Abstract
Detection of early acute myocardial ischemia/infarction prior to neutrophilic infiltration in autopsy myocardium poses a diagnostic dilemma to the surgical pathologist. Morphological changes can be subtle or not identified at all on the hematoxylin and eosin stain. To evaluate the Masson's trichrome stain and immunohistochemical stains, desmin and myoglobin, in detecting acute myocardial ischemia/infarction in autopsy myocardium. We reviewed the autopsy files of the New York Harbor Healthcare System and retrieved 25 cases of early acute myocardial infarction. Three autopsy hearts of non-cardiac related deaths were used as controls. Sections from grossly suspected early acute myocardial infaction areas were stained by a Masson's trichrome stain technique and with desmin by a standard immnunohistochemical method. The ischemic zone surrounding myocardial infarction and the acute infarct itself in 23/25(92%) were detected by desmin depletion, and in all cases with Masson's trichrome color changes. No change in staining for desmin or Masson's trichrome were seen in the three controls. Desmin and Masson's trichrome together are valuable tools when faced with the problem of postmortem detection of early myocardial infarction/ischemia.
Keywords: Desmin, Masson's trichrome stain, myocardial infarction, autopsy
Introduction
Detecting an acute myocardial infarction in autopsy myocardium prior to neutrophilic infiltration is often very subtle on hematoxylin and eosin staining.
Area of wavy fibers and loss of cross striations may be easily missed especially if they are small. Many methods including special stains [1–7], measuring Na/K ratio [8], and immnunohistochemistry [9–11] were developed to detect these early changes. However the dilemma is not yet resolved.
Masson's trichrome stain may be used to differentiate necrotic myocardium (blue cytoplasm) from viable myocardium (red cytoplasm) often with purple myocardial cytoplasm surrounding a necrotic area [12] presuming that this is the ischemic border. However, the results are dependent on which method is used [11].
Desmin is a cytoplasmic protein involved in striated muscle contraction. In human hearts removed from heart transplant recipients, in myocardial ischemia, desmin begins to disappear from the myocardial cytoplasm within 30 minutes of the ischemic insult, and is completely gone in 90-120 minutes [12].
The purpose of the present study is to investigate the utility of Masson's trichrome, and immnunohistochemical markers for the myocyte protein, desmin in detection of acute myocardial infarction and ischemia in a hospital based population.
Materials and methods
This study was approved by the Institutional Review Board of the New York Harbor Healthcare System. Twenty five consecutive autopsy cases of acute myocardial ischemia and infarction were retrieved from the files of Brooklyn VA Medical Center, Brooklyn, NY. All patients were male. The age range was 57 to 88 years. Acute myocardial ischemia and infarction was documented by serum cardiac enzyme study, ECG, if available, and/or gross evidence at the time of autopsy. Gross evidence consisted of pale or yellow areas of myocardium with or without a hype-remic border. These cases had infarcts ranging in age from less than 12 hours to several days. The time from death to autopsy ranged from 18 hours to 21 days. Autopsy tissue blocks taken of myocardium from these acutely infracted areas were fixed in 10% formalin chosen from each autopsy case. Serial 6 micron sections were stained with the following: Hematoxylin and eosin (H&E), Masson's trichrome, and desmin immunohistochemical stain.
Masson's Trichrome stain (Poly Scientific, Bay Shore, NY) were performed according to kit directions except as follows: Aniline Blue-Solution I-90 minutes incubation. The prolonged incubation is a standard procedure in our laboratory for trichrome stains of muscle.
Immunohistochemical stains of desmin (D33, DAKO Santa Barbara, CA), were performed on formalin-fixed, paraffin-embedded tissue using epitope retrieval, and a two step polymer method conjugated to peroxidase (Envision Plus-DAKO). DAB Plus (Dako) was the chromogen. An automated stainer was used (Dako Autostainer). Negative controls were performed by substituting the primary antibodiy with non-specific mouse immunoglobulin with comparable concentrations. In addition, irrelevant mouse monoclonal antibodies, caspase-3 (CPP32, DAKO) and BCL-2 (124, DAKO) were also used in substitution of the primary antibody as additional negative controls. Positive controls consisted of a multi-tissue control block containing smooth and striated muscle, and internal positive control of non-infarcted myocardium, and three autopsy cases in which there was no evidence of myocardial ischemia/ infarction either clinically or at autopsy. In each section, the decreased desmin immunoreactivity and detection of blue and purple color change in the myocardium on trichrome stain in the myocardium was graded on a scale of 0–2+ with 0 (dark brown in myocardial cells for desmin and red in the myocardial cells for trichrome stain), 1+ (light brown in myocardial cell cytoplasm or purple for trichrome stain), or 2+ (lack of staining or white in myocardial cell cytoplasm or purple for trichrome stain). This was compared to any histologic changes of early ischemia (glycogenolysis) or early infarction (wavy fibers, coagulation necrosis, or neutrophils, loss of myocardial nuclei) on the H&E stain. H & E stains, Masson's trichrome stains, and desmin slides were reviewed independently by two surgical pathologists. All disagreements were resolved by reviewing the slides together under the multiple-headed microscope.
Results
The results are summarized in Table 1. Twenty three of 25 cases (92%) had a history of ischemic heart disease. Of the remaining two, one was a sudden death and the other had an acute case of severe hemoptysis. Both cases had no prior history of ischemic heart disease. Ten of 25 cases (40%) had positive cardiac enzyme studies. One case (4%) had EKG evidence alone for MI. All cases had gross morphologic evidence of early MI. Review of the H&E sections showed no histological changes in 2 of 25 cases (8%). Twelve cases (48%) had very focal changes of early infarction (wavy fibers and contraction bands). One case (4%) had focal glycogenolysis only. Six cases (24%) had neutrophilic infiltration (two-mild, three-moderate, one-heavy infiltration).
Table 1.
Patient's clinical information, histology, trichrome and Desmin immunostaining results
| # | History | Cardiac enzymes | Death to post | Infarct location | H&E | Trichrome | Desmin |
|---|---|---|---|---|---|---|---|
| 1 | Ischemic heart disease. s/pCABG x2, | Troponin I+ 4.5 hours premortem | 3 hours | Acute MI IVS inferior lateral wall | No change | 1+ | 2+ |
| 2 | Ischemic heart disease, s/p CABG, CHF | Troponin T+ 2 wks antemortem | 13 days | Acute MI anteroseptal and lateral | No change | 1+ | 2+ |
| 3 | Ischemic heart disease; terminal cancer patient | CK-MB + 24 hours premortem | 12 days | Acute MI IVS | Glycogenolysis | 1+ | 0 |
| 4 | Ischemic heart disease, cardiac arrest 48 hours before death | Troponin I + 48 hours and CK-MB + after arrest | 8 days | Old and acute MI anteroseptal and lateral | Rare wavy fibers | 2+ | 2+ |
| 5 | Ischemic heart disease, s/p CABG, CHF | Troponin I + 72 hours premortem | 3 days | Acute MI left ventricle, nos | Rare wavy fibers | 2+ | 1+ |
| 6 | EKG T- wave inversions 48 hours | No | 2 days | Acute MI papillary muscle | Rare wavy fibers | 2+ | 1+ |
| 7 | Multiple MI | No | 16 days | Acute MI papillary muscle | Rare wavy fibers | 2+ | 0 |
| 8 | Old MI × 2; CABG × 2 | No | 22.5 hours | Acute MI site, nos | Rare wavy fibers | 2+ | 2+ |
| 9 | Ischemic heart disease | No | 6 hours | Old and acute MI lateral apical | Rare wavy fibers | 2+ | 2+ |
| 10 | Ischemic heart disease, sudden death | No | 18 hours | Acute MI post papillary muscle and left ventrical, nos | Rare wavy fibers | 1+ | 2+ |
| 11 | Ischemic heart diseas, respiratory distress | No | 3 days | Acute MI bundle of His | Rare wavy fibers | 1+ | 2+ |
| 12 | Ischemic heart disease, sudden death | No | 3 days | Acute MI IVS and lateral | Rare wavy fibers | 1+ | 2+ |
| 13 | Ischemic heart disease, CHF | Troponin T+ <24 hours premortem | 2 days | Acute MI anteroseptal and lateral | Rare wavy fibers | 1+ | 2+ |
| 14 | Ischemic heart disease, CHF | CK-MB and Troponin T + 24 hours premortem | 5 days | Acute MI site nos | Wavy fibers | 2+ | 2+ |
| 15 | CABG x 4 | Troponin I + 24 hours premortem | 22 hours | Old and acute MI ante rose ptal MI | Rare wavy fibers; contraction bands | 2+ | 2+ |
| 16 | Severe hemoptysis | No | 3 days | Acute MI IVS | Rare wavy fibers; coagulation necrosis | 1+ | 2+ |
| 17 | Ischemic heart disease, sudden death | No | 21 days | Acute MI papillary muscle | Wavy fibers, coagulation necrosis | 1+ | 2+ |
| 18 | Sudden death | No | 2 days | Old and acute posteroseptal MI | Contraction bands, loss of nuclei, rare polys | 2+ | 2+ |
| 19 | CHF 48 hours premortem | Troponin I + 48 hours premortem, new EKG Q- waves | 2 days | Acute MI posterolateral wall | Glycogenolysis, coagulation necrosis, wavy fibers | 2+ | 2+ |
| 20 | Ischemic heart disease, sudden death | No | 6 days | Acute MI papillary muscle | Coagulation necrosis | 2+ | 2+ |
| 21 | Ischemic heart disease | No | 10 days | Acute MI left ventricle, nos | Wavy fibers; coagulation necrosis; mild polys | 2+ | 2+ |
| 22 | Ischemic heart disease; old MI | No | 7 days | Thrombus right coronary; acute MI ante rose ptal | Contraction bands, loss of nuclei, moderate polys | 2+ | 2+ |
| 23 | Unstable angina | Troponin I + 33 days premortem | 13 days | Acute MI IVS and anterior wall | Contraction bands, loss of nuclei, moderate polys | 1+ | 2+ |
| 24 | Ischemic heart disease, CHF | CK-MB + 4 days premortem | 2 days | Acute MI lateral wall and posterior papillary muscle | Wavy fibers, coagulation necrosis, moderate polys | 2+ | 2+ |
| 25 | EKG changes <48 hours before death | No | 2 days | Acute MI IVS | Early granulation tissue, coagulation necrosis, heavy polys | 1+ | 2+ |
MI = myocardial infarct, EKG = electrocardiogram, CHF = congestive heart failure, CK-MB = creatine kinase- MB isoenzyme, IVS = interventricular septum, CABG = coronary artery bypass graft, nos = not otherwise specified, s/p = status post, Polys = polymorphonuclear cells.
Viable areas of all cases were dark brown on desmin (Figure 1A), and red on Masson's trichrome stain. (Figure 1B). These are excellent internal staining controls.
Figure 1.
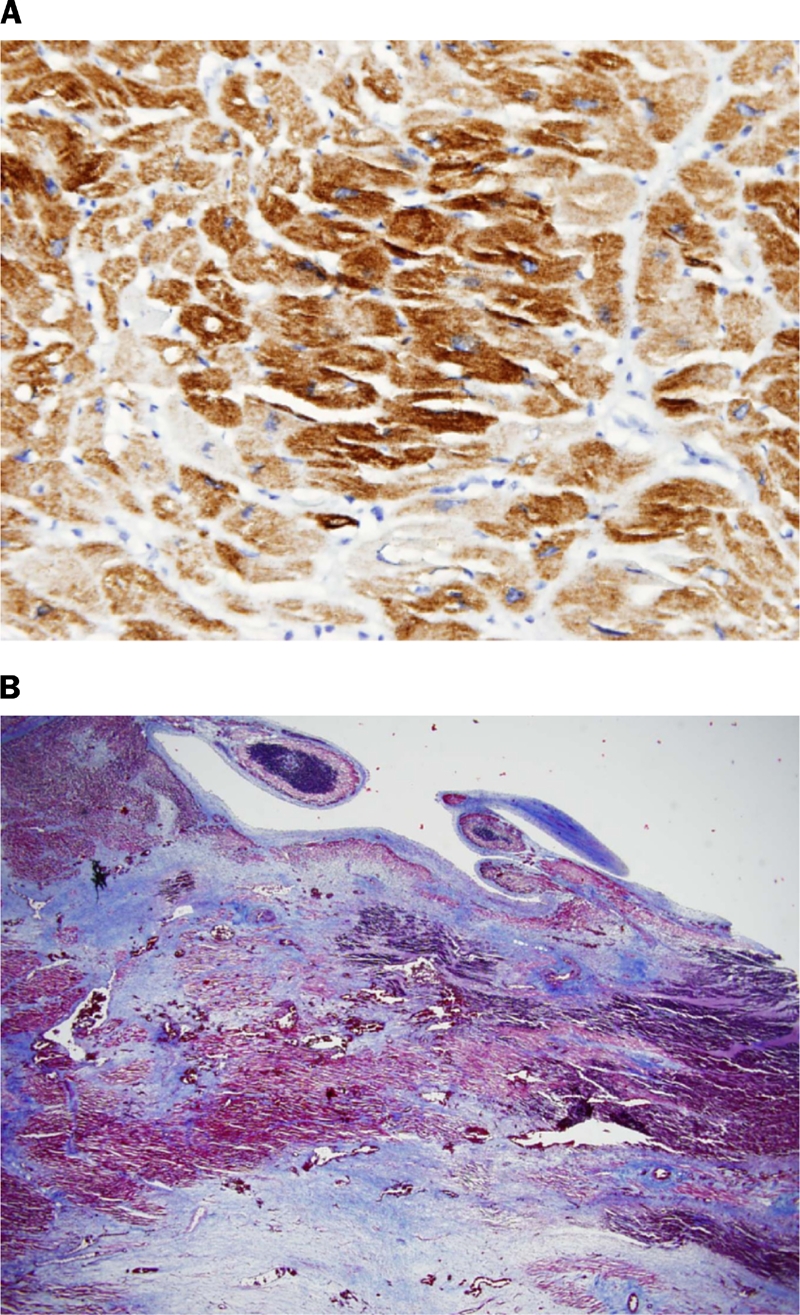
(A) Normal myocardium adjacent to infarct (case 16). (Desmin with Hematoxylin counterstain 400X). (B) Normal areas stain bright red. The areas of infarct are blue (Case 17). (Trichrome 20X).
The ischemic/infarcted areas showed decreased immunoreactivity for desmin in 23 of 25 cases (92%) (Figure 2A), and purple/blue color change on Masson's trichrome stain (Figure 2B) in all cases. All control cases (those that died of causes other than a myocardial infarction) showed uniform bright red positivity on Masson's trichrome, and were uniformly dark brown on desmin stain. All negative controls were negative in both viable and infracted/ ischemic myocardium.
Figure 2.
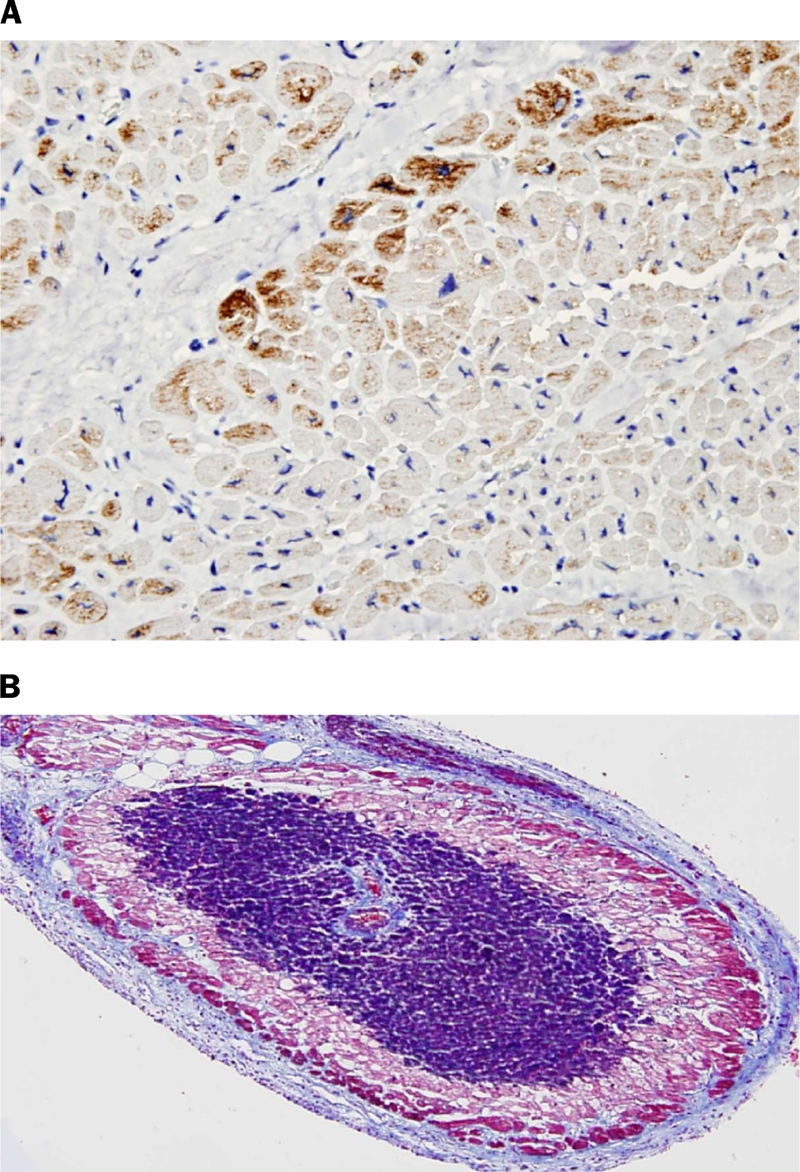
(A) Early infarct (case 16). Note the loss of desmin or negative stain. (Desmin with Hematoxylin counterstain 400X). (B) Early infarct in papillary muscle (case 17). Surrounding these areas are different shades of purple representing various stages in ischemia/infarction. (Trichrome 100X).
Both stains showed larger areas of involvement making the visualization of ischemia/ infarction easier. However, when comparing desmin 2+ (complete loss of cytoplasmic desmin or a negative stain) to Masson's trichrome 2+ (blue cytoplasmic stain), desmin was more sensitive in detecting infarction in 11/25 (44%) cases.
Discussion
Detecting an acute myocardial infarction in autopsy myocardium prior to neutrophilic infiltration is often very subtle on hematoxylin and eosin staining. According to Lilly [13] after 20–24 minutes of myocardial ischemia, irreversible cellular injury occurs which is marked by leaky cell membranes and release of proteolytic enzymes and other molecules including troponin. In the myocardial interstitium, after 20–24 minutes, increased vascular permeability adds to the increased intercellular oncotic pressure, and intercellular edema.
Wavy fibers are seen one to three hours after irreversible ischemic injury, and are the earliest change seen on routine H&E histologic sections. These wavy fibers are thought to be due to intercellular edema separating the dead myocytes as the surrounding normal contracting myocardium pulls on them. Contraction bands with loss of cross striations may be seen around the same time as wavy fibers, and are present at the edge of the infarct. After 4 to 12 hours, neutrophilic infiltration begins which peaks at two to four days. From 18 hours on, true coagulation necrosis in the myocardium with pyknotic nuclei and eosinophilic cytoplasm is seen. Nuclei are lost after 2–4 days of infarction.
Desmin is an intermediate filament expressed in striated muscle (including cardiac muscle) and smooth muscle. In striated muscle, desmin filaments surround the Z discs connecting them to each other, and connecting the entire contractile assembly to the cytoskeleton, nucleus and cytoplasmic organelles [14].
In human hearts removed from heart transplant recipients, in myocardial ischemia, desmin begins to disappear from the myocardial cytoplasm within 30 minutes of the ischemic insult, and is completely gone in 90–120 minutes [12].
Desmin depletion has been also detected with other myocyte proteins (myoglobin, Troponin C, Troponin T, FABP) when there is acute myocardial infarct in autopsy myocardium [8].
Our study is the first to use desmin and Masson's trichrome together in hospital autopsy cases of early infarction. In this study, desmin proved superior in 23 of 25 (92%) cases as compared to the H&E stain in detecting areas of ischemia/infarction. The two cases of normal dark brown desmin stain showed glyocogenolysis (case 3) and rare wavy fibers (case 7) respectively on H&E stain. We can postulate that reperfusion may be necessary after infarction to allow desmin depletion as reperfusion is known to be necessary for myocardial injury after ischemia in animal models [15]. This may account for the lack of desmin depletion in at least one of these two cases because the patient was dead on arrival in the emergency room. It is interesting to note that the interval from the time of death to autopsy did not adversely affect the immunohistochemical or histochemical changes in this study.
Masson's trichrome was superior to the H&E stain and desmin in all cases. However, in those 23 cases that showed desmin depletion, the contrast between dark brown (normal myocardium), light brown (rim of infarct) and white negative areas (infarction) is more easily discerned than the subtle color changes of shades of red to purple to blue on the trichrome stain, or the different shades of red on the H&E stain. The areas of ischemia and infarction with desmin and Masson's trichrome are larger than a corresponding serial section of H&E stain.
Other immunohistochemical markers have been used to detect myocardial ischemia/infarction including C5b-9 complex, fibronectin, troponin and myoglobin. [8, 16–18]. Although myoglobin has been suggested by Ortman, et al [8], to be more sensitive than desmin in early myocardial damage, desmin is more likely to be stocked in the hospital immunohistochemistry laboratory than myoglobin or C5b-9 complex, fibronectin, or troponin. In addition, in our hands, one particular marker, myoglobin did not show as clear delineation between normal to ischemia to infarction.
In summary, desmin and Masson's trichrome are valuable tools for the anatomic pathologist when faced with the question of early myocardial ischemia/infarction in autopsy material. It is important to emphasize that these stains are valuable tools to detect ischemia/infarction, but cannot discern whether these changes are the primary cause of death or are agonal changes in a heart affected by atherosclerotic heart disease.
References
- 1.Adegboyega PA, Adesokan A, Haque AK, Boor PJ. Sensitivity and specificity of triphenyl tetrazolium chloride in the gross diagnosis of acute myocardial infarcts. Arch Pathol Lab Med. 1997;121:1063–1068. [PubMed] [Google Scholar]
- 2.Al-Rufaie HK, Florio RA, Olsen EG. Comparison of the haematoxylin basic fuchsin picric acid method and the fluorescence of haematoxylin and eosin stained sections for the identification of early myocardial infarction. J Clin Pathol. 1983;36:646–649. doi: 10.1136/jcp.36.6.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armiger LC, Wheeler EE, Geraghty DE, Herdson PB. An experimental evaluation of staining techniques for the detection of early ischaemic injury to the myocardium. Pathology. 1977;9:161–171. doi: 10.3109/00313027709085254. [DOI] [PubMed] [Google Scholar]
- 4.Bouchardy B, Majno G. A new approach to the histologic diagnosis of early myocardial infarcts. Cardiology. 1971;56:327–332. doi: 10.1159/000169377. [DOI] [PubMed] [Google Scholar]
- 5.Van Reempts J, Borgers M, Reneman RS. Early myocardial ischaemia: evaluation of the histochemical haematoxylin-basic fuchsin-picric acid (HBFP) staining technique. Cardiovasc Res. 1976;10:262–267. doi: 10.1093/cvr/10.2.262. [DOI] [PubMed] [Google Scholar]
- 6.Rajs J, Jones DP, Jakobsson SW. Comparison of anoxic changes in isolated rat cardiac myocytes in suspension and in histological sections. Acta Pathol Microbiol Scand [A] 1978;86A:401–408. doi: 10.1111/j.1699-0463.1978.tb02064.x. [DOI] [PubMed] [Google Scholar]
- 7.Zugibe FT, Bell P, Jr., Conley T, Standish ML. Determination of myocardial alterations at autopsy in the absence of gross and microscopic changes. Arch Pathol. 1966;81:409–411. [PubMed] [Google Scholar]
- 8.Ortmann C, Pfeiffer H, Brinkmann B. A comparative study on the immunohistochemical detection of early myocardial damage. Int J Legal Med. 2000;113:215–220. doi: 10.1007/s004149900094. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara H, Fujiwara T, Tanaka M, Onodera T, Miyazaki S, Wu DJ, Matsuda M, Sasayama S, Kawai C. Detection of early myocardial infarction in formalin-fixed, paraffin-embedded tissue. Am J Cardiovasc Pathol. 1988;2:57–61. [PubMed] [Google Scholar]
- 10.Ribeiro-Silva A, CC SM, Rossi MA. Is immunohistochemistry a useful tool in the postmortem recognition of myocardial hypoxia in human tissue with no morphological evidence of necrosis? Am J Forensic Med Pathol. 2002;23:72–77. doi: 10.1097/00000433-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Edston E, Kawa K. Immunohistochemical detection of early myocardial infarction. An evaluation of antibodies against the terminal complement complex (C5b-9) Int J Legal Med. 1995;108:27–30. doi: 10.1007/BF01845613. [DOI] [PubMed] [Google Scholar]
- 12.Hein S, Scheffold T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg. 1995;110:89–98. doi: 10.1016/S0022-5223(05)80013-3. [DOI] [PubMed] [Google Scholar]
- 13.Naik H, Sabatine M, Lilly L. Ischemic Heart Disease and Acute Coronary Syndromes. In: Lily LS, editor. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. pp. 141–196. [Google Scholar]
- 14.Milner DJ, Taffet GE, Wang X, Pham T, Tamura T, Hartley C, Gerdes AM, Capetanaki Y. The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolicfunction. J Mol Cell Cardiol. 1999;31:2063–2076. doi: 10.1006/jmcc.1999.1037. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan JM, Cook JA, Hake PW, O'Connor M, Burroughs TJ, Zingarelli B. 15-Deoxy-delta(12,14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock. 2005;24:59–65. doi: 10.1097/01.shk.0000167108.88376.f2. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann B, Sepulchre MA, Fechner G. The application of selected histochemical and immunohistochemical markers and procedures to the diagnosis of early myocardial damage. Int J Legal Med. 1993;106:135–141. doi: 10.1007/BF01225234. [DOI] [PubMed] [Google Scholar]
- 17.Campobasso CP, Dell'Erba AS, Addante A, Zotti F, Marzullo A, Colonna MF. Sudden cardiac death and myocardial ischemia indicators: a comparative study of four immunohistochemical markers. Am J Forensic Med Pathol. 2008;29:154–161. doi: 10.1097/PAF.0b013e318177eab7. [DOI] [PubMed] [Google Scholar]
- 18.Martinez Diaz F, Rodriguez-Morlensin M, Perez-Carceles MD, Noguera J, Luna A, Osuna E. Biochemical analysis and immunohistochemical determination of cardiac troponin for the postmortem diagnosis of myocardial damage. Histol Histopathol. 2005;20:475–481. doi: 10.14670/HH-20.475. [DOI] [PubMed] [Google Scholar]


