Abstract
Histone deacetetylases (HDACs) are a group of corepressors of transcriptional activators and their levels of expression are potentially dysregulated in prostate cancer. Certain inhibitors of histone deacetylases show anti-tumor activity in prostate cancer cell lines. Here, we systemically studied the expression of HDACs in human prostate cancer and the suppression of prostate cancer growth and invasion by HDAC inhibitor SAHA. HDAC1-5 showed increased expression using a combination of DNA microarray, in-situ hybridization, and immunohistochemistry in benign and malignant human prostate tissue as well as RT-PCR and Western blot analysis on various PCa cell lines. Importantly, HDAC inhibitor SAHA suppressed, in particular, prostate cancer cell growth and invasion determined using cell proliferation and Matrigel invasion assays. The findings of this study show that the expression of HDACs and their associated corepressors are increased in prostate cancer in humans and HDAC inhibitor SAHA could serve as a potential therapeutic agent in prostate cancer in addition to anti-androgens.
Keywords: Prostate cancer, hormone receptor, androgen, corepressor, microarray, in situ hybridization, histone deacetylase
Introduction
The hormone therapy for prostate cancer targets the ligand-binding domain of the androgen receptor using drugs that reduce the serum level of testosterone. This is often administered in combination with competitive androgen receptor (AR) antagonists. The outcome of such a treatment follows a predictable course comprised of an initial response, a period of quiescence during which the tumor does not proliferate, followed by progression of the disease. This indicates that ligand directed anti-androgen therapy alone is not sufficient for achieving long-term remission, thus identification of other targets that can be used in conjunction with the current hormonal treatment is warranted [1, 2].
Histone deacetetylases (HDACs) are a group of corepressors of transcriptional activators, including AR [3–5]. These set of proteins regulate gene expression by altering nucleosome conformation at the chromatin level and the stability of several large complexes of transcription factors. The class I HDACs which include HDAC1 and HDAC2 associated with Sin3A and Sin3B and several other proteins to form the Sin3 complex [6]. This complex is thought to deacetylate histones near Sin3 regulated promoter regions leading to a repressed chromatin structure. Similarly, the class II HDACs, HDAC 4–5, have been shown to form complexes with the corepressors, N-CoR and SMRT [7]. HDAC3 associates with another large complex of coregulatory proteins to form the HDAC3/GPS/TBL/NCoR/SMRT complex [8]. These complexes are associated with the ligand bound androgen receptor dimers and are involved in the regulation of AR responsive genes [9].
In this study, we examined expression of HDAC and the effects of anti-proliferation and -invasion in prostate cancer by SAHA, an HDAC inhibitor. Our data show that the expression of known HDAC members are increased and correlate with poor prognosis including the, Gleason's score, stage of the disease, recurrence and metastasis. Importantly, SAHA, an HDAC inhibitor, significantly inhibited prostate cancer cell proliferation and invasion. These observations suggest that elevated levels of HDACs are associated with progression of prostate cancer, thus, the HDAC inhibitors could potentially have a future role in the treatment of prostate cancer.
Materials and Methods
Specimens
The expression of HDAC was studied in benign and malignant prostate tissues. The use of the tissues was approved by the institutional committee on the Use of Human Subjects in Medical Research. One group of 98 specimens was obtained as fresh tissue from prostate cancer patients who underwent radical retropubic radical prostatectomy (RRP) from Memorial Sloan Kettering Cancer Center. Among these, there were 42 cases of primary prostate cancer. PSA was undetectable after a >5 year follow-up. Thirty seven cases were obtained from patients with recurrent prostate cancer during a follow-up period. Eight samples were obtained from metastatic tumors. Eleven samples used as controls were derived from the peripheral zone of radical prostatectomy specimens that did not show cancer under microscope and were composed of benign prostatic tissue. This group of specimens was used for DNA microarray analysis [10].
A second group of samples were derived as formalin fixed, paraffin embedded tissue from 48 prostate cancer patients who underwent RRP from NYU/VA Medical Center. The pathological stage was T2 in 38 cases (79%), and T3 in 10 cases (21%). The mean Gleason's score in this group of specimen was 6.9 (SD ± 1.0). This group of specimens was used for in situ hybridization and immunohistochemiscal analysis.
Microarray analysis
DNA microarray analyses were performed as described [10]. Gene expression was calculated from the CEL files using the robust multi-array averaging method after quantile normalization. The expression levels of each transcript between any two groups of prostate tissues were compared using the two-sample t-test. All tests were two-sided. Comparisons resulting in p-values < 0.05 are declared statistically significant. The p-values were not adjusted for multiple comparisons due to the exploratory nature of this study.
In situ hybridization (ISH)
cDNAs of HDACs and associated corepressor were first subcloned into the pBluescript SK+ (Stratagene) expression vector either by direct cloning or PCR amplification (Table 1). The probes were labeled with digoxigennin using T7 and T3 promoter regions flanking the multiple cloning site of the vector to create respectively, the sense or antisense probes. The yield of the probes was assessed by dot blot hybridization using known concentrations of standard mRNA. For each target mRNA, in situ hybridization was performed using a previously described procedure [11].
Table 1.
List of primers and cloning strategy of HDACs and associated corepressors
| Corepressor | Probe/RE Site Positions | Cloning Primers |
|---|---|---|
| HDAC1 | NotI/1 – HincII/782 | N/A |
| HDAC2 | HindIII/759 -- EcoRI/1460 | N/A |
| HDAC3 | EcoRI/1 -- HindIII/585 | N/A |
| HDAC4 | SacII/2106 – XhoI/2914 | N/A |
| HDAC5 | EcoR1/776 – SalI/1390 | N/A |
| GPS2 | BamH1/240 – EcoR1/775 | U:cgggatccaaggaagaaaaaggagatggaaga L:cggaattcctgtggctgtggctgaagataggta |
| Sin3A | BamH1/1443 – EcoR1/2000 | U:cgggatccaaatttcctacgctgtcttgtat L:cggaatctttctatggatgacttctgatgtg |
| Sin3B | XbaI/618 – SmaI/1237 | U:gctctagaaggcatgtctgaagaggaggtgtt L:tcccccgggacttgggctgctggtaggttttg |
Immunohistochemistry
Immunohistochemcial staining was performed using the NexES, automated immunostainer and detection system (Ventana Medical Systems, Tucson, AZ, USA) as described previously [11]. Sin3A and Sin3B primary antibodies were each diluted 1:80. The chromogen, 3,3′-diaminobenzidine/hydrogen peroxide mix was applied for 8 minutes and then enhanced with copper sulfate for 4 minutes. Slides were then counterstained with hematoxylin, dehydrated in alcohol and mounted with permanent media.
Real time RT-PCR
The level of HDAC mRNA was examined by real time RT-PCR analysis in eight prostate cancer cell lines (RC165/hTert, RC170/hTert [12], LNCaP, LNCaP-AI, LAPC4, PC3, PC3AR and DU145). RC165/hTert and RC170/hTert are benign immortalized prostate cell lines with catalytic subunit of telomerase [12]. LNCaP is AR dependent prostatic cancer cell line with a T877A mutation. LNCaP-AI is derivative of LNCaP rendering androgen-independent growth [13] . LAPC4 is prostate cancer cell line with wild type AR. PC3 and DU145 are AR negative, thus androgen independent prostate cancer cell lines. PC3AR is a derivative of PC3 stably transfected with wild type AR.
Total RNA was prepared by using an RNAqueous-4 PCR kit (Ambion, Austin, TX) and reverse transcribed by using a Reverse transcription (RT)-PCR Reagents kit (Applied Biosystems). Primers for HDAC1, HDAC3, HDAC5 and β-actin were purchased from Superarray Company (Frederick, MD). Using the Rotor-Gene 3000 (Corbett Life Science), quantitative real-time PCR was performed in triplicate in 25 μL reaction volume consisting of 1xSYBR Green I Master Mix buffer (Qiagen, Valencia, CA), 400 nM, each of forward and reverse primer, and cDNA. Forty cycles of PCR was carried out as follows: 95°C 15 minutes for one cycle, 94°C for 15 seconds, 60°C for 30 seconds, 72°C for 30 seconds. The β-actin gene was used for normalization of data. The threshold cycle (Ct) values for each sample were determined with the amplification plots within the logarithmic phase. The efficiency of amplification for the primers was comparable, as indicated by the lines in plots of ΔCt values against diluted cDNA. Data were analyzed by using 2−ΔΔCt method described by Livak and Schmittgen. Statistical data are presented as mean ± SD.
Western blot analysis
Western blot analysis was performed in prostate cell lines, RC165/hTert, RC170/hTert, LNCaP, LNCaP-AI, LAPC4, PC3, PC3AR and DU145. Whole cell extracts from the above cell lines were subjected to SDS-PAGE. Proteins were then transferred to a nitrocellulose membrane. Blots were incubated with primary antibodies to HDAC 1–5 (Cell Signaling) for 2 hr at room temperature, washed with TBST three times, and incubated for 1.5 hr with the secondary antibody (1:5,000) (Amersham Biosciences, Piscataway, NJ). Antibodies were diluted in 2% BSA in TBST. The protein bands were detected by enhanced chemiluminesence (Amersham Biosciences, Piscataway, NJ).
Cell culture, cell proliferation and Matrigel invasion assays
Prostate cell lines were maintained as previously described [14]. To evaluate effects of SAHA on cell proliferation, LNCaP and LNCaP-AI cells at exponential growth phase were seeded into 96-well dishes at density of 2500 cells/well. Twenty-four h later, the cells were exposed to serial diluted concentrations of SAHA for 7 days, and the cell growth was then determined by MTT [15]. Briefly, after solubilization, the absorbance of the formazan, reduced from MTT, was measured with a microplate absorbance reader. To examine whether SAHA inhibits invasive potential of prostate cancer cells, invasive activity of LNCaP and LNCAP-AI cells was determined via the transwell matriget invasion assay [16]. Matrigel inserts were coated with 8 micron pore size matrigel. LNCaP and LNCaP-AI cells at exponential growth phase were added to the upper chamber at density of 1x104/per well in 500μl medium in the presence or absence of indicated concentration of SAHA and incubated at 37°C for 36 h. After the incubation, the non-invading cells were removed from upper chamber with a cotton swab, and the invading cells adherent to the bottom of membrane were fixed, stained, and counted by tallying the number of cells in 3 random fields under microscope. Data are adjusted by growth condition, and expressed as mean of migrating cells in 3 fields +/- SD.
Results
The goal of this investigation was to determine the expression of major classes I and II histone deacetylases and their associated corepressors in human prostate cancer and explore the efficacy of SAHA in inhibiting prostate cancer growth and invasion.
Increased HDAC expression in human prostate cancer and prostate cancer cell lines
We studied the expression of multiple HDACs and several associated coregulatory factors at mRNA and protein levels in benign and malignant prostatic tissue as well as cell lines. Increased HDAC expression in human prostate cancer by Affymetric oligonucleotide microarray: We compared the levels of HDAC1, 3, 4, 5 and GPS2 between groups of primary, recurrent, metastatic and benign prostate tissues in 98 RRP specimens from MSKCC as described in Material and Method section. The microarray studies showed that multiple HDACs were overexpressed in both primary CaP and recurrent CaP versus benign prostate. The expression levels of each transcript between any two groups were statistically analyzed using the two-sample t-test. The results of a comparison between the gene expressions of benign prostate, primary CaP, recurrent and metastatic prostate CaP is shown in Table 2. There were significantly increased levels of expression in HDAC1 (p=0.0024) and HDAC4 (p=0.03) in primary prostate cancer compared to benign prostatic tissue. HDACs 1 (p=0.0036), 3 (p=0.05), 4 (p=0.0095), and 5 (p=0.0004) were all overexpressed in recurrent CaP as compared with benign tissues. There was a trend toward an overexpression of GPS. However it did not reach statistical significance (p=0.07). HDAC 5 was significantly overexpressed in recurrent CaP vs. primary CaP (p=0.004) and HDAC1 was overexpressed in metastatic CaP compared to primary cancer (p=0.0026).
Table 2.
Significance of increase of expression levels of HDAC in cancer by oligonucleotide microarray
| GENE | Benign vs. CaP | Benign vs. Recurrent CaP | Benign vs. Met CaP | CaP vs. Recurrent CaP | CaP vs. Met CaP |
|---|---|---|---|---|---|
| (HDAC1) | 0.0024 | 0.0036 | 0.0941 | 0.7833 | 0.0026 |
| (HDAC3) | 0.161 | 0.0502 | 0.0321 | 0.3391 | 0.0995 |
| (HDAC4) | 0.0300 | 0.0095 | 0.3367 | 0.2129 | 0.3877 |
| (HDAC5) | 0.8730 | 0.0004 | 0.4719 | 0.0000 | 0.5089 |
| (GPS2) | 0.7582 | 0.0658 | 0.3787 | 0.1132 | 0.4491 |
Increased HDAC expression in human prostate cancer by in situ hybridization: In situ hybridization was carried out in the 48 RRP specimens from the NYU/VAMC. Expression of HDACs in prostate cancer compared to benign was categorized as either overexpressed or not overexpressed based on the scoring of the majority of prostate cancer glands on the slide as read by urologic pathologist. The levels of expression were as either not expressed or overexpressed compared to benign glands.
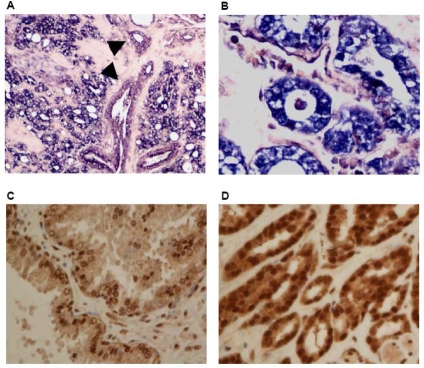
Examples of the ISH slides are shown in Figure 1A and 1B. The percentages of cases showing overexpression in PIN and cancer, compared to benign prostate, are determined. The in situ data showed that all the HDACs and corepressors studied were overexpressed in CaP vs. benign prostate tissue. In prostate cancer HDAC-5, and GPS showed the largest percentage of increased expression with 48% (23/48) and 72% (33/46) respectively. Sin3A and Sin3B were overexpressed in 33% and 28% of CaP cases, respectively. In PIN, HDAC5 and GPS also showed the highest levels of overexpression with 48% and 54% of cases respectively. We then stratified the expression of the corepressors in prostate cancer vs. benign glands by, Gleason score, PSA, and pathologic stage, and we found that HDAC 2–5 were all overexpressed in at least twice as many cases in stage T3 disease than in stage T2 disease.
Figure 1.
The expression of HDAC3 by in situ hybridization and Sin3A by immunohistochemistry. Increased expression of HDAC3 (A, 200x, B, 400X) in cancer by in situ hybridization compared with adjacent benign prostate gland (A, arrowhead). Increased Sin3A expression in cancer (D, 400x) compared with adjacent benign prostate (C, 400x).
To confirm the results obtained from in situ hybridization, immunohistochemical analysis was performed for Sin3A and Sin 3B. The results showed increased expression of both Sin 3A and 3B (Figure 1C and 1D). Antibodies suitable for immunohistochemistry of other target proteins of interest were not available.
HDAC expression in benign and malignant prostate cancer cell lines by real time RT-PCR and Western blot analysis: To further evaluate the observations above, we performed real time RT-PCR for HDAC1, HDAC2, HDAC3, HDAC4 and HDAC5 in several prostate cancer cell lines including RC165/hTert, RC170/hTert, LNCaP, LNCaP-AI, LAPC4, PC3, PC3AR and DU145. The levels of expression for each HDAC1, 3 and 5 are shown in Figure 2 after normalized with β-actin. The data confirmed low levels of mRNA expression of HDAC1, HDAC3 and HDAC5 in benign prostate RC165 and RC170 cells. They were expressed at moderate levels in androgen dependent LNCaP and LAPC4 prostate cancer cells. A higher level of expression was found in androgen independent PC3, DU145 and LNCaP-AI cells. However, there were several exceptions. HDAC1 was expressed at relatively high levels in RC170. HDAC5 had the highest level of expression in LAPC4 cells and HDAC3 was expressed at a low level in DU145 cells. HDAC2 and HDAC4 were expressed at comparable levels in all cell lines (data not shown).
Figure 2.
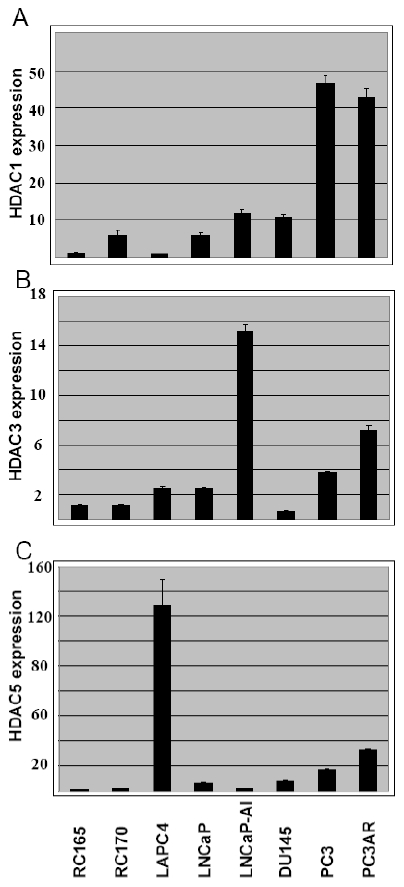
Relative levels of expression of HDACs in prostate cancer cell lines by real time RTPCR. The level of expression for each HDACs is normalized to β-actin.
We next performed Western blot analysis to detect the expression of HDAC1, HDAC3, and HDAC5 at protein level in the above cell lines. Using the Western blot, we confirmed higher levels of expression for HDAC1 in PC3, PC3AR, LNCaPAI and DU145 cells (Figure 3), which is similar to the RT-PCR results showing that HDAC 1 expressed at highest levels in PC3 and PC3AR cells. Notably, these are androgen independent cell lines. HDAC3 was expressed at the highest levels in LNCaP-AI cells (Figure 3) which was also confirmed with RT-PCR. HDAC5 was expressed at the highest level in LAPC4, an androgen dependent cell line, and again, this correlates with the RT-PCR results. We also examined the expression of HDAC2 and HDAC4 in these cell lines, however the difference in their expression was insignificant (data not shown). These data are comparable with the data obtained by DNA microarray and show that, these HDACS are overexpressed in prostate cancer including metastatic tumors as compared to benign prostate cells.
Figure 3.
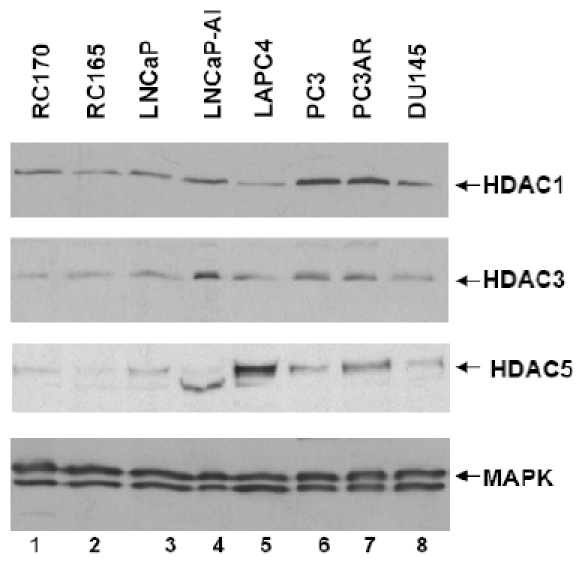
Expression of HDACs in prostate cancer cell lines by Western blot analysis. HDAC1 is overexpressed in the androgen-independent cell lines PC3, PC3AR, LNCaPAI, and DU145. HDAC3 showed the highest expression in LNCaP-AI and HDAC5 in LAPC4.
SAHA inhibits androgen-independent prostate cancer growth and invasion
Since the levels of several HADCs are increased in prostate cancer, we decided to determine the functional relevance of increased levels of HDACs in prostate cancer using HDAC inhibitor SAHA by in vitro cell proliferation and Marigel invasion assays in LNCaP and LNCaP-AI, an androgen-independent variant of LNCaP cells.
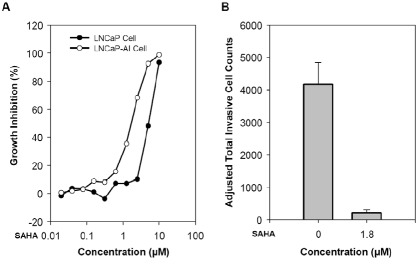
Cell proliferation assays were performed using MTT assays, measuring the doubling time directly from 2000 to 250,000 cells. SAHA was added from 0.01 to 100 μM and the efficacy of growth inhibition is determined by IC50 on day 7. HDAC inhibitor SAHA suppressed the growth of both LNCaP and its androgen-independent variant LNCaP-AI cells, but to a different degree. As shown in Figure 4A, we observed that IC50 for LNCaP-AI cells was found to be approximately 1.8 μM, much more sensitive to SAHA than that of LNCaP cells with IC50 as 5.1 μM. This observation is distinct from other chemotherapeutic agents to which LNCaP-AI cells are resistant [13, 17, 18]. The above finding is consistent with the fact that overall levels of HDAC are higher in LNCaP-AI cells than that in LNCaP cells.
Figure 4.
HDAC inhibitor SAHA inhibits growth and invasion of LNCaP and LNCaP-AI cells. A. SAHA suppresses the growth of LNCaP and LNCaP-AI with IC50 of 5.1 and 1.8 μM respectively. B. SAHA completely inhibited invasion of LNCaP-AI cells at 1.8 μM.
As reported previously, LNCaP cells are minimally invasive in Matrigel invasion assays [19]. In contrast, LNCaP-AI cells showed sharply increased degree of invasive potentials. Over 4000 cells /field invaded though Matrigel layer during 24 h period in the presence of androgen (10 nM R1881). In Matrigel invasion assays, the invasive capacity of LNCaP-AI cells was almost completely blocked by the presence of 1.8 μM (Figure 4B), which represents the dosage IC50 for growth inhibition. The number of invasive cells was adjusted to total number of cells on day 2 since SAHA also inhibited the cell growth. This finding parallels the result that protein levels of HDAC1, 3 and 5 were higher in LNCaP-AI than that in LNCaP cells (Figure 3).
The results of the above studies indicate HDAC inhibitor SAHA suppresses the growth and invasion of, in particular, androgen-independent prostate cancer LNCaP-AI cells.
Discussion
Prostate cancer begins as a hormone sensitive tumor and its growth is stimulated by the activation of AR in conjunction with multiple nuclear coregulatory proteins such as histone deacetylases. The histone deacetylases modulate transcriptional activity of hormonal receptors including AR by altering the stability of the transcriptional pre-initiation complex and/or modifying the chromatin structure[5]. HDACs associate with other co-repressors and form large protein complexes including Sin3 (Sin3A/Sin3B/HDAC1/HDAC2), HDAC3/GPS/ TBL/NCoR/SMRT, and the HDAC4/HDAC5/ SMRT/NCoR complex [9]. Several HDAC inhibitors have been developed for treatment of prostate cancer.
Limited reports indicate HDACs are increased in prostate cancer and their expression may regulate cancer growth. We showed HDAC1 is overexpressed in 35% of prostate cancer, in metastatic tumors and in androgen-independent cell lines. These findings are consistent with the previously reported increase in HDAC1 activity, mRNA expression, and protein expression in prostate cancer cell lines [20] and cancers that are refractory to hormone treatment [21]. HDAC2 has been found to be increased in human colon cancer [22] and we show it is overexpressed in about 30% of CaP cases. HDAC3 and GPS2, are members of a protein complex that also includes NCOR and SMRT. The expression of these genes has not been previously studied in prostate cancer [23]. Here, we show that HDAC 3 is overexpressed in 25% while GPS2 was increased in 72% of prostate cancers. Since HDAC3 is expressed at high levels in metastatic tumors, HDAC 3 might be involved in tumor progression. GPS2 not only facilitates the assembly of the GPS2-TBL1-N-CoR-HDAC3 complex, but also appears to inhibit the intracellular c-Jun N-terminal kinase (JNK) pathway, regulating the phosphorylation of AP-1 which modulates cellular apoptosis. Since treatment of HeLa cells with HDAC3 siRNA increases the percentage of apoptotic cells the GPS2-TBL1-N-CoR-HDAC3 corepressor complex could potentially be involved in alteration of apoptosis via the JNK pathway. HDAC4 is overexpressed in 23% of prostate cancer specimens studied in this report. A recent report has shown that there is altered intracellular localization of HDAC4 in prostate cancers that have become refractory to hormone treatment [24], suggesting that HDAC4 may be involved in the late events of prostate cancer progression. HDAC5 expression was increased in 57% of prostate cancer specimens as well as in about one half of the cases showing PIN. Since HDAC5 is able to associate with known AR corepressors such as SMRT and N-CoR [8], it may also play a role in tumorigenesis of prostate cancer. In prostate cancer cell lines, HDACs are increased in malignant cells compared to benign immortalized prostate cells. The increase in HDAC expression could represent an active role of HDACs in prostate cancer or a cancer epiphenomone. The results of cell proliferation assays show inhibition of prostate cancer growth by HDAC inhibitor SAHA, strongly indicating a functional relevance of increased HDACs in prostate cancer rather than cancer epiphenomone.
HDACs have been recently shown to be involved in cell migration and invasion [25]. HDACs regulate expression of many genes known participated in cancer invasion and metastasis, such as extracellular matrix (ECM), metastasis-associated proteins (MTAs), metastasis suppressor gene KAI1 and NF-kB, etc., thereby increases cancer cell invasion capacity [26–29]. For example, in advanced gastric cancer, the non-invasive port of the tumor express certain level of acetylated Histone H4, whereas the deeply invasive part of the tumor shows reduction in histone acetylation, suggesting that global Histone deacetylation may participate in cancer invasion and metastasis [30]. It has been shown that treatment with HDAC inhibitors represses cancer cell invasion and metastasis both in vitro and in vivo [29, 31–34]. We observed that LNCaP-AI, androgen-independent derivative of LNCaP cell line, possess significantly increased its potential for invasion as compared with its androgen-dependent parental cell line. In this report, we showed that the LNCaP-AI cells were more sensitive to SAHA than that of LNCaP cells. Significantly, the increased cell proliferation potential of the LNCaP-AI cells were completed inhibited by SAHA. These data parallel the findings that HDAC1, HDAC3, and HDAC5 were significantly higher in LNCaP-AI cells than that in LNCaP cells. Our observations further support that increased levels of HDACs play an important role in tumor invasion and metastasis.
It is of great interest to further investigation of the role of individual HDACs in their association with prostate cancer tumorigenesis and progression including development of androgen independence. Such studies are likely to provide a rationale for novel therapeutic targets in prostate cancer in clinical setting.
Acknowledgments
This study was supported by a VA Merit Review Grant and DOD Prostate Cancer Research Award to PL and IO. LAPC4 and LNCaP-AI were kindly provided by Dr. S. Logan, and Dr. Anna Ferrari, respectively. We would like to thank Dr. Liying Yang for technical assistance. We would also like to thank Hong Zhong for technical assistance.
References
- 1.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–15. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Culig Z. Role of the androgen receptor axis in prostate cancer. Urology. 2003;62:21–6. doi: 10.1016/s0090-4295(03)00698-8. [DOI] [PubMed] [Google Scholar]
- 3.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Hsu CL, Chang C. Androgen receptor corepressors: an overview. Prostate. 2005;63:117–30. doi: 10.1002/pros.20170. [DOI] [PubMed] [Google Scholar]
- 5.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–87. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Mei Q, Zielinska-Kwiatkowska A, Matsui Y, Blackburn ML, Benedetti D, Krumm AA, Taborsky GJ, Jr, Chansky HA. An ERG (ets-related gene)-associated histone methyltransferase interacts with histone deacetylases 1/2 and transcription co-repressors mSin3A/B. Biochem J. 2003;369:651–7. doi: 10.1042/BJ20020854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertos NR, Wang AH, Yang XJ. Class II histone deacetylases: structure, function, and regulation. Biochem Cell Biol. 2001;79:243–52. [PubMed] [Google Scholar]
- 8.Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 9.Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184:1–16. doi: 10.1002/(SICI)1097-4652(200007)184:1<1::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, Reuter V, Gerald WL. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002;62:4499–506. [PubMed] [Google Scholar]
- 11.Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19:1792–802. doi: 10.1210/me.2004-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, Li H, Miki J, Kim KH, Furusato B, Sesterhenn IA, Chu WS, McLeod DG, Srivastava S, Ewing CM, Isaacs WB, Rhim JS. Phenotypic characterization of telomerase-immortalized primary non-malignant and malignant tumor-derived human prostate epithelial cell lines. Exp Cell Res. 2006;312:831–43. doi: 10.1016/j.yexcr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Wang LG, Ossowski L, Ferrari AC. Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res. 2001;61:7544–51. [PubMed] [Google Scholar]
- 14.Peng Y, Chen F, Melamed J, Chiriboga L, Wei J, Kong X, McLeod M, Li Y, Li CX, Feng A, Garabedian MJ, Wang Z, Roeder RG, Lee P. Distinct nuclear and cytoplasmic functions of androgen receptor cofactor p44 and association with androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2008;105:5236–41. doi: 10.1073/pnas.0712262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LG, Liu XM, Chiao JW. Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis. 2006;27:2124–32. doi: 10.1093/carcin/bgl075. [DOI] [PubMed] [Google Scholar]
- 16.Cai CQ, Peng Y, Buckley MT, Wei J, Chen F, Liebes L, Gerald WL, Pincus MR, Osman I, Lee P. Epidermal growth factor receptor activation in prostate cancer by three novel missense mutations. Oncogene. 2008 doi: 10.1038/sj.onc.1210983. [DOI] [PubMed] [Google Scholar]
- 17.Gao M, Ossowski L, Ferrari AC. Activation of Rb and decline in androgen receptor protein precede retinoic acid-induced apoptosis in androgen-dependent LNCaP cells and their androgen-independent derivative. J Cell Physiol. 1999;179:336–46. doi: 10.1002/(SICI)1097-4652(199906)179:3<336::AID-JCP11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Liu XM, Jiang JD, Ferrari AC, Budman DR, Wang LG. Unique induction of p21(WAF1/CIP1)expression by vinorelbine in androgen-independent prostate cancer cells. Br J Cancer. 2003;89:1566–73. doi: 10.1038/sj.bjc.6601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Y, Li CX, Chen F, Wang Z, Ligr M, Melamed J, Wei J, Gerald W, Pagano M, Garabedian MJ, Lee P. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70. Am J Pathol. 2008;172:225–35. doi: 10.2353/ajpath.2008.070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra SK, Patra A, Dahiya R. Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem Biophys Res Commun. 2001;287:705–13. doi: 10.1006/bbrc.2001.5639. [DOI] [PubMed] [Google Scholar]
- 21.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–89. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 22.Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Göttlicher M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5:455–63. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. Embo J. 2000;19:4342–50. doi: 10.1093/emboj/19.16.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halkidou K, Cook S, Leung HY, Neal DE, Robson CN. Nuclear accumulation of histone deacetylase 4 (HDAC4) coincides with the loss of androgen sensitivity in hormone refractory cancer of the prostate. Eur Urol. 2004;45:382–9. doi: 10.1016/j.eururo.2003.10.005. author reply 389. [DOI] [PubMed] [Google Scholar]
- 25.Liu T, Kuljaca S, Tee A, Marshall GM. Histone deacetylase inhibitors: multifunctional anticancer agents. Cancer Treat Rev. 2006;32:157–65. doi: 10.1016/j.ctrv.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Whetstine JR, Ceron J, Ladd B, Dufourcq P, Reinke V, Shi Y. Regulation of tissue-specific and extracellular matrix-related genes by a class I histone deacetylase. Mol Cell. 2005;18:483–90. doi: 10.1016/j.molcel.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Wang RA, Bagheri-Yarmand R. Emerging roles of MTA family members in human cancers. Semin Oncol. 2003;30:30–7. doi: 10.1053/j.seminoncol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, Chen C, Chung CH, Huber O, Rose DW, Sawyers CL, Rosenfeld MG, Baek SH. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature. 2005;434:921–6. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 29.Takada Y, Gillenwater A, Ichikawa H, Aggarwal BB. Aggarwal, Suberoylanilide hydroxamic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing nuclear factor-kappaB activation. J Biol Chem. 2006;281:5612–22. doi: 10.1074/jbc.M507213200. [DOI] [PubMed] [Google Scholar]
- 30.Yasui W, Oue N, Ono S, Mitani Y, Ito R, Nakayama H. Histone acetylation and gastrointestinal carcinogenesis. Ann N Y Acad Sci. 2003;983:220–31. doi: 10.1111/j.1749-6632.2003.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 31.McGarry LC, Winnie JN, Ozanne BW. Invasion of v-Fos(FBR)-transformed cells is dependent upon histone deacetylase activity and suppression of histone deacetylase regulated genes. Oncogene. 2004;23:5284–92. doi: 10.1038/sj.onc.1207687. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Ahn S, Han JW, Lee HW, Lee HY, Lee YW, Kim MR, Kim KW, Kim WB, Hong S. Apicidin is a histone deacetylase inhibitor with anti-invasive and anti-angiogenic potentials. Biochem Biophys Res Commun. 2004;315:964–70. doi: 10.1016/j.bbrc.2004.01.149. [DOI] [PubMed] [Google Scholar]
- 33.Eyüpoglu IY, Hahnen E, Buslei R, Siebzehnrübl FA, Savaskan NE, Lüders M, Tränkle C, Wick W, Weller M, Fahlbusch R, Blümcke I. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93:992–9. doi: 10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 34.Coradini D, Zorzet S, Rossin R, Scarlata I, Pellizzaro C, Turrin C, Bello M, Cantoni S, Speranza A, Sava G, Mazzi U, Perbellini A. Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res. 2004;10:4822–30. doi: 10.1158/1078-0432.CCR-04-0349. [DOI] [PubMed] [Google Scholar]