Abstract
The effectiveness of hair bundle motility in mammalian and avian ears is studied by examining energy balance for a small sinusoidal displacement of the hair bundle. The condition that the energy generated by a hair bundle must be greater than energy loss due to the shear in the subtectorial gap per hair bundle leads to a limiting frequency that can be supported by hair-bundle motility. Limiting frequencies are obtained for two motile mechanisms for fast adaptation, the channel re-closure model and a model that assumes that fast adaptation is an interplay between gating of the channel and the myosin motor. The limiting frequency obtained for each of these models is an increasing function of a factor that is determined by the morphology of hair bundles and the cochlea. Primarily due to the higher density of hair cells in the avian inner ear, this factor is ∼10-fold greater for the avian ear than the mammalian ear, which has much higher auditory frequency limit. This result is consistent with a much greater significance of hair bundle motility in the avian ear than that in the mammalian ear.
Introduction
With the mechanoelectric transducer (MET) channel strategically placed in their hair bundles, hair cells effectively convert mechanical signal into electrical signal. This transduction is supported by reverse transduction in hair cells that generates force in response to mechanical stimuli. Such a reciprocal process has been predicted by Gold (1) in 1948 as the requirement for counteracting viscous damping for the ear's performance. In recent studies, this effect is recognized as the basis of the cochlear amplifier (2,3), which is critical for the sensitivity and frequency selectivity of the ear in mammals (4–7) as well as in other vertebrates (8,9). Those motile responses of hair cells include electromotility in the cell body of outer hair cells (10–12), which is specific to the mammalian ear, and the motility called fast adaptation in hair bundles themselves (13–17), which is not specific to any animal species.
For the mammalian ear where outer hair cells with two motile mechanisms could be involved in reverse transduction, the relative significance of the two mechanisms is an important issue (18,19). The importance of electromotility is supported by the hearing deficit of mice, which have mutant prestin, the protein essential for electromotility (20), with its functional range outside the physiological range of the membrane potential (21). The significance of fast adaptation is supported by an innbsp;vitro experiment that showed the importance of Ca2+ entry through the transducer channels into hair cells on the vibration of the basilar membrane (19). Because the ear of nonmammalian vertebrates lacks electromotility, it has been assumed that hair-bundle motility is the basis of the cochlear amplifier in those animals (8,9).
Here we examine the effectiveness of two models for hair-bundle motility, which can function as the cochlear amplifier in the mammalian ear and the avian ear. One of the motile mechanisms, which is usually referred to as the channel re-closure model, assumes that Ca2+-binding to the cytosolic side of the MET channel on channel opening leads to closing of the channel (22). With this model, spontaneous oscillation and signal amplification by an individual hair bundle are described (22). This motile mechanism uses chemical energy in the form of Ca2+ gradient across the plasma membrane. This fast mechanism, which is called fast adaptation, is separate from myosin-based slow motility or slow adaptation (23–25), which controls the operating point of the MET channel.
Another model proposed by Tinevez etnbsp;al. (26) assumes that fast adaptation is not an independent phenomenon but it is the result of interplay between gating of the MET channel and ATP-dependent myosin motor, which is responsible for slow adaptation (23–25). This model (26) specifically assumes that myosin is a force generator with a built-in viscoelastic property. Let us tentatively refer to this model, for brevity, as the interplay model.
To study the effectiveness with which hair bundles function as an amplifier, previous treatments used equations of motion for the hair bundle and followed the amplitude (22,26). In those treatments, the amplitude is determined by a nonlinear term that appears in the local resonance. In this report, instead of solving equations of motion, we impose a small sinusoidal displacement on a hair bundle with a certain frequency and evaluate the work done by the motor in the hair bundle. If the work done by hair bundles exceeds energy loss by viscous damping, energy output is greater than energy input and the hair bundle can function as an amplifier. The validity of this condition is not limited to local resonance (27). The method of this comparison is similar to a previous attempt to examine the efficiency of electromotility (28).
This approach has several advantages. First, it is much simpler because we need to consider only linear terms in the perturbation method. Second, the effect of cochlear amplifier is significant only where the amplitude is small. Third, with small amplitude stimulation, the operating point will not be subjected to the effect of slow adaptation. This justifies omitting slow adaptation in the model. The main disadvantage is that with this method we only consider a necessary condition for amplification and not the sufficient condition. In addition, we cannot obtain such details of the amplifier gain, which depends on the nonlinearity of the system. When energy output exceeds energy input at a small amplitude, the oscillation grows out of linear range until the growth is stopped by the nonlinearity of the system.
Initially we describe the basic assumptions and an outline of the method. Next, we examine the effectiveness of a simplified channel re-closure model and interplay model as the cochlear amplifier. After examining these two mechanisms, we discuss their implications.
Assumptions
In the following we list and briefly describe our major assumptions. The assumptions specific to each model for hair bundle motility are described later.
Regarding the geometry of the hair bundle and the mechano-electric transducer (MET) channel, we make the following assumptions:
-
1.
The structure of the hair bundle imposes an equal displacement to each tip-link in the bundle. This assumption allows our describing displacement of the hair bundle as if it has one tip-link (29). This condition can lead to negative stiffness of the hair bundles (30). To be precise, this displacement of tip-link is a displacement of tip-link assembly, which includes elements associated with tip link such as the MET and elastic elements other than tip-link itself. We do not discuss how the complex structure of a hair bundle can have such a property (31–33).
-
2.
One MET channel with two states, open and closed, is associated with each tip link. This assumption is required to explain gating compliance and is in line with most theoretical treatments (26,29,34,35). Some experimental data are analyzed with one MET channel with three states, two closed and one open (36,37), which can be re-interpreted as two interacting two state channels (38). We do not consider such complex models here.
-
3.
An MET is in series connection with a myosin motor, which interacts with actin filaments in the hair bundle and maintains the operating point of the MET channel.
In addition, we make the following assumptions to simplify our analysis regarding the effectiveness of hair bundle motility:
-
4.The amplitude of tip-link displacement is periodic and small. We impose a tip-link displacement of angular frequency ω and amplitude δX,
where is the steady-state value before stimulation and . The force Fhb elicited in the hair bundle depends on the model, as will be described later. The leading term is linear in each model for the small amplitude stimulation. The mechanical energy generated by the hair bundle per cycle is then(1)
which is proportional to δX2.(2) -
5.
The dominant viscous drag is due to shear in the gap between the tectorial membrane and the reticular lamina (subtectorial gap). That has been suggested for the mammalian cochlea (39), where surface of the tectorial membrane that faces the reticular lamina is smooth and planar. The morphology of the avian tectorial membrane is not as certain. Electron micrographs show cavities (domes) in the avian tectorial membrane near its contactnbsp;points with hair bundles and a thin structure of the tectorial membrane that descends to the microvilli surrounding each hair cell (40). However, fixation artifacts in those electron micrograph preparations of the avian tectorial membrane have been suggested (41,42). Here we evaluate the viscous loss in the avian ear in a manner similar to the mammalian ear. The assumptions involved would be that the thin structure of the tectorial membrane that surrounds each hair cell is thin enough not to have a significant internal shear, and that the dome above each hair bundle has no significant effect on viscous drag. The former assumption would lead to underestimation and the latter to overestimation of the drag.
-
The tallest row stereocilia in the hair bundles of mammalian outer hair cells and the tallest row stereocilia of all avian hair cells are firmly attached to the tectorial membrane, capable of exerting force generated by those hair bundles. In those systems, the shear of the subtectorial gap is the same as the displacement Xs at the tip of the hair bundle, which is related to the displacement X at the tip-link by a geometrical factor g, i.e., Xs = X/g. For small displacement, we may use g ≈ s/h where s is rootlet separation and h the height of the tallest stereocilia (43).
The gap, which can be approximated with the height h ofnbsp;the tallest stereocilia, is less than the thickness of a boundary layer (28). Thus, viscous drag Fd of the subtectorial gap per hair cell is proportional to the shear velocity, - Here η is the viscosity of an external medium and A the area of the subtectorial gap per hair cell. For sinusoidal hair bundle displacement X in Eq. 1, the viscous loss Ed, energy loss by viscous damping during one cycle of the displacement is
(3) -
6.Hair bundle energy must be greater than viscous loss. This condition,
(4) leads to a frequency limit flim for the hair-bundle motility to be able to counteract viscous drag. Note that it does not depend on the amplitude because both Ehb and Ed are proportional to δX2. If this frequency limit does not exceed the auditory frequency, the motile mechanism described by the model does not satisfy a necessary condition for the cochlear amplifier.
Channel re-closure model
Now we examine the channel re-closure model (22). This model assumes that elevation of cytosolic Ca2+ concentration due to opening of the MET channel, which does not have cation selectivity, leads to the binding of Ca2+ to the cytosolic side of the channel, which in turn leads to channel re-closure (14,15,17,23,29), thereby increasing the tension on the tip-link. This delayed tension increase can have amplifying effect on an oscillator with which the tip-link is in contact. Indeed, this mechanism can lead to spontaneous oscillation of hair bundles (22). The operating point of the MET channel is determined by a myosin motor, which is also triggered by an elevation of cytosolic Ca2+ concentration caused by channel opening.
Here we assume that the MET channel has only one Ca2+ binding site instead of two (22) for simplicity. For convenience, we assign each channel state with a number (Fig.nbsp;1). The channel in the open state may be either Ca-unbound (state 2) or Ca-bound (state 3). Closed state is either Ca-unbound (state 1) or Ca-bound (state 4).
Figure 1.
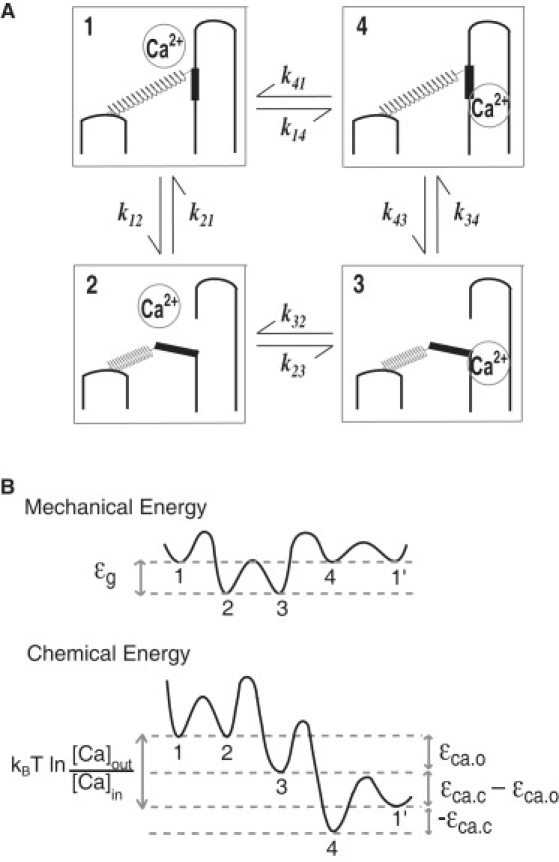
Channel re-closure model. (A) Schematic representation of transitions, which the mechanoelectric transducer channel undergoes. An opening of the channel (state 2) elevates Ca2+ concentration on the cytosolic side of the channel. The resulting binding of Ca2+ to a binding site leads to closure of the channel (state 3). Closure of the channel (state 4) reduces the cytosolic Ca2+ concentration, leading to dissociation (state 1). (B) Schematic representation of energy levels of these states. The mechanical energy level of open states 2 and 3 are the same and differs from the closed states 1 and 4 by gating energy ɛg. States 3 and 4 differs in the binding energy. These levels differ from unbound states 1 and 4. Directed transitions of the channels are supported by the expenditure of the chemical energy kBT ln([Ca]out/[Ca]in) of Ca2+ after one cycle.
The probability Pi of the MET channel being in the ith state follows a set of differential equations,
| (5) |
where the index i runs from 1 to 4. Here the index value 0 and 5 are, respectively, identical to 4 and 1. The quantity kij represents the transition rate from ith state to jth state. Open probability Po is given by P2 + P3. Here the transition rates are to be determined by the energy barriers (Fig.nbsp;1 B) in the manner consistent with Arrhenius-Eyring equation (44,45), which is used for nonequilibrium systems (45,46).
The transitions between states 1 and 2 involve gating of the MET channel. The ratio of the transition rates is given by the difference of the free energy in the two states,
| (6) |
where β = 1/(kBT) with Boltzmann's constant kB and the temperature T. Gating energy ɛg can be expressed as
| (7) |
Here X is the displacement at the tip-link, Kg is the stiffness of gating spring, and Xg is the gating distance.
Both states 2 and 3 are open states. The transitions between them involve Ca2+ binding and unbinding. We have
| (8) |
assuming that the cytosolic Ca2+ concentration near the channel can be approximated by that of the external medium, [Ca]out. Ca2+ binding energy of the open configuration is represented by −ɛca, o (<0).
The transitions between states 3 and 4, both of which are Ca2+ bound, involve gating of the MET channel. However, the free energy of Ca2+ binding may depend on the conformation and may differ in these two states. On closing of the channel, Ca2+ level inside the cell ([Ca]in) should immediately drop due to diffusion and Ca2+ buffers, aided by Ca2+ pumps (47,48), which maintains the low cytosolic Ca2+ concentration. Thus, we obtain
| (9) |
where −ɛca, o (<0) is Ca2+ binding energy of the channel when it is in closed configuration.
Transitions between states 4 and 1, which are both closed, involve only Ca2+ binding and unbinding. They are similar to transitions between states 2 and 3 except that the Ca2+ concentration is lower because the channel is closed. The ratio of the transition rates is given by
| (10) |
The free energy change ɛcycle after one cycle is then
| (11) |
Substitution of the transition rates using Eqs. 6 and 8–10 leads to
| (12) |
which is the difference of Ca2+ chemical potential inside and the outside of the cell. This result is consistent with nonequilibrium condition of this system, which uses chemical energy of a single Ca2+ per cycle.
These relationships between the transition rates allow usnbsp;to replace eight transition rates by six new parameters k1, k2, k3, k4, r3, and r4:
Response of the MET channel
A small sinusoidal displacement X (See Eq. 1) of the hair bundle elicits a small periodic response in the channel. The probability Pi of the channel being in state i is, to the first-order terms,
| (13) |
where ϕi is the phase, the probability of i state at the operating point (i.e., δX = 0), and the amplitude for inbsp;= 1, 2, 3, and 4.
The applied displacement changes the transition rates k21nbsp;and k34 through its effect on the gating energy Δɛg (≡nbsp;KgXgδX sin ωt). The linearized forms are given by
| (14) |
where = κij and with κij being a prefactor.
With Eqs. 13 and 14, Eq. 5 leads to
where θi is a constant. The open probability Po (= P2 + P3) is then expressed by
| (15) |
Constants θo and ϕo depend on the rates k1, …, k4, r3, r4, thenbsp;ratio [Ca]out/[Ca]in, and the open probability at the operating point.
Limiting frequency
The force Fhb produced by a hair bundle with N tip-links in response to the displacement X at each tip-link is expressed by
where Xg is the gating distance, Kg is the stiffness of the gating spring of each MET channel, and Ks is the stiffness due to rootlet and side links.
The work Ehb done by the hair bundle during one cycle of stimulation is
| (16) |
because the work done by elastic elements drops off at the end of one cycle, leaving components of force with phase shifts. With Eq. 15, we obtain
| (17) |
where Φ = πθo sin ϕo, which we call the phase factor.
With the aid of Eq. 3, the Assumption 6 that mechanical energy Ehb produced by the hair bundle must be larger than viscous loss Ed leads to a condition for the limiting frequency flim,
| (18) |
below which viscous drag can be counteracted. This limiting frequency is obtained by dividing the corresponding angular frequency by 2π. A factor M is defined by
| (19) |
Notice that M can be determined by morphological data alone. For this reason, we call it the morphological factor.
The limiting frequency flim (Eq. 18) for the channel re-closure model is determined by gating force KgXg, the morphological factor M, and the phase factor Φ. Among these factors, the limiting frequency is particularly sensitive to gating force because of its second power dependence.
Optimal value of the phase factor
Since we are interested in the frequency limit, here we attempt to obtain the maximum value for the phase factor Φ. The numerical calculation is done using SciLab program (http://www.scilab.org) on Biowulf at the National Institutes of Health (http://biowulf.nih.gov), after normalizing four parameters k1, k2, k3, and k4, with respect to the operating frequency ω. The range of parameter values examined is shown in the Supporting Material.
Since the value for the steady-state open probability is fixed, the steady-state bundle displacement must be determined by solving a nonlinear equation for every set of transition rate values and the steady-state open probability . We found that inclusion of displacement dependence in k1 and k3 renders the solution too lengthy to be accepted by the computer program. For this reason, we assume that k1 and k3 are constants. That is equivalent to assuming that the energy barriers for channel opening do not change with the tip-link displacement and would be somewhat unreasonable (36). This assumption may have significant effect where channel gating is rate-limiting. However, it will be much less significant where the phase factor is insensitive to the details of gating.
Preliminary trials show that the phase factor Φ is a monotonically increasing function of k1/ω and k3/ω, although it is virtually flat beyond 106. That is illustrated by plotting Φ under the constraints of k1 = k3, parameters involved in gating, and k2 = k4, parameters involved in Ca2+-binding and unbinding (Fig.nbsp;2). The figure shows that gating with a rate close to the operating frequency has a damping effect. That is intuitively obvious, because gating is a mechanical relaxation. On the other hand, Ca2+-binding and unbinding at a rate near the operating frequency is effective in making a phase delay that has an amplifying effect.
Figure 2.
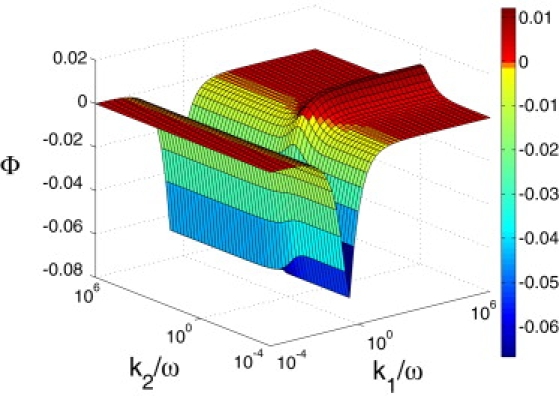
The phase factor Φ of the channel re-closure model. The phase factor Φ is plotted against k1/ω and k2/ω under the constraints k3 = k1 and k4nbsp;= k2. The range of the parameter values is in the Supporting Material. Each point represents the maximum value with respect to r3 and r4. The maximum value of Φ is 0.0086 at k2/ω = 1.00. [Ca]out/[Ca]in = 100 and = 0.1.
For this reason, we can maximize Φ with respect to only four parameters, while giving large fixed values for k1/ω and k3/ω (i.e., k1/ω = 106, k3/ω = 106). The grid is iteratively recast in the optimal zone until the relative increment of the maximum value for Φ between the iteration falls below 1%.
If we assume that open probability at the operating point is of 0.1, the optimal value of Φ is 0.05 for the mammalian ear, which has the Ca concentration ratio of 102 (Table 1). The optimal parameter values k2, k4, r3, and r4 are 2.1, 0.3, 5 × 10−4, and 0.26. For the avian ear, which has ∼10 times higher endolymphatic Ca concentration (49), the optimal value is 0.07. The corresponding optimal parameter values are 2.5, 0.4, 5 × 10−4, and 0.08. The phase factor Φ also depends on the operating point. It has a maximum at ofnbsp;0.5, and the values are higher by ∼25% than that for of 0.1.
Table 1.
Parameter values for the basal end of the cochlea
| Notation | Quantity | Mammal | Chicken | Unit |
|---|---|---|---|---|
| KgXg | Gating force | 4.4∗ | > 0.43† | pN |
| h | Height of tallest cilia | 0.7 (61) | 1.54 (53) | μm |
| s | Rootlet separation | 0.5 (62) | 0.45 (54) | μm |
| N | Number of tip-links/cell | 60‡ | 187§ | |
| A | Subtectorial gap area/hair cell | 125 (61) | 19 (neural) (53) | μm2 |
| 23 (abneural) (53) | ||||
| [Ca]out | Endolymphatic [Ca2+] | 23.7 (63) | 240 (49) | μM |
| [Ca]in | Cytosolic [Ca2+] | 0.2¶ | 0.2¶ | μM |
The viscosity coefficient η of the medium is assumed 1 mPa·s, same as water.
Obtained from 500 fN force (37) at the tip of mouse outer hair cell and the geometric factor for h ≃ 4.4 μm (64).
Obtained from the value 40 fN (58,59) at the tip and h ≃ 4.8 μm (at the apex).
At the midturn of the cochlea (65).
Median of estimated 60 nM and 300 nM (48).
Interplay model
The model proposed by Tinevez etnbsp;al. (26), which we refer to as the interplay model, assumes that fast adaptation is not based on a special structure or a mechanism but is a result of interplay between the MET channel and the myosin motor, which is a force generator and responsible for slow adaptation. While a release model (50,51) assumes that an element, which links the myosin motor with the MET channel, undergoes Ca2+-activated fast elongation, the interplay model assumes the actomyosin system that produces force has viscoelasticity. In the following we give a brief description of this model.
The distance Xa of the myosin motor from actin filaments obeys a differential equation,
| (20) |
where the myosin motor which generates isometric force Fa has an intrinsic pre-stressed Voigt-type viscoelastic element with viscosity λa and stiffness Ke (26) (Fig.nbsp;3). The degree of pre-stress is represented by Xe, which can serve as an adjustable parameter.
Figure 3.
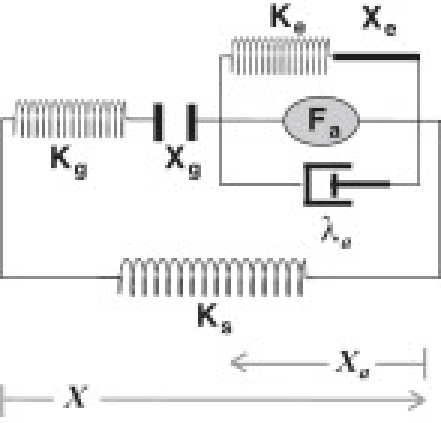
Interplay model. Gating of the MET channel is characterized by the stiffness Kg and distance Xg of the gate. Myosin, which produces force Fa, is connected with the MET channel through an element with stiffness Ke and friction coefficient λa. A parallel element has stiffness Ks.
The force Fa generated by unconventional myosin is a decreasing function of the cytosolic Ca2+ concentration, which is approximately proportional to open probability Po of the MET channel (26). Hence it can be expressed to the first-order term,
| (21) |
The coefficient S can be expressed by
| (22) |
The channel's open probability Po, that affects the cytosolic Ca2+ concentration, is expressed by a two-state Boltzmann function (26),
| (23) |
where B determines the open probability at rest.
Linearized response
A small sinusoidal displacement of the hair bundle, represented by Eq. 1, should give rise to a small displacement on the position Xa of the motor. This can be expressed as
| (24) |
where is the position at rest. For small periodical displacements, Eq. 23 becomes
| (25) |
where the open probability at the operating point is given by
Substitution of Xa and Po into the differential equation Eq.nbsp;20, using Eqs. 24 and 25, leads to an expression fornbsp;the amplitude δXa of the motor displacement,
| (26) |
where
| (27) |
which is the familiar form of gating stiffness (29) and can take negative values. The quantity Kca defined by
| (28) |
is the sensitivity of force production by the myosin motor to displacement. That is because a displacement Xg at the tip-link leads to an increase of in the open probability, which in turn causes a rise in the cytosolic Ca2+ concentration as described by Eq. 21, decreasing the motor force by the efficiency factor of SFmax.
Energy balance and frequency limit
In response to a tip-link displacement X, N tip-links of a hair bundle produce total force Fhb,
| (29) |
For small periodical displacement as shown in Eq. 1, the work done by the hair bundle per cycle depends only on terms that include Xa and Po, because elastic terms do not contribute. Only the term that is proportional to δXa is nonelastic and remains in Eq. 25 after a full cycle. Thus, we have
| (30) |
With the aid of Eq. 26, we obtain
| (31) |
Note that the work Ehb done by the hair bundle can be negative. Under such conditions, the hair bundle behaves asnbsp;a damper. The condition Ehb > 0 requires . Since Kca > 0 as shown in Eq. 28, This condition leads to
| (32) |
Namely, negative stiffness is a necessary condition for the hair bundle to function as an amplifier.
The condition for being an amplifier Ehb > Ed leads to ω > 2π flim with a linear-limiting frequency flim,
| (33) |
For a limiting frequency flim to exist, the terms inside the square root must be positive,
| (34) |
Factors that determine the limiting frequency
The frequency limit for the interplay model depends on many parameters. Its sensitivity to operating point enters through and Kca. An example of the dependence on the operating point is shown in Fig.nbsp;4. Notice that Ehb has a symmetry axis = 0.5, where it has the maximum. The hair bundle energy Ehb is negative if is small. For a limiting frequency flim to exist, must be large enough to make Ehb > Ed, which is positive. The dependence of flim on its parameters is illustrated in Fig.nbsp;5.
Figure 4.
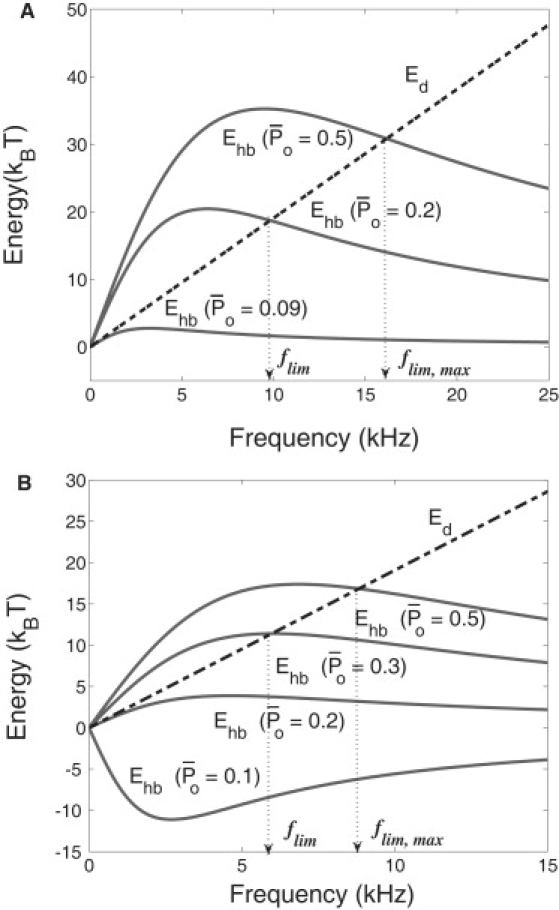
Hair bundle work Ehb and viscous loss Ed. Examples of Ed (dotted) and Ehb. (A) = 0.09 (or 0.91), 0.2 (or 0.8), and 0.5. Ehb > 0 requires 0.08 < < 0.92. Ehb > Ed requires 0.09 < < 0.91. (B) nbsp;= 0.1 (or 0.9), 0.2 (or 0.8), 0.3 (or 0.7), and 0.5. Ehb > 0 requires 0.17 < < 0.83. The value Ehb > Ed requires 0.2 < < 0.8. Assumed parameter values: N = 60, Kg = 1.2 mN/m, Xg = 7 nm for panel A, 5 nm for panel B, Fm = 3 pN, S = 4, λa = 0.1 μN.s/m, Ke = 0.13 mN/m, η = 1 mPa.s, A = 100 μm2, h = 1 μm, and s = 0.5 μm.
Figure 5.
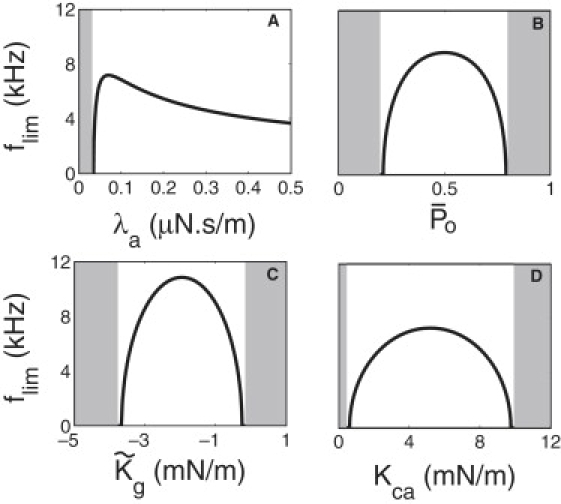
An example of parameter dependence of limiting frequency. The dependence on (A) λa, (B) , (C) , and (D) Kca. Limiting frequency flim does not exist in the shaded areas. Assumed parameter values are same as those for Fig.nbsp;4B.
The morphological factor M (Eq. 19) that appears in the channel re-closure model also appears in the frequency limit of the interplay model. Here we observe that the limiting frequency is an increasing function of this factor. The limiting frequency obtained is, however, quite sensitive to other factors (Fig.nbsp;5).
Morphological factor
The morphological features of the cochlea are represented by a single factor M in the limiting frequency for each of the two models. How do the values for this factor in the mammalian ear compare with those in the avian ear? The mammalian ear differs from the avian ear in having lower bundle height h and smaller number N of tip-links per bundle (see Tablenbsp;1). These two factors do not have significant influences on the morphological factor M because they work in the opposite directions in Eq. 19. The most important factor is the area A of the subtectorial gap per hair cell, which is much larger in the mammalian ear. For this reason, the morphological factor M for the avian cochlea is ∼10 times of that for the mammalian cochlea.
This large morphological factor M for the avian ear appears to indicate hair-bundle motility is more important in the avian ear than in the mammalian ear, when it is combined with the avian ear's lower auditory frequency limit, which is ∼1/10th of the mammalian one. Our result is that the limiting frequency is an increasing function of the morphological factor in the two models.
For this reason it is of interest to compare the mapping of the morphological factor along the avian basilar papilla with that of the characteristic frequency. In the chicken cochlea, each quantity that contributes to this factor has been carefully mapped along its longitudinal axis (52,53), although morphological data obtained from electron microscopy may need correction for sample shrinkage, which could be ∼30% in length (54,55) for scanning electron microscopy.
It has been shown that the height h of the tallest cilia in a hair bundle shows monotonic decrease from the apex to the base (Fig.nbsp;6 B). The number of stereocilia shows monotonic increases (53). The number N of tip-links in a hair bundle, which can be estimated by multiplying a factor 0.8 (52) to the number of stereocilia, is therefore an increasing function of the distance from the apex (Fig.nbsp;6 A). The surface area A of a single hair cell on the reticular lamina does not show monotonic dependence on the distance from the apex (Fig.nbsp;6 C) (53). It has a maximum at ∼50% from the apex on the abneural side (i.e., abneural hair cells) and it is relatively constant on the neural side (neural hair cells) (53).
Figure 6.
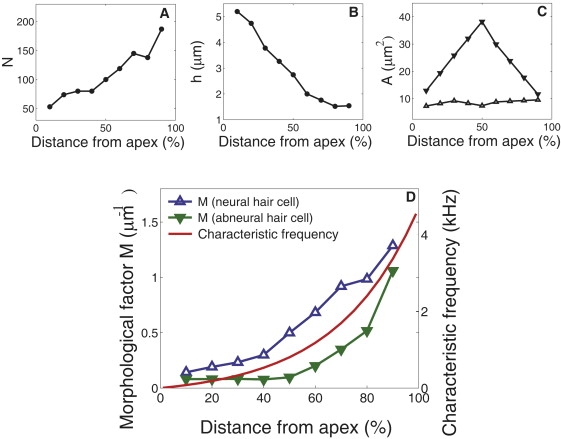
Morphological factor of the chicken cochlea. (A) Hair bundle height h, (B) the number N of tip-links, and (C) apical surface area A per hair cell on the neural (up-pointing open triangle) and abneural (down-pointing solid triangle) sides are plotted against the distance from the apex. Adopted from Tilney and Saunders (53). N is obtained by multiplying a factor 0.8 (52) to the number of stereocilia (53). Shrinkage factor is not considered. (D) Morphological factor M (= Ns2/A h) on the neural (blue) and abneural sides (green) are plotted together with the best frequency (red line). The value s is assumed to be 0.45 μm (54) (Tablenbsp;1). The best frequencies are adopted from Gleich and Manley (66).
The morphological factor M for the avian ear obtained from these experimental data is an increasing function of the distance from the apical end (Fig.nbsp;6 D), similar to the mapping of the characteristic frequency (Fig.nbsp;6 D). This observation is somewhat surprising because our condition to introduce the morphological factor is simply a necessary condition for an upper bound of characteristic frequency. However, this factor is always greater on the neural side than the abneural side at each longitudinal location. If one assumes that the role of abneural hair cells is similar to that of outer hair cells, this observation is somewhat paradoxical, because larger values of the morphological factor correspond to a greater effectiveness as the amplifier if other parameters are the same.
If we assume that achieving high frequency sensitivity is biologically costly, limiting frequency may have a correlation with the characteristic frequency. Then a relatively good correspondence of the morphological factor and the characteristic frequency along the longitudinal axis of the chicken ear appears consistent with the channel re-closure model, which predicts that the limiting frequency is proportional to the morphological factor. The difference in the morphological factor for neural and abneural cells could be attributed to either systematic difference in gating force in those cells or that in the open probability of the MET channel. Namely, gating force KgXg or the resting open probability of the channel could be somewhat larger in abneural cells than in neural cells.
The implication of the mapping of the morphological factor on the interplay model is more equivocal. The square-root dependence of the limiting frequency on the morphological factor indicates that the frequency limit predicted by the interplay model does not rise as sharply as the characteristic frequency along the longitudinal axis if all other parameters are constant throughout the cochlea. However, with so many parameters to which the limiting frequency is sensitive, such an assumption could be unrealistic. Rather, it could imply that other parameters need to change along the axis to make the rise of the limiting frequency sharper.
Alternatively, it is possible that the limiting frequencies that we obtained could be significantly higher than the characteristic frequency. If that is the case, our analysis is more effective in examining the validity of the re-closure model than in examining the interplay model because the re-closure model is more constrained by a smaller number of parameters.
Limiting frequency
For the channel re-closure model, gating force KgXg and the morphological factor M can be used to predict the limiting frequency if we can assume that the phase factor Φ is optimized. For the basal turn of the mammalian ear, gating force KgXg is 4.4 pN for mice (37), and the morphological factor at the base is 0.17 μm−1 for the chinchilla. If we can use the value for mouse gating force for chinchilla, the limiting frequency is ∼2 kHz, much lower than the auditory frequency, which is 20 kHz for the chinchilla, 40 kHz for guinea pigs andnbsp;gerbil. It is also lower than the limiting frequency of ∼10 kHz, obtained from the condition for electromotility of outer hair cells to locally counteract viscous drag (28,56).
If we can assume that gating force for the chicken is ∼4.3nbsp;pN, similar to that of other animals, including ∼5.2 pN for frog (34) and ∼4.1 pN for turtle (57) (these values are based on s/h = 0.11), we obtain the limiting frequency of 20 kHz on the neural side and 17 kHz for the abneural side at the basal end. These frequencies are higher than the auditory range of ∼4 kHz, even after correcting for EM sample shrinkage that would reduce the morphological factor and thereby the limiting frequency by 50%. This observation is consistent with the hypothesis that hair bundles function as the cochlear amplifier in the avian ear. However, experimental values for the gating force measured at the tip of the hair bundle of the chicken are ∼40 fN (58,59), ∼1/10 of those in other animals, leading to 0.43 pN at the tip-link by considering the geometrical factor g ≈ s/h (Table 1). This lower value for gating force leads to the limiting frequency of 200 Hz for the neural side and 170 Hz for the abneural side, without correcting for sample shrinkage.
Why are the values for gating force of the chicken hair bundle so far obtained so small compared with those from other animals? A possible reason could be technical difficulties in experiments, specifically the time resolution must be high enough compared with measuring gating force in frogs (D. Bozovic, personal communication, 2008). Alternatively, it is possible that gating of channels in a single hair bundle may not be synchronous in the preparation, broadening channel opening with respect to bundle displacement (31,32). If the channel re-closure model, which predicts second power dependence of the limiting frequency on gating force, is correct, the gating force of chicken hair cells must be as high as that of other animals.
The predictions of the interplay model on the limiting frequency are difficult to make. It depends on a greater number of parameters such as λa, Fm, and S, which are related to the myosin motor and show considerable variability in their values (26). In addition, a relatively small uncertainty in parameter values tends to lead to a large uncertainty in the limiting frequency. For example, a 10% difference in either gating distance or gating stiffness can lead to >100% difference in the limiting frequency.
Conclusions
We assumed that viscous drag in the subtectorial gap must be counteracted by hair-bundle motility for small periodic stimulation and derived limiting frequencies of the ear for two models, the channel re-closure model (22) and the interplay model (26), of hair-bundle motility, each of which has been shown to work as an amplifier.
The limiting frequency obtained from the channel re-closure model is proportional to the phase factor, the morphological factor, and the square of gating force. The limiting frequency obtained from the interplay model is much more complex. Although it depends on the morphological factor, it is very sensitive to factors that characterize the gating of the mechanoelectric transducer channel as well as force production by the myosin motor.
The morphological factor is much larger for the avian ear than for the mammalian ear. For the chicken ear, this factor shows a longitudinal dependence similar to the tonotopic map. Such properties of the morphological factor can be derived by the channel re-closure model. However, gating force for the chicken must be larger than reported values. The limiting frequency predicted by the interplay model is not as specific, involving numerous parameters.
Supporting Material
One table is available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01425-8.
Supporting Material
Acknowledgments
We thank Dr. Christine Köppl and Dr. James Saunders for discussion on the morphology of the avian cochlea and Dr. Dolores Bozovic for discussion on gating force in the avian ear.
This work was supported by the intramural program of the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, MD.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons-Attribution Noncommercial License (http://creativecommons.org/licenses/by-nc/2.0/), which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Gold T. Hearing. II. The physical basis of the action of the cochlea. Proc. R. Soc. Lond. B. Biol. Sci. 1948;135:492–498. [Google Scholar]
- 2.Zwicker E. A model describing nonlinearities in hearing by active processes with saturation at 40 dB. Biol. Cybern. 1979;35:243–250. doi: 10.1007/BF00344207. [DOI] [PubMed] [Google Scholar]
- 3.Davis H. An active process in cochlear mechanics. Hear. Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.O., Neely S.T., Molnar C.E., Matthews J.W. An active cochlear model with negative damping in the partition: comparison with Rhode's ante- and post-mortem observations. In: van den Brink G., Bilsen F.A., editors. Psychological, Physiological and Behavioral Studies of Hearing. Delft University Press; Delft, The Netherlands: 1980. [Google Scholar]
- 5.Liberman M.C., Dodds L.W. Single neuron labeling and chronic cochlear pathology. III. stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- 6.Neely S.T., Kim D.O. A model for active elements in cochlear biomechanics. J. Acoust. Soc. Am. 1986;79:1472–1480. doi: 10.1121/1.393674. [DOI] [PubMed] [Google Scholar]
- 7.Zweig G. Finding the impedance of the organ of Corti. J. Acoust. Soc. Am. 1991;89:1229–1254. doi: 10.1121/1.400653. [DOI] [PubMed] [Google Scholar]
- 8.Manley G.A., Kirk D.L., Köppl C., Yates G.K. In vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proc. Natl. Acad. Sci. USA. 2001;98:2826–2831. doi: 10.1073/pnas.041604998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manley G.A. Evidence for an active process and a cochlear amplifier in nonmammals. J. Neurophysiol. 2001;86:541–549. doi: 10.1152/jn.2001.86.2.541. [DOI] [PubMed] [Google Scholar]
- 10.Brownell W., Bader C., Bertrand D., Ribaupierre Y. Evoked mechanical responses of isolated outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 11.Ashmore J.F. A fast motile response in guinea-pig outer hair cells: the molecular basis of the cochlear amplifier. J. Physiol. 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos-Sacchi J., Dilger J.P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear. Res. 1988;65:143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- 13.Crawford A.C., Evans M.G., Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J. Physiol. 1989;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benser M.E., Marquis R.E., Hudspeth A.J. Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus. J. Neurosci. 1996;16:5629–5643. doi: 10.1523/JNEUROSCI.16-18-05629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci A.J., Crawford A.C., Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J.nbsp;Neurosci. 2000;20:7131–7142. doi: 10.1523/JNEUROSCI.20-19-07131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy H.J., Evans M.G., Crawford A.C., Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat. Neurosci. 2003;6:832–836. doi: 10.1038/nn1089. [DOI] [PubMed] [Google Scholar]
- 17.Cheung E.L., Corey D.P. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys. J. 2006;90:124–139. doi: 10.1529/biophysj.105.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberman M.C., Gao J., He D.Z., Wu X., Jia S. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 19.Chan D.K., Hudspeth A.J. Ca2+ current-driven nonlinear amplification by the mammalian cochlea innbsp;vitro. Nat. Neurosci. 2005;8:149–155. doi: 10.1038/nn1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J., Shen W., He D.Z.-Z., Long K.B., Madison L.D. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 21.Dallos P., Wu X., Cheatham M.A., Gao J., Zheng J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe Y., Magnasco M.O., Hudspeth A.J. A model for amplification of hair-bundle motion by cyclical binding of Ca2+ to mechanoelectrical-transduction channel. Proc. Natl. Acad. Sci. USA. 1998;95:15321–15326. doi: 10.1073/pnas.95.26.15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard J., Hudspeth A.J. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc. Natl. Acad. Sci. USA. 1987;84:3064–3068. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hacohen N., Assad J.A., Smith W.J., Corey D.P. Regulation of tension on hair-cell transduction channels: displacement and calcium dependence. J. Neurosci. 1989;9:3988–3997. doi: 10.1523/JNEUROSCI.09-11-03988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillespie P.G., Wagner M.C., Hudspeth A.J. Identification of a 120 kD hair-bundle myosin located near stereociliary tips. Neuron. 1993;11:581–594. doi: 10.1016/0896-6273(93)90071-x. [DOI] [PubMed] [Google Scholar]
- 26.Tinevez J.-Y., Jülicher F., Martin P. Unifying the various incarnations of active hair-bundle motility by the vertebrate hair cell. Biophys. J. 2007;93:4053–4067. doi: 10.1529/biophysj.107.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shera C.A. Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves. J. Acoust. Soc. Am. 2003;114:244–262. doi: 10.1121/1.1575750. [DOI] [PubMed] [Google Scholar]
- 28.Ospeck M., Dong X.-X., Iwasa K.H. Limiting frequency ofnbsp;the cochlear amplifier based on electromotility of outer hair cells. Biophys. J. 2003;84:739–749. doi: 10.1016/S0006-3495(03)74893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard J., Hudspeth A.J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron. 1988;1:189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 30.Iwasa K.H., Ehrenstein G. Cooperative interaction as the physical basis of the negative stiffness in hair cell stereocilia. J. Acoust. Soc. Am. 2002;111:2208–2212. doi: 10.1121/1.1466864. [DOI] [PubMed] [Google Scholar]
- 31.Duncan R.K., Hernandez H.N., Saunders J.C. Relative stereocilia motion of chick cochlear hair cells during high-frequency water-jet stimulation. J. Aud. Neurosci. 1995;1:321–329. [Google Scholar]
- 32.Duncan R.K., Eisen M.D., Saunders J.C. Distal separation of chick cochlear hair cell stereocilia: analysis of contact-constraint models. Hear. Res. 1999;127:22–30. doi: 10.1016/s0378-5955(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 33.Kozlov A.S., Risler T., Hudspeth A.J. Coherent motion of stereocilia assures the concerted gating of hair-cell transduction channels. Nat. Neurosci. 2007;10:87–92. doi: 10.1038/nn1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Goff L., Bozovic D., Hudspeth A.J. Adaptive shift in the domain of negative stiffness during spontaneous oscillation by hair bundles from the internal ear. Proc. Natl. Acad. Sci. USA. 2005;102:16996–17001. doi: 10.1073/pnas.0508731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy H.J., Crawford A.C., Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- 36.Corey D.P., Hudspeth A.J. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 1983;3:962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Netten S.M., Kros C.J. Gating energies and forces of the mammalian hair cell transducer channel and related hair bundle mechanics. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1915–1923. doi: 10.1098/rspb.2000.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Netten S.M., Meulenberg C.J.W., Lennan G.W.T., Kros C.J. Pairwise coupling of hair cell transducer channels links auditory sensitivity and dynamic range. Pflugers Arch. 2009;458:273–281. doi: 10.1007/s00424-008-0617-z. [DOI] [PubMed] [Google Scholar]
- 39.Allen J. Cochlear micromechanics—a physical model of transduction. J. Acoust. Soc. Am. 1980;68:1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- 40.Goodyear R.J., Richardson G.P. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J. Neurobiol. 2002;53:212–227. doi: 10.1002/neu.10097. [DOI] [PubMed] [Google Scholar]
- 41.Runhaar G. The surface morphology of the avian tectorial membrane. Hear. Res. 1989;37:179–187. doi: 10.1016/0378-5955(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 42.Cotanche D.A. Video-enhanced DIC images of the noise-damaged and regenerated chick tectorial membrane. Exp. Neurol. 1992;115:23–26. doi: 10.1016/0014-4886(92)90215-c. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs R.A., Hudspeth A.J. Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog's internal ear. Cold Spring Harb. Symp. Quant. Biol. 1990;55:547–561. doi: 10.1101/sqb.1990.055.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Atkins P., de Paula J. Oxford University Press; 2002. Atkins' Physical Chemistry. [Google Scholar]
- 45.Scott S.K. Oxford University Press; USA: 1994. Oscillations, Waves, and Chaos in Chemical Kinetics. [Google Scholar]
- 46.Läuger P. Dynamics of ion transport systems in membranes. Physiol. Rev. 1987;67:1296–1331. doi: 10.1152/physrev.1987.67.4.1296. [DOI] [PubMed] [Google Scholar]
- 47.Yamoah E.N., Lumpkin E.A., Dumont R.A., Smith P.J., Hudspeth A.J. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J. Neurosci. 1998;18:610–624. doi: 10.1523/JNEUROSCI.18-02-00610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumpkin E.A., Hudspeth A.J. Regulation of free Ca2+ concentration in hair-cell stereocilia. J. Neurosci. 1998;18:6300–6318. doi: 10.1523/JNEUROSCI.18-16-06300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauer G., Richter C.P., Klinke R. Sodium, potassium, chloride and calcium concentrations measured in pigeon perilymph and endolymph. Hear. Res. 1999;129:1–6. doi: 10.1016/s0378-5955(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 50.Stauffer E.A., Scarborough J.D., Hirono M., Miller E.D., Shah K. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron. 2005;47:541–553. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LeMasurier M., Gillespie P.G. Hair-cell mechanotransduction and cochlear amplification. Neuron. 2005;48:403–415. doi: 10.1016/j.neuron.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Tilney L.G., Cotanche D.A., Tilney M.S. Actin filaments, stereocilia and hair cells of the bird cochlea. VI. How the number and arrangement of stereocilia are determined. Development. 1992;116:213–226. doi: 10.1242/dev.116.1.213. [DOI] [PubMed] [Google Scholar]
- 53.Tilney L.G., Saunders J.C. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J. Cell Biol. 1983;96:807–821. doi: 10.1083/jcb.96.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köppl C., Iwasa K.H., Sul B. Big and powerful: a model of the contribution of bundle motility to mechanical amplification in hair cells of the bird basilar papilla. In: Cooper N.P., Kemp T.D., editors. Concepts and Challenges in the Biophysics of Hearing. World Scientific; Singapore: 2009. [Google Scholar]
- 55.Duncan R.K., Ile K.E., Dubin M.G., Saunders J.C. Hair bundle profiles along the chick basilar papilla. J. Anat. 2001;198:103–116. doi: 10.1046/j.1469-7580.2001.19810103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwasa K.H., Sul B. Effect of the cochlear microphonic on the limiting frequency of the mammalian ear. J. Acoust. Soc. Am. 2008;124:1607. doi: 10.1121/1.2953317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricci A.J., Crawford A.C., Fettiplace R. Mechanisms of active hair bundle motion in auditory hair cells. J. Neurosci. 2002;22:44–52. doi: 10.1523/JNEUROSCI.22-01-00044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y., Yamoah E.N., Gillespie P.G. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc. Natl. Acad. Sci. USA. 1996;93:15469–15474. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Si F., Brodie H., Gillespie P.G., Vazquez A.E., Yamoah E.N. Developmental assembly of transduction apparatus in chick basilar papilla. J. Neurosci. 2003;23:10815–10826. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reference deleted in proof.
- 61.Lim D.J. Cochlear anatomy related to cochlear micromechanics. a review. J. Acoust. Soc. Am. 1980;67:1686–1695. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- 62.Pickles J.O. A model for the mechanics of the stereociliar bundle on acousticolateral hair cells. Hear. Res. 1993;68:159–172. doi: 10.1016/0378-5955(93)90120-p. [DOI] [PubMed] [Google Scholar]
- 63.Salt A.N., Inamura N., Thalmann R., Vora A. Calcium gradients in inner ear endolymph. Am. J. Otol. 1989;10:371–375. doi: 10.1016/0196-0709(89)90030-6. [DOI] [PubMed] [Google Scholar]
- 64.Géléoc G.S., Lennan G.W., Richardson G.P., Kros C.J. Anbsp;quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc. Biol. Sci. 1997;264:611–621. doi: 10.1098/rspb.1997.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beurg M., Evans M.G., Hackney C.M., Fettiplace R. Anbsp;large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J. Neurosci. 2006;26:10992–11000. doi: 10.1523/JNEUROSCI.2188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gleich O., Manley G.A. The hearing organ of birds and crocodilia. In: Dooling R.J., Fay R.R., Popper A.N., editors. Comparative Hearing: Birds and Reptiles. Springer; New York: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


