Summary
Mechanochemical proteins rely on ATP-hydrolysis to establish the different functional states required for their biological output. Studying the transient functional intermediate states these proteins adopt as they progress through the ATP-hydrolysis cycle is key to understanding the molecular basis of their mechanism. Many of these intermediates have been successfully ‘trapped’ and functionally characterised using ATP analogues. Here, we present a new nucleotide analogue, AMP-AlFx, which ‘traps’ PspF, a bacterial Enhancer Binding Protein, in a stable complex with the σ54-RNA polymerase holoenzyme. The crystal structure of AMP-AlFx•PspF1-275 provides new information on protein-nucleotide interactions and suggests that the β and γ phosphates are more important than the α phosphate in terms of sensing nucleotide bound states. In addition, functional data obtained with AMP-AlFx establish distinct roles for the conserved catalytic AAA+ residues suggesting that AMP-AlFx is a powerful new tool to study AAA+ protein family members and more generally Walker motif ATPases.
Keywords: AAA+ ATPase, nucleotide analogue, transcription, bEBP, PspF
In bacteria, the major variant sigma factor, sigma54 (σ54), forms a transcriptionally silent closed complex (CC) with RNA polymerase (E) at σ54-dependent promoters. ATP-hydrolysis by a σ54 activator protein that belongs to the large AAA+ (ATPases Associated with various cellular Activities) protein family is required to actively remodel the CC to a transcriptionally competent open complex (OC) 1; 2; 3. σ54-activators are also termed bacterial Enhancer Binding Proteins (bEBP; 4) as they often bind Upstream Activating Sequences (UAS) located approximately 100 bp upstream from the σ54 promoter. bEBP, like most AAA+ proteins, first need to oligomerise (usually to form hexamers) to be functionally active. What distinguishes bEBPs from other AAA+ family members is the presence of a highly conserved GAFTGA motif, shown to be essential for σ54 binding, found at the tip of the L1 loop 5; 6; 7; 8; 9. Structurally characterised bEBPs include NtrC1, PspF, ZraR and NtrC 7; 8; 9; 10. The AAA+ domains of NtrC1 and PspF form functionally relevant stable complexes with σ54 or σ54 in the presence of the nucleotide analogues ADP-BeFx and ADP-AlFx thought to mimic the ATP-bound state prior to, or at the point of, ATP-hydrolysis, respectively 8; 11; 12; 13. The complexes formed using these nucleotide analogues have been proposed to closely represent otherwise transient intermediates formed with ATP. However, the precise number and arrangement of the fluoride ions in the AlFx or BeFx moieties within the nucleotide-binding pockets of these bEBPs remains unknown, making the exact assignment of the respective complexes to the different nucleotide states (ground or transition state) somewhat uncertain. We previously proposed that sensing the nucleotide-bound state of PspF involves the Walker B residue E108 and that via its interaction with residue N64 the ATP can affect the conformation of the σ54 interacting L1 loop 14. We proposed that in the ADP-bound state the L1 loop is locked in a non-productive conformation, unable to interact with σ54. Upon ATP binding, an interaction between E108 and N64 promotes the release of the L1 loop, enabling the interaction with σ54 to take place 12; 14. At the point of ATP-hydrolysis, the L1 loop is further exposed allowing more stable interactions with σ54 to occur.
Nucleotide analogues are routinely used to capture proteins in transient conformational states; they are thus indispensable tools to successfully study and dissect the different stages of nucleotide hydrolysis driven molecular mechanisms. Here, we report the identification and functional characterisation of a new stable complex between the AAA+ domain of PspF (residues 1 to 275, called hereafter PspF1-275) and σ54 or Eσ54 using the new nucleotide analogue AMP-AlFx formed in situ. In addition, using site-directed mutagenesis and biochemical approaches, we assign the residues in PspF1-275 that are responsible for forming either the ADP-AlFx- or the AMP-AlFx-dependent complexes. Detailed analysis of our data suggests that the highly conserved AAA+ catalytic residues, including the Walker B motif residues and the putative arginine finger (R-finger) residues, have distinct roles in catalysis and may therefore be implicated in PspF functionality at different stages of the hydrolysis cycle.
AMP-AlFx complex formation
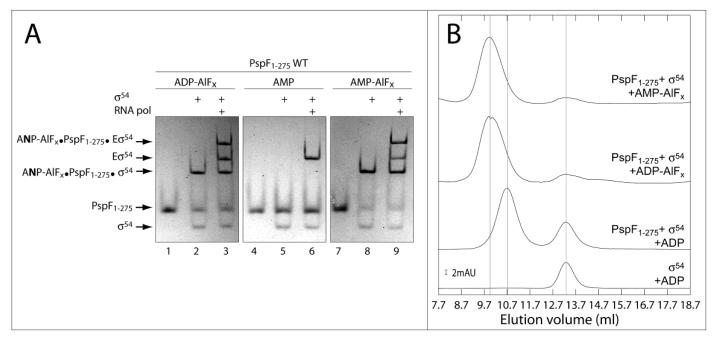
To investigate whether nucleotide analogues other than ADP-AlFx support formation of stable complexes between the activator protein and σ54 or Eσ54, we performed “trapping experiments” as described by 11; 15, in which we replaced ADP with AMP. Incubation of PspF1-275 Wild Type (WT) with σ54 or Eσ54 in the presence of AMP, AlCl3 and NaF lead to formation of stable AMP-AlFx•PspF1-275WT•σ54 or AMP-AlFx•PspF1-275•Eσ54 complexes which migrate with the same mobility as ADP-AlFx dependent complexes on native gels suggesting that they are very similar (Figure 1A lanes 2, 3, 8 and 9). As a control, we demonstrated that AMP alone did not support formation of stable complexes between PspF1-275 and σ54 (or Eσ54) (Figure 1A lanes 4-6). Interestingly, we noted that when using the histidine-tagged form of PspF1-275WT [(His)6-PspF1-275WT] we observed a stable homo-hexamer of (His)6-PspF1-275WT in the presence of ADP-AlFx but not AMP-AlFx, suggesting differences between PspF1-275WT subunits exist in the presence of these two nucleotide analogues (Table 1).
Figure 1. Identification and characterisation of new stable PspF1-275 WT•σ54 or Eσ54 complexes in the presence of AMP-AlFx.
A- Native gel showing the complexes formed by PspF1-275 WT (5 μM) ± σ54 (1 μM) ± RNA polymerase (0.15 μM) in the presence of AlFx reactant (AlC3 (0.4 mM) + NaF (5 mM)) and different nucleotides (4 mM, as indicated). The sample was loaded onto native PAGE 4.5% and proteins were detected by Coomassie Blue staining. ANP indicates AMP or ADP.
B- Gel filtration profiles of samples containing PspF1–275 WT (64 μM) ± σ54 (30 μM) ± AMP-AlFx or ADP-AlFx (as indicated) chromatographed through a Superdex 200 column (10×300 mm, 24 ml, GE Healthcare) at 4 °C. The scale bars give the scale of the ordinate axis; absorption units (AU) correspond to an A280nm of 1.
Table 1.
Summary of the native gel analysis demonstrating whether “trapped” complexes were obtained using PspF1-275 variants and different nucleotides in the presence of AlCl3 and NaF.
| PspF1-275 | Nucleotide + AlCl3+ NaF |
alone | σ 54 | Eσ54 |
|---|---|---|---|---|
| His-WT | ADP | + | + | + |
| ATP | + | + | + | |
| AMPPNP | − | − | − | |
| AMP | − | + | + | |
|
| ||||
| WT | ADP | − | + | + |
| ATP | − | + | + | |
| AMPPNP | − | − | − | |
| AMP | − | + | + | |
|
| ||||
| K42A | ADP | − | − | − |
| ATP | − | − | − | |
| AMPPNP | − | − | − | |
| AMP | − | − | − | |
|
| ||||
| L44A | ADP | − | − | − |
| ATP | − | − | − | |
| AMPPNP | − | − | − | |
| AMP | − | − | − | |
|
| ||||
| D107A | ADP | − | + | + |
| ATP | − | − | − | |
| AMPPNP | − | − | − | |
| AMP | − | − | − | |
|
| ||||
| E108A | ADP | − | − | − |
| ATP | − | +* | +* | |
| AMPPNP | − | − | − | |
| AMP | − | − | − | |
|
| ||||
| R162A | ADP | − | + | + |
| ATP | − | − | − | |
| AMPPNP | − | − | − | |
| AMP | − | − | − | |
|
| ||||
| R168A | ADP | − | − | − |
| ATP | − | − | − | |
| AMPPNP | − | − | − | |
| AMP | − | − | − | |
“−” no complex observed on native gel.
“+” complex observed on native gel.
“+*” complex observed on native gel but not dependent on AlCl3 + NaF 17.
AMP-AlFx trapped complex characterisation
To further characterise the new AMP-AlFx•PspF1-275•σ54 (or Eσ54) complex, we performed gel filtration experiments. Elution profiles of the PspF1-275•σ54 complexes obtained using either ADP-AlFx- or AMP-AlFx are very similar (Figure 1B). We thus conclude from the gel filtration and band shift assays that the ADP-AlFx- and AMP-AlFx-dependent complexes are similar in terms of size and stability. In addition, DNA binding assays, using fluorescent probes mimicking different promoter DNA conformations 16, demonstrated no significant differences in the DNA binding properties of the two types of ‘trapped’ complexes (data not shown). Having established that the AMP-AlFx-dependent complexes can bind DNA, we investigated whether the AMP-AlFx•PspF1-275•Eσ54 complex was able to activate transcription in vitro from the supercoiled S. meliloti nifH promoter. Similar to the ADP-AlFx•PspF1-275•Eσ54 complex, transcription from the AMP-AlFx•PspF1-275•Eσ54 complex was not observed (data not shown). Finally, using in-solution DNA crosslinking (as described in 17) we found no significant differences between the ADP-AlFx- and AMP-AlFx-dependent complexes formed with σ54, suggesting that the protein•DNA interactions are similar when using the two AlFx dependent nucleotide analogues (P. Burrows and N. Joly, unpublished result).
Taken together, these results indicate that both AMP-AlFx- and ADP-AlFx-dependent complexes are likely to have similar conformations and stability although some subtle differences do exist.
Structure of Mg-AMP bound PspF1-275
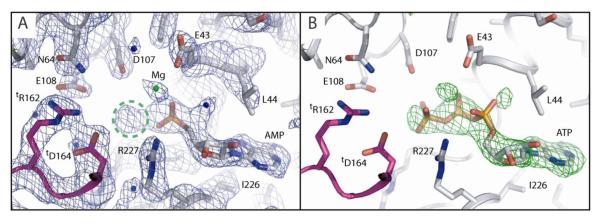
To establish the detailed organisation of Mg-AMP-AlFx-bound PspF1-275, and to provide a structural basis for the distinct determinants implicated in forming ADP-AlFx versus AMP-AlFx complexes, we soaked PspF1-275 crystals with MgCl2, AMP, AlCl3 and NaF (Table 2). These crystals diffracted to 2.85 Å at ESRF and the structure was solved by molecular replacement using the structure of apo PspF1-275 WT as a search model. Inspection of the first FoFc map contoured at 3 sigma (3σ), revealed clear densities for both the AMP and the Mg moieties and an extra density located at the tip of the α-phosphate of the AMP moiety (Figure 2A), which is too large to be a water molecule. We believe this density corresponds to the AlFx moiety but because of the low resolution we cannot establish this unambiguously and are thus unable to conclude anything regarding the precise coordination of the Al ion. The structure however clearly shows that the torsion angles around the ribose moiety and the phosphate backbone have significantly changed when compared with those in either the AMPPNP-, ATP- or ADP-bound structures 14. The torsion angle of the C4′-C5′ bond changes 114°, from 38° in the ATP-bound structure to 152° in the Mg-AMP-bound structure and as a result the ribose plane is translated some 3 Å towards the Walker B motif allowing the α-phosphate of AMP to occupy the canonical position of the β-phosphate of ATP (Figure 2). The extra electron density (potentially the AlFx moiety) occupies a similar position to that of the γ–phosphate of ATP in the ATP-bound structure (Figure 2; 14). We also observe a water molecule sitting where the apical oxygen (O) of the α-phosphate of ATP lies in the ATP-bound structure (Figure 2; 14). Interestingly, the N-terminus of PspF appears more structured and has moved 0.5 Å towards the Walker B motif when compared to the ATP-bound structure. This movement of the N-terminus could promote or facilitate the translation of AMP to compensate for the missing β-phosphate.
Table 2.
Crystallographic Data and Refinement Statistics of Mg-AMP-bound PspF1-275.
| Structure | Mg-AMP-PspF1-275 |
|---|---|
| Space group | P65 |
| Unit Cell (Å) | a = b = 113.6; c = 39.3; α = β = 90°; γ = 120 |
| A. Data reduction statistics | |
| λ (Å) | 0.97570 |
| Resolution (Å) | 98.39-2.85 (3.0-2.85) |
| Unique reflections | 6998 |
| Redundancy | 10.4 (10.7) |
| I/σ | 4.7 (1.9) |
| Mean ((I)/sd(I)) | 18.2 (6.5) |
| Completeness (%) | 100 (100) |
| Rmeas (%) | 14.3 (38.3) |
| Rpim (%) | 4.4 (11.6) |
| Rsym (%) | 13.6 (36.4) |
| B. Refinement Statistics | |
| Reflections (work/free) | 6985/348 |
| Number of atoms | 2093 (114 water molecules) |
| Rwork (%) | 18.6 |
| Rfree (%) | 24.1 |
| Ramachandran plot (%) | |
| Favoured regions | 90.1 |
| Additional allowed regions | 9.4 |
| Generously allowed regions | 0.5 |
| Disallowed | 0 |
| RMS Deviation from ideality | |
| Bond lengths (Å) | 0.013 |
| Bond angles (°) | 1.4 |
Figure 2. Final 2FoFc and omit difference FoFc electron density maps of the nucleotide binding pocket of PspF1-275.
A- Final 2FoFc map of the Mg-AMP-PspF1-275 structure at 2.85 Å resolution contoured at 1σ. The neighbouring subunit is coloured blue. Important intra-molecular catalytic residues are highlighted and important inter-molecular catalytic residues offered by the neighbouring subunit are denoted “t” for “trans”. Note how the extra electron density encircled in green dashed lines occupies the position of the γ-phosphate in ATP-bound structures of PspF1-275 and is connected to the electron density of the AMP moiety.
B- Omit difference FoFc map of the Mg-AMP-PspF1-275 structure contoured at 3σ. The PspF1-275-ATP-bound structure was superimposed onto the P-loop of the PspF1-275-Mg-AMP-bound structure and the result is displayed in this figure. Note that ATP fits convincingly into the difference map. Further note how the γ-phosphate of ATP fits into the extra density encircled in figure 2A.
Data was collect at ESRF beamline ID-29. Refinement of the structure was performed as described 14 and the nucleotide and the Mg ion were refined with unit occupancy. Map inspection, model building and water molecule picking were done using Coot 20. The average temperature factors in this structure are: for the protein: 30, for the AMP: 60 and for the Mg: 57. For analysis, all the liganded (ATP: 2C96; ADP: 2C98; AMPPNP: 2C99; Mg-ATP-PspF1-275R227A: 2C9C) and unliganded (Apo: 2BJW) PspF structures were aligned onto their P-loops (residues 35 to 43). All figures were prepared using Pymol (Delano, W.L. The PyMOL Molecular Graphics System (2002) on World Wide Web http://www.pymol.org).
When superposing the ATP-bound structure onto the Mg-AMP-bound structure, ATP fits convincingly into the FoFc difference map contoured at 3σ (Figure 2B). We note that the α-phosphate of ATP lies outside the density whereas the β-phosphate and γ-phosphate fit inside it. This observation suggests that the AMP-AlFx analogue, regardless of the coordination of the Al, mimics more closely a tri-phosphate nucleoside rather than a di-phosphate one. Furthermore, when comparing the Mg-AMP-bound structure to the AMPPNP-, Mg-ATP- and ADP-bound structures14 we notice that certain key residues and secondary structure elements adopt conformations that are closer to those previously observed in the tri-phosphate nucleoside bound structures. Indeed, the crucial Walker B E108 residue adopts in the Mg-AMP-bound structure a conformation midway between that observed in the AMPPNP-bound structure and that observed in the Mg-ATP-bound structure. Also, the L1 loop in the Mg-AMP-bound structure is as ordered and points in the same direction as in the AMPPNP-bound structure. L1 loop is not as disordered as in the Mg-ATP-bound structure and it points up instead of pointing down as it does in the ADP-bound structure. Finally, in the Mg-AMP-bound structure the N-terminus of PspF appears more structured and has moved 0.5 Å towards the Walker B motif when compared to the N-terminus in the Mg-ATP-bound structure and 1.2 Å when compared to the ADP-bound structure. This movement of the N-terminus could promote or facilitate the translation of the AMP to compensate for the missing β-phosphate.
Translation of the AMP is consistent with results using the human Guanylate Binding Protein 1 (hGBP1), which showed a clear di-phosphatase activity and revealed that GMP-AlF4 adopts a conformation that permits the AlF4 moiety to occupy the canonical position of the γ-phosphate of GTP 18. Interestingly, for hydrolysis of GDP to occur, the GDP moiety has to be translated in the binding pocket, and the torsion angle between the ribose moiety and the phosphate backbone needs to change so that the β-phosphate of GDP occupies the canonical position of the γ-phosphate of GTP whilst the α-phosphate occupies that of the β-phosphate, similar to the Mg-AMP-bound structure presented here (Figure 2). We did not detect hydrolysis of ATP (or ADP) to AMP by PspF1-275.
Determinants for ADP-AlFx versus AMP-AlFx complex formation
In order to better understand the organisation of AMP and ADP within AlFx-containing complexes, and to probe any intrinsic differences between these two complexes, we chose to mutate residues implicated in ATP binding and hydrolysis. More specifically, residues that contact the γ-phosphate and the β-phosphate of ATP, as well as the potential ‘trans’ (provided by the neighbouring subunit) R-finger residues involved in inter-subunit ATP-hydrolysis were substituted using site-directed mutagenesis. Using the same experimental approach as described in Figure 1A, we screened for complexes formed with PspF1-275 variants. Two distinct classes of residues emerged: (i) residues required for forming both ADP-AlFx and AMP-AlFx-dependent complexes; and (ii) residues essential only for AMP-AlFx-dependent complex formation.
Residues implicated in both ADP-AlFx- and AMP-AlFx-dependent complex formation include the Walker A residue K42, the ribose interacting residue L44, the Walker B residue E108 and the ‘trans’ R-finger residue R168 (tR168) (Table 1). Substitution of these residues with alanine eliminated both ADP-AlFx- and AMP-AlFx-dependent complex formation. Specific determinants for AMP-AlFx complex formation are the Walker B D107 residue and the ‘trans’ R-finger residue R162 (tR162) (Table 1).
The Walker B motif residues D107 and E108 and the R-finger residues R162 and R168 are conserved features among AAA+ proteins. They are categorized as catalytic residues since their substitution results in significantly reduced ATP-hydrolysis ability 19. Interestingly, the integrity of D107 is required for AMP-AlFx-dependent complex formation, but not for ADP-AlFx-dependent complex formation (Table 1). We infer that D107 is probably involved first in γ-phosphate coordination and then in hydrolysis per se 17. Mutating D107 would thus affect interaction with the γ-phosphate, but not the latter state when the β-γ phosphate bond has been cleaved, as it is no longer involved in nucleotide interaction due to the change in valence of the γ-phosphate of ATP after nucleophilic attack. Integrity of E108 is required for formation of ADP- and AMP-AlFx dependent complexes, consistent with its role in sensing the state of the γ-phosphate (either cleaved or non-cleaved) and transmitting changes to the σ54 interacting L1 loop 17. Mutation of E108 presumably impairs the ability to detect the γ-phosphate, hence affecting complex formation when using either AMP-AlFx or ADP-AlFx (Table 1). Importantly, to relay the state of the γ-phosphate in one subunit to the remaining hexameric assembly, thereby enabling hydrolysis, tR162 contributes to formation of the catalytic centre in the neighbouring subunit. Since the integrity of R162 is required for AMP-AlFx- but not ADP-AlFx-dependent complex formation, we propose that tR162 is also involved in γ-phosphate coordination and catalysis. As integrity of R168 is required for both AMP-AlFx- and ADP-AlFx-dependent complex formation, tR168 is probably involved in interactions with other parts of the nucleotide (namely the C2′ and C3′ hydroxyl groups), acting as a nucleotide-dependent self-association determinant rather than a catalytic residue per se 14. It is interesting to note that we were unable to identify a mutation which supports formation of a stable AMP-AlFx complex and not an ADP-AlFx complex.
In this study, we identified and characterised a new nucleotide analogue supporting formation of a stable bEBP•σ54 (Eσ54) complex. In the crystal structure of PspF1-275•AMP-AlFx we observe an extra density (probably due to the AlFx moiety) linked to the AMP moiety, which occupies the same position as the γ-phosphate in the PspF1-275-ATP-bound structure. Furthermore, in the Mg-AMP-bound structure presented here, the α-phosphate (of AMP) occupies the same position as the β-phosphate of ATP in the PspF1-275-ATP-bound structure. These data taken together suggest that occupancy of the α-phosphate position is not as important as occupancy of the β-phosphate and γ-phosphate positions for stimulating stable complex formation. The change in the ribose-phosphate bond observed for AMP (compared to AMPPNP, ATP and ADP) resembles that observed for hGBP1 suggesting a level of conservation between nucleotide binding pocket properties. We propose that AMP-AlFx will be a useful tool to study the ATP binding pockets of AAA+ proteins and more specifically to demonstrate how residues of the AAA+ catalytic centre are implicated in nucleotide stabilisation and utilisation. The apparent adaptability of AMP in this complex suggests that AMP-AlFx could enable identification of new functional states of AAA+ proteins in complex with their natural target. Finally, to our knowledge, this is the first time that AMP-AlFx has been reported to act as a nucleotide analogue. It can therefore be regarded as a powerful new tool to study the mechanisms of other AAA+ protein family members and more generally Walker motif ATPases.
Supplementary Material
Acknowledgements
We thank Dr P. Burrows for comments on the manuscript. We are grateful to members of Prof. Buck's and Dr Zhang's labs for helpful discussions and friendly support. This work was supported by grants from the BBSRC and Wellcome Trust to M.B. and X.Z. M.B. acknowledges fellowship support from the Leverhulm trust. N.J. was the recipient of an EMBO fellowship (ALTF 387-2005).
References
- 1.Rappas M, Bose D, Zhang X. Bacterial enhancer-binding proteins: unlocking sigma(54)-dependent gene transcription. Curr Opin Struct Biol. 2007;17:110–6. doi: 10.1016/j.sbi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher J, Joly N, Rappas M, Zhang X, Buck M. Structures and organisation of AAA+ enhancer binding proteins in transcriptional activation. J Struct Biol. 2006 doi: 10.1016/j.jsb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Wigneshweraraj SR, Burrows PC, Bordes P, Schumacher J, Rappas M, Finn RD, Cannon WV, Zhang X, Buck M. The second paradigm for activation of transcription. Prog Nucleic Acid Res Mol Biol. 2005;79:339–69. doi: 10.1016/S0079-6603(04)79007-8. [DOI] [PubMed] [Google Scholar]
- 4.Wedel A, Weiss DS, Popham D, Droge P, Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990;248:486–90. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- 5.Bordes P, Wigneshweraraj SR, Chaney M, Dago AE, Morett E, Buck M. Communication between Esigma(54), promoter DNA and the conserved threonine residue in the GAFTGA motif of the PspF sigma-dependent activator during transcription activation. Mol Microbiol. 2004;54:489–506. doi: 10.1111/j.1365-2958.2004.04280.x. [DOI] [PubMed] [Google Scholar]
- 6.Bordes P, Wigneshweraraj SR, Schumacher J, Zhang X, Chaney M, Buck M. The ATP hydrolyzing transcription activator phage shock protein F of Escherichia coli: identifying a surface that binds sigma 54. Proc Natl Acad Sci U S A. 2003;100:2278–83. doi: 10.1073/pnas.0537525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SY, De La Torre A, Yan D, Kustu S, Nixon BT, Wemmer DE. Regulation of the transcriptional activator NtrC1: structural studies of the regulatory and AAA+ ATPase domains. Genes Dev. 2003;17:2552–63. doi: 10.1101/gad.1125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rappas M, Schumacher J, Beuron F, Niwa H, Bordes P, Wigneshweraraj S, Keetch CA, Robinson CV, Buck M, Zhang X. Structural insights into the activity of enhancer-binding proteins. Science. 2005;307:1972–5. doi: 10.1126/science.1105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallai L, Tucker PA. Crystal structure of the central and C-terminal domain of the sigma(54)-activator ZraR. J Struct Biol. 2005;151:160–70. doi: 10.1016/j.jsb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.De Carlo S, Chen B, Hoover TR, Kondrashkina E, Nogales E, Nixon BT. The structural basis for regulated assembly and function of the transcriptional activator NtrC. Genes Dev. 2006;20:1485–95. doi: 10.1101/gad.1418306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaney M, Grande R, Wigneshweraraj SR, Cannon W, Casaz P, Gallegos MT, Schumacher J, Jones S, Elderkin S, Dago AE, Morett E, Buck M. Binding of transcriptional activators to sigma 54 in the presence of the transition state analog ADP-aluminum fluoride: insights into activator mechanochemical action. Genes Dev. 2001;15:2282–94. doi: 10.1101/gad.205501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, Doucleff M, Wemmer DE, De Carlo S, Huang HH, Nogales E, Hoover TR, Kondrashkina E, Guo L, Nixon B,T. ATP Ground- and Transition States of Bacterial Enhancer Binding AAA+ ATPases Support Complex Formation with Their Target Protein, sigma54. Structure. 2007;15:429–440. doi: 10.1016/j.str.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittinghofer A. Signaling mechanistics: aluminum fluoride for molecule of the year. Curr Biol. 1997;7:R682–5. doi: 10.1016/s0960-9822(06)00355-1. [DOI] [PubMed] [Google Scholar]
- 14.Rappas M, Schumacher J, Niwa H, Buck M, Zhang X. Structural basis of the nucleotide driven conformational changes in the AAA+ domain of transcription activator PspF. J Mol Biol. 2006;357:481–92. doi: 10.1016/j.jmb.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Joly N, Schumacher J, Buck M. Heterogeneous nucleotide occupancy stimulates functionality of phage shock protein F, an AAA+ transcriptional activator. J Biol Chem. 2006;281:34997–5007. doi: 10.1074/jbc.M606628200. [DOI] [PubMed] [Google Scholar]
- 16.Cannon W, Bordes P, Wigneshweraraj SR, Buck M. Nucleotide-dependent triggering of RNA polymerase-DNA interactions by an AAA regulator of transcription. J Biol Chem. 2003;278:19815–25. doi: 10.1074/jbc.M301296200. [DOI] [PubMed] [Google Scholar]
- 17.Joly N, Rappas M, Wigneshweraraj SR, Zhang X, Buck M. Coupling nucleotide hydrolysis to transcription activation performance in a bacterial enhancer binding protein. Mol Microbiol. 2007;66:583–595. doi: 10.1111/j.1365-2958.2007.05901.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature. 2006;440:101–4. doi: 10.1038/nature04510. [DOI] [PubMed] [Google Scholar]
- 19.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–29. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 20.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.