Abstract
Evidence suggests that multiple tumors, including pancreatic adenocarcinoma, display heterogeneity in parameters that are critical for tumor formation, progression and metastasis. Understanding heterogeneity in solid tumors is increasingly providing a plethora of new diagnostic and therapeutic approaches. In this study, a particular focus was put on identifying a subpopulation of stem cell-like, slow cycling tumor cells in a pancreas adenocarcinoma cell lines. Using a label retention technique a subpopulation of slow cycling cells (DiI+/SCC) was identified and further evaluated in the BxPC-3 and Panc03.27 cell lines. These slowly cycling cells managed to retain the lipophilic labeling dye DiI, while the bulk of the cells (>94%) did not. The DiI+/SCC population, showed only a partial overlap with the CSC markers CD24+/CD44+, CD133+ and ALDH but they survived chemotherapeutic treatment, and were able to recreate the initial heterogeneous tumor cell population. DiI+/SCCs exhibited an increased invasive potential as compared with their non-label retaining, faster cycling cells (DiI−/FCC). They also had increased tumorigenic potential and morphological changes resembling cells that have undergone an epithelial to mesenchymal transition (EMT). Analysis of DiI+/SCC cells by real time PCR revealed a selective up-regulation of tell tale components of the Hedgehog/TGFβ pathways, as well as a down-regulation of EGFR, combined with a shift in crucial components implied in EMT. The presented findings offer an expanded mechanistic understanding that associates tumor initiating potential with cycling speed and EMT in pancreatic cancer cell lines.
Electronic supplementary material
The online version of this article (doi:10.1007/s10585-009-9260-0) contains supplementary material, which is available to authorized users.
Keywords: Cancer stem cell, EMT, Gli 1, Pancreatic adenocarcinoma, Slow cycling, Sonic hedgehog (Shh)
Introduction
Pancreatic cancer is known for its extensive local tumor invasion and early systemic dissemination. The molecular basis for these characteristics is not yet fully understood. Therefore, despite advances in surgical and medical therapy, little effect has been made on the mortality rate of this disease [1].
Most attempts to better understand the characteristics of pancreatic adenocarcinoma have focused on studying gene and protein expression profiles of total samples of pancreatic adenocarcinoma, and have not taken into account the heterogeneity of cells within a particular tumor. However, based on the concept of cancer stem cells (CSC), claims are made that the ability of a tumor to grow and propagate may depend on small subsets of cells, as only a minority of cancer cells showed extensive proliferation when examined for their proliferative potential in various in vitro or in vivo assays [2–4]. In particular cells that are either positive for the surface antigens CD24 and CD44, CD133 or display ALDH activity have been reported to exhibit increased self renewal and tumor initiating potential, as well as to give rise to renewed heterogeneity in pancreas adenocarcinoma [3–7]. Cells with such properties are termed by various authors “CSC” or “tumor initiating cells”.
Interestingly, the CD24+/CD44+ cells in pancreas adenocarcinoma also display increased expression of components of the sonic hedgehog (Shh) stem cell signaling pathway [3, 7]. However, the role of the Hh signaling pathway and its mediator, the zinc finger transcription factor Gli1 [8], in etiology and progression of pancreas adenocarcinoma is not fully understood, although there is evidence to support a role of the Hh pathway in this tumor. The central importance of Gli1 mediated signaling was confirmed by a broad genetic analysis of pancreatic cancer that identified genetic alterations in 100% of samples, together with changes in KRAS, TGF, and Wnt/Notch. [9]. All 4 pathways are central in developmental and stem cell biology where they control cell cycle, developmental potential, cell adhesiveness and apoptosis [8, 10]. Although the involvement of these pathways in tumors is well established, less is known about their role and involvement in tumor heterogeneity or subpopulations. Analysis of the correlation of pathways to subpopulations will be of substantial significance.
It is believed that subpopulations of cells within the tumor microenvironment can undergo changes and are the main contributor to metastatic disease. Interestingly, a recent study where a chemo-resistant population of pancreatic cancer cells was created, suggested that the resistant population of cells had undergone EMT changes and was now of a more motile invasive phenotype as compared to the parental line [11]. EMT, as controlled by developmental pathways, is a key player in cancer metastasis as it allows cells to migrate and invade surrounding issues and escape into the bloodstream, en route to establishing metastasis. Once these metastatic cells reach their destination, they can undergo reverse EMT—mesenchymal epithelial transition (MET), to establish secondary tumors.
Consequently, EMT stimuli can generate cells with properties of stem or progenitor cells. In a recent report, normal mammary epithelial cells were induced to acquire the CD44high/CD24low breast CSC phenotype after exposure to TGFβ [12]. This implies that expression of TGFβ either converts CD44low/CD24high cells to the CD44high/CD24low CSC phenotype, thereby suggesting that the majority of cells have the possibility to switch to this CSC phenotype; or that the EMT stimuli inhibits the proliferation of the epithelial tumor cell population and induces the proliferation or stops the differentiation of the CSC. As a result, it has been suggested that CSCs may actually not be distinct entities, but rather tumor cells that transiently acquire stem cell-like properties as a consequence of EMT [13]. Such transient subpopulations of cancer cells with stem cell character may not only have an increased innate ability to resist chemo and radio therapies [14–16], but may carry a specific profile of developmental signals, which could make them amenable to therapeutic approaches through specific pathway antagonists.
In this report, a subpopulation of DiI+/SCC in the pancreatic adenocarcinoma cell lines BxPC-3 and Panc03.27 was identified, selected upon and sorted by cell cycle speed. The resulting population (DiI+/SCC) was overlapping, but not identical, with the previously reported pancreatic population of tumor initiating cells (CD133+, ALDH+, or CD24+/CD44+). DiI+/SCCs were morphologically distinct and showed a more malignant, invasive and neoplastic phenotype when compared to the fast cycling bulk of cells (DiI−/FCC). Quantitative RT-PCR profiling confirmed that DiI+/SCC showed alterations in key developmental and stem cell signaling pathways, and an expression profile consistent with EMT conversion.
Materials and methods
Cells and culture conditions
BxPC-3 and Panc03.27 pancreatic adenocarcinoma cells were obtained from ATCC and were cultured in RPMI media (Sigma–Aldrich) containing 10% FBS (Invitrogen) with 2 mmol/l glutamine and penicillin/streptomycin (BioWhittaker) either 10 μl ITS (BxPC-3) or 500 μl ITS (Panc03.27) (Sigma–Aldrich) at 37°C in a humidified atmosphere of 5% CO2.
Labeling and sorting of cell populations
Vybrant® DiI cell-labeling solution (Invitrogen/Molecular Probes), was used to label cells, according to the protocol for attached cells [17]. The labeled cells were then allowed to grow for 4–6 weeks (passaging when confluent, and retaining all cells through the period), and were analyzed and sorted using a BD FACS Aria (VWR). As positive controls, freshly labeled cells were used, whereas the negative control consisted of cells that never came in contact with the dye. When sorting, the brightest positive population was selected and used in further analysis. Data represent means ± SD; n = 20 (BxPC-3); n = 9 (Panc03.27).
Flow cytometry
Previously DiI labeled cells were trypsinized (Trypsin-EDTA, Biowhittaker), resuspended in PBS and sorted on BD FACS Aria using BD FACS Diva software.
To analyze expression of CSC markers, freshly sorted cell populations were immediately stained with mouse anti-human CD44-PE/Cy7 and CD24-PE or CD24-FITC (both from Abcam) as such; cells were washed and re-suspended in PBS with 10%FBS, then incubated with 10 μl of each antibody in 100 μl for 1 h (in the dark, on ice). Cells were then washed 3 times, resuspended in PBS with 10% FBS, and immediately re-analyzed by FACS.
To analyze CD133 expression, 4–6 weeks DiI labeled, unsorted cells were incubated with rabbit anti-CD133 (Abcam) (1/200 dilution in PBS 10%FBS) for 30 min (in the dark, on ice), then washed 3 times in PBS with 10%FBS. For secondary antibody, goat anti-rabbit Alexa 488 (Invitrogen) was used (1/200 dilution in PBS 10%FBS) for 30 min (in the dark, on ice), then washed 3 times in PBS with 10%FBS and analyzed.
To examine ALDH expression 4–6 weeks DiI labeled, unsorted cells were stained using the Aldefluor kit (StemCell Technologies) according to manufacturer’s instructions. Data represent means ± SD;n = 3 per assay.
Chemotherapy resistance assay
Cells were labeled with DiI and seeded into six well plates at 300,000 cells/well (BD Falcon, VWR) 24 h prior, then treated with a 5-Fluorouracil (5-FU) (Sigma) at 0, 5, 50, 100, 150, 200 μg/ml. After 3 days, media was replaced, and cells were followed for growth. Additionally FACS sorted DiI+/SCC were plated, allowed to adhere overnight, then challenged with 5-FU in a similar fashion as above.
Images were obtained on an Axiovert 200 M microscope (Zeiss) using Axiovision software. Electronic images were further processed using Adobe Photoshop.
Data represent means ± SD; n = 3 per assay.
Soft agar assay
The colony formation capability of the DiI+/SCC and DiI−/FCC populations in soft agar was investigated. Briefly, 5000 freshly sorted cells were resuspended in 1.5 ml growth media containing 0.35% agar (Oxoid), and plated in triplicate over a 1.5 ml base layer containing 0.5% agar in six-well plates. The plates were incubated for 14 (Panc03.27) or 21 (BxPC-3) days, then stained for 1 h with 1 ml of 0.02% crystal violet (Sigma), and colonies were counted. Colony diameters larger than 75 μm or colony cells more than 50 cells were counted as 1 positive colony. Images were obtained on an Axiovert 200 M microscope (Zeiss) using Axiovision software. Electronic images were further processed using Adobe Photoshop. Data represent means ± SD; n = 3.
Invasion assay
BD BioCoat matrigel invasion chamber 24-well plates; 8.0 μm pore size (VWR) were used in the experiment as such; 25,000 cells were plated in the top chamber in 500 μl of media containing 1% FBS. The bottom wells contained 750 μl of complete media (10% FBS). The cells were allowed to grow and invade for 20 or 44 h, then the non-invading cells were scraped from the upper surface of the membrane with a cotton swab, and the cells on the lower side were stained with 0.02% crystal violet in formaldehyde (Sigma). The number of invading cells were counted through the entire surface area (about 9 microscopic fields at 10×). Data represent means ± SD; n = 3 for each data point.
Animals
About 4–6 weeks old female CB17/SCID mice were used throughout this study. All mice were housed and used under the approved protocols in accordance with the National Institute of Health guide for the care and use of laboratory animals, and all efforts were made to minimize the number and suffering of animals.
In vivo tumor establishment
Anesthesia was induced briefly using open drop exposure to isoflurane (Baxter) [18], then the lower flank was shaved and freshly sorted (DiI+/SCC vs. DiI−/FCC) were carefully injected sub cue in GFR matrigel (BD Biosciences) in a total volume of 200 μl (1:1 matrigel:PBS) in the following amounts; DiI−/FCC: 1 × 106 (n = 3); 5 × 105 (n = 3), 1 × 105 (n = 4), 5 × 104 (n = 4), 1 × 104 (n = 4), 5 × 103 (n = 4) DiI +/SCC: 1 × 106 (n = 3), 5 × 105 (n = 3), 1 × 105 (n = 4), 5 × 104 (n = 4), 1 × 104 (n = 4), 5 × 103 (n = 4). Mice were followed over a 4 weeks period and tumor size (if present) was measured using calipers (mm2).
RT-PCR
Total RNA was isolated using the GeneElute miniprep kit (Sigma) following the manufacturer’s instructions. cDNA was synthesized using the Retroscript kit (Ambion), and real-time PCR was carried out using the SYBR Green PCR master mix (Stratagene) according to the manufacturer’s instructions with an Mx3000P cycler (Stratagene). The general amplification protocol (50 cycles) was set as follows: initial denaturation for 5 min at 95°C, then denaturation for 1 min at 95°C, specific primer annealing temperature for 30 sec (Supplementary Table 2), and amplification at 72°C for 1 min. The concentrations of cDNA present in each sample were calculated by the MxPro software (Stratagene). To normalize the amount of cDNA in each sample and to guarantee the comparability of the calculated mRNA expression in all samples analyzed, the housekeeping gene GAPDH was also analyzed. Primers and specific annealing temperatures are listed in Supplementary Table S2. Data represent means ± SD; n ≥ 4.
Results
Isolation of slow cycling cells
To isolate slower cycling cells, a label retention method was used with the pancreas adenocarcinoma cell lines Panc03.27 and BxPC-3. Cells were labeled with the long term lipophilic tracer dye, DiI (C59H97ClN2O4), which is structurally identical to PKH26, a dye which has numerous references regarding its use to trace cell divisions and identify slow cycling populations such as long-term culture-initiating cells (LTC-IC) [19–23]. Advantages of DiI to PKH26 include simple uniform cellular labeling (no requirement for special osmolarity agents or salt in the staining buffer), low cytotoxicity (DiI does not affect cell viability, development, or other basic physiological properties) and high resistance to intercellular transfer. DiI, like PKH26 has also been used to trace cells throughout divisions in vitro, and has been detected throughout cell passages for as long as 7 weeks in bone marrow stromal cells [24]. The strong, photostable fluorescence and excellent cellular retention of DiI make it highly favorable for a number of applications, including long-term labeling and tracking of cells [17].
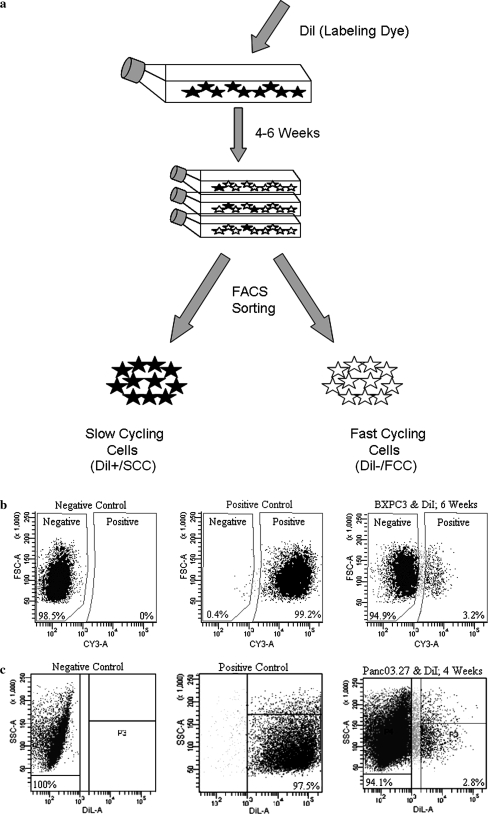
As seen in Fig. 1, cells exhibited clear heterogeneity with respect to cell cycle length, and after 6 weeks of growth (BxPC-3; only 4 weeks were required to identify a DiI+/SCC population with Panc03.27), a segregated, bright DiI+ retaining population of cells (DiI+/SCC) correlating to ~3% (mean = 2.9% ± 1.7 BxPC-3; 2.4% ± 1.8 Panc03.27) of the total population was visible, traceable, and easy to sort by FACS (Fig. 1). Nearly all DiI+/SCCs showed striking morphological changes when compared to DiI−/FCCs. The cells exhibited an elongated fibroblast like cell shape, regardless whether they were in a cellular context, or growing isolated. In contrast non label retaining cells (DiI−/FCC) displayed a more compact and epithelial morphology (Fig. 2a).
Fig. 1.
Cell labeling, preparation and sorting a Schematic illustration of labeling, expansion and sorting of cells. b FACS analysis of BxPC-3 and Panc03.27 cells after 6 and 4 weeks growth, with 3.2 and 2.8% positive cells (DiI+/SCC), respectively
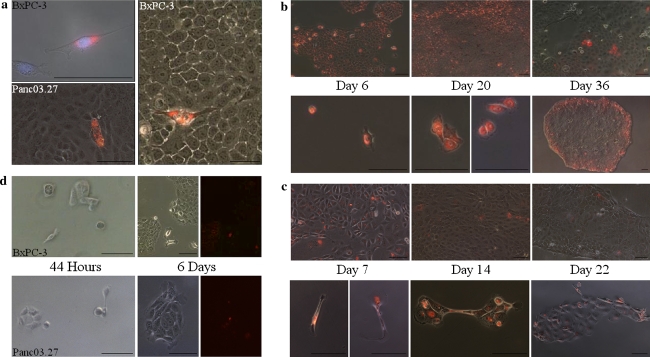
Fig. 2.
Cell growth after DiI labeling. a Image of DiI+/SCC and DiI−/FCC cells after 4 (Panc03.27) or 6 (BxPC-3) weeks growth; before and after (BxPC-3) sorting (plated for high resolution imagery and taken after 24 h). b BxPC-3 normal growth (top) or with the addition of 100 μg/ml 5-FU (treated with 5-FU for 3 days) (bottom) of cells followed through 36 days. c Panc03.27 normal growth (top) or with the addition of 100 μg/ml 5-FU (treated with 5-FU for 3 days) (bottom) of cells followed through 22 days. d Growth of plated DiI+/SCC after sorting (times represent growth duration after plating). Scale bars represent 100 μm
Chemotherapy resistance selects for repopulating DiI+slow cycling cells
Slow cycling cells would be predicted to show increased resistance to standard chemotherapy. However, resistance to chemotherapy may also be due to other mechanisms, including highly active drug pumps [15]. To test possible therapeutic relevance we asked whether chemotherapy applied to pancreas adenocarcinoma cells would selectively or preferably target the DiI+/SCC or DiI−/FCC subpopulations. After labelling, BxPC-3 and Panc03.27 cells were exposed to various doses of the base analogue 5-FU (5- Fluorouracil). 5-FU or gemcitabine is commonly used as therapy in pancreas adenocarcinoma [25]. As seen in Fig. 2, after a 3 day regime of 100 μg/ml 5-FU, only a few BxPC-3 (3.5% ± 1.0; Fig. 2b) or Panc03.27 (3.3% ± 1.1; Fig. 2c) cells survived.
Next, to determine whether the DiI+/SCCs that survived 5-FU treatment could recreate the pre-selection tumor cell culture, selective pressure was released by reverting cells into standard growth medium. As seen in Fig. 2, single cells started to divide by 14 days (Panc03.27; Fig. 2c) or 20 days (BxPC-3; Fig. 2b), and after 22 or 36 days, respectively, heterogeneous colonies of slow and fast dividing cells had emerged from each of the cells. Similarly, when sorted according to cell cycle speed, and re-plated, DiI+/SCC were also able to regenerate a pre-sorting population (Fig. 2d; data not shown). These colonies were heterogeneous in regards to cell cycle, as displayed by the varying intensities of DiI, indicating that slow cycling cells can be founder cells for fast cycling cells. Accordingly, colonies emerging from the FACS sorted (DiI+/SCC) or 5-FU treatment survivors showed the same pattern of fast and slow cycling cells as the cell cultures before, strongly indicating that the chemotherapy resistant, slow cycling cells have tumor initiating and repopulating potential. These findings are consistent with previously published data in which the selection of a chemoresistant subpopulation of cells following gemcitabine treatment had increased tumor initiating potential [11].
Interestingly, not only were DiI+/SCC able to give rise to DiI−/FCC, but also the reverse mechanism was present. Thus, when DiI−/FCCs were replated after FACS sorting, exposed to DiI, and followed using the same protocol, a distinct population of DiI+/SCC reemerged (data not shown). In conclusion, the pancreatic adenocarcinoma cell lines Panc03.27 and BxPC-3 contain distinct fast and slow cycling cell populations that can revert into each other.
Slow cycling cells have increased tumorigenic and invasive potential
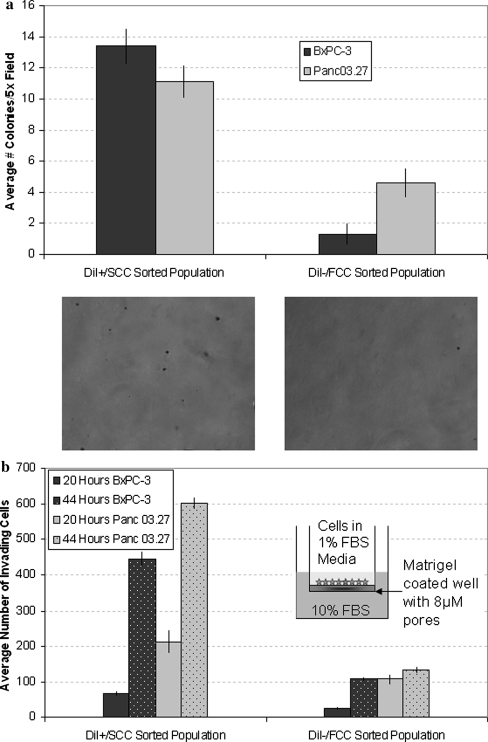
Isolated DiI+/SCC could re-establish colonies of tumor tissue that were visually indistinguishable from the pre-selection cell population. To test whether such a repopulating capability may go along with increased tumorigenicity, the capability to form colonies in soft agar between DiI+/SCC and DiI−/FCC was compared. The sorted cell populations were seeded in soft agar and followed over 14 (Panc03.27) or 21 (BxPC-3) days growth. By 8 days it was already visible that DiI+/SCC colonies were more numerous than the DiI−/FCC counterparts (data not shown). At the conclusion of the assay, the rate of colony formation had increased by ten fold in the BxPC-3 DiI+/SCC population (DiI+/SCC 13.4 ± 1.1; DiI−/FCC 1.3 ± 1.0) and 2.5-fold in the Panc03.27 DiI+/SCC population (DiI+/SCC 11.4 ± 1.0; DiI−/FCC 4.6 ± 1.0), as compared to their corresponding DiI−/FCC population (Fig. 3a).
Fig. 3.
Invasion and Colony Forming Assays a Soft agar assay for colony formation. Graph represents results after 14 (Panc03.27) or 21 (BxPC-3) days of culture with DiI+/SCC or DiI−/FCC sorted populations. An increase in colony formation was seen with the DiI+/SCC population. b Invasion assay run with freshly sorted DiI+/SCC vs. DiI−/FCC populations. Results show increased invasive potential of the DiI+/SCC population over the DiI−/FCC population after 20 and 44 hours
Next, the invasive potential of DiI−/FCC and DiI+/SCC populations was compared. Using BD matrigel coated invasion plates, freshly sorted DiI+/SCC and DiI−/FCC were allowed to grow and invade for 20 or 44 hours. Already after 20 h, the BxPC-3 DiI+/SCC population had a 2.5-fold increase in invading cells versus the DiI−/FCC population (DiI+/SCC 66 ± 14.1; DiI−/FCC 26 ± 4.2), while the Panc03.27 DiI+/SCC population had a two fold increase (DiI+/SCC 213 ± 22.6; DiI−/FCC 107 ± 22.3) (Fig. 3b). At 40 h, the invasive potential of BxPC-3 DiI+/SCC increased to four fold over the DiI−/FCC population (DiI+/SCC 445 ± 52.4; DiI−/FCC 109 ± 13.4) and 4.5-fold in Panc03.27 DiI+/SCC (DiI+/SCC 602.5 ± 13.4; DiI−/FCC 131.5 ± 17.7). In summary, these results suggest that the DiI+/SCC population consists of highly tumorigenic cells with increased invasive and metastatic potential compared to the DiI−/FCC population.
Slow cycling cells have increased tumor formation ability
Several reports show a strongly increased potential of selected populations in pancreas adenocarcinoma and other solid tumors to initiate tumor growth in SCID mice [3, 26–28]. To examine tumorigenicity freshly sorted DiI+/SCC and DiI−/FCC populations from BxPC-3 were injected to CB17/SCID mice. BxPC-3 cells are described in the literature to require 107 cells for tumor formation in nude mice [29]. In our hands, 5 × 106 BxPC-3 cells were capable of forming tumors in CB17/SCID mice, and in a preliminary pilot study to compare the two subpopulations in vivo, similar tumorigenicity was observed when tumors were initiated by injecting cells to as little as 2 × 106. It was only at injections of cell numbers below this level that suggested an increased tumorigenicity of the DiI+/SCC population (data not shown). Therefore, we further examined the tumor initiating potential of DiI+/SCC vs. DiI−/FCC at 1 × 106 and below in this study. As seen on Table 1, injection of DiI+/SCCs resulted in an enhanced tumorigenic potential when compared to the DiI−/FCC population. After injection of 104 cells, 75% (3/4) of mice receiving DiI+/SCC had established tumors, while no tumors formed in mice receiving DiI−/FCCs. Similarly, tumors formed after injection of as few as 5 × 103 DiI+/SCCs (1/4), while no tumor established from DiI−/FCCs until at least 5 × 104 cells were injected. This represents a ten fold increase in tumorigenic potential and supports our previous in vitro results.
Table 1.
Tumor formation ability of freshly sorted DiI+/SCC versus DiI−/FCC subpopulations injected subcutaneously into SCID mice
| Cell population/Cell number | 1 × 106 | 5 × 105 | 1 × 105 | 5 × 104 | 1 × 104 | 5 × 103 |
|---|---|---|---|---|---|---|
| DiI−/FCC | 3/3 | 3/3 | 2/4 | 2/4 | 0/4 | 0/4 |
| DiI+/SCC | 3/3 | 3/3 | 4/4 | 3/4 | 3/4 | 1/4 |
Slow cycling cells display increased expression of cancer stem cell markers
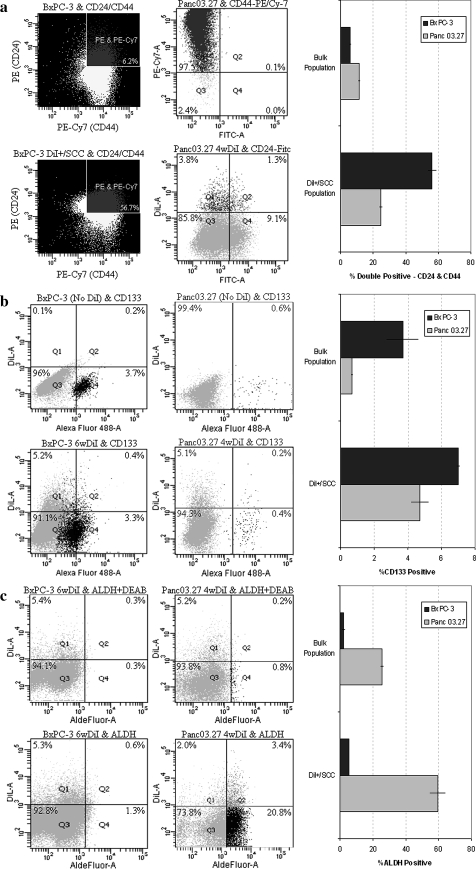
Previously published data report the existence of a phenotypically distinct and relatively rare population (0.5–2%) of CD24+/CD44+ CSC in primary pancreatic adenocarcinoma that have an enhanced ability to form tumors when compared with the bulk population of tumor cells or CD24−/CD44− populations [3]. As seen in Fig. 4a, 6.2% (mean = 6.0% ± 0.9) of the total BxPC-3 cell population and 10.4% (mean = 11.3% ± 2.4) of the total Panc03.27 cell population was double positive for CD24+/CD44+. Interestingly, when the sorted DiI+/SCC fraction was analyzed, the number of CD24+/CD44+ cells increased almost ten fold in BxPC-3 to 56.7% (mean = 56.2% ± 8.9) and greater than two fold in Panc03.27 to 25.5% (mean = 24.5 ± 1.4), demonstrating an overlap between the already described CD24+/CD44+ population, and the here presented DiI+/SCC population. Interestingly, although all cells are CD44+, only a fraction of the DiI+/SCC are CD24+, while others remain CD24−.
Fig. 4.
Expression of CSC markers a FACS analysis of percent CD24+/CD44+ cells in the bulk verses DiI+/SCC populations. b FACS analysis of percent CD133+ cells in the bulk and DiI+/SCC populations. c FACS analysis of percent ALDH+ cells in the bulk and DiI+/SCC populations
Next, we examined the expression of CD133, another CSC marker that has been reported in pancreatic carcinomas, to determine if any correlation with DiI+/SCC exists [5]. As seen in Fig. 4b, 3.9% (mean = 3.7% ± 1.2) of the total BxPC-3 cell population and 0.6% (mean = 0.7% ± 0.1) of the total Panc03.27 cell population was positive for CD133. When examining the DiI+/SCC fraction in detail, 7.1% (mean = 7.0% ± 0.2) of BxPC-3 and 3.8% (mean = 4.7% ± 1.3) of Panc03.27 DiI+/SCCs expressed CD133. This indicates an overlap between the known CD133+ CSC population and the here presented DiI+/SCC population again.
Finally, we looked at aldehyde dehydrogenase (ALDH) activity, a marker which has been used to identify both normal and malignant stem cells [6, 7]. Using the aldefluor assay, we were able to investigate if there was a correlation between ALDH positive cells and DiI+/SCC. As shown in Fig. 4c, just 1.3% (mean = 2.1% ± 1.5) of total BxPC-3 cells were ALDH+, while 24.2% (mean = 25.5% ± 1.8) of all Panc03.27 cells were ALDH+. This correlates to 5.4% (mean = 5.3% ± 0.1) of the BxPC-3 DiI+/SCC population and 63.0% (mean = 59.6% ± 4.8) of the Panc03.27 DiI+/SCC being ALDH+. These results therefore suggest that although overlap between the previously identified CSC markers exists, slowly cycling DiI+ cells are a distinct and unique population of cancer stem-like cells.
DiI+/SCCs express markers associated with EMT, stem cell regulation and metastatic potential
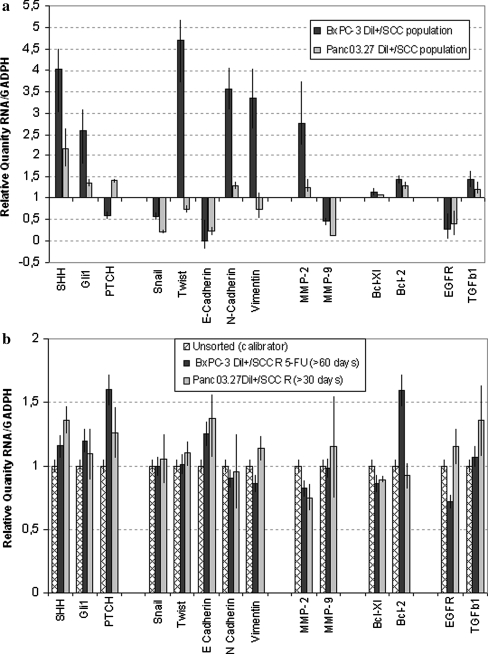
To further determine molecular characteristics of the DiI+/SCC and DiI−/FCC subpopulations, quantitative RT-PCR profiling was performed. Since factors specifically associated with the conversion of early stage tumors into invasive malignancies through an EMT were particularly of interest, the gene expression profile of Snail, Twist, E-Cadherin, N-Cadherin, and Vimentin was analyzed. As seen in Fig. 5a, DiI+/SCC showed decreases in snail and E-cadherin, while the expression level of N-cadherin was increased when compared to the DiI−/FCC population. Additionally, twist and vimentin were strongly increased in BxPC-3 DiI+/SCC. These alterations are characteristic for cells undergoing EMT [30, 31], and support the observed morphological change along with the increased invasiveness and metastatic potential of the DiI+/SCC population.
Fig. 5.
Gene Expression Analysis a Change in gene expression, obtained from RT-PCR analysis, in DiI+/SCC as compared to DiI−/FCC populations. b RT-PCR profiling displaying return of gene expression levels to that of the original population after 30 (Panc03.27) or 60 (BxPC-3) days of repopulating growth by DiI+/SCC
Next indicative markers for increased metastatic potential (MMP-2, MMP-9), and apoptosis protection (Bcl-xl, BCL-2) were analyzed. Both features are prominent in aggressive tumor progression and therapeutic resistance. Thus, upregulation of MMP-2, Bcl-xl and Bcl-2 imply metastatic potential in these cells. Basement membrane degradation through MMPs and subsequent protection from apoptosis (Bcl-xl, Bcl-2) after detachment from the basement membrane occur throughout stages of metastasis [32]. Real time PCR on the DiI+/SCC population revealed a clear increase of MMP-2, but a moderate decrease of MMP-9. Furthermore, expression levels of Bcl-xl and Bcl-2 were moderately increased (Fig. 5a).
Stem cell pathways have shown to play a crucial role in pancreas adenocarcinoma, and components of the Hedgehog, Wnt/Notch, KRAS and TGFb pathways are found to be de-regulated in 100% of analyzed pancreas adenocarcinoma samples [9]. We therefore asked whether members of these central pathways are differentially expressed in fast cycling, and slow cycling pancreas adenocarcinoma cells. Indeed, when compared to the DiI−/FCC population, Shh was increased nearly four fold in BxPC-3 and two fold in Panc03.27 and the zinc finger transcription factor Gli1, was up-regulated 2.5-fold in BxPC-3 and 1.5-fold in the Panc03.27 DiI+/SCC populations. In contrast, PTCH1, the receptor for Shh and a negative regulator of the pathway was found to be slightly down regulated in BxPC-3 DiI+/SCC and slightly increased in the Panc03.27 DiI+/SCC population. Finally, TGFβ, which regulates a diverse array of cellular functions is a key participant in many tumor microenvironments [33], and has been found to induce the expression of the zinc finger transcription factors Gli1 and Gli2 in various human cell types [34], was found to be upregulated in DiI+/SCCs. TGFβ receptors are known to be expressed by epithelial cells undergoing EMT [34], and TGFβ alone is known to induce EMT in vitro [12]. Moreover, EGFR transcripts were found down regulated in DiI+/SCCs demonstrating differential activity of core stem cell pathways between DiI+/SCCs and DiI−/FCCs. EGFR receptor down-regulation was further confirmed through immunohistochemistry (data not shown).
Finally we tested whether colonies that were derived from DiI+/SCCs were similar to pre-selection colonies. We previously observed that DiI+/SCCs, either after 5-FU chemotherapy or after FACS sorting, were able to re-establish colonies of morphological diverse tumor tissue, which were indistinguishable from the pre-selection material after 30 (Panc03.27) or 60 (BxPC-3) days of growth. As shown in Fig. 5b, RT-PCR analysis indicates that the re-established colony of pancreas adenocarcinoma cells is nearly identical to the pre-selection parental population.
Taken together, these results from the real time PCR analysis confirmed the stem cell character of the DiI+/SCC population and its increased invasive potential through undergoing EMT.
Discussion
In this report we identified and characterized an intrinsic system of slow and fast cycling cells in pancreas adenocarcinoma cell lines. Notably, the variations in cycling speed were associated with a whole panel of tumor relevant alterations. These include the increased presence of CD24/CD44 and CD133 surface markers and ALDH expression in DiI+/SCCs, the morphological and genetic finger print of EMT including increased invasiveness and tumor initiating potential, a shift in the activity of stem cell pathways, and a shift in sensitivity to chemotherapy. While several of these features have been reported to be present in both primary and established pancreas adenocarcinoma cell lines, as well as in other solid tumors, this report shows all these characteristics to coincide in an easily identifiable and traceable subpopulation of cells. The characteristics of DiI+/SCCs would qualify them to be defined as tumor stem cells. However, such a term may be misleading as it implies that tumor stem cells are founder cells with a broader self renewal and lineage potential compared to the bulk population of tumor cells. While we confirm the increased potential of DiI+/SCCs with regard to central parameters including tumor initiation, chemotherapy resistance and invasiveness, we also observed that not only can DiI+/SCCs give rise to DiI−/FCCs, but vice versa, the population of DiI−/FCCs can segregate DiI+/SCCs. Thus, in contrast to the classical tumor stem cell concept that ascribes CSCs as lying at the apex of a hierarchy [35] the here described cells support a model of a bidirectional potential in both subpopulation and bulk cells. If generally applicable, this would expand the current unidirectional concept of CSC to a more flexible scheme with context dependent state transitions. Such a scheme would accommodate both MET and EMT, which is observed in vivo during metastasis. However, it may well be that both models are valid, depending on cancer type and progression. Although we report heterogeneity in cell cycle speed in pancreas adenocarcinoma lines, it is likely that the phenomenon is of more general validity. Likewise, while we report an increased tumorigenic potential in the DiI+/SCC population, we also note that the DiI+/SCC can transition to DiI−/FCC and vice versa. Therefore, hypothetically, DiI−/FCC may be capable of generating tumors similar to the DiI+/SCC population after a long lag phase. While we did not observe this, one can not dismiss the possibility of this occurrence after a long term investigation.
Recent reports describe a CD24+/CD44+ population of cells in both primary pancreas adenocarcinoma cell lines, and in the established cell line Panc1 [4]. These cells have also shown to be of higher tumor initiating potential, and are positive for the stem cell pathway Hh [3]. Independent research has also established that pancreas adenocarcinoma can undergo EMT, and that this process may be regulated positively by Hh and/or TGFβ signaling. Finally, chemotherapy resistance has been associated with CD24+/CD44+ cells in pancreas adenocarcinoma. Similarly, as chemotherapy resistance and metastasis are general issues in solid tumors, heterogeneity in key features of cell cycle control and EMT have been reported in a multitude of tumor models [12, 13, 31, 36–38].
There is further evidence for heterogeneity within highly tumorigenic subpopulations in solid tumors. Previous reports have found variability in cell surface phenotypes used to classify CSC. Specifically in pancreatic cancer, although overlap was found between CD133+ and CD24+/CD44+ cells, it was highly variable and only 10–40% of CD24+/CD44+ cells were found to also express CD133 [5]. In other reports, high aldehyde dehydrogenase 1 (ALDH1A1) activity was shown to identify the tumorigenic fraction [6, 7]. This ALDH1A1+ population though, was found to have a surprisingly small overlap with the previously described breast CSC (CD44+/CD24−/low) phenotype [26]. In this study, an overlap of SCC and CD24+/CD44+, CD133+ and ALDH was found; the nature of the CD24−/CD44+, CD133− and ALDH− SCC population is yet still to be determined.
Using DiI to identify slow cycling cancer stem-like cell populations is a simple procedure that yields an easily identifiable, traceable and isolatable population. Yet, as with any label retention technique, it has some weaknesses. The downside to using this method is simply that one must wait for the bulk of the population to cycle the dye out before identification of the label retaining cells can occur. This can mean weeks of cell growth and expansion while keeping large numbers of cells in culture in order to obtain a sufficient number of DiI+/SCC after sorting the populations. Moreover, although label retention could be used to identify SCC in primary cell cultures, one final drawback is that it would require these cells to be passaged a number of times; potentially altering the characteristics of the original primary tissue. However, when comparing this technique of cancer stem-like cell purification to classical ones (such as direct antibody targeting on primary tissue) this method yields the possibility of obtaining much more material, thereby allowing many more functional assays to be carried out. In conclusion, the DiI label retention technique employed to isolate slowly cycling cancer cells could aid the understanding of tumor heterogeneity and the development of therapeutic tools. This study represents a novel step in better defining the biological activities of tumorigenic subpopulations within the vast heterogeneous tumor microenvironment.
Of note, during the review process of our manuscript, the connection between SCC and CSC has also been explored and strongly supported by the Bieberich laboratory [39], in which SCC in the breast cancer cell line 4T1 were found to have CSC-like properties in vitro and in vivo. These data further support our findings and suggest that they are not limited to pancreatic adenocarcinoma cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(a) CD24/CD44 expression verses DiI intensity. BxPC-3 DiI+/SCC population was further grouped by DiI intensity (P5: DiI+/lowSCC; P6: DiI+/midSCC; P7: DiI+/highSCC) then analyzed against CD24 and CD44 expression. Additionally, the DiI+/SCC population was segregated to CD24−/CD44+ (P8) and CD24+/CD44+ (P10) subpopulations and the location of each group was visualized against DiI intensity. Results show no correlation of DiI intensity to CD24 expression in DiI+/SCC, but rather an overlap of the CD24−/CD44+ and CD24+/CD44+ subpopulations. (TIFF 314 kb)
Acknowledgments
We thank James Booth and Nomdo Westerdaal for technical assistance in FACS analysis. This work was supported by the Norwegian research council.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- 5-FU
5-Fluorouracil
- EMT
Epithelial to mesenchymal transition
- MET
Mesenchymal to epithelial transition
- CSC
Cancer stem cell
- Shh
Sonic hedgehog
- Hh
Hedgehog
- Gli1
Glioma-associated oncogene homolog 1
- PTCH
Patched protein homolog 1
- TGFβ
Transforming growth factor beta
- EGFR
Epidermal growth factor receptor
- MMP-2
Matrix metalloproteinase-2
- MMP-9
Matrix metalloproteinase-9
- ALDH
Aldehyde dehydrogenase
- DiI+/SCC
DiI positive (label retaining), slow cycling cell
- DiI−/FCC
DiI negative (label lost), fast cycling cell
References
- 1.Hoyert DL, Heron MP, Murphy SL, et al. Deaths: final data for 2003. Nat Vital Stat Rep. 2006;54:1–120. [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 4.Huang P, Wang CY, Gou SM, et al. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroenterol. 2008;14:3903–3907. doi: 10.3748/wjg.14.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimeno A, Feldmann G, Suarez-Gauthier A, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement V, Sanchez P, de TN, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: sonic hedgehog, notch, and bone morphogenic proteins. J Cell Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- 11.Shah AN, Summy JM, Zhang J, et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 12.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson EW, Williams ED. EMT and MET in carcinoma–clinical observations, regulatory pathways and new models. Clin Exp Metastasis. 2008;25:591–592. doi: 10.1007/s10585-008-9189-8. [DOI] [PubMed] [Google Scholar]
- 14.Costello RT, Mallet F, Gaugler B, et al. Human acute myeloid leukemia CD34+/ Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- 15.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 16.Guzman ML, Swiderski CF, Howard DS, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Invitrogen - Molecular Probes.Vybrant Cell-Labeling Solutions. 2001. http://probes.invitrogen.com/media/pis/mp22885.pdf
- 18.Rodent Anesthesia using Open-Drop Exposure to Isoflurane. 2004. http://iacuc.yale.edu/procedures/opendrop.html
- 19.Verfaillie CM, Miller JS. A novel single-cell proliferation assay shows that long-term culture-initiating cell (LTC-IC) maintenance over time results from the extensive proliferation of a small fraction of LTC-IC. Blood. 1995;86:2137–2145. [PubMed] [Google Scholar]
- 20.Liu CM, Yu CH, Chang CH, et al. Hyaluronan substratum holds mesenchymal stem cells in slow-cycling mode by prolonging G(1) phase. Cell Tissue Res. 2008;334:435–443. doi: 10.1007/s00441-008-0699-0. [DOI] [PubMed] [Google Scholar]
- 21.Fairley EA, Riddell A, Ellis JA, et al. The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype. J Cell Sci. 2002;115:341–354. doi: 10.1242/jcs.115.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Batard P, Monier MN, Fortunel N, et al. TGF-(beta)1 maintains hematopoietic immaturity by a reversible negative control of cell cycle and induces CD34 antigen up-modulation. J Cell Sci. 2000;113(Pt 3):383–390. doi: 10.1242/jcs.113.3.383. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Law P, Francis K, et al. Symmetry of initial cell divisions among primitive hematopoietic progenitors is independent of ontogenic age and regulatory molecules. Blood. 1999;94:2595–2604. [PubMed] [Google Scholar]
- 24.Li N, Yang H, Lu L, et al. Comparison of the labeling efficiency of BrdU, DiI and FISH labeling techniques in bone marrow stromal cells. Brain Res. 2008;1215:11–19. doi: 10.1016/j.brainres.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 25.Chemotherapy for Pancreatic Cancer. (2003). Alimentary Pharmacology & Therapeutics,18(11), 1049–1069. doi:10.1111/j.1365-2036.2003.01781.x [DOI] [PubMed]
- 26.Al-Hajj M, Wicha MS, ito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 28.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 29.Promochem, L.G.C. Cell Biology Collection; BXPC3. 2006. http://www.lgcpromochem-atcc.com
- 30.Cates JM, Byrd RH, Fohn LE, et al. Epithelial-mesenchymal transition markers in pancreatic ductal adenocarcinoma. Pancreas. 2008;38:1–6. doi: 10.1097/MPA.0b013e3181878b7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 32.Frankel A, Rosen K, Filmus J, et al. Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L) Cancer Res. 2001;61:4837–4841. [PubMed] [Google Scholar]
- 33.Dembinski J, Spaeth E, Sasser K, et al. Mesenchymal stem cell (MSC) edification; tumor microenvironment drives MSC differentation into tumor-associated fibroblasts that contribute to fibrovascular network expansion. PLoS ONE. 2009;4(4):e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennler S, Andre J, Alexaki I, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 36.Zemskova M, Sahakian E, Bashkirova S, et al. The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. J Biol Chem. 2008;283:20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton JP, Mongeau ME, Klimstra DS, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 39.Krishnamurthy K, Wang G, Rokhfeld D, et al. Deoxycholate promotes survival of breast cancer cells by reducing the level of pro-apoptotic ceramide. Breast Cancer Res. 2008;10:R106. doi: 10.1186/bcr2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) CD24/CD44 expression verses DiI intensity. BxPC-3 DiI+/SCC population was further grouped by DiI intensity (P5: DiI+/lowSCC; P6: DiI+/midSCC; P7: DiI+/highSCC) then analyzed against CD24 and CD44 expression. Additionally, the DiI+/SCC population was segregated to CD24−/CD44+ (P8) and CD24+/CD44+ (P10) subpopulations and the location of each group was visualized against DiI intensity. Results show no correlation of DiI intensity to CD24 expression in DiI+/SCC, but rather an overlap of the CD24−/CD44+ and CD24+/CD44+ subpopulations. (TIFF 314 kb)