Abstract
A systematic histologic analysis of 62 gastric fundic gland polyps (FGP) was carried out. All FGP (100%) showed foveolar cells with hypertrophic cytoplasm. In 95% of the FGP, parietal cells ballooned into the lumen and in 93%, exfoliated anucleated structures with eosinophilic granules were found. Plugs of anucleated structures with eosinophilic granules, most likely derived from exfoliated parietal cells, were found to clog the outlets of the glands in 86% of the FGP. None of the 30 control gastric biopsies without FGP had similar cellular aberrations. FGP seems to evolve by cellular aberrations affecting parietal cells. This is not surprising considering that genetic mutations are recorded in FGP with a common APC/b-catenin pathway in both FAP and sporadic cases. The genetic mutations in FGP might alter the biological behavior of the parietal cells, leading to increased exfoliation with clogging of the outlets of the glands. Thus, the blocking of the glandular outflow by plugs of anucleated structures with eosinophilic granules is the most likely cause for the cystic accumulation of “normal” glandular secretions.
Keywords: Fundic gland polyps, eosinophilic plugs, cystic formations
Introduction
Fundic gland polyps (FGP) are common benign epithelial expansions of the fundic mucosa in patients having either hereditary diseases, such as familial adenomatous polyposis (FAP), Gartner's syndrome, attenuated familial adenomatous polyposis syndromes, Peutz-Jeghers syndrome, Cowden's syndrome, juvenile polyposis, or non-hereditary disorders (sporadic or somatic) such as Zollinger-Ellison syndrome, atrophic gastritis and medication with proton-pump inhibitors [1–23].
First described by Japanese researchers [24, 25], FGP are the most common gastric lesion in patients with FAP, with a reported prevalence between 27% and 73% [9, 10]. In the non-FAP population the prevalence of FGP is estimated to be between 0.8% and 1.4% [13, 21].
Lately, while reading gastric biopsies [26] having FGP, we found additional histological alterations to those previously reported in the literature.
The purpose of the present work was to describe and illustrate these not previously reported histological features in FGP and to assess their frequency in a cohort of patients with FAP and in patients without that genetic association.
Materials and methods
A total of 62 FGP removed at gastroscopy were analyzed: 33 were from FAP patients (hereditary FGP) and the remaining 29 were from patients without that genetic association (sporadic or somatic FGP). None of the patients received PPI medication. Fifteen sets of gastric biopsies from patients with Helicobacter pylori induced gastritis and 15 from patients with dyspeptic symptoms having normal gastric biopsies, were also reviewed.
Gastric biopsies were fixed in 4% neutral formalin; sections were stained with hematoxylin-eosin (H&E). Twenty of the 62 FGP sections were also stained with PAS.
All sections were scrutinized at high power microscopy (x40).
As FGPs were regarded focal areas of the fundic mucosa with cystic dilatations lined with parietal, chief cells and occasional mucous foveolar cells.
A systematic histologic analysis of the FGP (Figure 1) was carried out starting from the top of the polyps as follows: the foveolar epithelium, the parietal cells subjacent to the foveolar epithelium, the outlets of glandular cysts and the cellular components wall-papering the fundic cysts.
Figure 1.
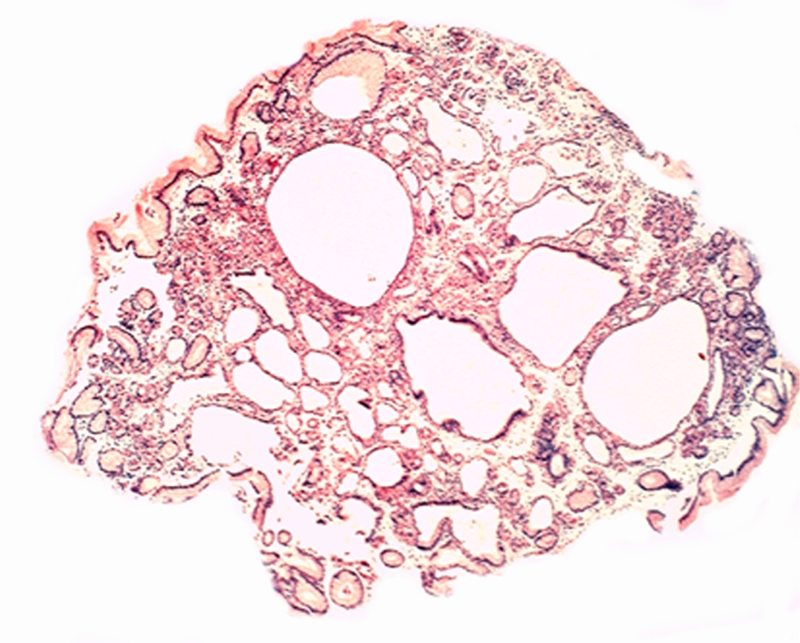
Fundic gland polyp (H&E, x2).
Results
Foveolar epithelium: The cytoplasm of the foveolar cells were abnormally tall in all 100% (62/62) of the FGP. The hypertrophic cytoplasm contained excessive mucin, easily demonstrated with PAS stain (Figure 2).
Figure 2.
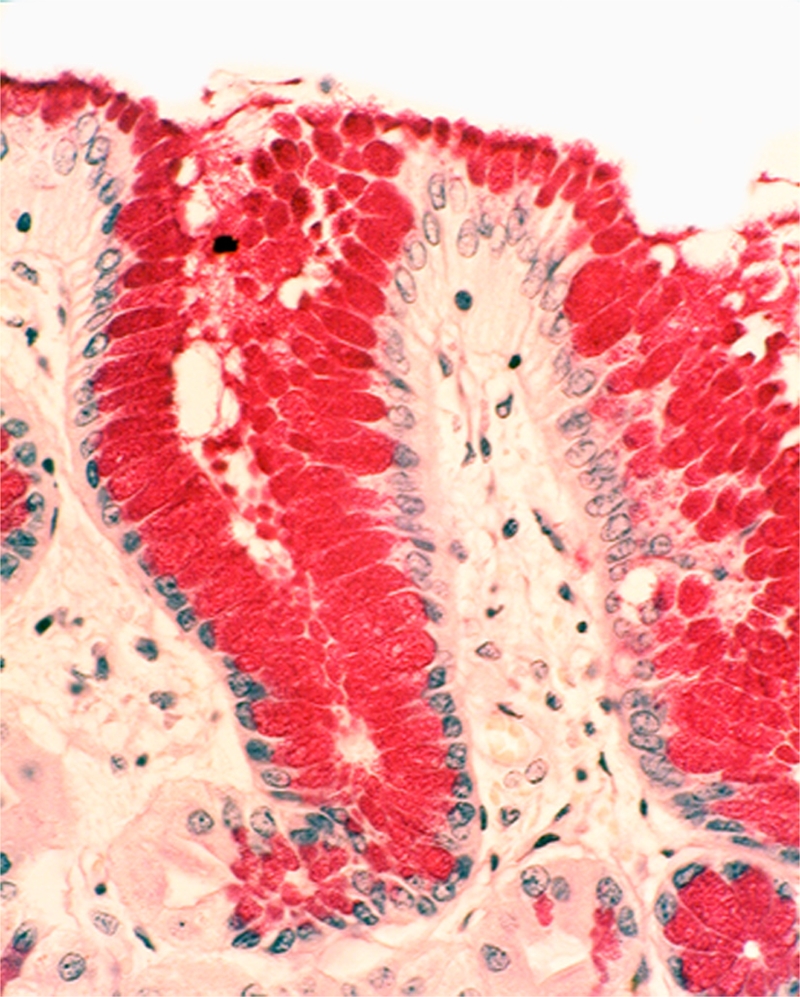
Foveolar cells with hypertrophic cytoplasm containing excessive mucin (PAS x20).
Parietal cells subjacent to the foveolar epithelium: Near the foveolar epithelium the parietal cells were found to protrude into the lumen in 95.2% (59/62) (Figure 3). In addition, exfoliated anucleated cells with eosinophoilic cytoplasmic granules, resembling the cytoplasm of parietal cells were found near the foveolar epithelium in 91.9% (57/62) of the FGP (Figure 4). No apoptotic granules were observed in these rounded eosinophilic structures.
Figure 3.
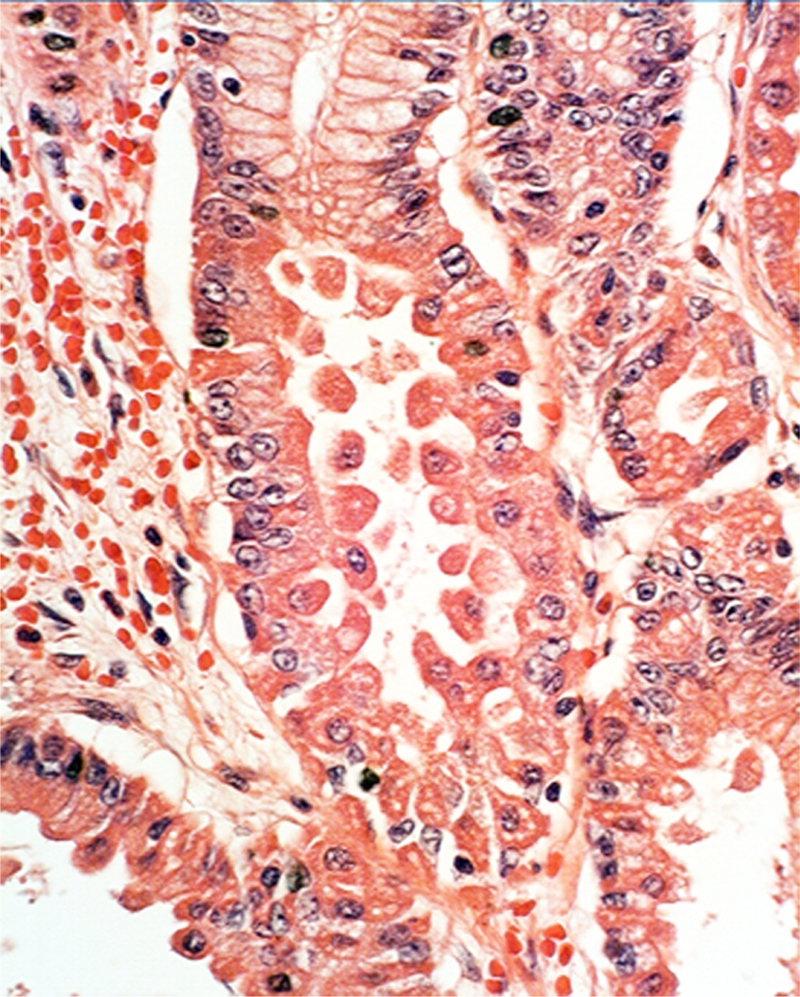
Ballooning parietal cells near the foveolar epithelium (H&E x
Figure 4.
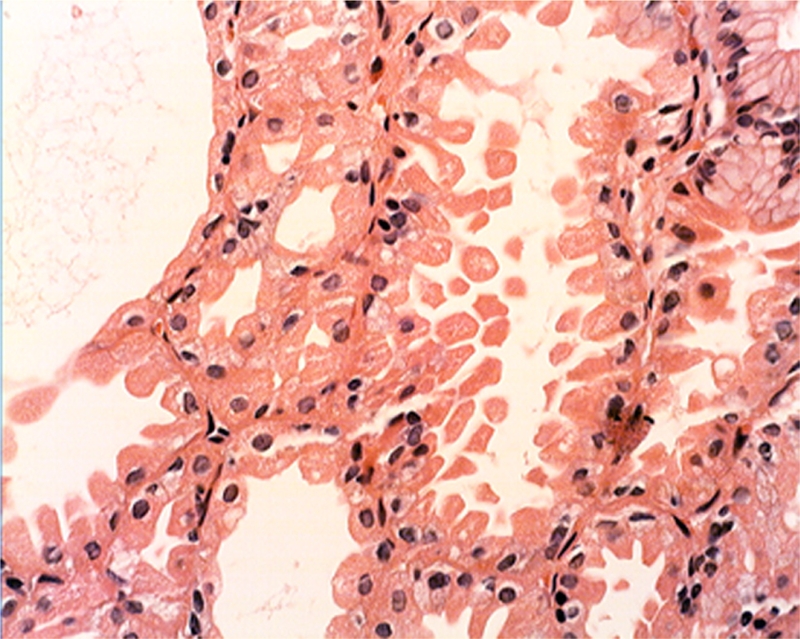
Exfoliating anucleated structures with eosinophoilic cytoplasmic granules, resembling the cytoplasm of parietal cells (H&E x20).
Plugs in the outlets of the glandular cysts: Masses of anucleated, eosinophilic, granulated material (most likely derived from exfoliated parietal cells) or of more homogeneous stuff, were seen in the plugs that clogged the outlets of the fundic glands in 85.5% (53/62) of the FGP (Figures 5–7).
Figure 5.
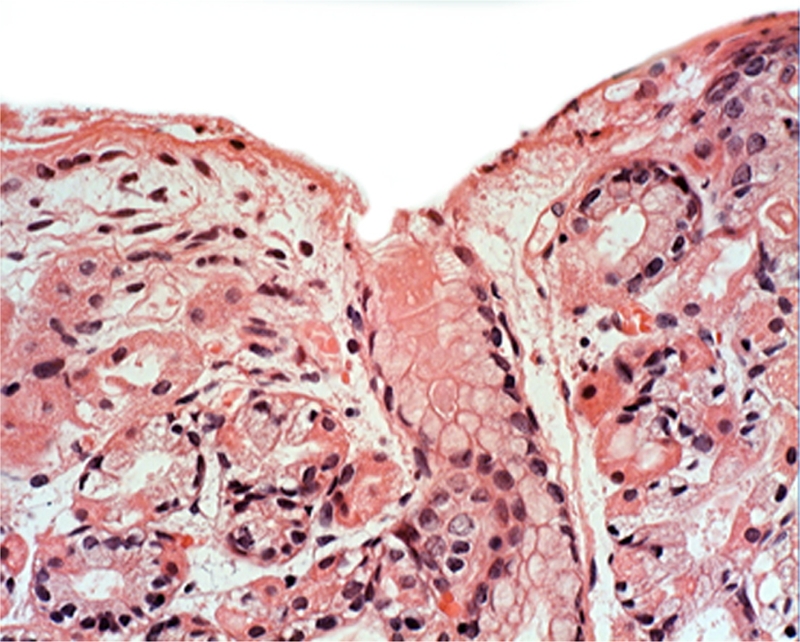
Plug clogging the outlet of a gatric gland (at arrow) (H&E x20).
Figure 7.
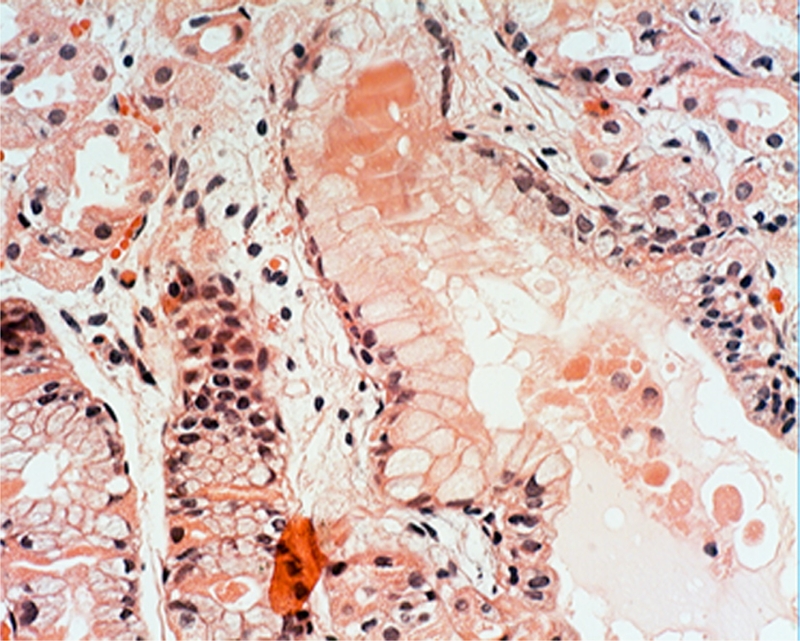
Plug clogging the outlet of another gatric gland. The plug is built of anucleated, eosinophilic, granulated material, most likely derived from exfoliated parietal cells (H&E x20).
Figure 6.

Another view showing a plug clogging the outlet of a gatric gland. (H&E x20).
Plugs outside the outlets of the glandular cysts. Plugs outside the luminal aspect of the outlet of the glands (Figure 8) were found in 5 of the remaining 9 FGP (3 in FAP and 2 in sporadic cases. In addition, isolated eosinophilic clumps replacing the normal glandular epithelium were recorded in the remaining 4 FGP (3 in FAP case and 1 in a sporadic case.
Figure 8.
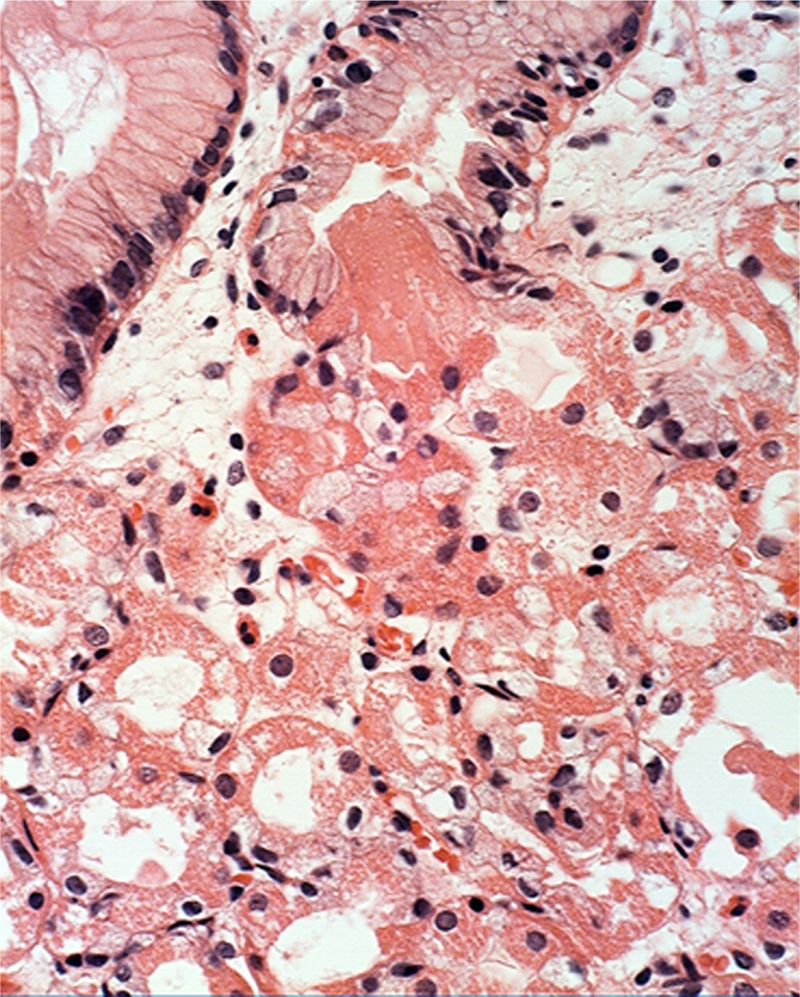
Plug, apparently outside the luminal aspect of the outlet of the glands. Note the anucleated, eosinophilic, granulated material in the plug, similar to the granulated cytoplasm in surrounding parietal cells (H&E x20).
Cellular components wall-papering fundic cysts: The parietal cells as well as other cells coating the cysts appeared flattened in the dilated fundic glands of all (100%) 62 FGP (Figure 1). The retained secretion seemed to have compressed the epithelium lining of the cysts.
No essential differences were found between the occurrence of the above-mentioned histological parameters in FAP cases and in sporadic cases.
None of the 30 control gastric biopsies without FGP, had similar cellular aberrations.
Discussion
The results of this investigation showed that in addition to the classically reported histological parameters characterizing FGP [1–25], several additional histological features were found such as the ballooning of the parietal cells, their abnormal exfoliation, the presence of anucleated, round structures with eosinophilic granules and the formation of plugs clogging the outlets of the glands.
The cystic formations in FGP would, therefore, evolve by the retention of the “normal” glandular secretions in obstructed glands. This obstruction appears to be the result of cellular alterations affecting parietal cells. This conclusion is based on the observation that parietal cells ballooned and subsequently exfoliated into the lumen of the glands. Once in the lumen, the exfoliated cells joined other exfoliated anucleated round formations with eosinophilic granules. These anucleated structures resembled the cytoplasm of parietal cells as no other cells in the area, had eosinophilic granules in their cytoplasm.
None of the 30 control gastric biopsies without FGP had similar cellular aberrations.
The finding of cellular aberrations in FGP is perhaps, not surprising considering that genetic mutations are often recorded in hereditary FGP (in the adenomatous polyposis coli gene (APC) and in sporadic FGP (in the b-catenin, a downstream target regulated by the APC protein) [27–36]. A common APC/b-catenin pathway seems to be involved in FGP through the targeting of different genes, both in FAP cases and in sporadic cases.
The gene ATP4A encodes the H+/K+ ATPase which is the major membrane constituent of parietal cells. Recently it was demonstrated that mice lacking H+/K+ ATPase (that is Atp4a(-/-) mice) developed countless gland cysts in the fundic mucosa [37]. Interestingly, the parietal cells in the knock-out Atp4a(-/-) mice are obviously affected, as these animals develop severe achlorhydria [37].
The claim that an obstructive mechanism is the cause for FGP is substantiated by the fact that a similar cell-obstructive mechanism was recorded in the glandular cysts often present in gastric adenomas [38]. The stratified dysplastic foveolar epithelium found at the luminal aspect of the gastric adenomas obstructed the glandular outlets of the normal gastric glands. Serial sections in gastric adenomas showed an inter-departmental communication between the labyrinths of cystically dilated glands [39]. The dysplastic epithelium initially detected at the luminal aspect of the adenomatous tissue, eventually replaced the entire epithelial layer of the cysts, in a downward growth fashion [38, 39]. More recently, we also found that the cause for the formation of retention cysts in colorectal adenomas was the obstruction of glandular outlets by dysplastic cells [40].
In the light of the present findings, several questions arise:
If FGP is conveyed by a genetic aberration of the parietal cells, why not all cells in the fundic mucosa is also affected in patients with FGP? To answer that question appears to be as difficult as to answer why only some foveolar cells develop dysplatic changes leading to a gastric adenoma, while the rest of the foveolar epithelium appears normal, despite that all foveolar cells should have been subjected to the same carcinogen.
Why the parietal cells in FGP apparently develop regressive changes and detach from the gland resulting in abnormal exfoliation?
Further studies are necessary to unveil whether the genetic mutations in FGP alter the biological behavior of the parietal cells, leading to increased parietal cells exfoliation, resulting in the clogging of the outlets of the glands.
References
- 1.Nishiura M, Hirota T, Itabashi M. A clinical and histopathological study of gastric polyps in familial polyposis coli. Am J Gastroenterol. 1984;79:98–103. [PubMed] [Google Scholar]
- 2.Lee RG, Burt RW. The histopathology of fundic gland polyps of the stomach. Am J Clin Pathol. 1986;86:498–503. doi: 10.1093/ajcp/86.4.498. [DOI] [PubMed] [Google Scholar]
- 3.Elster K. Histologic classification of gastric polyps. Curr Top Pathol. 1976;63:77–93. doi: 10.1007/978-3-642-66481-6_3. [DOI] [PubMed] [Google Scholar]
- 4.Lee RG, Burt RW. The histopathology of fundic gland polyps of the stomach. Am J Clin Pathol. 1986;86:498–503. doi: 10.1093/ajcp/86.4.498. [DOI] [PubMed] [Google Scholar]
- 5.Domizio P, Talbot IC, Spigelman AD. Upper gastrointestinal pathology in familial adenomatous polyposis: Results from a prospective study of 102 patients. J Clin Pathol. 1990;43:738–743. doi: 10.1136/jcp.43.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: A morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol. 1996;27:896–903. doi: 10.1016/s0046-8177(96)90215-4. [DOI] [PubMed] [Google Scholar]
- 7.Odze RD. Gastric fundic gland polyps: Are they preneoplastic lesions? Gastroenterology. 1998;114:422–423. doi: 10.1016/s0016-5085(98)70514-1. [DOI] [PubMed] [Google Scholar]
- 8.Declich P, Isimbaidi G, Sironi M. Sporadic fundic gland polyps: An immunohistochemical study of their antigenic profile. Pathol Res Pract. 1996;192:808–815. doi: 10.1016/S0344-0338(96)80054-9. [DOI] [PubMed] [Google Scholar]
- 9.Marcial MA, Villafana M, Hernandez-Denton J. Fundic gland polyps: Prevalence and clinicopathologic features. Am J Gastroenterol. 1993;88:1711–1713. [PubMed] [Google Scholar]
- 10.Iida M, Yao T, Watanabe H, Itoh H, Iwashita A. Fundic gland polyposis in patients without familial adenomatosis coli: its incidence and clinical features. Gastroenterology. 1984;86:1437–1442. [PubMed] [Google Scholar]
- 11.Hizawa K, Iida M, Matsumoto T, Aoyagi K, Yao T, Fujishima M. Natural history of fundic gland polyposis without familial adenomatosis coli: follow-up observations in 31 patients. Radiology. 1993;189:429–432. doi: 10.1148/radiology.189.2.8210371. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchikame N, Ishimaru Y, Ohshima S, Takahashi M. Three familial cases of fundic gland polyposis without polyposis coli. Virchows Arch A Pathol Anat Histopathol. 1993;422:337–340. doi: 10.1007/BF01608345. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita Y, Tojo M, Yano T, Kitajima N, Itoh T, Nishiyama K, et al. Incidence of fundic gland polyps in patients without familial adenomatous polyposis. Gastrointest Endosc. 1993;39:161–163. doi: 10.1016/s0016-5107(93)70057-7. [DOI] [PubMed] [Google Scholar]
- 14.Choudhry U, Boyce HW, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol. 1998;110:615–621. doi: 10.1093/ajcp/110.5.615. [DOI] [PubMed] [Google Scholar]
- 15.Graham JR. Omeprazole and gastric polyposis in humans. Gastroenterology. 1993;104:1584–1586. doi: 10.1016/0016-5085(93)90396-t. [DOI] [PubMed] [Google Scholar]
- 16.el-Zimaity HM, Jackson FW, Graham DY. Fundic gland polyps developing during omeprazole therapy. Am J Gastroenterol. 1997;92:1858–1860. [PubMed] [Google Scholar]
- 17.Declich P, Ambrosiani L, Grassini R, Tavani E, Bellone S, Bortoli A. Fundic gland polyps: a still elusive entity on the eve of the year 2000. Pol J Pathol. 2000;51:3–8. [PubMed] [Google Scholar]
- 18.Burt WR. Gastric fundic gland polyps. Gastroenterology. 2003;125:1462–1469. doi: 10.1016/j.gastro.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Sebastian S, Qasim A, McLoughlin R, O'Morain C, O'Connor H. Fundic gland polyps: not so trivial entity and worth evaluation. Gastroenterology. 2004;126:1497–8. doi: 10.1053/j.gastro.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Jung A, Vieth M, Meir O, Stolte M. Fundic gland polyps (Elsters cysts) of the gastric mucosa. A marker for colorectal epithelial neoplasia? Pathol Res Pract. 2002;198:731–734. doi: 10.1078/0344-0338-00328. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita Y, Tojo M, Yano T. Incidence of fundic gland polyps in patients without familial adenomatous polyposis. Gastrointest. Endosc. 1993;39:161–163. doi: 10.1016/s0016-5107(93)70057-7. [DOI] [PubMed] [Google Scholar]
- 22.Declich P, Bellone S, Ambrosiani L, Bortoli A, Gozzini C, Tavani E, Grassini R, Prada A. Fundic gland polyps: do they arise as a byproduct of hypergastrinemia in patients with Zollinger-Ellison syndrome? Hum Pathol. 2000;31:889–90. doi: 10.1053/hupa.2000.8908. [DOI] [PubMed] [Google Scholar]
- 23.Freeman HJ. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J Gastroenterol. 2008;14:1318–20. doi: 10.3748/wjg.14.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utsunomiya J, Maki T, Iwama T, Matsunaga Y, Ichikawa T. Gastric lesions of familial polyposis of the colon. Nippon Shokakibyo Gakkai Zasshi. 1974;71:86–95. [PubMed] [Google Scholar]
- 25.Watanabe H, Enjoji M, Yao T. Gastric lesions in familial adenomatosis coli: Their incidence and histologic analysis. Hum Pathol. 1978;9:269–283. doi: 10.1016/s0046-8177(78)80085-9. [DOI] [PubMed] [Google Scholar]
- 26.Rubio CA. My approach to reporting a gastric biopsy. J Clin Pathol. 2007;60:160–6. doi: 10.1136/jcp.2006.039008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001;158:1005–1010. doi: 10.1016/s0002-9440(10)64047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham SC, Park SJ, Mugartehui L, Hamilton SR, Wu TT. Sporadic fundic gland polyps with epithelial dysplasia (evidence for preferential targeting for mutations in the adenomatous polyposis coli gene) Am J Pathol. 2002;161:1735–1742. doi: 10.1016/S0002-9440(10)64450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham SC, Park SJ, Cruz-Correa M, Houlihan PS, Half EE, Lynch PM, Wu TT. Frequent CpG island methylation in sporadic and syndromic gastric fundic gland polyps. Am J Clin Pathol. 2004;122:740–746. doi: 10.1309/4QUN-J4F2-7QK7-RR0G. [DOI] [PubMed] [Google Scholar]
- 30.Hassan A, Yerian LM, Kuan SF, Xiao SY, Hart J, Wang HL. Immunohistochemical evaluation of adenomatous polyposis coli, beta-catenin, c-Myc, cyclin D1, p53, and retinoblastoma protein expression in syndromic and sporadic fundic gland polyps. Hum Pathol. 2004;35:328–34. doi: 10.1016/j.humpath.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Sekine S, Shibata T, Yamauchi Y, Nakanishi Y, Shimoda T, Sakamoto M, Hirohashi S. Beta-catenin mutations in sporadic fundic gland polyps. Virchows Arch. 2002;440:381–386. doi: 10.1007/s004280100527. [DOI] [PubMed] [Google Scholar]
- 32.Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol. 2000;157:747–754. doi: 10.1016/S0002-9440(10)64588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001;158:1005–1010. doi: 10.1016/s0002-9440(10)64047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 35.Torbenson M, Lee J-H, Cruz-Correa M, Ravich W, Rastgar K, Abraham S, Wu T. Sporadic fundic gland polyposis: A clinical, histological, and molecular analysis. Mod Pathol. 2002;15:718–723. doi: 10.1097/01.MP.0000018976.15044.9B. [DOI] [PubMed] [Google Scholar]
- 36.Groden J, Thilveris A, Samovitz W. Identification and characterisation of the Familial Adenomatous Polyposis Coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 37.Judd LM, Andringa A, Rubio CA, Spicer Z, Shull GE, Miller ML. Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(-/-)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol. 2005;20:1266–1278. doi: 10.1111/j.1440-1746.2005.03867.x. [DOI] [PubMed] [Google Scholar]
- 38.Rubio CA, Kato Y, Sugano H, Hirota T. The intramucosal cysts of the stomach. VII: A pathway of gastric carcinogenesis? J Surg Oncol. 1986;32:214–9. doi: 10.1002/jso.2930320407. [DOI] [PubMed] [Google Scholar]
- 39.Rubio CA, Kato Y, Sugano H. The intramucosal cysts of the stomach. IV Their quantitative and qualitative characteristics in focal (elevated) neoplastic lesions. Pathol Res Pract. 1984;179:105–109. doi: 10.1016/S0344-0338(84)80070-9. [DOI] [PubMed] [Google Scholar]
- 40.Rubio CA. Possible mechanism pertinent to mucosal invasion in sporadic colonic adenomas. Anticancer Res. 2004;24:2033–2036. [PubMed] [Google Scholar]


