Abstract
Our knowledge and understanding of the P2 receptor signalling system in the kidney have increased significantly in the last ten years. The broad range of physiological roles proposed for this receptor system and the variety of P2 receptor subtypes found in the kidney suggest that any disturbance of function may contribute to several pathological processes. So far, most reports of a possible pathophysiological role for this system in the kidney have focussed on polycystic kidney disease, where abnormal P2 receptor signalling might be involved in cyst expansion and disease progression, and on the P2X7 receptor, a unique P2X subtype, which when activated enhances inflammatory cytokine release and production, and also cell death. Expression of this particular receptor is upregulated in some forms of chronic renal injury and inflammatory diseases. Further studies of adenosine triphosphate signalling and P2 receptor expression in renal disorders could provide us with novel insights into the role of these receptors in both normal and abnormal kidney function.
Keywords: ATP, Cytokines, Glomerulonephritis, Polycystic kidney disease, Proliferation
Introduction
Extracellular adenosine triphosphate (ATP) and its metabolites (ADP, AMP and adenosine) are now considered essential autocrine and paracrine modulators of renal cell function. As indicated elsewhere in this Special Issue, roles as diverse as alteration of renal blood flow and glomerular filtration rate, control of renin release, and regulation of renal tubular transport have all been proposed. As might be expected, receptors for ATP (ionotropic P2X or metabotropic P2Y receptors) have a wide and varying pattern of expression in the kidney, including glomerular cells, renal tubular cells (at both the apical and basolateral membranes), renal vascular (endothelial and smooth muscle) cells, and interstitial cells [1–5] (and see articles by Inscho [6] and Bailey and Shirley [7] in this Special Issue). Virtually every cell expresses at least one subtype of P2 receptor. ATP can be released from almost every cell in the kidney and is found in tubular fluid and the final urine [8–10]. The intraluminal concentration of ATP in the proximal tubule is around 200 nM, which is within the physiological range for P2 receptor activation [9], although concentrations of ATP at the cell surface, and therefore adjacent to any P2 receptor, may be even higher. Consequently, the tubular lumen is a microenvironment in which P2 receptor signalling can occur, and one which is also under the control of several nucleotidases expressed at cell surfaces and secreted into tubular fluid [11, 12] (and see article by Shirley et al. [13] in this Special Issue). The discovery of the pyrimidine-favouring P2Y receptors raises the possibility that uracil nucleotides can also function as signalling molecules.
It is likely that controlled signalling involves a dynamic interrelationship between nucleotide release, receptor activation and nucleotide breakdown. There is also evidence to suggest that this delicate balance can be disturbed, for example, when the renal tubule becomes an enclosed cyst. This has been a focus of interest of several laboratories, including our own [10, 14–18]. We have also been interested in the P2X7 receptor, which is not normally expressed by healthy kidney tissue, though it is highly expressed at sites of tissue damage and inflammation, when ATP is released from injured and dying cells [5, 14, 19].
Polycystic kidney disease
Polycystic kidney disease (PKD) is associated with abnormal and unregulated proliferation of the normally quiescent tubular cells of the adult nephron. This leads to progressive distension and dilatation of tubules, which eventually become encapsulated fluid-filled cysts that compress and destroy neighbouring tissue. Inside a cyst the fluid is trapped, creating an enclosed microenvironment containing secreted ATP able to act on the surface P2 receptors expressed by the lining cells. The concept that ATP is present in cyst fluid at elevated concentrations has been proposed previously [10]. In both cultured cyst cells harvested from autosomal dominant polycystic kidney disease (ADPKD) patients and from cpk/cpk mouse collecting duct monolayers, ATP release is enhanced predominantly from the apical surface [10, 16]. Also, ATP degradation by ecto-ATPases in ADPKD cells occurs at a much slower rate than in normal renal tissue [16]. We have shown, using a three-dimensional (3D) cell culture system in which Madin Darby canine kidney (MDCK) cells grow as rounded cysts, that ATP, acting via mainly P2Y receptor stimulation, drives cyst growth and expansion. In this in vitro system, the use of mainly non-subtype-specific P2Y receptor antagonists, and the ATP scavenger apyrase, markedly reduced the growth of MDCK microcysts. Furthermore, inhibition of the extracellular signal related kinase (ERK) pathway also significantly reduced cyst growth [18]. The ERK pathway can be activated by both P2X and P2Y receptors [20–22], and it is already well established that P2 receptor ligands can stimulate renal cell proliferation by activating mitogenic intracellular signalling pathways and/or growth factors [23–28].
When the released ATP and other P2 receptor ligands are enclosed within the cyst lumen, the relationship between autocrine and paracrine P2 receptor signalling and activation of mitogenic intracellular signalling pathways becomes critically important in abnormal cellular proliferation and, consequently, cyst growth and expansion. Indeed, we and others have shown expression of P2 receptors at, or near, the lining of renal cysts in rodent models of polycystic kidney disease and in ADPKD cell monolayers [16, 17, 29]. In fact, P2Y2, P2Y6 and P2X7 receptor expression was increased in the Han:SPRD cy rat model of ADPKD [17], and expression of P2X7 receptor messenger RNA (mRNA) and protein was also detected in the cystic epithelium of the cpk/cpk mouse model of autosomal recessive PKD [29]. Surprisingly, activation of the P2X7 receptor in a 3D suspension model of cpk/cpk cell cysts reduced the number of cysts formed, suggesting another function for this receptor, perhaps in cell turnover and tissue remodelling [14].
In addition to abnormal cellular proliferation, cyst cells exhibit altered polarity of transport proteins and receptors and abnormal secretion of fluid and electrolytes. This is in contrast to the normal vectorial transport of fluid along the nephron, when most of the glomerular filtrate is reabsorbed. However, in secretory epithelia, such as the airway epithelium, net fluid secretion depends on transepithelial cyclic adenosine monophosphate (cAMP)-stimulated Cl− secretion. It has been well documented that MDCK cells, as well as distal tubule and collecting duct cell lines, secrete Cl− via P2Y receptor-mediated increases in intracellular Ca2+ or cAMP, which drives Cl− channel activity [30–36]. In renal cysts, both in cell culture systems and in mouse models, fluid secretion is driven by excessive cAMP activity [37–39]. Moreover, P2 receptor-mediated increases in cell Ca2+ or cAMP drive Cl− secretion in ADPKD primary cultures [16, 40]. The functional significance of P2 receptors in controlling Cl− secretion can be extrapolated from studies of airway epithelia, where P2Y receptor activation is coupled to the cystic fibrosis chloride conductance regulator (CFTR). Currently, P2Y receptor agonists are being explored as possible treatments for cystic fibrosis, to improve mucus hydration and mucociliary clearance of the airways [41].
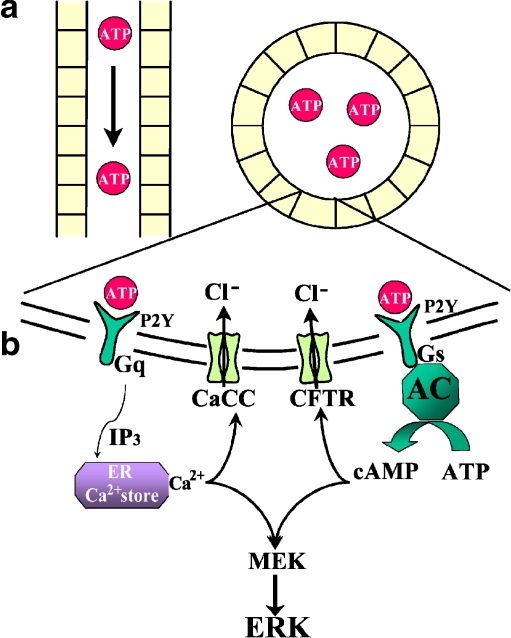
The CFTR Cl− channel is expressed in abundance in the kidney [42] and it is often expressed by cyst-lining renal epithelial cells [43]. It is controlled by cAMP, and inhibition of CFTR slows the growth of MDCK cysts [44]. The ability of P2Y receptors to alter cAMP production via coupling to G proteins, and either stimulation or inhibition of adenylate cyclase activity, is well documented [45]. There is also some evidence to suggest that polycystin-1, mutated in the majority of cases of ADPKD, is in some way linked to enhanced Cl− secretion. Expression of the COOH-terminal tail of polycystin-1 has been shown to enhance ATP-induced Ca2+ release in human kidney cells [46] and to promote ATP-stimulated Cl− secretion in a mouse-collecting duct cell line [47]. Furthermore, dysfunction of polycystin-1 appears to enhance the expression of CFTR in MDCK cells, augmenting the Cl− secretory mechanism [48]. Therefore, it is possible that over-stimulation of P2Y receptors, perhaps due to the presence of a large amount of agonist trapped within the lumen of cysts, can drive aberrant, osmotically driven, fluid secretion via increases in intracellular Ca2+ and cAMP and subsequent activation of Cl− ion channels, including CFTR (see Fig. 1). Modification of P2Y receptor signalling is an attractive prospect in the treatment of PKD.
Fig. 1.
P2Y receptor signalling could enhance cyst growth. a Remodelling of the normal renal tubule (top left) into an enclosed cyst (top right) allows ATP to accumulate. Coupled with perhaps reduced nucleotidase activity and the large representation of P2 receptor subtypes likely to be present in these cyst-lining epithelial cells, an amplifying loop of autocrine signalling could occur that promotes osmotically driven fluid accumulation and cellular proliferation. b Exploded view of the apical cell membrane lining a hypothetical renal cyst. G-protein-coupled P2Y receptor activation triggers release of Ca2+ from the endoplasmic reticulum or production of cAMP via adenylyl cyclase (AC) and subsequent activation of calcium-sensitive chloride channels (CaCC) or CFTR, respectively. The facilitated transcellular transport of Cl− creates a solute gradient that promotes the osmotic flow of water into the cyst lumen. The increase in [Ca2+]i and cAMP can modulate the ERK pathway and consequently cellular proliferation
However, the participation of P2X receptors in cyst pathophysiology should not be discounted, since they may also contribute to movement of fluid and electrolytes across epithelia. P2X receptors are themselves ion channels and can mediate rapid flux of Na+, Ca2+ and K+ ions, and they can also regulate several other ion channels [49]. In renal epithelium, nucleotides have been shown to inhibit Na+ reabsorption in M1 cells and in rat collecting duct, to inhibit Mg2+ reabsorption in a mouse distal tubule cell line and to inhibit the small conductance K+ channel in mouse cortical collecting ducts (see article by Bailey and Shirley [7] in this Special Issue). Reduced ion transport from lumen to blood could enhance osmotically driven fluid accumulation into cysts.
In summary, abundant evidence now exists to suggest that P2 receptor signalling may be detrimental in causing growth of renal cysts by increases in cellular proliferation and fluid secretion (Fig. 1). Any secretagogue or mitogen, such as ATP, released into an encapsulated cyst could establish a vicious autocrine/paracrine cycle of fluid accumulation, cellular growth and proliferation and cyst enlargement. Nevertheless, more studies are needed before the P2 receptor signalling system can be considered as a suitable target for therapeutic intervention. Manipulation of ecto-ATPases to reduce the effects of ATP is also another potential target.
Renal inflammation and fibrosis
ATP is known to be involved in the inflammatory process via histamine release [50] and cytokine production and release from immune cells [51–54]. The P2X7 receptor plays a central role in the latter process: its activation has broad pro-inflammatory effects, suggesting involvement in a wide variety of disease states, which is strongly supported by evidence that mice lacking P2X7 are resistant to experimentally induced inflammatory arthritis [55, 56]. Despite this, the role of P2X7 in other diseases, including those with kidney involvement, is still unclear. Factors that have contributed to this dearth of information on the expression pattern of P2X7 include a lack of specific agonists/antagonists, as well as scepticism that levels of ATP high enough (EC50 ≈ 300 μM) to stimulate the receptor are encountered in vivo, especially given the rapid rate of degradation of extracellular ATP. Surprisingly, the availability of mice deficient in P2X7 has, in some ways, increased the level of confusion. In particular, despite many studies indicating a neuronal function for P2X7 [57], two reports have shown similar staining of parental and P2X7-deficient brain tissue with anti-P2X7 antibodies [58, 59]. While staining for P2X7 in extra-neuronal tissue, such as salivary gland and lung, showed the expected loss of P2X7 protein in gene-targeted mice [59], these studies have cast doubt on the specificity of the antibodies used to detect P2X7. Despite this, it is still possible that the protein detected by anti-P2X7 antibodies in neuronal tissue of gene-targeted mice is a splice variant of P2X7 [60] that is capable of retaining significant ‘normal’ function [61].
In general, it is accepted that P2X7 is constitutively expressed on the majority of cells of the immune system, and several studies indicate expression on other cell types too, including endothelium, although its function in these non-immune tissues is still unclear. In non-immune cells, inflammatory mediators may upregulate expression of P2X7, for example, TNFα stimulates glomerular mesangial expression of P2X7 receptor mRNA [23]. Indeed, perhaps as important as its constitutive expression pattern is the evidence for altered distribution of P2X7 in diseased tissue. Despite its virtual absence from healthy kidneys, upregulated P2X7 receptor expression has been detected in the glomeruli of three different rodent models of renal disease and in lupus nephritis in humans. In streptozotocin-induced diabetic rats, increased P2X7 receptor expression was co-localised mainly in glomerular podocytes, and to some extent in mesangial cells and endothelial cells [19]. Glomerular expression of P2X7 was also reported in transgenic rats with renin-dependent and severe hypertension; increased expression of P2X7 receptor was also detected in mouse and rat models of anti-glomerular basement antibody-mediated glomerulonephritis in intrinsic glomerular cells and infiltrating macrophages [19, 62]. In rat glomerulonephritis, increased P2X7 mRNA expression coincided with elevated IL-1β mRNA and with the onset of glomerular damage in this model [62]. It is, therefore, the activity of P2X7 in tissue in pathological conditions that may be of greatest interest and value in beginning to understand its function. In a study of ureteric obstruction, TGFβ expression, macrophage infiltration and tubular apoptosis were all decreased in P2X7 knockout mice [63]. Further investigations with selective P2X7 receptor antagonists and/or in knockout mice should provide more direct evidence of an important role for this receptor in kidney disease.
Given the usually low levels of extracellular ATP, activation of P2X7 is not favoured under normal conditions. This appears to be true, even though the level of nucleotide in the pericellular environment is difficult to measure or estimate reliably, and it may be considerably higher near the receptor itself. However, while some ATP can be released by normal renal epithelial cells (though by still poorly understood pathways) [8], its concentration is likely to increase markedly, if only transiently, in disease states, because of leakage from damaged or necrotic cells, and release from nucleotide-rich granules in platelets recruited to the initial site of any injury and damage [64, 65]. Indeed, once activated, P2X7-stimulated cells themselves may become a significant and self-activating source of ATP, as not only can concentrations released at the cell surface of living cells reach 100–200 µM [17], but prolonged stimulation leads to cytolysis [66] and uncontrolled release of ATP from dying cells, which could reach millimolar concentrations. Moreover, the effective ATP concentration at sites of tissue damage may also be raised by a reduced rate of breakdown of ATP by the ectonucleotidase CD39, which exhibits decreased activity in inflammation [67]. Importantly, not only is the level of its agonist relatively high at sites of tissue damage, there is also evidence that the activity of the P2X7 receptor itself is increased under pathological conditions. This appears to reflect a broader tissue distribution (see above) and an increase in protein expression under the influence of inflammatory cytokines or bacterial products [68, 69], and, perhaps more surprisingly, a decrease in activation threshold of the receptor in conditions of hypoxia [70–72]. Taken together, the data suggest that activation of P2X7 in disease states is likely to occur more readily than might have been predicted from studies done under more conventional in vitro conditions.
The current literature provides increasing evidence for an association between P2X7 receptor activation, macrophage chemotaxis and glomerular inflammation (Fig. 2). This may be a mechanism for deleting damaged cells, remodelling of extracellular matrix and eventual tissue repair. Currently, its potential role is perhaps best indicated by its association with known downstream activities of receptor activation in renal disease. Work is currently under way to determine a functional link with the P2X7 receptor in experimental glomerulonephritis and diabetes mellitus by using P2X7 knockout mice.
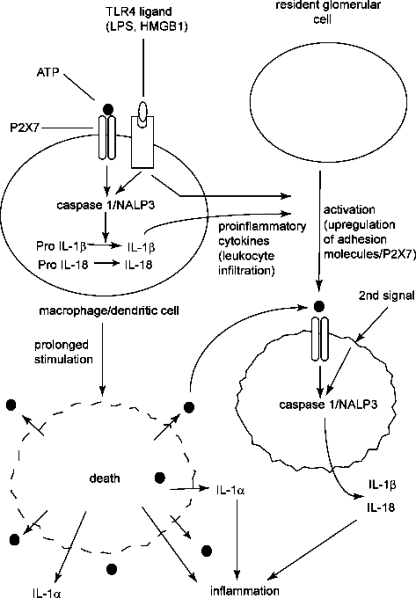
Fig. 2.
Hypothetical role for the P2X7 receptor in inflammatory glomerular disease. Following initial injury to the kidney, ATP released from damaged cells, probably together with endogenous Toll-like receptor 4 (TLR4) ligands such as high mobility group box 1 (HMGB1) and lipopolysaccharide (LPS), stimulates the NALP3 inflammasome. Inflammasome activation leads to the maturation of caspase 1, which in turn promotes cleavage, maturation and release of IL-1β and IL-18 from resident macrophages. Released cytokines promote leukocyte influx and stimulate upregulation of P2X7 on intrinsic renal cells. Prolonged P2X7 stimulation results in cell death with release of intracellular pro-inflammatory mediators such as ATP, IL-1α and HMGB1, resulting in further rounds of P2X7 stimulation
Discovery of a naturally occurring ligand for the P2X7 receptor, LL37 [73], may provide more clues as to the function and role of the P2X7 receptor in kidney and other organs. LL37 is a potent antimicrobial peptide produced predominantly by neutrophils and epithelial cells. It protects epithelia in the airway and urinary tract against bacterial colonisation. It has been shown to activate the P2X7 receptor at much lower concentrations than ATP, and it promotes IL-1β processing and release without causing cytotoxicity [73]. Understanding how P2X7 receptor activity is regulated may provide a new means of modulating the inflammatory response.
Other considerations
There are recent reports of several P2X7 receptor polymorphisms with various allele frequencies in the population that confer either loss or reduced P2X7 receptor function. Initial reports indicate increased susceptibility to tuberculosis infection due to impaired ATP-induced mycobacterial killing [74]. This field is only just beginning to be examined in detail; it has important implications for the pathogenesis of renal disease, and it could be relevant to chronic inflammatory disorders such as glomerulonephritis. Moreover, the P2X7 receptor has also been proposed as a candidate gene for susceptibility to systemic lupus erythematosus (SLE), which is based on its properties as an inflammatory mediator and its genetic location within an identified SLE susceptibility locus [75]. SLE is a multisystem autoimmune disease that can include an immune complex-mediated form of glomerulonephritis.
Consideration should also be given to other, as yet unidentified, polymorphisms in P2 receptor subtypes that might alter its function. The physiological role of P2 receptors in the kidney raises the possibility that any genetic variability that affects function could serve as a disease modifier. For example, a recently identified polymorphism in the P2Y2 gene appears to alter intracellular Ca2+ release and Cl− secretion, which might be relevant to the rate of progression of PKD [76]. More recently, both the P2Y1 and P2Y12 subtypes have been implicated in chronic renal disease. In a model of passive nephrotoxic nephritis, P2Y1 gene-deficient mice had better long-term survival and less chronic injury than wild-type mice. Loss of the P2Y1 receptor appeared to have a protective effect by safeguarding against capillary loss, fibrosis and renal failure, and preserving renal function, although the mechanisms for this are still unclear [77]. Inhibition of the P2Y12 receptor with clopidogrel in a rat model of angiotensin II-induced hypertension reduced cellular proliferation and macrophage infiltration and lessened tissue injury [78]. This suggests that the P2Y12 receptor could be involved in the cascade of events that lead to vascular and glomerular injury in this form of hypertension.
Another newly emerging area of potential interest concerns P2 receptor signalling in epithelial cell cancer. At present, studies have focussed on basal and squamous cell tumours, intestinal cell carcinomas, colorectal, prostate and bladder cancers. However, the role that P2 receptors might play is not yet very well defined, since P2X and P2Y receptors have been reported to have both proliferative and apoptotic effects [79–81]. Generally, P2Y1 and P2Y2 receptors mediate proliferation or anti-proliferation, P2X5 receptors mediate differentiation and P2X7 receptors mediate apoptotic, necrotic or ‘aponecrotic’ cell death [80, 82, 83]. These effects may be relevant to renal cell cancer, given the large representation of P2 receptors in renal epithelial cells. Targeting these receptors may also be a means of characterising and eventually treating these forms of cancer.
Conclusion
Clearly, much work still needs to be done, and more functional studies are required to establish the role of P2 receptor signalling in renal disease, before this system can be considered to be a useful therapeutic target. ATP complexed with MgCl2 has long been used as an effective treatment for protecting kidneys against ischaemic damage. The results of phase II trials for a P2X7 antagonist in the treatment of rheumatoid arthritis and chronic obstructive pulmonary disease are awaited with great interest. If successful, these trials would indicate the potential use of P2X7 inhibitors in a range of inflammatory diseases, including those affecting the kidney. Indeed, several major pharmaceutical companies consider this receptor system of such potential importance in health and disease that they are making a significant effort to develop selective and safe P2 receptor agonists and antagonists, which will also help us in defining their function.
Acknowledgements
Some of the authors’ work referred to in this review was supported by the MRC, Wellcome Trust and St. Peter’s Trust for Prostate, Bladder and Kidney Research. CMT is supported by the Wellcome Trust.
Abbreviations
- ADPKD
Autosomal dominant polycystic kidney disease
- cAMP
Cyclic adenosine monophosphate
- CFTR
Cystic fibrosis chloride conductance regulator
- ERK
Extracellular signal related kinase
- IL-1β
Interleukin-1β
- MDCK
Madin Darby canine kidney
- TGFβ
Transforming growth factor β
- TNFα
Tumour necrosis factor α
Footnotes
James I. Elliott and Frederick W. K. Tam contributed equally to this work.
References
- 1.Bailey MA, Imbert-Teboul M, Turner C et al (2000) Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58:1893–1901 [DOI] [PubMed]
- 2.Bailey MA, Turner CM, Hus-Citharel A et al (2004) P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiol 96:79–90 [DOI] [PubMed]
- 3.Deetjen P, Thomas J, Lehrmann H et al (2000) The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol 11:1798–1806 [DOI] [PubMed]
- 4.Kishore BK, Ginns SM, Krane CM et al (2000) Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278:F43–F51 [DOI] [PubMed]
- 5.Turner CM, Vonend O, Chan C et al (2003) The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175:105–117 [DOI] [PubMed]
- 6.Inscho EW (2008) ATP, P2 receptors and the renal microcirculation. Pur Sig (in press) [DOI] [PMC free article] [PubMed]
- 7.Bailey MA, Shirley DG (2009) Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signalling. doi:10.1007/s11302-009-9149-z [DOI] [PMC free article] [PubMed]
- 8.Schwiebert EM (2001) ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol 28:340–350 [DOI] [PubMed]
- 9.Vekaria RM, Unwin RJ, Shirley DG (2006) Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17:1841–1847 [DOI] [PubMed]
- 10.Wilson PD, Hovater JS, Casey CC et al (1999) ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10:218–229 [DOI] [PubMed]
- 11.Dawson TP, Gandhi R, Le Hir M et al (1989) Ecto-5′-nucleotidase: localization in rat kidney by light microscopic histochemical and immunohistochemical methods. J Histochem Cytochem 37:39–47 [DOI] [PubMed]
- 12.Vekaria RM, Shirley DG, Sévigny J et al (2006) Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290:F550–F560 [DOI] [PubMed]
- 13.Shirley DG, Vekaria RM, Sévigny J (2009) Ectonucleotidases in the kidney. Purinergic Signalling. doi:10.1007/s11302-009-9152-4 [DOI] [PMC free article] [PubMed]
- 14.Hillman KA, Woolf AS, Johnson TM et al (2004) The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun 322:434–439 [DOI] [PubMed]
- 15.Hooper KM, Unwin RJ, Sutters M (2003) The isolated C-terminus of polycystin-1 promotes increased ATP-stimulated chloride secretion in a collecting duct cell line. Clin Sci 104:217–221 [DOI] [PubMed]
- 16.Schwiebert EM, Wallace DP, Braunstein GM et al (2002) Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282:F763–F775 [DOI] [PubMed]
- 17.Turner CM, Ramesh B, Srai SK et al (2004) Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs 178:168–179 [DOI] [PubMed]
- 18.Turner CM, King BF, Srai KS et al (2007) Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Renal Physiol 292:F15–F25 [DOI] [PubMed]
- 19.Vonend O, Turner CM, Chan CM et al (2004) Glomerular expression of the ATP-sensitive P2X7 receptor in diabetic and hypertensive rat models. Kidney Int 66:157–166 [DOI] [PubMed]
- 20.Amstrup J, Novak I (2003) P2X7 receptor activates extracellular signal-regulated kinases ERK1 and ERK2 independently of Ca2+ influx. Biochem J 374:51–61 [DOI] [PMC free article] [PubMed]
- 21.May C, Weigl L, Karel A et al (2006) Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1 receptor in a Ca2+ independent manner in differentiated human skeletal muscle cells. Biochem Pharmacol 71:1497–1509 [DOI] [PubMed]
- 22.Neary JT, Kang Y, Willoughby KA et al (2003) Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci 23:2348–2356 [DOI] [PMC free article] [PubMed]
- 23.Harada H, Chan CM, Loesch A et al (2000) Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int 57:949–958 [DOI] [PubMed]
- 24.Huwiler A, Pfeilschifter J (1994) Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br J Pharmacol 113:1455–1463 [DOI] [PMC free article] [PubMed]
- 25.Ishikawa S, Higashiyama M, Kusaka I et al (1997) Extracellular ATP promotes cellular growth of renal inner medullary collecting duct cells mediated via P2u receptors. Nephron 76:208–214 [DOI] [PubMed]
- 26.Lee YJ, Han HJ (2006) Role of ATP in DNA synthesis of renal proximal tubule cells: involvement of calcium, MAPKs, and CDKs. Am J Physiol Renal Physiol 291:F98–106 [DOI] [PubMed]
- 27.Schulze-Lohoff E, Schagerl S, Ogilvie A et al (1995) Extracellular ATP augments mesangial cell growth induced by multiple growth factors. Nephrol Dial Transplant 10:2027–2034 [PubMed]
- 28.Vonend O, Grote T, Oberhauser V et al (2003) P2Y-receptors stimulating the proliferation of human mesangial cells through the MAPK(42/44) pathway. Br J Pharmacol 139:1119–1126 [DOI] [PMC free article] [PubMed]
- 29.Hillman KA, Johnson TM, Winyard PJ et al (2002) P2X7 receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol 10:34–42 [DOI] [PubMed]
- 30.Akimova AO, Bourcier N, Taurin S et al (2005) Cl− secretion in ATP-treated renal epithelial C7-MDCK cells is mediated by activation of P2Y1 receptors, phospholipase A2 and protein kinase A. J Physiol 568:789–801 [DOI] [PMC free article] [PubMed]
- 31.Boese SH, Glanville M, Aziz O et al (2000) Ca2+ and cAMP-activated Cl− conductances mediate Cl− secretion in a mouse renal inner medullary collecting duct cell line. J Physiol 523:325–338 [DOI] [PMC free article] [PubMed]
- 32.Cuffe JE, Bielfeld-Ackermann A, Thomas J et al (2000) ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524:77–90 [DOI] [PMC free article] [PubMed]
- 33.Kolb HA, Brown CD, Murer H (1985) Identification of a voltage-dependent anion channel in the apical membrane of a Cl−-secretory epithelium (MDCK). Pflügers Arch 403:262–265 [DOI] [PubMed]
- 34.Mohamed A, Ferguson D, Seibert FS et al (1997) Functional expression and apical localization of the cystic fibrosis transmembrane conductance regulator in MDCK I cells. Biochem J 322:259–265 [DOI] [PMC free article] [PubMed]
- 35.Simmons NL (1981) Stimulation of Cl− secretion by exogenous ATP in cultured MDCK epithelial monolayers. Biochim Biophys Acta 646:231–242 [DOI] [PubMed]
- 36.Zegarra-Moran O, Romeo G, Galietta LJ (1995) Regulation of transepithelial ion transport by two different purinoceptors in the apical membrane of canine kidney (MDCK) cells. Br J Pharmacol 114:1052–1056 [DOI] [PMC free article] [PubMed]
- 37.Hanaoka K, Guggino WB (2000) cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11:1179–1187 [DOI] [PubMed]
- 38.Mangoo-Karim R, Ye M, Wallace DP et al (1995) Anion secretion drives fluid secretion by monolayers of cultured human polycystic cells. Am J Physiol 269:F381–F388 [DOI] [PubMed]
- 39.Yamaguchi T, Nagao S, Kasahara M et al (1997) Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis 30:703–709 [DOI] [PubMed]
- 40.Mangoo-Karim R, Uchic M, Lechene C et al (1989) Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci U S A 86:6007–6011 [DOI] [PMC free article] [PubMed]
- 41.Marcet B, Boeynaems JM (2006) Relationships between cystic fibrosis transmembrane conductance regulator, extracellular nucleotides and cystic fibrosis. Pharmacol Ther 112:719–732 [DOI] [PubMed]
- 42.Morales MM, Falkenstein D, Lopes AG (2000) The cystic fibrosis transmembrane regulator (CFTR) in the kidney. An Acad Bras Cienc 72:399–406 [DOI] [PubMed]
- 43.Hanaoka K, Devuyst O, Schwiebert EM et al (1996) A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol 270:C389–C399 [DOI] [PubMed]
- 44.Li H, Findlay IA, Sheppard DN (2004) The relationship between cell proliferation, Cl− secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int 66:1926–1938 [DOI] [PubMed]
- 45.Von Kugelgen I, Wetter A (2000) Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol 362:310–323 [DOI] [PubMed]
- 46.Aguiari G, Campanella M, Manzati E et al (2003) Expression of polycystin-1 C-terminal fragment enhances the ATP-induced Ca2+ release in human kidney cells. Biochem Biophys Res Commun 301:657–664 [DOI] [PubMed]
- 47.Wildman SS, Hooper KM, Turner CM et al (2003) The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl− conductance through increased Ca2+ entry. Am J Physiol Renal Physiol 285:F1168–F1178 [DOI] [PubMed]
- 48.Ikeda M, Fong P, Cheng J et al (2006) A regulatory role of polycystin-1 on cystic fibrosis transmembrane conductance regulator plasma membrane expression. Cell Physiol Biochem 18:9–20 [DOI] [PubMed]
- 49.McCoy DE, Taylor AL, Kudlow BA et al (1999) Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol 277:F552–F559 [DOI] [PubMed]
- 50.Schulman ES, Glaum MC, Post T et al (1999) ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol 20:530–537 [DOI] [PubMed]
- 51.Lister MF, Sharkey J, Sawatzky DA et al (2007) The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 4:5–18 [DOI] [PMC free article] [PubMed]
- 52.MacKenzie A, Wilson HL, Kiss-Toth E et al (2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15:825–835 [DOI] [PubMed]
- 53.Mehta VB, Hart J, Wewers MD (2001) ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem 276:3820–3826 [DOI] [PubMed]
- 54.Mariathasan S, Weiss DS, Newton K et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232 [DOI] [PubMed]
- 55.Chessell IP, Hatcher JP, Bountra C et al (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114:386–396 [DOI] [PubMed]
- 56.Labasi JM, Petrushova N, Donovan C et al (2002) Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168:6436–6445 [DOI] [PubMed]
- 57.Sperlagh B, Vizi ES, Wirkner K et al (2006) P2X7 receptors in the nervous system. Prog Neurobiol 78:327–346 [DOI] [PubMed]
- 58.Kukley M, Stausberg P, Adelmann G et al (2004) Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP. J Neurosci 24:7128–7139 [DOI] [PMC free article] [PubMed]
- 59.Sim JA, Young MT, Sung HY et al (2004) Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24:6307–6314 [DOI] [PMC free article] [PubMed]
- 60.Cheewatrakoolpong B, Gilchrest H, Anthes JC et al (2005) Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun 332:17–27 [DOI] [PubMed]
- 61.Sanchez-Nogueiro J, Marin-Garcia P, Miras-Portugal MT (2005) Characterization of a functional P2X7-like receptor in cerebellar granule neurons from P2X7 knockout mice. FEBS Lett 579:3783–3788 [DOI] [PubMed]
- 62.Turner CM, Tam FW, Lai PC et al (2007) Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22:386–395 [DOI] [PubMed]
- 63.Goncalves RG, Gabrich L, Rosario A Jr et al (2006) The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70:1599–1606 [DOI] [PubMed]
- 64.Beigi R, Kobatake E, Aizawa M et al (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol 276:C267–C278 [DOI] [PubMed]
- 65.Bodin P, Burnstock G (2001) Purinergic signalling: ATP release. Neurochem Res 26:959–969 [DOI] [PubMed]
- 66.Surprenant A, Rassendren F, Kawashima E et al (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738 [DOI] [PubMed]
- 67.Robson SC, Kaczmarek E, Siegel JB et al (1997) Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med 185:153–163 [DOI] [PMC free article] [PubMed]
- 68.Humphreys BD, Dubyak GR (1998) Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 64:265–273 [DOI] [PubMed]
- 69.Narcisse L, Scemes E, Zhao Y et al (2005) The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49:245–258 [DOI] [PMC free article] [PubMed]
- 70.Franke H, Gunther A, Grosche J et al (2004) P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol 63:686–699 [DOI] [PubMed]
- 71.Milius D, Groger-Arndt H, Stanchev D et al (2007) Oxygen/glucose deprivation increases the integration of recombinant P2X7 receptors into the plasma membrane of HEK293 cells. Toxicology 238:60–69 [DOI] [PubMed]
- 72.Wirkner K, Kofalvi A, Fischer W et al (2005) Supersensitivity of P2X receptors in cerebrocortical cell cultures after in vitro ischemia. J Neurochem 95:1421–1437 [DOI] [PubMed]
- 73.Elssner A, Duncan M, Gavrilin M et al (2004) A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol 172:4987–4994 [DOI] [PubMed]
- 74.Shemon AN, Sluyter R, Fernando SL et al (2006) A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 281:2079–2086 [DOI] [PubMed]
- 75.Elliott JI, McVey JH, Higgins CF (2005) The P2X7 receptor is a candidate product of murine and human lupus susceptibility loci: a hypothesis and comparison of murine allelic products. Arthritis Res Ther 7:R468–R475 [DOI] [PMC free article] [PubMed]
- 76.Buscher R, Hoerning A, Patel HH et al (2006) P2Y2 receptor polymorphisms and haplotypes in cystic fibrosis and their impact on Ca2+ influx. Pharmacogenet Genomics 16:199–205 [DOI] [PubMed]
- 77.Hohenstein B, Renk S, Lang K et al (2007) P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J Am Soc Nephrol 18:494–505 [DOI] [PubMed]
- 78.Graciano ML, Nishiyama A, Jackson K et al (2008) Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294:F161–F169 [DOI] [PMC free article] [PubMed]
- 79.Chen L, He HY, Li HM et al (2004) ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett 215:239–247 [DOI] [PubMed]
- 80.Coutinho-Silva R, Stahl L, Cheung KK et al (2005) P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol 288:G1024–G1035 [DOI] [PubMed]
- 81.Janssens R, Boeynaems JM (2001) Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol 132:536–546 [DOI] [PMC free article] [PubMed]
- 82.Greig AV, Linge C, Terenghi G et al (2003) Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol 120:1007–1015 [DOI] [PubMed]
- 83.Taylor SR, Gonzalez-Begne M, Dewhurst S et al (2007) Sequential shrinkage and swelling underlie P2X7-stimulated lymphocyte phosphatidylserine exposure and death. J Immunol 180:300–308 [DOI] [PubMed]