Abstract
Chemo- or radioresistance markedly impairs the efficacy of cancer therapy and involves anti-apoptotic signal transduction pathways that prevent cell death. In resistant cancer cells, both inhibitors of apoptosis proteins (IAPs) and nuclear factor-kappa B (NF-κB) play a pivotal role in preventing apoptosis triggered by a variety of stresses, facilitating them as potential targets in cancer treatment. Furthermore, mounting evidences have established the crosstalks between IAPs (eg. XIAP, cIAP-1, cIAP-2) and proteins involved in NF-κB signaling (eg. TRAF2, RIP1, TAB1). Second mitochondria-derived activator of caspases (Smac) is a mitochondrial protein that released into cytoplasm upon apoptotic stimuli. As Smac functions as an endogenous IAP inhibitor, small molecule Smac-mimetics are believed to neutralize IAPs function that results in liberating caspase activity and promoting apoptosis. Moreover, recent studies show that Smac-mimetics may kill cancer cells in a different manner, which involves inducing ubiquitination of cIAPs, regulating NF-κB signaling and facilitating TNFα-triggered, caspase-8-mediated apoptosis in a certain cancer cell types. In other cancer cells that are resistant to TNFα or chemo/radiotherapy, Smac-mimetic IAP-inhibitors can enhance ionizing radiation or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, indicating the potential role of Smac-mimetics in overcoming acquired therapy-resistance. Such findings provide important impetus for utilizing IAP-inhibitors as novel adjuvant therapy for the TNFα–resistant, NF-κB constitutively active cancers that account for the majority of patients who are refractory to current therapeutic approaches.
Keywords: Chemoresistance, inhibitors of apoptosis proteins, NF-κB, small molecule inhibitors
The aggressive cancer cell phenotype is the result of a variety of genetic and epigenetic alterations leading to deregulation of intracellular signaling pathways, including an impaired ability of the cancer cell to undergo apoptosis [1, 2]. Most of the current anticancer therapies work, at least in part, through inducing apoptosis in cancer cells [3–6]. Lack of appropriate apoptosis due to defects in the normal apoptosis machinery plays a crucial role in the resistance of cancer cells to a wide variety of current anticancer therapies [7, 8]. Chemo- or radioresistance markedly impairs the efficacy of cancer therapy and involves anti-apoptotic signal transduction pathways that prevent cell death [9–11]. For example, primary or acquired resistance of hormone-refractory prostate cancer to current treatment protocols has been associated with apoptosis-resistance of cancer cells and is linked to the failure of therapies [12–14].
Current and future efforts toward designing new therapies to improve survival and quality of life of cancer patients must include strategies that specifically target cancer cell resistance to current chemo/radiotherapies [13, 15]. In this review article, we will summarize the state of our knowledge for the role of both IAPs and NF-κB in relation to cancer therapeutics resistance. Furthermore, we will discuss the potential role of small molecule candidates that target apoptosis and/or NF-κB signaling pathway on the sensitization of conventional cancer therapy.
The inhibitor of apoptosis proteins are potent negative regulators of apoptosis and related to apoptosis-resistance
Cancer cells will acquire resistance to apoptosis by upregulating multiple pro-survival factors. The inhibitors of apoptosis proteins (IAPs) are a pivotal class of intrinsic cellular inhibitors of apoptosis [16–19]. IAPs widely and potently suppress apoptosis against a large variety of apoptotic stimuli, including chemotherapeutic agents, radiation and immunotherapy in cancer cells [20, 21]. In human, eight IAPs were identified so far (Table 1), all can block caspase cascade, but only some of them directly interact with caspases [22]. IAPs are characterized by the presence of one to three domains known as baculoviral IAP repeat (BIR) domains and belong to a larger family of proteins, called the BIR-domain-containing proteins (BIRPs) [16].
Table 1.
The inhibitor of apoptosis proteins family
| Gene | Protein |
|---|---|
| BIRC 1 | NAIP |
| BIRC 2 | cIAP-1 |
| BIRC 3 | cIAP-2 |
| BIRC 4 | XIAP (ILP1) |
| BIRC 5 | Survivin |
| BIRC 6 | Bruce (Apollon) |
| BIRC 7 | ML-IAP (livin, K-IAP) |
| BIRC 8 | ILP2 (TsIAP) |
Since the IAPs function at the convergence of both mitochondria pathway and death receptor pathway, they are described as an apoptosis “brake” and IAP antagonists function to release the “brake” [14, 23]. Most components of the major cell death regulatory pathways have been implicated in radiation-induced cell death [24]. Some of these apoptosis pathway proteins have overlapping functions and compensatory pathways, and these apoptosis pathways have extensive cross-talks [24]. Inside a live cell upon irradiation, multiple apoptosis pathway proteins are involved in the shifting of the balance of life and death signals. In the context of IAP-inhibitor treatment and most conventional therapy, the relative levels of individual apoptosis pathway proteins and their roles in the process of irradiation-induced cell death dictate the outcome of the cell's response to therapy. Therefore, investigation of the potential role of apoptosis pathway proteins in IAP-inhibitor-mediated sensitization will provide critical information as to how the IAP-inhibitors work in the context of radiation, and what types of cells may respond better to the therapy. The latter has clear clinical relevance in that the information will be useful to predict or select the patients who will benefit the most from the molecular therapy targeting IAPs [14, 23].
Although these BIRP proteins were all initially called IAP proteins, it is apparent that they are divided into two distinct groups based upon their binding properties to caspases and inhibition of caspase activity. The first group of mammalian BIRPs includes XIAP (BIRC4), cIAP-1 (BIRC2), cIAP-2 (BIRC3), ML-IAP (BIRC7), NAIP (BIRC1) and ILP2 (BIRC8) (Table 1). These IAP proteins potently bind to and inhibit caspase-3, -7 and -9 and function as potent apoptosis inhibitors (Figure 1). The second group of BIRPs includes the mammalian proteins Survivin (BIRC5) and Bruce (BIRC6) as well as BIR-containing proteins in yeasts and C. elegans [16] (Table 1). In contrast to the first group of BIRPs, these Survivin-like BIRPs don't bind to caspases. In addition to their potent anti-apoptotic activity, these Survivin-like BIRPs also regulate cytokinesis and mitotic spindle formation [25, 26].
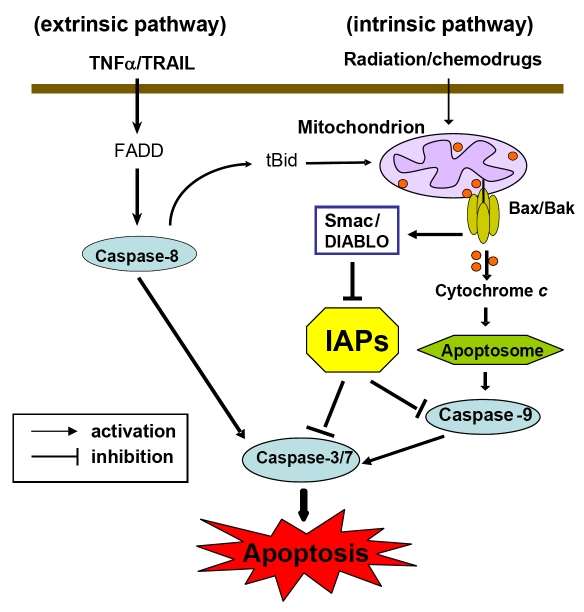
Figure 1.
Apoptosis pathways in mammalian cells. Apoptosis activation by the extrinsic pathway involves the binding of extracellular death ligands (such as TNF ligand/TRAIL) to death receptors, provoking the recruitment of adaptor proteins, such as the Fas-associated death domain protein (FADD) and recruiting caspase-8. Active caspase-8 then activates effector caspase-3 and/or -7. In some situations, extrinsic death signals can crosstalk with the intrinsic pathway through caspase-8-mediated proteolysis of BID (BH3-interacting domain death agonist). Truncated BID (tBID) can promote mitochondrial cytochrome c release and assembly of the apoptosome. In the intrinsic pathway, stresses such as radiation or chemotherapeutic agents target mitochondria and induce efflux of intermembrane space proteins, such as cytochrome c and Smac/DIABLO, into the cytosol, by the formation of BAK–BAX oligomers on mitochondrial outer membranes. On release from mitochondria, cytochrome c can join the apoptosome assembly. Active caspase-9 then propagates a cascade of further caspase activation events. The inhibitors of apoptosis proteins (IAPs) is an important class of intrinsic cellular apoptosis inhibitors that function as potent endogenous apoptosis inhibitors by directly binding to and effectively inhibiting both initiator caspase-9 and effector caspases -3/-7. Smac can neutralize IAP inhibition of caspases thus functions as an endogenous IAP-antagonist. Functional blockade of IAPs by Smac results in facilitating caspase activation and apoptosis.
X-linked IAP protein (XIAP) is the first well-characterized IAP family member due to its potent anti-apoptosis activity [17, 19, 27]. XIAP protein was found to be expressed in most of the NCI 60 human cancer cell lines [28]. Analysis of tumor samples in 78 previously untreated patients showed that those with lower levels of XIAP had significantly longer survival [28]. Two regions in XIAP confer different specificity in the inhibition of caspase-3, -7, and -9. The third BIR domain (BIR3) of XIAP selectively targets caspase-9, whereas the linker region between BIR1 and BIR2 of XIAP inhibits both caspase-3 and caspase-7, the effector caspases that triggers downstream apoptosis [23, 29] (Figure 1). While XIAP prevents the activation of all three caspases, it was shown that the interaction of XIAP with caspase-9 is the most critical for its inhibition of apoptosis [16].
In contrast to XIAP, cIAP-1 and cIAP-2 are weak caspase inhibitors in vitro [30]. Although the role of cIAP-1 and -2 in apoptosis is less defined, their function on the cellular responses other than apoptosis are widely reported. Over ten years before, several studies have proposed that both cIAP-1 and cIAP-2 were associated with the TNF receptor 1 signaling complex [31–33]. Moreover, the cIAP-1 and -2 do not directly contact TNF-receptor 2, but rather physiologically interact with TNF-receptor associated factors (TRAFs) [32], and regulate their function by mutually ubiquitination [34, 35]. Through binding to TRAF2, cIAPs are recruited to TNFR signaling complexes where they regulate the activation of caspase-8 [32, 36]. Also, cIAP-1 and cIAP-2 directly ubiquitinate RIP1 and induce constitutive RIP1 ubiquitination in cancer cells and demonstrate that constitutively ubiquitinated RIP1 associates with the prosurvival kinase TAK1 [37]. Collectively, these studies elucidate the potential role of cIAPs on regulating TNFα-induced both apoptosis and NF-κB signaling.
Second mitochondria-derived activator of Caspases (Smac) was identified as a pro-apoptotic factor released from mitochondria into the cytosol triggered by multiple apoptosis stimuli [38–41]. Upon stimulation, the released Smac physically interacts with XIAP through the N-terminal four conserved amino acid residues (AVPI) that bind to the baculoviral IAP repeat 3 (BIR3) domain of XIAP, and eliminate the inhibitory effect of XIAP on caspase activation [42–44]. Therefore, Smac functions as an endogenous IAP-antagonist. Due to the potent pro-apoptotic role of Smac, synthetic small molecule Smac-mimicking compounds (Smac-mimetics) are being developed to sensitize apoptosis-resistant cancer cells to various apoptotic stimuli [45, 46]. Smac-mimetic IAP-antagonists induce TNFα-dependent apoptosis in several transformed cell lines [47–49]. Other reports show that small molecule Smac-mimetics successfully sensitize TRAIL-induced apoptosis by blocking functions of IAPs in multiple cancer cells [38, 50–52]. Also, Smac-mimetic tetrapeptide pSmac-8c significantly sensitized androgen-independent prostate cancer cells to chemotherapeutic agents [53, 54]. These studies manifest that mimicking Smac may represent a promising strategy for restoring defective apoptosis signaling in human cancer therapy. Furthermore, it has recently been reported that Smac can potentiate apoptosis by simultaneously antagonizing caspase-IAP interactions and repressing IAP ubiquitin ligase activities [55]. Yoon, et al [56] identified a Smac-binding protein, NADE. The interaction between Smac and NADE regulates apoptosis through the inhibition of Smac ubiquitination [56]. Dr. Duckett's group reported that some cytoprotective IAPs can inhibit apoptosis through the neutralization of IAP antagonists, such as Smac, rather than by directly inhibiting caspases [27]. These recent studies suggest that endogenous Smac protein plays more complicated roles than expected in apoptosis. In a subset of highly sensitive tumor cell lines, activity of Smac mimetic compounds is dependent on TNFα signaling. Mechanistic studies indicate that in the system they tested, XIAP is a positive modulator of TNFα induction whereas cIAP-1 negatively regulates TNFα-mediated apoptosis, indicating the opposite effect of XIAP versus cIAP-1 on modulation TNFα signaling [57]. Also, Smac-mimic IAP-antagonists sensitize TRAIL-induced apoptosis by blocking XIAP function in multiple tumor models, including breast cancer [50], multiple myeloma [52], glioblastoma [38], and ovarian cancer [51].
Inhibitor of apoptosis proteins are attractive molecular targets for designing novel therapy for human cancers
XIAP is so far the most potent inhibitor of apoptosis among all the IAP proteins [17]. XIAP effectively inhibits both intrinsic and extrinsic apoptosis pathways by binding and inhibiting the initiator caspase-9 and effector caspases (caspase-3 and -7), whose activity is crucial for the execution of apoptosis [17, 58]. Because effector caspase activity is both necessary and sufficient for irrevocable programmed cell death, XIAP functions as a gatekeeper to this final stage of the process [38]. XIAP is overexpressed in many cancer cell lines and tumor tissues but not in normal cells, and a high level of XIAP results in apoptosis-resistance of cancer cells to a wide variety of therapeutic agents [59]. The multiple biological activities of XIAP, its unique translational and post-translational control and the centrality of the caspase cascade make XIAP an exceptionally promising molecular target for modulating apoptosis [14, 19, 60]. For example, overexpression of XIAP increases resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, while downregulation of XIAP restores cell response to TRAIL [61–63].
Since IAPs block apoptosis at the down-stream effector phase, a point where multiple apoptosis signaling pathways converge, strategies targeting IAP may prove to be highly effective for overcoming apoptosis-resistance in human cancers that overexpress IAPs. The link between therapy resistance and IAPs is supported by recent studies in which the suppression of XIAP levels by RNA interference or antisense indeed sensitized XIAP-overexpressing cancer cells to death receptor-induced apoptosis as well as radiation [64, 65]. Combination of irradiation and inhibition of XIAP through the antisense approach resulted in improved tumor control by radiotherapy in vivo [66], advocating a distinct role for XIAP in radiation resistant phenotype of human cancers, and providing a proof-of-concept that IAPs may be a novel and promising target for chemo/ radiosensitization of human cancers. Loss of XIAP by RNAi also sensitized cancer cells to a certain chemotherapeutic agents and TRAIL, and the increased sensitivity of the XIAP shRNA cells was correlated with enhanced Caspase activation [67]. We have found that embelin, the first non-peptidic natural XIAP inhibitor identified by us, induces apoptosis in prostate cancer cells [68]. Embelin sensitized TRAIL-induced apoptosis in pancreatic cancer cells [69]. These findings provide a strong rationale that downmodulation of overexpressed XIAP will achieve sensitization on current therapeutic modality.
Through computational structure-based 3D-database search and rational design, a series of small molecule inhibitors of IAPs have been discovered and synthesized, which show potent therapeutic activity to overcome apoptosis-resistance in vitro in cancer cells overexpressing IAPs while minimize side-effect on normal cells with relative low level of IAPs [38, 51]. Based on the high-resolution experimental 3D structure of Smac in complex with the XIAP BIR3 domain, Wang and his colleagues have designed and synthesized a group of potent non-peptidic compounds that mimic the tetra-peptide at the N-terminal of natural Smac [53, 54]. These cell-permeable compounds show at least 20-fold more potential than the natural Smac peptide in binding to the XIAP BIR3 domain in a cell-free system [53, 54, 70]. It has been shown that SH130, one of the most potential compounds, enhances ionizing radiation-induced apoptosis in vitro and combination therapy achieves significant tumor regression in hormone-refractory prostate cancer models [71]. Also, SH122, another potential compound, promotes TRAIL-mediated cell death in several human prostate cancer cell lines (Dai et al., manuscript submitted). These findings suggest that Smac-mimetic IAP-antagonist can sensitize cell-killing effect by either radiation or death-receptor therapy.
However, little is known about the role of endogenous Smac in cells treated with Smac-mimetic IAP-inhibitors and irradiation. In multiple human cancer models, full-length Smac enhanced gamma-irradiation-induced apoptosis by loss of mitochondrial membrane potential, cytochrome c release, and activation of a serial of caspases [72]. Takasawa et al. shows that the sustained release of Smac/DIABLO from mitochondria is an important event for the onset of apoptosis in keratinocytes exposed to UVB irradiation [40]. In hormone refractory prostate cancer, small molecule IAP inhibitors exhibit a promising therapeutic potential to overcome resistance of prostate cancer cells to radiation-induced growth inhibition, both in vitro and in vivo in xenograft tumor models, suggesting that targeting IAPs may be a promising approach for radiosensitization of human prostate cancer with high levels of IAPs [71], and provides important impetus for utilizing IAP-inhibitors as an adjuvant therapy for the TNFα–resistant cancers that account for the majority of patients who are refractory to radiation/chemotherapy.
NF-κB is a pro-survival factor that blocks apoptosis and promote therapeutic resistance
The transcription factor NF-κB pathway plays important roles in the control of cell proliferation, apoptosis, inflammation, cell signaling transduction, and other physiological processes [73, 74]. Because the disorder of these physiological processes has been linked with the onset of cancers, NF-κB has been described as a major culprit in cancer [74]. Current evidences have also shown that NF-κB participates in the processes of angiogenesis, invasion, and metastasis [73, 75, 76]. Moreover, NF-κB is critically involved in the processes of development and progression of cancers, raising its importance in cancer research [77]. NF-κB is constitutively activated in most of human cancers, suggesting that the activation of NF-κB is involved in the process of carcinogenesis. Therefore, targeting NF-κB is attracting more attention as a novel preventive and therapeutic strategy against human cancers.
Besides death receptors, genotoxic stress also activates NF-κB signaling pathway that attenuates apoptotic responses. Blocking NF-κB pathway can sensitize cancer cells to chemotherapeutic agents and radiation [78–82]. It has been shown that NF-κB is activated by ionizing radiation (IR)-induced double-strand breaks (DSB) [81–84] (Figure 2). IR-induced DSB directly activates ATM and PIDD (p53-inducible death domain –containing protein), which further recruit NEMO, a member of IKKs signalsome, by serial of posttranslational modification [85, 86]. Modified NEMO participates to the formation of IKK complex, and thus activates downstream NF-κB pathway through degradation of IκBα and liberate NF-κB heterodimer from cytosol into nucleus [87, 88]. On the other side, IR can directly trigger intrinsic apoptosis pathway through activation of pro-apoptotic Bcl-2 family members Bax and Bak [89, 90], and activates downstream caspase cascade and apoptosis (Figure 2).
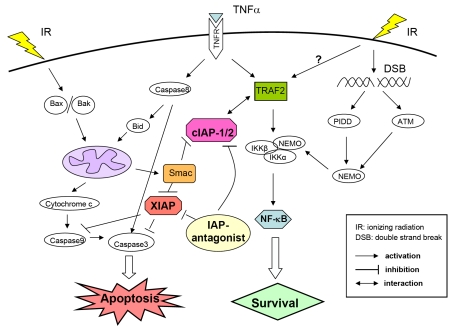
Figure 2.
Crosstalk between ionizing radiation-induced apoptosis and NF-κB pathway. IR activates both apoptosis and NF-κB signaling pathway. IR triggers instrinsic apoptosis pathway as described in Figure 1. Simultaneously, NF-κB pathway is activated by IR-induced DNA damage. IR induced double-strand breaks (DSB) directly activates initiator kinase ATM and PIDD, which further recruit NEMO, also known as IKKγ, by multiple steps of posttranslational modification. Modified NEMO joins formation of IKKs signalosome and further activates classical NF-κB pathway. Upon apoptotic stimuli (IR or TNFα), natural Smac can bind to both XIAP and cIAPs, and predominantly neutralize the suppression effect of XIAP on caspases. It has been shown that cIAP-1 physically binds to TRAF2 and regulates its function through ubiquitination in TNFα signaling. Smac-mimetics that function as IAP-antagonists, not only promote caspase activation by neutralizing IAPs, but also interferes the stability and interaction between cIAP-1/2 and TRAF2, thus modulate the balance between apoptosis and NF-κB signaling pathway.
It has been established that NF-κB is the major survival factor in preventing apoptosis, and inhibition of this transcription factor may improve the efficacy of apoptosis-inducing cancer therapies [74, 91, 92]. Due to its constitutive or treatment-induced activity, NF-κB functions mainly as an inhibitor of apoptosis and is prerequisite for cell survival. NF-κB-mediated protection of lymphoid cells from antigen receptor- and death receptor-induced apoptosis plays an instrumental role in activation of the immune response [93]. In addition, NF-κB may also protect cells from cellular stresses, such as DNA damage, which activate the mitochondrial-dependent “intrinsic” pathway [93–96]. Suppression of NF-κB by genetic or chemical inhibitors induces the apoptosis and/or restores the apoptotic response after treatment with chemotherapeutic agents or radiation in various tumor cells, thus overcoming NF-κB-mediated chemo-/radioresistance [95, 97, 98]. Moreover, the mechanisms of action of several more ancient drugs have been re-evaluated and some of them were discovered to act at least partially through NF-κB inhibition. However, the role of NF-κB in the control of apoptosis is not unambiguous and this factor is also, in some experimental conditions, required for the induction of apoptosis either of mutated cells or in response to anti-cancer agents [98–101]. Therefore, a precise knowledge of the signaling pathways controlling NF-κB as well as of the NF-κB target genes is essential in order to define who will benefit the most from the anti-NF-κB therapies. More preclinical studies are needed to determine a tolerable and efficacious dose and schedule for NF-κB inhibitors [73, 102–104]. Other NF-κB inhibitors might also have a greater effect on sensitizing anticancer agents. It is important to clarify what type of NF-κB inhibitors is most effective and least toxic.
Molecular targeting of NF-κB pathway in sensitization of conventional therapies
Many chemotherapeutic agents trigger the cell-death process through activation of the tumor-suppressor protein p53 [105]. However, NF-κB is also activated in response to treatment that attenuates p53-medieated cell death [106] with chemotherapy and radiation therapy. The NF-κB pathway thus impinges on many aspects of cell survival. Recent studies indicate that the effects of conventional cancer therapeutics could be enhanced by natural and synthetic NF-κB inhibitors, suggesting that down-regulation of NF-κB could sensitize cancer cells to conventional therapeutics [74, 103]. For example, genistein, one of three main isoflavones found in soybeans, inactivates NF-κB and leads to increased growth inhibition and apoptosis induced by various chemotherapeutic agents and promote drug-induced cell-killing effect in many types of cancers [107, 108]. Indole-3-carbinol that is produced from cruciferous vegetables, significantly inhibited NF-κB DNA binding activity in prostate and breast cancer cells, corresponding with the inhibition of cell proliferation and the induction of apoptosis [109, 110].
As it is well-established that in classical NF-κB pathway, proteasomes are responsible for IκBα degradation, which facilitates NF-κB nuclear translocation and activates multiple target gene expression [111], modulation of proteasomal function with specific inhibitors has been demonstrated as a promising strategy for the treatment of human cancers, presumably by suppression NF-κB [112, 113]. In preclinical cancer models, proteasome inhibitors alone induce apoptosis [113–115], as well as overcome radioresistance of tumor cells and enhance radiation–mediated response, typically apoptosis [114]. Bortezomib (Velcade; PS-341), the first proteasome inhibitor to be used in clinical applications, has demonstrated impressive antitumor activity, both as a single agent and in combination with conventional therapies [116–118]. More significantly, in preclinical studies, bortezomib in combination with radiation therapy has been shown to achieve potential therapeutic benefits in various cancers [119, 120]. Besides bortezomib, many other proteasome inhibitory candidates are under investigation in preclinical models, either alone or in combination with conventional therapies [121, 122]. We have found that Celastrol, a natural proteasome inhibitor, enhances therapeutic efficacy of ionizing radiation both in vitro and in vivo, typically by increasing apoptosis and decreasing both primary and acquired NF-κB activity (Dai et al., manuscript in revision). These studies provide substantial evidence that proteasome inhibitors can potentiate response of cancer cells to chemotherapeutic agents or radiation.
Crosstalks between apoptosis and NF-κB signaling by IAP-antagonist
As NF-κB has been proved to play a pivotal role in mediating cell survival, blockade of NF-κB activation can shift the tumor survival/death balance towards apoptosis, suggesting that targeting IAPs and/or NF-κB may become a potential approach to overcoming resistance of human cancer.
The cytokine TNFα elicits a wide range of biological responses, including inflammation, cell proliferation, differentiation, and apoptosis. Although the molecular mechanisms of TNF signaling have been largely elucidated, the principle that regulates the balance of life and death is still unknown. Several reviews have focused on the crosstalk that exists between proteins of the TNF receptor (TNF-R) which are involved in the initiation of NF-κB activation or apoptosis [106, 123, 124]. At least three different mechanisms of regulation can be distinguished: (i) NF-κB-mediated recruitment of TNF-R complex [125]; (ii) NF-κB-independent protection against apoptosis by the TRAF2-mediated recruitment of antiapoptotic proteins [126]; (iii) dual activation of apoptosis and NF-κB by a single molecule, such as TNFα, TRAIL and radiation [74, 98]. Overall, the multiple facets of crosstalk have been established between the apoptosis and NF-κB signaling pathways. A direct evidence is that cIAP-1 physically binds to TRAF2 through its BIR1 domain and regulates its biological function through ubiquitination [32, 127–130], suggesting a potential role of cIAP-1 on linking apoptosis and NF-κB pathways (Figure 2). Interestingly, a recent study shows that TRAF2-knockdown by siRNA indeed radiosensitizes cancer cells via reduced NF-κB activation, suggesting that TRAF2 is an attractive drug target for anticancer therapy and radiosensitization [131]. Function as an adaptor protein in NF-κB signaling, TRAF2 may thus play a potential role in protecting radiation-induced cell death, and establish the crosstalk between NF-κB and DNA damage-induced apoptosis (Figure 2).
Studies in recent two years tend to elucidate the potential mechanism in TNFα signaling by Smac-mimetics in cancer cells. It has been demonstrated that Smac-mimetics stimulate autoubiquitination of cIAPs, resulting in their proteasomal degradation [30, 48] (Figure 3). This in turn leads to NIK stabilization and facilitates RIP1 recruitment [49, 132] (Figure 3). Moreover, cIAP-1 and cIAP-2 promote cancer cell survival by functioning as E3 ubiquitin ligases that maintain constitutive ubiquitination of the RIP1 adaptor protein, suggesting that constitutively ubiquitinated RIP1 associates with the prosurvival kinase TAK1 [37]. Collective reports demonstrate that either cIAP-1 or 2 is required for proper RIP1 polyubiquitination and NF-κB activation upon TNFα treatment [37, 132] (Figure 3). This results in the activation of the noncanonical and canonical NF-κB pathways, causing autocrine TNFα production in a substantial number of tumor cells. Besides cIAPs, a recent study proposes that BIR1 domain of XIAP, which has no previously ascribed function, directly interacts with TAB1 to induce NF-κB activation [133] (Figure 3). Smac, the antagonist for caspase inhibition by XIAP, also inhibits the XIAP/TAB1 interaction. Disruption of BIR1 dimerization abolishes XIAP-mediated NF-κB activation, implicating a proximity-induced mechanism for TAK1 activation [133]. Taken together, mounting experimental evidence indicate that IAPs function as “bridging” molecules that mediate cross-talk between the apoptosis pathway and NF-κB pathway.
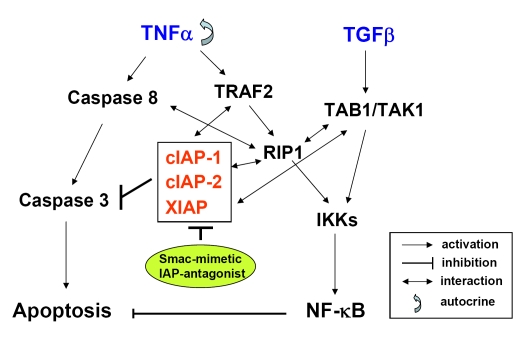
Figure 3.
Crosstalk between apoptosis and NF-κB signaling by IAP-antagonist. TNFα induces both apoptosis and NF-κB activation in cancer cells. TNFα induces extrinsic apoptosis via activation of caspase cascade that involves initiator Caspase-8 and effector Caspase-3. IAPs (XIAP, cIAP-1 and cIAP-2) are anti-apoptotic proteins that block caspase-mediated apoptosis. Paradoxically, TNFα still activates NF-κB and promotes cell survival that counteracts with apoptosis. The classical NF-κB pathway that is mediated by IKKs signalosome can be triggered either by TNFα via TRAF2 and RIP1, or alternatively, by TGFβ via TAB1 and TAK1. Multiple studies have established crosstalk between IAPs and proteins that mediate NF-κB signaling pathway (see text). Smac mimetics that function as IAP antagonists can modulate the interaction between IAP proteins and NF-κB signaling proteins, which leads to TNFα-triggered apoptosis primarily via an autocrine manner (see text). TNFα, tumor necrosis factor alpha; TGFβ, transforming growth factor beta; TRAF2, TNF receptor-associated factor 2; RIP1, receptor interacting protein 1; TAK1, TGFβ activated kinase 1; TAB1, TAK1 bind protein 1; IKK, IκBα kinase.
As multiple recent studies suggest the role of IAPs on the crosstalks between apoptosis and NF-κB pathway [37, 47–49, 57, 132–134] (Figure 3), it is reasonable to speculate that an IAP inhibitor may also function as a modulator in regulating such crosstalk. Indeed, several Smac-mimetic IAP-inhibitors can induce TNFα-dependent apoptosis in several transformed cell lines, via cIAP-1 down-regulation and NF-κB activation [47–49]. Blocking NF-κB activation reduced TNF production and protected cells from Smac-mimetics-induced cell death (Figure 2). Ahn et al. suggests that embelin sequentially inhibits NF-κB activation induced by TNFα at the IκBα kinase (IKK), IκBα degradation, and RelA nuclear translocation levels in several cancer cell lines [135]. Other studies show that Smac mimetics lead to sensitivity to TNFα-induced cell death, likely through the degradation of cIAPs and by favoring the formation of a RIP1-dependent caspase-8-activating complex [136]. Highlighting the potential of Smac mimetics for clinical application will validate that cancer cells that are sensitive to Smac-mimetic treatment in vitro are also responsive to the same treatment in an in vivo mouse model. AEG40730, a Smac mimetic dimer compound, binds to cIAP-1 and cIAP-2, facilitates their autoubiquitination and proteasomal degradation, and causes a dramatic reduction in RIP1 ubiquitination [37]. When deubiquitinated by AEG40730 treatment, RIP1 binds caspase-8 and induces apoptosis in cancer cells [37]. In addition, in Smac-mimetic sensitive cell lines, apoptosis caused by Smac-mimetics is blocked by the caspase-8 inhibitor crmA and that IAP antagonists activate NF-κB signaling via inhibition of cIAP-1 [48]. In those transformed tumor lines, IAP antagonist induced NF-κB-stimulated production of TNFα that killed cells in an autocrine fashion. Inhibition of NF-κB reduced TNFα production, and blocking NF-κB activation or TNFα allowed tumor cells to survive Smac-mimetic-induced apoptosis [48]. Moreover, in a subset of highly sensitive tumor cell lines, XIAP is a positive modulator of TNFα production whereas cIAP-1 negatively regulates TNFα-mediated apoptosis [57]. It is interesting that Smac mimetics induced degradation of cIAPs in certain types of cancer cell lines, suggesting that additional switch points control the sensitivity to Smac mimetics [30].
In a vast amount of solid tumors that are resistant to therapeutics, for example, most of androgen-independent human prostate cancers exert highly constitutive NF-κB activity [75, 137] that may result in resistance to TNFα. How these majority resistant cancer cells respond to IAP-inhibitors remains to be investigated. In our recent publication [71], we found that those androgen-independent prostate cancer cells are highly resistant to Smac-mimetics, which has a IAP-binding affinity comparable to that of the compounds used in other reports [47–49]. Surprisingly, although Smac-mimetics alone hardly show any cell-killing effect both in vitro and in vivo, they potently sensitize those TNFα–resistant cells to radiation-induced growth inhibition and apoptosis, which might involve blocking radiation-induced NF-κB activation [71]. Moreover, such Smac-mimetic compound treatment does not induce cIAP-1 degradation, TNFα upregulation and NF-κB activation when used alone in those resistant cells [71]. This mode of action of Smac-mimetic IAP-inhibitor is distinct from that in TNFα–sensitive cells reported recently [47–49] . Similarly, another study indicates that blockade of IAPs by a small molecule Smac-mimetic compound promotes TRAIL-induced apoptosis in TRAIL-resistant prostate cancer cells, via modulating both the apoptosis pathway and NF-κB pathway (Dai et al, manuscript submitted). This discrepancy of the mechanisms of Smac-mimetic IAP-inhibitors in chemo/radiosensitization has significant clinical implications and provides important impetus for utilizing IAP-inhibitors as an adjuvant therapy for the TNFα–resistant, NF-κB constitutively active cancers that account for the majority of patients who are refractory to current therapeutic approaches.
Conclusion and perspective
Conventional chemo/radiotherapies the most commonly used current therapies for cancer patients, however primary or acquired resistance remains to be a major challenge in clinic and is emerging as a significant impediment to effective cancer treatment. Although both IAPs and NF-κB pathways have been subjected to intense preclinical and clinical studies, the detailed clinically relevant correlation between these two classes of proteins still remains unclear. Answers to such question will provide a clear rationale for combining Smac-mimetic IAP-antagonists with conventional therapy that will achieve a significantly improved therapeutic outcome.
Mounting evidences have established the potential crosstalks between IAPs (eg. XIAP, cIAP-1, cIAP-2) and the proteins that are involved in NF-κB signaling (eg. TRAF2, RIP1, TAB1). As Smac functions as an endogenous IAP inhibitor, small molecule Smac-mimetics are believed to neutralize IAPs function that promotes apoptosis. However, Smac-mimetics may kill cancer cells in a different manner, which involves inducing ubiquitination of cIAPs, regulating NF-κB signaling and facilitating TNFα-triggered, caspase-8-mediated apoptosis in a certain cancer cell types. In other cancer cells that are resistant to TNFα or chemo/radiotherapy, exhibit a promising therapeutic potential to overcome resistance of cancer cells to radiation therapy, at least in part, by suppressing NF-κB activation. For example, in hormone-refractory prostate cancer, molecular modulation of IAPs and/or NF-κB has been proven to be a novel adjuvant approach to enhance the efficacy of radiation therapy [14, 15, 71, 73, 75, 138]. Therefore, IAP antagonists may be developed as a personalized medicine in IAP-targeting molecular therapy and establish promising novel strategy to overcome the resistance to current therapies, with the ultimate goal of improving the survival of cancer patients who will benefit the most from the individualized therapy modality. This strategy may also benefit patients of other malignancies with high levels of IAPs and high constitutive NF-κB activity in a clinical setting.
In the clinic, continued treatment with potent IAP-inhibitors throughout the cycles of chemo/radiotherapy may help to further reduce or eliminate the “minimal residual disease”, thus may reduce the risk of tumor local recurrence as well as metastasis. A rationale patient screen based on individual genetic background will help to optimize the personalized therapeutic advantage. Therefore, the combination of IAP-targeting molecular therapy and conventional chemo/ radiotherapy may become a promising novel strategy to enhance the efficacy of current cancer treatments and ultimately improve the survival of cancer patients.
Acknowledgments
This study was supported in part by grants from Department of Defense Prostate Cancer Research Program W81XWH-06-1-0010 (to L. X.), NIH R01 CA121830-01 and R21 CA128220-01 (to L. X.).
References
- 1.Ponder BA. Cancer genetics. Nature. 2001;411:336–41. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- 2.Kumar MV, Shirley R, Ma Y, Lewis RW. Role of genomics-based strategies in overcoming chemotherapeutic resistance. Curr Pharm Biotechnol. 2004;5:471–80. doi: 10.2174/1389201043376698. [DOI] [PubMed] [Google Scholar]
- 3.Hall EJ. Radiobiology for the Radiologist. Fourth edition. Philadelphia: J.B.Lippincott Company; 1994. [Google Scholar]
- 4.Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends Cell Biol. 2001;11:S22–6. doi: 10.1016/s0962-8924(01)02124-9. [DOI] [PubMed] [Google Scholar]
- 5.Ikuta K, Takemura K, Kihara M, Naito S, Lee E, Shimizu E, Yamauchi A. Defects in apoptotic signal transduction in cisplatin-resistant non-small cell lung cancer cells. Oncol Rep. 2005;13:1229–34. [PubMed] [Google Scholar]
- 6.Li J, Feng Q, Kim JM, Schneiderman D, Liston P, Li M, Vanderhyden B, Faught W, Fung MF, Senterman M, Korneluk RG, Tsang BK. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–80. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 7.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 8.Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–21. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence TS, Davis MA, Hough A, Rehemtulla A. The Role of Apoptosis in 2′,2′-Difluoro-2′-deoxycytidine (Gemcitabine)-mediated Radiosensitization. Clin Cancer Res. 2001;7:314–9. [PubMed] [Google Scholar]
- 10.Algan O, Stobbe CC, Helt AM, Hanks GE, Chapman JD. Radiation inactivation of human prostate cancer cells: the role of apoptosis. Radiat Res. 1996;146:267–75. [PubMed] [Google Scholar]
- 11.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–7. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM, Huang W, Cruz I, Yin Y, Chang EH. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther. 2002;13:469–81. doi: 10.1089/10430340252792594. [DOI] [PubMed] [Google Scholar]
- 13.DiPaola RS, Patel J, Rafi MM. Targeting apoptosis in prostate cancer. Hematol Oncol Clin North Am. 2001;15:509–24. doi: 10.1016/s0889-8588(05)70229-x. [DOI] [PubMed] [Google Scholar]
- 14.Devi GR. XIAP as target for therapeutic apoptosis in prostate cancer. Drug News Perspect. 2004;17:127–34. doi: 10.1358/dnp.2004.17.2.829046. [DOI] [PubMed] [Google Scholar]
- 15.Watson RW, Fitzpatrick JM. Targeting apoptosis in prostate cancer: focus on caspases and inhibitors of apoptosis proteins. BJU Int. 2005;96 Suppl 2:30–4. doi: 10.1111/j.1464-410X.2005.05944.x. [DOI] [PubMed] [Google Scholar]
- 16.Schimmer AD. Inhibitor of Apoptosis Proteins: Translating Basic Knowledge into Clinical Practice. Cancer Res. 2004;64:7183–90. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 17.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–4. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 18.Deveraux QL, Stennicke HR, Salvesen GS, Reed JC. Endogenous inhibitors of caspases. J Clin Immunol. 1999;19:388–98. doi: 10.1023/a:1020502800208. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell. 2008;30:123–35. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 21.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–68. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 22.Zangemeister-Wittke U, Simon HU. An IAP in action: the multiple roles of survivin in differentiation, immunity and malignancy. Cell Cycle. 2004;3:1121–3. [PubMed] [Google Scholar]
- 23.Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–61. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Yuan R, Serggio L. Molecular mechanisms of irradiation-induced apoptosis. Front Biosci. 2003;8:d9–19. doi: 10.2741/927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Welm A, Bishop JM. Cell division and cell survival in the absence of survivin. Proc Natl Acad Sci U S A. 2004;101:15100–5. doi: 10.1073/pnas.0406665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–76. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol. 2004;24:7003–14. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamm I, Trepel M, Cardó-Vila M, Sun Y, Welsh K, Cabezas E, Swatterthwait A, Arap W, Reed JC, Pasqualini R. Peptides targeting caspase inhibitors. J Biol Chem. 2003;278:14401–5. doi: 10.1074/jbc.M210133200. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. Journal of Biological Chemistry. 1998;273:7787–90. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, Tschopp J, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison? Cell. 2007;131:655–8. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci U S A. 1996;93:4974–8. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–52. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins CJ, Ekert PG, Uren AG, Holmgreen SP, Vaux DL. Anti-apoptotic potential of insect cellular and viral IAPs in mammalian cells. Cell Death Differ. 1998;5:569–76. doi: 10.1038/sj.cdd.4400389. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–7. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 35.Samuel T WK, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–90. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 36.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 37.Bertrand MJ, Milutinovic S, Dickson KM, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 39.Irmler M, Steiner V, Ruegg C, Wajant H, Tschopp J. Caspase-induced inactivation of the anti-apoptotic TRAF1 during Fas ligand-mediated apoptosis. FEBS Lett. 2000;468:129–33. doi: 10.1016/s0014-5793(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 40.Takasawa R, Tanuma S. Sustained release of Smac/DIABLO from mitochondria commits to undergo UVB-induced apoptosis. Apoptosis. 2003;8:291–9. doi: 10.1023/a:1023629023696. [DOI] [PubMed] [Google Scholar]
- 41.Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–85. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- 42.Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–61. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 43.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–12. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–8. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 45.Bucur O, Ray S, Bucur MC, Almasan A. APO2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in prostate cancer therapy. Front Biosci. 2006;11:1549–68. doi: 10.2741/1903. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasula SM, Ashwell JD. IAPs: What's in a Name? Mol Cell. 2008;30:123–35. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 49.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–8. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 51.Petrucci E, Pasquini L, Petronelli A, Saulle E, Mariani G, Riccioni R, Biffoni M, Ferretti G, Benedetti-Panici P, Cognetti F, Scambia G, Humphreys R, Peschle C, Testa U. A small molecule Smac mimic potentiates TRAIL-mediated cell death of ovarian cancer cells. Gynecol Oncol. 2007;105:481–92. doi: 10.1016/j.ygyno.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–7. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Tomita Y, Krajewski K, Roller PP, Wang S. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–50. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Liu M, Tomita Y, Pan H, Yoshioka Y, Krajewski K, Roller PP, Wang S. Structure-based design of potent, conformationally constrained Smac mimetics. J Am Chem Soc. 2004;126:16686–7. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]
- 55.Creagh EM, Murphy BM, Duriez PJ, Duckett CS, Martin SJ. Smac/Diablo antagonizes ubiquitin ligase activity of inhibitor of apoptosis proteins. J Biol Chem. 2004;279:26906–14. doi: 10.1074/jbc.M313859200. [DOI] [PubMed] [Google Scholar]
- 56.Yoon K, Jang HD, Lee SY. Direct interaction of Smac with NADE promotes TRAIL-induced apoptosis. Biochem Biophys Res Commun. 2004;319:649–54. doi: 10.1016/j.bbrc.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 57.Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, Sage D, Slisz J, Tran M, Straub C, Ramsey T, Iourgenko V, Huang A, Chen Y, Schlegel R, Labow M, Fawell S, Sellers WR, Zawel L. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–8. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis.[comment][erratum appears in Nature 2001 Jun 28;411(6841):1081] Nature. 2001;410:112–6. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 59.Huang Q, Deveraux QL, Maeda S, Stennicke HR, Hammock BD, Reed JC. Cloning and characterization of an inhibitor of apoptosis protein (IAP) from Bombyx mori. Biochim Biophys Acta. 2001;1499:191–8. doi: 10.1016/s0167-4889(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 60.Vucic D. Targeting IAP (inhibitor of apoptosis) proteins for therapeutic intervention in tumors. Curr Cancer Drug Targets. 2008;8:110–7. doi: 10.2174/156800908783769373. [DOI] [PubMed] [Google Scholar]
- 61.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–23. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 63.Johnson RW, A.J. F, M.J. S The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 64.Ohnishi K, Scuric Z, Schiestl RH, Okamoto N, Takahashi A, Ohnishi T. siRNA targeting NBS1 or XIAP increases radiation sensitivity of human cancer cells independent of TP53 status. Radiat Res. 2006;166:454–62. doi: 10.1667/RR3606.1. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi Y, Shiraki K, Fuke H, Inoue T, Miyashita K, Yamanaka Y, Saitou Y, Sugimoto K, Nakano T. Targeting of X-linked inhibitor of apoptosis protein or survivin by short interfering RNAs sensitize hepatoma cells to TNF-related apoptosis-inducing ligand- and chemotherapeutic agent-induced cell death. Oncol Rep. 2005;14:1311–6. [PubMed] [Google Scholar]
- 66.Cao C, Mu Y, Hallahan DE, Lu B. XIAP and survivin as therapeutic targets for radiation sensitization in preclinical models of lung cancer. Oncogene. 2004;23:7047–52. doi: 10.1038/sj.onc.1207929. [DOI] [PubMed] [Google Scholar]
- 67.McManus DC, Lefebvre CA, Cherton-Horvat G, et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105–17. doi: 10.1038/sj.onc.1207967. [DOI] [PubMed] [Google Scholar]
- 68.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, Lippman ME, Yang D, Wang S. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–40. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 69.Naumann U, Bähr O, Wolburg H, Altenberend S, Wick W, Liston P, Ashkenazi A, Weller M. Adenoviral expression of XIAP antisense RNA induces apoptosis in glioma cells and suppresses the growth of xenografts in nude mice. Gene Ther. 2007;14:147–61. doi: 10.1038/sj.gt.3302845. [DOI] [PubMed] [Google Scholar]
- 70.Sun H, Nikolovska-Coleska Z, Lu J, Qiu S, Yang CY, Gao W, Meagher J, Stuckey J, Wang S. Design, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimetic. J Med Chem. 2006;49:7916–20. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]
- 71.Dai Y, Liu ML, Tang WH, et al. Molecularly Targeted Radiosensitization of Human Prostate Cancer by Modulating Inhibitor of Apoptosis. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0188. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S. Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res. 2005;65:10502–13. doi: 10.1158/0008-5472.CAN-05-0866. [DOI] [PubMed] [Google Scholar]
- 73.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarkar FH, Li Y. NF-kappaB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–9. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 75.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–17. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 76.Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–8. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 77.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 78.Magne N TR, Bottero V, Didelot C, Houtte PV, Gerard JP, Peyron JF. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–68. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Voboril R, Weberova-Voborilova J. Constitutive NF-kappaB activity in colorectal cancer cells: impact on radiation-induced NF-kappaB activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53:518–23. [PubMed] [Google Scholar]
- 80.Rho HS, Kim SH, Lee CE. Mechanism of NF-kappaB activation induced by gamma-irradiation in B lymphoma cells: role of Ras. J Toxicol Environ Health A. 2005;68:2019–31. doi: 10.1080/15287390491009129. [DOI] [PubMed] [Google Scholar]
- 81.Kim BY, Kim KA, Kwon O, Kim SO, Kim MS, Kim BS, Oh WK, Kim GD, Jung M, Ahn JS. NF-kappaB inhibition radiosensitizes Ki-Ras-transformed cells to ionizing radiation. Carcinogenesis. 2005;26:1395–403. doi: 10.1093/carcin/bgi081. [DOI] [PubMed] [Google Scholar]
- 82.Habraken Y, Piette J. NF-kappaB activation by double-strand breaks. Biochem Pharmacol. 2006;72:1132–41. doi: 10.1016/j.bcp.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 83.Kim KM, Zhang Y, Kim BY, Jeong SJ, Lee SA, Kim GD, Dritschilo A, Jung M. The p65 subunit of nuclear factor-kappaB is a molecular target for radiation sensitization of human squamous carcinoma cells. Mol Cancer Ther. 2004;3:693–8. [PubMed] [Google Scholar]
- 84.Russo SM, Tepper JE, Baldwin AS, Jr, Liu R, Adams J, Elliott P, Cusack JC., Jr Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50:183–93. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 85.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell. 2005;123:1079–92. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed KM, Li JJ. ATM-NF-kappaB connection as a target for tumor radiosensitization. Curr Cancer Drug Targets. 2007;7:335–42. doi: 10.2174/156800907780809769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wuerzberger-Davis SM, Nakamura Y, Seufzer BJ, Miyamoto S. NF-kappaB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene. 2007;26:641–51. doi: 10.1038/sj.onc.1209815. [DOI] [PubMed] [Google Scholar]
- 88.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–6. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 89.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death.[see comment] Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–4. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 92.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer–role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–82. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 93.Ravi R, Bedi A. NF-kappaB in cancer–a friend turned foe. Drug Resist Updat. 2004;7:53–67. doi: 10.1016/j.drup.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 95.Wang CY, Cusack JC, Jr., Liu R, Baldwin AS., Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 96.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–9. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bentires-Alj M, Barbu V, Fillet M, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–7. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 98.Olivier S, Robe P, Bours V. Can NF-kappaB be a target for novel and efficient anti-cancer agents? Biochem Pharmacol. 2006;72:1054–68. doi: 10.1016/j.bcp.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 99.Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol Cell. 2004;13:853–65. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 100.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–43. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 101.Ho WC, Dickson KM, Barker PA. Nuclear factor-kappaB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-kappaB-dependent transcription in cancer cells. Cancer Res. 2005;65:4273–81. doi: 10.1158/0008-5472.CAN-04-3494. [DOI] [PubMed] [Google Scholar]
- 102.Tabruyn SP, Griffioen AW. NF-kappa B: a new player in angiostatic therapy. Angiogenesis. 2008;11:101–6. doi: 10.1007/s10456-008-9094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 104.Montagut C, Rovira A, Albanell J. The proteasome: a novel target for anticancer therapy. Clin Transl Oncol. 2006;8:313–7. doi: 10.1007/s12094-006-0176-8. [DOI] [PubMed] [Google Scholar]
- 105.Chen L, Yin H, Farooqi B, Sebti S, Hamilton AD, Chen J. p53 {alpha}-Helix mimetics antagonize p53/MDM2 interaction and activate p53. Mol Cancer Ther. 2005;4:1019–25. doi: 10.1158/1535-7163.MCT-04-0342. [DOI] [PubMed] [Google Scholar]
- 106.Tergaonkar V, Perkins ND. p53 and NF-kappaB crosstalk: IKKalpha tips the balance. Mol Cell. 2007;26:158–9. doi: 10.1016/j.molcel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 107.Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–72. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–42. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci. 2005;10:236–43. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- 110.Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–36. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 111.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 112.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 113.Zavrski I, Kleeberg L, Kaiser M, Fleissner C, Heider U, Sterz J, Jakob C, Sezer O. Proteasome as an emerging therapeutic target in cancer. Curr Pharm Des. 2007;13:471–85. doi: 10.2174/138161207780162908. [DOI] [PubMed] [Google Scholar]
- 114.Voorhees PM, Dees EC, O'Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–25. [PubMed] [Google Scholar]
- 115.Moran E, Nencioni A. The role of proteasome in malignant diseases. J Buon. 2007;12 Suppl 1:S95–9. [PubMed] [Google Scholar]
- 116.Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P. Proteasome inhibitors: antitumor effects and beyond. Leukemia. 2007;21:30–6. doi: 10.1038/sj.leu.2404444. [DOI] [PubMed] [Google Scholar]
- 117.Montagut C, Rovira A, Mellado B, Gascon P, Ross JS, Albanell J. Preclinical and clinical development of the proteasome inhibitor bortezomib in cancer treatment. Drugs Today (Barc) 2005;41:299–315. doi: 10.1358/dot.2005.41.5.893706. [DOI] [PubMed] [Google Scholar]
- 118.Milano A, Iaffaioli RV, Caponigro F. The proteasome: a worthwhile target for the treatment of solid tumours? Eur J Cancer. 2007;43:1125–33. doi: 10.1016/j.ejca.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Saylor M, Wen S, Silva MD, Rolfe M, Bolen J, Muir C, Reimer C, Chandra S. Longitudinally quantitative 2-deoxy-2-[18F]fluoro-D-glucose micro positron emission tomography imaging for efficacy of new anticancer drugs: a case study with bortezomib in prostate cancer murine model. Mol Imaging Biol. 2006;8:300–8. doi: 10.1007/s11307-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 120.Papandreou CN, Logothetis CJ. Bortezomib as a potential treatment for prostate cancer. Cancer Res. 2004;64:5036–43. doi: 10.1158/0008-5472.CAN-03-2707. [DOI] [PubMed] [Google Scholar]
- 121.Sorolla A, Yeramian A, Dolcet X, Pérez de Santos AM, Llobet D, Schoenenberger JA, Casanova JM, Soria X, Egido R, Llombart A, Vilella R, Matias-Guiu X, Marti RM. Effect of proteasome inhibitors on proliferation and apoptosis of human cutaneous melanoma-derived cell lines. Br J Dermatol. 2008;158:496–504. doi: 10.1111/j.1365-2133.2007.08390.x. [DOI] [PubMed] [Google Scholar]
- 122.Grimes KR, Daosukho C, Zhao Y, Meigooni A, St Clair W. Proteasome inhibition improves fractionated radiation treatment against non-small cell lung cancer: an antioxidant connection. Int J Oncol. 2005;27:1047–52. [PubMed] [Google Scholar]
- 123.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 124.Heyninck K, Beyaert R. Crosstalk between NF-kappaB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol Cell Biol Res Commun. 2001;4:259–65. doi: 10.1006/mcbr.2001.0295. [DOI] [PubMed] [Google Scholar]
- 125.Sheikh MS, Huang Y. Death receptor activation complexes: it takes two to activate TNF receptor 1. Cell Cycle. 2003;2:550–2. [PubMed] [Google Scholar]
- 126.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 127.Wu CJ, Conze DB, Li X, Ying SX, Hanover JA, Ashwell JD. TNF-alpha induced c-IAP1/TRAF2 complex translocation to a Ubc6-containing compartment and TRAF2 ubiquitination. EMBO J. 2005;24:1886–98. doi: 10.1038/sj.emboj.7600649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–7. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 129.Xia ZP, Chen ZJ. TRAF2: a double-edged sword? Sci STKE. 2005;272:pe7. doi: 10.1126/stke.2722005pe7. [DOI] [PubMed] [Google Scholar]
- 130.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng M, Morgan-Lappe SE, Yang J, Bockbrader KM, Pamarthy D, Thomas D, Fesik SW, Sun Y. Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008;68:7570–8. doi: 10.1158/0008-5472.CAN-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 Are Critical Mediators of Tumor Necrosis Factor {alpha} (TNF{alpha})-induced NF-{kappa}B Activation. J Biol Chem. 2008;283:24295–9. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu M, Lin SC, Huang Y, Kang YJ, Rich R, Lo YC, Myszka D, Han J, Wu H. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–83. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–19. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 136.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 137.Lee EC, Tenniswood M. Programmed cell death and survival pathways in prostate cancer cells. Arch Androl. 2004;50:27–32. doi: 10.1080/01485010490250498. [DOI] [PubMed] [Google Scholar]
- 138.Chi KN, Gleave ME. Antisense approaches in prostate cancer. Expert Opin Biol Ther. 2004;4:927–36. doi: 10.1517/14712598.4.6.927. [DOI] [PubMed] [Google Scholar]