependent on the presence of divalent cations (Ca2+, Mg2+ or Zn2+). Ectonucleotidases located in the vasculature and in specific segments of the nephron will have a profound influence on the stimulation of purinoceptors, not only because the availability of nucleotide agonists is controlled by their hydrolysis but also because the generation of different nucleotides (e.g. ADP) may preferentially target different P2 receptor subtypes, while the nucleoside derivative adenosine can either target P1 (adenosine) receptors or re-enter cells via adenosine transporters (Fig. 1). In this short review, we will examine the characteristics of the four families of ectonucleotidases, describe current knowledge about their intrarenal distribution, and speculate on their functional significance.
Table 1.
Hydrolysis pathways of the ectonucleotidases expressed in the kidney
Major pathways are shown in black, others in grey. N.B. For most of these enzymes, nucleotides derived from other bases (UTP, GTP, TTP, CTP, UDP, GDP, TDP and CDP) can also act as substrates
Fig. 1.
Potential effects of renal ectonucleotidases and consequences for activation of purinoceptor subtypes. The major enzymes involved in each degradative pathway are shown in bold print; for more detail, see text. The information given in the figure indicates the relative potencies of ATP and ADP with respect to P2X and P2Y receptor subtypes. At sufficiently high concentrations, ATP can activate all P2 receptors other than P2Y6 and P2Y14. It is important to note that nucleotides derived from other bases are also hydrolysed/synthesised by these enzymes, but have been omitted for clarity. Uracil-based nucleotides are particularly significant: UTP is a potent agonist of P2Y2 and P2Y4 subtypes, and its dinucleotide derivative UDP is the major naturally occurring agonist of the P2Y6 subtype. The mechanism(s) of ATP exit from renal cells has/have not been defined (see article by Leipziger [7] in this Special Issue). NDP, nucleoside diphosphate; NTP, nucleoside triphosphate
Ectonucleoside triphosphate diphosphohydrolases (NTPDases; EC 3.6.1.5)
The E-NTPDase family comprises eight members. Four of these, NTPDases 1, 2, 3 and 8, hydrolyse extracellular nucleotides. As NTPDases 4–7 are mainly intracellular enzymes, they will not be considered here. NTPDases 1–3 and 8 are anchored to the cell membrane via two transmembrane domains. These enzymes hydrolyse adenine-based nucleotides, as well as other nucleotides such as UTP, GTP, ITP, CTP and their respective nucleoside diphosphates, leading ultimately to the generation of the corresponding nucleoside monophosphates as final products.
NTPDase1 (CD39) hydrolyses ATP and ADP with almost equal preference [8]. Although ATP is dephosphorylated one phosphate at a time, the processing step is favoured, leading to the rapid generation of AMP with little ADP accumulation in the milieu [9–11]. Interestingly, and of possible physiological importance, the kinetics are different when UTP is used as substrate, as UDP accumulates transiently, being completely hydrolyzed only after the concentration of UTP has decreased [10]. NTPDase2 has a much greater preference for the hydrolysis of triphosphonucleosides (e.g., ATP) than the diphosphate derivatives (e.g., ADP), and therefore causes accumulation of the latter; NTPDase3 and NTPDase8 also have a preference for the hydrolysis of tri- over diphosphonucleosides, though in these cases the preference is less marked [8, 12]. Thus, NTPDases 3 and 8 generate only a transient accumulation of diphosphonucleosides [10, 13, 14].
Ectonucleotide pyrophosphatase/phosphodiesterases (NPPs; EC 3.1.4.1; EC 3.6.1.9)
The E-NPP family comprises seven members, but only NPPs 1–3 are able to hydrolyse nucleotides. NPP1 and NPP3 are anchored to the cell membrane by a single transmembrane domain, although these enzymes may be proteolytically cleaved and released in soluble form; whilst NPP2 exists only in secreted form [15].
NPPs 1–3 have a wide range of enzymatic activity. They are able to catalyse the hydrolysis of ATP and ADP to AMP, but, in contrast to NTPDases, they always release the monophosphate derivative (here AMP) along with the remaining moiety (pyrophosphate, when using ATP as a substrate). Importantly, NPPs can also hydrolyse diadenosine polyphosphates (again to AMP) and other dinucleoside polyphosphates, as well as UDP-glucose (an agonist of P2Y14 receptors). NPP1 and NPP3 hydrolyze nucleotides more effectively than does NPP2 [16], the latter preferring phospholipids as substrates (as do NPPs 4-7). It is noteworthy that NPPs prefer alkaline pHs for activity. In our hands, recombinant human NPP1 and NPP3 had only low ATPase activities (compared with those of NTPDases) [17], suggesting that the hydrolysis of dinucleoside polyphosphates may be a more important physiological function of NPPs.
Ecto-5′-nucleotidase (CD73; EC 3.1.3.5)
Ecto-5′-nucleotidase catalyses the hydrolysis of nucleoside monophosphates to nucleosides (e.g., AMP → adenosine). Interestingly, this enzyme is inhibited by ATP and ADP, suggesting that the rate of adenosine production may depend on which other ectonucleotidase(s) is/are present in the proximity of ecto-5′-nucleotidase. For example, NTPDase1 will convert ATP through to AMP and thereby supply substrate for ecto-5′-nucleotidase and at the same time deplete the enzyme inhibitors, whereas NTPDase2 preferentially converts ATP to ADP, so provides little substrate for ecto-5′-nucleotidase, while the enzyme inhibitor ADP accumulates. Another feature of ecto-5′-nucleotidase is that it is attached to the cell membrane via a glycosylphosphatidyl inositol (GPI) anchor, which may be cleaved to release a soluble form.
Alkaline phosphatases (EC 3.1.3.1)
This family of enzymes has a broad substrate specificity, capable of catabolising nucleoside tri-, di- or monophosphates down to the related nucleoside. Thus, one enzyme can, in theory, catalyse the entire hydrolysis chain [8, 18]. However, the Km values (i.e., the concentration of substrate required to attain half-maximal velocity for the reaction) for all these substrates are high, i.e. in the low millimolar range. Together with the fact that these enzymes prefer alkaline conditions, this raises questions about their physiological significance in relation to nucleotide degradation. Indeed, ATP is a rather poor substrate for these enzymes [18]. Nevertheless, there are reports showing that tissue-nonspecific alkaline phosphatase, together with ecto-5′-nucleotidase, is involved in AMP hydrolysis [19, 20]. Like ecto-5′-nucleotidase, alkaline phosphatases are GPI-anchored proteins, suggesting that they can also exist as soluble forms.
Intrarenal distribution of ectonucleotidases
NTPDases 1–3 and 8
NTPDases 1–3 and 8 have all been found in the kidney at the protein and/or mRNA level [12, 21–24].
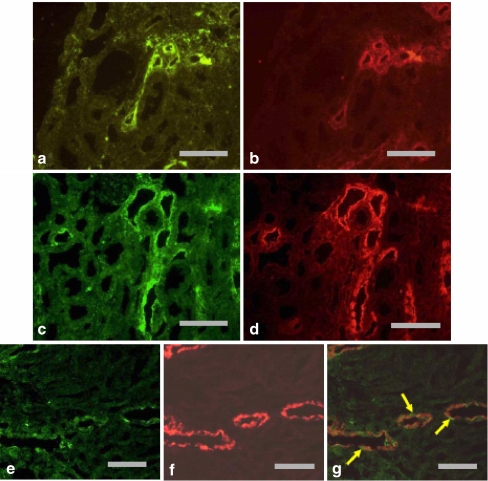
Immunohistochemical studies show that NTPDase1 is prominent in the renal vasculature of rats and mice: it is present in interlobular arteries (endothelial layer and smooth muscle cells), afferent (but not efferent) arterioles and glomerular capillaries and mesangial cells [22, 23; J. Sévigny, unpublished observations]. Kishore et al. [22] also located NTPDase1 in peritubular capillaries (Fig. 2). A degree of interspecies agreement exists in that Lemmens et al. identified the porcine homologue of NTPDase1 in glomerular and peritubular capillaries of the pig kidney [21, 24]. As far as tubular structures are concerned, the only region of the kidney staining for NTPDase1 is the inner medulla, where it has been found in the rat thin ascending limb of Henle (identified as thin segments of tubule that did not stain for AQP1) [22] and (very-low-level staining only) collecting duct (identified using AQP2 as a marker) [23].
Fig. 2.
Immunohistochemical labelling of NTPDase1 in murine kidneys. a, b Labelling of medium-sized blood vessels (denoted by arrows) and peritubular capillaries (denoted by arrowheads) in mouse kidney cortex. c Labelling of glomerular mesangial cells and/or capillary membrane (denoted by arrow) and peritubular capillaries (denoted by arrowheads) in mouse kidney cortex. d Labelling of small blood vessels (denoted by arrows) and glomerular capillaries (denoted by arrowheads) in rat kidney cortex. e Labelling of peritubular capillaries in mouse kidney cortex (denoted by arrows), which was not seen when the specific antibody was substituted with pre-immune serum (f). Reproduced, with permission, from Kishore et al. [22]
NTPDase2 protein has been immunolocalised in the adventitial layer of blood vessels and in Bowman’s capsules in mice and rats [22] and in rat thick ascending limb of Henle (TALH; using Tamm–Horsfall protein as a marker) and distal tubules (using calbindin-D28k as a marker), with again some low-level expression in the inner medullary collecting duct [23].
The intrarenal expression of NTPDase3 has been investigated only in the rat, where it was found in all post-proximal nephron segments examined: the TALH, the distal tubule and the entire collecting duct [23] (Fig. 3).
Fig. 3.
Immunohistochemical labelling of NTPDase3 in rat kidney. a Labelling of thick ascending limbs. b Same section as a, stained for Tamm–Horsfall protein. Scale bar 100 µm. c Labelling of distal tubules. d Same section as c, stained for calbindin-D28k. Scale bar 100 µm. e Labelling of outer medullary collecting ducts. f Same section as e, stained for aquaporin-2. g Merged image of e and f, stained for NTPDase3 (green) and aquaporin-2 (red); arrows indicate presumed intercalated cells. Scale bar 50 µm. Reproduced, with permission, from Vekaria et al [23]
Firm information on NTPDase8 localisation in the kidney is scant. Using a monoclonal antibody raised against a liver ATP diphosphohydrolase, since identified as NTPDase8 [14], the enzyme was immunolocalised in porcine renal tubules, on brush-border membranes (presumably, therefore, proximal tubules) [21], but its exact distribution remains unknown. NTPDase8 was not detected in the renal vasculature [21].
It is worth mentioning that the study of Lemmens et al., which documented the immunolocalisation of NTPDase1 in the vasculature of porcine kidneys, also used a polyclonal antibody (named ‘Ringo’) that must have recognised additional members of the E-NTPDase family because its epitope is part of the apyrase conserved region IV of porcine NTPDase1, although this was unknown at the time [J. Sévigny, unpublished observation]. This polyclonal antibody stained a number of tubular structures: Bowman’s capsule, proximal and distal tubules, ascending segments of Henle’s loop and papillary collecting ducts [24]. Furthermore, Lemmens et al. used an enzyme histochemical method to detect ATPase and ADPase activities along the nephron. Electron microscopy revealed strong enzyme activity in proximal tubular brush-border membranes, with some activity also on the basolateral membrane [24]. At the light microscopic level, the authors also showed strong ATPase activity in the proximal convoluted tubules and blood vessels, lower ATPase activity in the glomeruli and no activity in the distal tubules.
NPPs 1–3
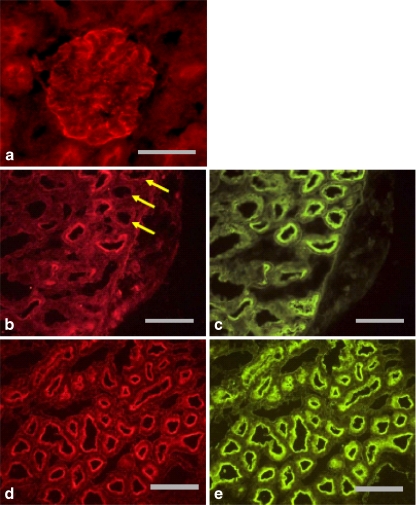
Only limited information is available concerning the presence and distribution of NPPs in the kidney. In the mouse, NPP1 protein has been identified in proximal and distal tubules but was absent from glomeruli [25]. Staining was much stronger in the distal tubules, where it was located in the basolateral membrane. Absence of suitable antibodies to rat NPP1 or NPP2 has thus far precluded an investigation of enzyme protein along the nephron in this species. However, a recent study in our laboratory has used a specific antibody to rat NPP3 to examine this enzyme’s intrarenal distribution [23]. Prominent staining for NPP3 was found in glomeruli and in the brush-border membrane of proximal straight tubules, identified using neutral endopeptidase antibody as a marker of the rat S3 segment (Fig. 4). NPP3 was absent from more distal regions of the nephron.
Fig. 4.
Immunohistochemical labelling of NPP3 in rat kidney. a Labelling of glomerulus. Scale bar 50 µm. b Labelling of proximal tubules. Arrows show positively stained proximal tubules lacking NPP3 expression. c Same section as b, stained for Phaseolus vulgaris erythroagglutinin, a marker of proximal tubules. Scale bar 100 µm. d Labelling of pars recta. e Same section as d, stained for neutral endopeptidase, a marker of rat proximal tubular S3 segment. Scale bar 100 µm. Reproduced, with permission, from Vekaria et al [23]
Ecto-5′-nucleotidase
Ecto-5′-nucleotidase is present in all tissues studied. It has a high level of expression in the kidney [26], where it is the most documented ectonucleotidase. Cole et al [6] were the first to report high ecto-5′-nucleotidase activities in rat proximal tubule. Subsequently, a comprehensive mapping of the enzyme protein in the kidney has been performed by Kaissling’s group, and more recently by Vekaria et al. , using immunohistochemical techniques. The enzyme has been detected in the brush-border membrane of the rat proximal tubule, mainly in the S1 and, to a lesser extent, S2 segments, and in the apical membrane of intercalated cells in the connecting tubule and collecting duct [23, 27–29]. It is also found outside the tubule, in the peritubular space—probably in interstitial fibroblasts [28]. In mice (but not rats) it is also present in glomerular mesangial cells [29].
Alkaline phosphatases
Alkaline phosphatases have not been adequately studied in the kidney. One iso-enzyme has been identified in the brush-border membrane of proximal tubules in the rat kidney [30], although the enzyme’s high Km values for adenine nucleotides (vide supra) suggests that its function there may be unconnected with nucleotide degradation.
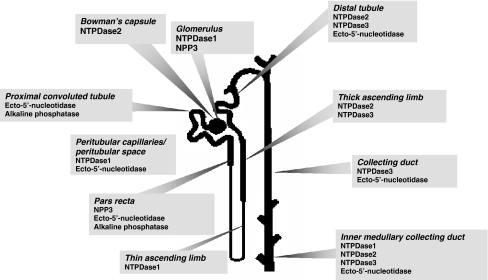
Figure 5 summarises existing knowledge of the distribution of ectonucleotidases along the rat nephron.
Fig. 5.
Distribution of ectonucleotidases along the rat nephron. Information compiled from Cole et al. [6], Kishore et al. [22], Vekaria et al. [23], Gandhi et al. [27], and Le Hir and Kaissling [28, 29]. Adapted, with permission, from Vekaria et al. [23]. N.B. No information is yet available concerning NPPs 1 and 2 in the rat. NTPDase8 has been detected in porcine tubules [21], but its exact location is not yet known
Functional significance of renal ectonucleotidases
The existence of a variety of P2, as well as P1, purinoceptors in the renal vasculature and along the nephron suggests that enzymatic modification of secreted or synthesised extracellular nucleotides will profoundly influence renal physiological and/or pathophysiological events. At present, since our knowledge of ectonucleotidase location is incomplete, and since there are uncertainties as to the cellular polarity (apical vs. basolateral) of some of the enzymes, it is not possible to describe the precise consequences of ectonucleotidase action. It seems likely, however, that the hydrolysis of extracellular nucleotides will result in a constantly shifting activation of different purinoceptor subtypes. Based on existing knowledge, we can make a number of—sometimes speculative—inferences.
Glomerular and pre-glomerular function
The NTPDase1 located in pre-glomerular vessels, in glomeruli and in peritubular capillaries is likely to fulfil a function of NTPDase1 found elsewhere in the vasculature: termination of the platelet aggregation response to extracellular ADP [31, 32]. In addition, NTPDase1 may specifically affect renal blood flow and glomerular filtration. Stimulation of P2X1 receptors by ATP in afferent arterioles causes vasoconstriction (see article by Inscho [33] in this Special Issue); hydrolysis of locally produced ATP may therefore be important in maintaining renal blood flow. In this context, circulating or locally produced diadenosine polyphosphates can also cause vasoconstriction of afferent arterioles [34], and NPPs can hydrolyse these compounds. However, although NPP3 has been detected in glomeruli, thus far it has not been described in pre-glomerular vessels.
In the glomerulus, ATP induces relaxation of pre-contracted mesangial cells [35]. NTPDase1 and NPP3 may modulate this activity, thereby influencing the capillary surface area available for filtration (Kf). Additionally, these enzymes may influence other glomerular purinoceptor responses such as P2Y-dependent cell proliferation of mesangial cells, or may serve in the protection of these cells by preventing ATP levels reaching concentrations that activate the apoptotic P2X7 receptor [36].
Tubuloglomerular feedback
As described elsewhere in this Special Issue (see articles by Inscho [33] and Bell et al. [37]), there is evidence that ectonucleotidases play a pivotal role in tubuloglomerular feedback (TGF), whereby changes in renal perfusion pressure, or other causes of altered NaCl delivery to the macula densa, ultimately cause compensatory changes in afferent arteriolar resistance so that glomerular filtration rate is regulated [38]. ATP concentrations in renal interstitial fluid increase in response to elevations in renal arterial perfusion pressure [39], and, as indicated above, ATP can act directly on P2X1 receptors on the afferent arteriole to induce constriction [40]. However, strong evidence also exists for adenosine (acting via A1 receptors) being the chemical mediator in this response [41, 42]. In this context, it is possible that NTPDase1, expressed in the peritubular space, and/or NPP1, expressed basolaterally in the distal nephron of mice [25], might convert any unbound or excess ATP into AMP. The latter, being a suitable substrate for ecto-5′-nucleotidase, also expressed in the peritubular space, could then be converted to adenosine to cause or augment the vasoconstrictive response in TGF. Strong support for this scheme comes from the observation that ecto-5′-nucleotidase ‘knockout’ mice display a blunted TGF response [43, 44]. Moreover, the response in wild-type mice is blocked by adenosine (A1) receptor blockade [44]. As yet, no information on TGF is available from NTPDase or NPP ‘knockout’ mice.
Proximal tubular function
Although NPP3 is present in the proximal straight tubule of the rat, thus facilitating degradation of ATP or ADP to AMP in this segment, the enzyme’s presence throughout the proximal convoluted tubule (PCT) could not be confirmed [23]. As indicated above, we do not yet know whether NPP1 or NPP2 is present, but a liver ATP diphosphohydrolase, now known to be NTPDase8, was detected in porcine tubules (most likely to be PCTs), predominantly on the luminal side [21]. In accord with this, ATPase and ADPase activities were described in the PCT brush-border and, to some extent, basolateral membranes [24]. It is uncertain whether proximal tubular alkaline phosphatase, with its high Km values for ATP and ADP, could be responsible for degrading ATP. However, it is clear that any AMP produced in the proximal tubule should be converted to adenosine by the ecto-5′-nucleotidase present throughout this segment. The adenosine would be expected to activate proximal tubular A1 receptors [45], causing increased sodium and water reabsorption [46]. Additionally or alternatively, adenosine may be taken up by epithelial cells for re-use: nucleoside transporters are present in both apical and basolateral membranes of proximal tubular cells [47]. It is worth noting that free-flow micropuncture studies in ecto-5′-nucleotidase ‘knockout’ mice failed to observe any disruption of PCT function under standard conditions [44], although the possibility of chronic compensatory mechanisms in these genetically engineered animals cannot be excluded.
Distal nephron function
Extracellular nucleotides have a number of major (largely inhibitory) effects on solute and water transport in the distal nephron, particularly in the collecting duct, acting from both apical and basolateral sides (see articles by Bailey and Shirley [48] and Kishore et al [49] in this Special Issue). Furthermore, activation of adenosine A1 receptors, found in medullary collecting ducts [45], reduces sodium reabsorption [50]. Therefore, the presence of NTPDases and ecto-5′-nucleotidase throughout the distal nephron (see Fig. 5) is likely to be of considerable functional significance. Indeed, adenosine formation in rat collecting ducts was reported to be reduced when ecto-5′-nucleotidase was inhibited with α,βMeADP [51], indicating that this enzyme (and, by implication, at least one AMP-producing enzyme) is tonically active. It remains to be seen how inhibition/deletion of this and other enzymes affects transport processes.
Renal ectonucleotidases under pathological conditions
Given that adenine nucleotides are in general pro-inflammatory [52], it is unsurprising to find that ectonucleotidases can have a renal protective function in a number of pathological situations. For example, in both type 1 and type 2 diabetes, there is evidence that patients with a polymorphism of the gene encoding NPP1 have more severe reductions in renal function than those seen in matched patients with a normal gene [53, 54]. NTPDase1 also appears to be important in this condition: NTPDase1 ‘knockout’ mice subjected to streptozotocin-induced diabetes exhibit increased proteinuria and more severe glomerular sclerosis compared with their wild-type counterparts [55]. This enzyme is also implicated in offsetting organ rejection processes [56]. It has been reported that the glomerular expression of ‘ecto-ATPase’ (a term used for enzymes that we now believe to belong mainly to NTPDase family members) is reduced in patients with chronic allograft nephropathy [57] and also in rats with experimental renal transplant failure [58]. Thus, down-regulation of NTPDases may be an unwanted consequence of the rejection process, contributing to its progression. A similar conclusion has been drawn with regard to ischaemia–reperfusion injury [59]. Set against this is the recent study of Grenz et al [60] in which mice were subjected to short periods of ischaemic preconditioning, which helped to protect renal function against a subsequent period of ischaemia. It was found that ischaemic preconditioning induced an increase in renal NTPDase1 (but not in NTPDases 2, 3 or 8) mRNA and protein; moreover, renal protection was absent in NTPDase1 ‘knockout’ mice.
As already indicated, the chief end-product of NTPDase activity is AMP, the substrate for ecto-5′-nucleotidase; this enzyme can also be up-regulated in pathological situations. Bakker and colleagues have reported increased glomerular expression of ecto-5′-nucleotidase in patients with chronic allograft nephropathy [57] and in those with glomerular ischaemia due to malignant hypertension [61]; increased activity of ecto-5′-nucleotidase in fibroblasts in the peritubular space of chronically hypoxic rats has also been reported [62]. A recent detailed study of the importance of ecto-5′-nucleotidase in protecting against renal ischaemia in mice demonstrated that short periods of ischaemic preconditioning caused increases in renal ecto-5′-nucleotidase mRNA and protein, and in renal tissue adenosine [63]. That these events were critical to the protective mechanisms was indicated by the findings that renal function after a subsequent period of renal ischaemia was severely compromised in mice subjected to either pharmacological inhibition of ecto-5′-nucleotidase or deletion of the gene encoding the enzyme [63].
The increased production of adenosine following up-regulation of ecto-5′-nucleotidase under pathological conditions may counteract the effects of ischaemic damage by several mechanisms: adenosine inhibits platelet aggregation, promotes vasodilatation, and scavenges reactive oxygen species [57]. Okusa [64] reported that stimulation of A2A receptors on renal endothelial cells significantly reduced the expression of endothelial intracellular adhesion molecule-1. This molecule is required for binding and activating neutrophils, a key mechanism for pathogenesis during ischaemia–reperfusion injury. Thus, under these conditions the increased levels of adenosine might serve to prevent inflammatory responses in the renal endothelium. Adenosine A3 receptors have been identified on mesangial cells of the glomerulus, the activation of which results in apoptosis [65]. During pathophysiological conditions, it could be argued that apoptosis should be favoured over necrosis, as the latter may augment inflammatory responses and result in further tissue damage.
Inhibitors of ectonucleotidases
The use of inhibitors of ectonucleotidases in attempts to determine the physiological roles of the enzymes is fraught with difficulty, partly because of the absence of selective and potent inhibitors for each individual enzyme, and partly because most inhibitors have other effects on the purinoceptor system. Thus, most agents used as P2 receptor antagonists, such as suramin and its derivatives, Reactive Blue 2 (RB2), Coomassie Brilliant Blue, and pyridoxal phosphate-6-azophenyl-2′, 4′-disulphonic acid (PPADS), also inhibit ‘ecto-ATPase’ [9, 66]. Indeed, RB2 and the suramin derivative NF279 are potent inhibitors of NTPDases, almost completely abrogating the activity of these enzymes at a concentration of 100 µM [66]. RB2 and PPADS also inhibit NPP1 [67]. PPADS and suramin have little effect on ecto-5′-nucleotidase; as indicated earlier, this enzyme is inhibited by α,βMeADP, a compound with no reported P2 receptor activity. The ATP analogue 6-N, N-diethyl-d-β,γ-dibromomethylene ATP (ARL 67156) is another commercially available ecto-ATPase inhibitor that does not affect significantly purinoceptors. Recent reports demonstrated that ARL 67156 is a weak competitive inhibitor of NTPDase1, NTPDase3 and NPP1, with Ki for the human enzymes of 11 ± 3, 18 ± 4 and 12 ± 3 µM, respectively [17, 68]. Another ATP analogue, 8-thiobutyladenosine 5′-triphosphate (8-BuS-ATP), and 1-naphthol-3,6-disulfonic acid (BG0136), appear of interest, but so far they have only been characterised on a NTPDase-active fraction from bovine spleen membranes [69, 70]. In addition, recent evidence indicates that some polyoxometalate anionic complexes inhibit rat NTPDases [71], that a uridine-5′-carboxamide derivative selectively inhibits human NTPDase2 [72] and that a novel monoclonal antibody selectively inhibits human NTPDase3 [73].
Finally, ion chelators (e.g., EDTA), by reducing the availability of the divalent cations necessary for ectonucleotidase activity, are potent inhibitors of all these enzymes. However, in addition to their lack of specificity with regard to the various ectonucleotidase families, their use in vivo is compromised by disruption of normal physiological processes. Similarly, inhibition of ectonucleotidases by NaN3 [74] is also inappropriate for in vivo studies, as azide is a more potent inhibitor of the essential enzyme mitochondrial ATPase [75].
Conclusions
Although our knowledge of the intrarenal location of the ectonucleotidase families is still incomplete, it is evident that their distribution varies significantly along the different segments of the nephron. It does not seem too far-fetched to propose that the enzymes are strategically distributed so as to influence the activity of P2 and P1 purinoceptors through the generation, or hydrolysis, of agonists such as ATP, ADP or adenosine. In addition, it is possible that ectonucleotidases prevent desensitisation of tubular P2 receptors, as has been demonstrated in other tissues [76–78]. Finally, it has been hypothesised that in conditions such as ischaemia, the ectonucleotidase-mediated modulation of the inhibitory effects of nucleotides on water and electrolyte reabsorption might be overwhelmed by excess ATP release, thereby reducing energy-consuming reabsorptive processes [79].
We emphasise that much remains to be learned. It is hoped that insights into the physiological/pathophysiological roles of individual ectonucleotidases will emerge from studying the phenotypes of genetically manipulated ‘knockout’ animals, although long-term compensatory changes often hamper interpretation of the results of such studies. An alternative/complementary strategy is the development of further iso-enzyme-specific inhibitors. A pessimistic view, however, is that the overlapping catalytic activities of the various family members may bedevil both approaches.
Acknowledgements
Work in the authors’ laboratories was supported by Kidney Research UK, St Peter’s Trust for Kidney, Bladder and Prostate Research and the Canadian Institutes of Health Research (CIHR). J.S. was also the recipient of a New Investigator award from the CIHR.
References
- 1.Schwiebert EM, Kishore BK (2001) Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280:F945–F963 [DOI] [PubMed]
- 2.Vekaria RM, Unwin RJ, Shirley DG (2006) Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17:1841–1847 [DOI] [PubMed]
- 3.Lazarowski ER, Boucher RC, Harden TK (2000) Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275:31061–31068 [DOI] [PubMed]
- 4.Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signaling cascade. Biochim Biophys Acta 1783:673–694 [DOI] [PubMed]
- 5.Kimura N, Shimada N, Nomura K, Watanabe K (1990) Isolation and characterization of a cDNA clone encoding rat nucleoside diphosphate kinase. J Biol Chem 265:15744–15749 [PubMed]
- 6.Cole BR, Hays AE, Boylan JG, Burch HB, Lowry OH (1982) Distribution of enzymes of adenylate and guanylate nucleotide metabolism in rat nephron. Am J Physiol 243:F349–F355 [DOI] [PubMed]
- 7.Praetorius HA, Leipziger J (2009) ATP release from non-excitable cells. Pur Sig in press [DOI] [PMC free article] [PubMed]
- 8.Zimmermann H (2001) Ecto-nucleotidases. In: Abbracchio MP, Williams M (eds) Purinergic and pyrimidinergic signalling. Handbook of experimental pharmacology, vol 151. Springer-Verlag, Berlin, pp 209–250
- 9.Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Nuanyn Schmiedebergs Arch Pharmacol 362:299–309 [DOI] [PubMed]
- 10.Kukulski F, Lévesque SA, Lavoie EG et al (2005) Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Pur Sig 1:193–204 [DOI] [PMC free article] [PubMed]
- 11.Laliberté JF, Beaudoin AR (1983) Sequential hydrolysis of the gamma- and beta-phosphate groups of ATP by the ATP diphosphohydrolase from pig pancreas. Biochim Biophys Acta 742:9–15 [DOI] [PubMed]
- 12.Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Pur Sig 2:409–430 [DOI] [PMC free article] [PubMed]
- 13.Bigonnesse F, Lévesque SA, Kukulski F et al (2004) Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry 43:5511–5519 [DOI] [PubMed]
- 14.Fausther M, Lecka J, Kukulski F et al (2007) Cloning, purification and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol 292:G785–G795 [DOI] [PMC free article] [PubMed]
- 15.Stefan C, Jansen S, Bollen M (2006) Modulation of purinergic signalling by NPP-type ectophosphodiesterases. Pur Sig 2:361–370 [DOI] [PMC free article] [PubMed]
- 16.Gijsbers R, Aoki J, Arai H, Bollen M (2003) The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS Lett 538:60–64 [DOI] [PubMed]
- 17.Lévesque SA, Lavoie EG, Lecka J et al (2007) Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152:141–150 [DOI] [PMC free article] [PubMed]
- 18.Millán JL (2006) Alkaline phosphatases. Pur Sig 2:335–341 [DOI] [PMC free article] [PubMed]
- 19.Ohkubo S, Kimura J, Matsuoka I (2000) Ecto-alkaline phosphatase in NG108–15 cells: a key enzyme mediating P1 antagonist-sensitive ATP response. Br J Pharmacol 131:1667–1672 [DOI] [PMC free article] [PubMed]
- 20.Picher M, Burch LH, Hirsh AJ et al (2003) Ecto 5'-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 278:13468–13479 [DOI] [PubMed]
- 21.Sévigny J, Robson SC, Waelkens E et al (2000) Identification and characterization of a novel hepatic canalicular ATP diphosphohydrolase. J Biol Chem 275:5640–5647 [DOI] [PubMed]
- 22.Kishore BK, Isaac J, Fausther M et al (2005) Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288:F1032–F1043 [DOI] [PubMed]
- 23.Vekaria RM, Shirley DG, Sévigny J, Unwin RJ (2006) Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290:F550–F560 [DOI] [PubMed]
- 24.Lemmens R, Kupers L, Sévigny J et al (2000) Purification, characterization, and localization of an ATP diphosphohydrolase in porcine kidney. Am J Physiol Renal Physiol 278:F978–F988 [DOI] [PubMed]
- 25.Harahap AR, Goding JW (1988) Distribution of the murine plasma cell antigen PC-1 in non-lymphoid tissues. J Immunol 141:2317–2320 [PubMed]
- 26.Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Pur Sig 2:351–360 [DOI] [PMC free article] [PubMed]
- 27.Gandhi R, Le Hir M, Kaissling B (1990) Immunolocalization of ecto-5′-nucleotidase in the kidney by a monoclonal antibody. Histochemistry 95:165–174 [DOI] [PubMed]
- 28.Le Hir M, Kaissling B (1989) Distribution of 5′-nucleotidase in the renal interstitium of the rat. Cell Tissue Res 258:177–182 [DOI] [PubMed]
- 29.Le Hir M, Kaissling B (1993) Distribution and regulation of renal ecto-5′-nucleotidase: implications for physiological functions of adenosine. Am J Physiol Renal Fluid Electrolyte Physiol 264:F377–F387 [DOI] [PubMed]
- 30.Beliveau R, Brunette MG, Strevey J (1983) Characterization of phosphate binding by alkaline phosphatase in rat kidney brush border membrane. Pflügers Arch 398:227–232 [DOI] [PubMed]
- 31.Kaczmarek E, Koziak K, Sévigny J et al (1996) Identification and characterization of CD39 vascular ATP diphosphohydrolase. J Biol Chem 271:33116–33122 [DOI] [PubMed]
- 32.Enjyoji K, Sévigny J, Lin Y et al (1999) Targeted disruption of CD39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5:1010–1017 [DOI] [PubMed]
- 33.Inscho EW (2009) ATP, P2 receptors and the renal microcirculation. Pur Sig in press [DOI] [PMC free article] [PubMed]
- 34.Flores NA, Stavrou BM, Sheridan DJ (1999) The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc Res 42:15–26 [DOI] [PubMed]
- 35.Jankowski M, Szczepanska-Konkel M, Kalinowski L, Angielski S (2001) The role of P2Y-receptors in the regulation of glomerular volume. Med Sci Monit 7:635–640 [PubMed]
- 36.Harada H, Chan CM, Loesch A, Unwin R, Burnstock G (2000) Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int 57:949–958 [DOI] [PubMed]
- 37.Bell PD, Komlosi P, Zhang Z-R (2009) ATP as a mediator of macula densa cell signaling. Pur Sig in press [DOI] [PMC free article] [PubMed]
- 38.Schnermann J (2003) The juxtaglomerular apparatus: from anatomical peculiarity to physiological relevance. J Am Soc Nephrol 14:1681–1694 [DOI] [PubMed]
- 39.Nishiyama A, Majid DS, Walker M et al (2001) Renal interstitial ATP responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension 37:753–759 [DOI] [PubMed]
- 40.Inscho EW, Cook AK, Imig JD et al (2003) Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112:1895–1905 [DOI] [PMC free article] [PubMed]
- 41.Osswald H, Muhlbauer B, Schenk F (1991) Adenosine mediates tubuloglomerular feedback response: an element of metabolic control of kidney function. Kidney Int Suppl 32:S128–S131 [PubMed]
- 42.Sun D, Samuelson LC, Yang T et al (2001) Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98:9983–9988 [DOI] [PMC free article] [PubMed]
- 43.Castrop H, Huang Y, Hashimoto S et al (2004) Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotide/CD73-deficient mice. J Clin Invest 114:634–642 [DOI] [PMC free article] [PubMed]
- 44.Huang DY, Vallon V, Zimmermann H et al (2006) Ecto-5′-nucleotidase (cd73)-dependent and –independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 291:F282–F288 [DOI] [PubMed]
- 45.Smith JA, Sivaprasadarao A, Munsey TS (2001) Immunolocalisation of adenosine A1 receptors in the rat kidney. Biochem Pharmacol 61:237–244 [DOI] [PubMed]
- 46.Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L (1999) Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol 10:714–720 [DOI] [PubMed]
- 47.Mangravite LM, Xiao G, Giacomini KM (2003) Localization of human equilibrative nucleoside transporters, hENT1 and hANT2, in renal epithelial cells. Am J Physiol Renal Physiol 284:F902–F910 [DOI] [PubMed]
- 48.Bailey MA, Shirley DG (2009) Effects of extracellular nucleotides on renal tubular solute transport. Pur Sig in press [DOI] [PMC free article] [PubMed]
- 49.Kishore BK, Nelson RD, Miller RL et al (2009) P2Y2 receptors and water transport in the kidney. Pur Sig in press [DOI] [PMC free article] [PubMed]
- 50.Yagil C, Katni G, Yagil Y (1994) The effects of adenosine on transepithelial resistance and sodium uptake in the inner medullary collecting duct. Pflügers Arch 427:225–232 [DOI] [PubMed]
- 51.Jackson EK, Mi Z, Zhu C, Dubey RK (2003) Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther 307:888–896 [DOI] [PubMed]
- 52.Poelstra K, Heynen ER, Baller JF et al (1992) Modulation of anti-Thy1 nephritis in the rat by adenine nucleotides. Evidence for an anti-inflammatory role for nucleotidases. Lab Invest 66:555–563 [PubMed]
- 53.Canani LH, Ng DP, Smiles A et al (2002) Polymorphism in ecto-nucleotide pyrophosphatase/phosphodiesterase1 gene (ENPP1/PC-1) and early development of advanced diabetic nephropathy in type 1 diabetes. Diabetes 51:1188–1193 [DOI] [PubMed]
- 54.De Cosmo S, Trevisan R, Dalla Vestra M et al (2003) PC-1 amino acid variant Q121 is associated with a lower glomerular filtration rate in type 2 diabetic patients with abnormal albumin excretion rates. Diabetes Care 26:2898–2902 [DOI] [PubMed]
- 55.Friedman DJ, Rennke HG, Csizmadia E et al (2007) The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes 56:2371–2379 [DOI] [PubMed]
- 56.Imai M, Takigami K, Guckelberger O et al (1999) Modulation of nucleoside triphosphate diphosphohydrolase-1 (NTPDase-1) cd39 in xenograft rejection. Mol Med 5:743–752 [PMC free article] [PubMed]
- 57.Mui KW, van Son WJ, Tiebosch TMG et al (2003) Clinical relevance of immunohistochemical staining for ecto-AMPase and ecto-ATPase in chronic allograft nephropathy (CAN). Nephrol Dial Transplant 18:153–163 [DOI] [PubMed]
- 58.Smit-van Oosten A, Bakker WW, van Goor H (2002) De-novo expression of vascular ecto-5′-nucleotidase and down-regulation of glomerular ecto-ATPase in experimental chronic renal transplant failure. Transpl Int 15:602–609 [DOI] [PubMed]
- 59.Candinas D, Koyamada N, Miyatake T et al (1996) Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb Haemost 76:807–812 [PubMed]
- 60.Grenz A, Zhang H, Hermes M et al (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21:2863–2873 [DOI] [PubMed]
- 61.Bakker WW, Mui KW, van Son WJ (2000) Detection of glomerular ischemia in chronic graft failure by the quantification of glomerular ecto 5′nucleotidase and ecto-ATPase. In: Vanduffel, Lemmens (ed) Ecto-ATPase and related ectonucleotidases. Shaker Publishers, Maastricht, pp 192–201
- 62.Kaissling B, Speiss S, Rinne B, Le Hir M (1993) Effects of anemia on morphology of rat renal cortex. Am J Physiol 264:F608–F617 [DOI] [PubMed]
- 63.Grenz A, Zhang H, Eckle T et al (2007) Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18:833–845 [DOI] [PubMed]
- 64.Okusa MD (2002) A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282:F10–F18 [DOI] [PubMed]
- 65.Zhao Z, Kapoian T, Shepard M, Lianos EA (2002) Adenosine-induced apoptosis in glomerular mesangial cells. Kidney Int 61:1276–1285 [DOI] [PubMed]
- 66.Munkonda MN, Kauffenstein G, Kukulski F et al (2007) Inhibition of human and mouse plasma membrane bound NTPDases by P2 receptor antagonists. Biochem Pharmacol 74:1524–1534 [DOI] [PubMed]
- 67.Grobben B, Claes P, Roymans D et al (2000) Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br J Pharmacol 130:139–145 [DOI] [PMC free article] [PubMed]
- 68.Iqbal J, Vollmayer P, Braun N et al (2005) A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and the analysis of inhibitors by in-capillary enzymatic microreaction. Pur Sig 1:349–358 [DOI] [PMC free article] [PubMed]
- 69.Gendron FP, Halbfinger E, Fischer B et al (2000) Novel inhibitors of nucleoside triphosphate diphosphohydrolases: chemical synthesis and biochemical and pharmacological characterizations. J Med Chem 43:2239–2247 [DOI] [PubMed]
- 70.Gendron FP, Benrezzak O, Krugh BW et al (2002) Purine signaling and potential new therapeutic approach: possible outcomes of NTPDase inhibition. Curr Drug Targets 3:229–245 [DOI] [PubMed]
- 71.Müller CE, Iqbal J, Bagi Y et al (2006) Polyoxometalates—a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett 16:5943–5947 [DOI] [PubMed]
- 72.Brunschweiger A, Iqbal J, Umbach F et al (2008) Selective nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) inhibitors: nucleoside mimetics derived from uridine-5′-carboxamide. J Med Chem 51:4518–4528 [DOI] [PMC free article] [PubMed]
- 73.Munkonda MN, Pelletier J, Ivanenkov VV et al (2009) Characterization of a monoclonal antibody as the first specific inhibitor of human nucleoside triphosphate diphosphohydrolase-3 (NTPDase3): partial characterization of the inhibitory epitope and potential applications. FEBS J 276:479–96 [DOI] [PMC free article] [PubMed]
- 74.Knowles AE, Nagy AK (1999) Inhibition of an ecto-ATP-diphosphohydrolase by azide. Eur J Biochem 262:349–357 [DOI] [PubMed]
- 75.Plesner L (1995) Ecto-ATPases: identities and functions. Int Rev Cytol 158:141–214 [DOI] [PubMed]
- 76.Enjyoji K, Sévigny J, Lin Y et al (1999) Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5:1010–1017 [DOI] [PubMed]
- 77.Cauwenberghs S, Feijge MA, Hageman G et al (2006) Plasma ectonucleotidases prevent desensitization of purinergic receptors in stored platelets: importance for platelet activity during thrombus formation. Transfusion 46:1018–1028 [DOI] [PubMed]
- 78.Schaefer U, Machida T, Broekman MJ et al (2007) Targeted deletion of ectonucleoside triphosphate diphosphohydrolase 1/CD39 leads to desensitization of pre- and postsynaptic purinergic P2 receptors. J Pharmacol Exp Ther 322:1269–1277 [DOI] [PubMed]
- 79.Leipziger J (2003) Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284:F419–F432 [DOI] [PubMed]