Abstract
Lung cancer leads all other cancers in both incidence and mortality. Recent advances in underlying molecular pathogenesis have validated a panel of protein tyrosine kinases as new targets in lung cancer treatment. Insulin-like growth factor-1 receptor (IGF-1R) is an important tyrosine kinase receptor involved in cell proliferation, differentiation, metabolism, apoptosis, and angiogenesis. Aberrant activation of IGF-1R is frequently found in patients with lung cancer and contributes to malignant transformation and poor prognosis for patients with lung cancer. In this review, we focused on recent progress in the research of IGF-1R's role in lung cancer development and progression, including its structure and biological function, potential mechanisms of aberrant activation, and related oncogenic effects. We also discussed effective IGF-1R antagonists that are currently registered for clinic trials or are undergoing preclinical study with special emphasis on their antibodies and small molecule tyrosine kinase inhibitors.
Keywords: Insulin-like growth factor, lung cancer, receptor, targeted therapy
Introduction
Epidemiological data show that lung cancer leads all other cancers in both incidence and mortality [3]. The high mortality rate is primarily owing to difficulty in the diagnosis of lung cancer in the early stages and rapid progression of the disease in the advanced stages. In the past 30 years, the survival rate of patients with non-small cell lung cancer (NSCLC) has increased slightly, from 13% to 15%, which is likely due to the relative effectiveness of platinum-based chemotherapy [5]. Given this limited improvement in this disease's treatment and survival, new therapeutic approaches such as target-based therapies are urgently needed to improve NSCLC treatment.
Recent advances in the understanding of molecular pathogenesis have identified a panel of protein tyrosine kinases as new targets in lung cancer treatment, such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), vascular endothelial cell growth factor receptor (VEGFR), mesen-chymal-epithelial transition factor (C-MET), Src and the insulin-like growth factor-1 receptor (IGF-1R) [13–19]. The IGF-1R is an important tyrosine kinase receptor that mediates IGF signaling and plays a crucial role in mitogenesis, angiogenesis, transformation, apoptosis, and cell motility. In contrast, insulin-like growth factor-2 receptor (IGF-2R), another IGF signaling receptor, is non-functional because it does not contain any kinase domain [19]. Numerous preclinical and epidemiological studies have identified a role of the IGF signaling in carcinogenesis, including cancers of the prostate, breast, colorectum, and lung [7, 9].
In this review, we will focus on recent progress in the research of the IGF-1R and NSCLC, including its structure and biological functions, and roles in lung cancer development and progression. In addition, we will summarize recent progress in IGF-1R-targeted therapies using immune-antibodies or tyrosine kinase inhibitors in the NSCLC treatment.
Structure and biology of IGF-1R
Structure
The IGF-1R gene, also known as CD221 or JTK13, is located on chromosome 15q26.3 and encodes a single polypeptide of 1,367 amino acids, which is constitutively expressed in most cells [21]. This gene is highly polymorphic with at least 1,964 variants, most of which are either not detectable or are of low frequency in the general population; however, no common variants (minor allele frequency > 0.05) that affect amino acids have been identified to date, according to the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/).
After translation, the polypeptide precursor is cleaved at the Arg-Lys-Arg-Arg proteolytic cleavage site to yield alpha and beta subunits of IGF-1 receptor, which then form heterotetrameric glycoproteins consisting of two extracellular ligand-binding α-subunits and two transmembrane catalytic ß-subunits [22]. The cytoplasmic portions of the ß-subunits contain a juxtamembrane region, a tyrosine kinase domain, and a C-terminal tail. The IGF-1 receptor is structurally homologous to the insulin receptor, with an 84% sequence homology in the tyrosine kinase domains, a 61% sequence homology in the juxtamembrane, and a 44% sequence homology in the C-terminal regions [23, 24]. This high degree of homology enables these two receptors to form hybrid receptors using similar proteins as their substrates [25].
Two IGF-1R isoforms known as CAG- and CAG+ differ in an amino acid coding sequence from Thr-Gly to Arg in the extracellular portion of the beta subunit, as a result of alternative mRNA splicing by deleting CAG nucleotides at position 2829 of the coding region [21]. Although they both bind to IGF-1 with similar affinities, the CAG- receptor is more potent than the CAG+ receptor in regulating signaling activity with a lower rate of receptor-mediated internalization [26]. However, it is unknown whether these two receptors are distributed differently in normal and cancer cells.
Activation and downstream Signalings
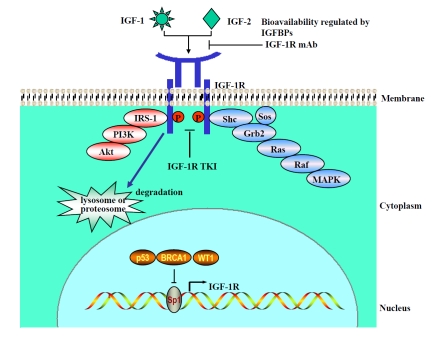
IGF-1R activation is ligand dependent (Figure 1). The two insulin-like growth factors, IGF-1 and IGF-2, are polypeptides with high sequence similarity to insulin and communicate with IGF-1R to regulate cell physiological functions. Binding of IGF-1 and IGF-2 (with a low affinity) to IGF-1R triggers structural rearrangement that results in receptor trans-autophosphory-lation (one kinase domain phosphorylating the other) and destabilizes the autoinhibitory conformation within the kinase domain [27]. This conformational change allows an unrestricted access of adenosine 5′-triphosphate (ATP) and protein substrates to the catalytic site [28] and induces a 120-fold increase in the kinase activity, compared with the basal state [22].
Figure 1.
IGF-1R activation and regulation. IGF ligand, IGF-1, or IGF-2 activates IGF-1R and its downstream signaling as modified from the picture in reference [1]. It also causes ligand binding-induced receptor internalization, which degrades IGF-1R through lysosome (normal cells) or proteosome (lung cancer cells) pathways. Sp1 is the major transcriptional factor of the IGF-1R gene and is negatively regulated by p53, BRCA1, and WT1. IGF-1R mAb or IGF-1R TKI blocks IGF-1R activation and inhibits tumor growth.
After the receptor activation, some scaffold proteins are recruited to the docking sites of the kinase domain, which initiates signaling transduction. Two major downstream pathways have been proposed. In the first pathway, insulin receptor substrate-1 (IRS-1) and IRS-2 act as substrates of IGF-1R to activate the phosphatidylinositol-3 kinase (PI3K)/Akt pathway after tyrosine phosphorylation [29, 30]. In the second pathway, scaffold proteins Shc, Grb2, and Sos form a protein complex and bring ras and raf proteins to the inner cell surface, which in turn activates the mitogen-activated protein kinase (MAPK) pathway [31, 32]. In some type of cells, a third JAK/STAT pathway exists, which can be activated through an unknown mechanism [33, 34]; however, this JAK/STAT pathway has not been identified to be the downstream of IGF-1R in NSCLC cells.
Physiological functions
In general, IGF signaling plays a critical role in the growth of essentially every normal organ (including the lungs) prenatally and postnatally [35]. Inactivation of IGF-1R in the embryonic stage could result in loss of vascular endothelial cells and apoptosis of mesenchymal cells [36]. An early study showed that bioengineered mice with complete loss of IGF-1R (Igf-1r[-/-]) developed lung hypoplasia and died at birth from respiratory failure [37], whereas mice with partial loss of IGF-1R were postnatally viable but resistant to mild hypoxia. The latter phenotype was different from their wild-type (WT) littermates [38], suggesting that IGF-1R may have additional functions involved in the regulation of oxidative stress. In the past two decades, IGF-1R has received increased attention for its critical role in a wide variety of physiological functions, including mitogenesis, angiogenesis, transformation, antiapoptosis and cell motility. Therefore, dysregulated IGF-1R signaling can enhance abnormal growth, conferring tumor growth-promoting properties in local tissues.
IGF-1R in lung cancer development and progression
The association of the IGF signaling with lung cancer risk has been extensively studied with mixed results (Table 1) [2, 4, 6, 8, 11, 12, 20]. Most of these studies analyzed serum IGF-1 concentrations in patients with lung cancer, but serum IGF-1 concentrations may not be an accurate estimation of the IGF-1R activation status, because local expression of IGF ligands and IGF-1R also contribute to overall IGF-1R activation in lung tissues. For example, Western blotting analysis confirmed that NSCLC cell lines had a much higher rate of IGF-1R activation than normal human bronchial epithelial cells had, which has been considered the rationale for the IGF-1R–targeted therapy in patients with NSCLC [39].
Table 1.
Summary of published circulating IGF-1 and lung cancer risks
| Study | Study Design | Sample Size (case/control) | Sample Medium | IGF-1 Test | Cancer Risk | P Value for Trend | OR Highest vs.Lowest Quartile (95% CI) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Yu 1999 | Hospital c/c | 204/218 | Plasma | ELISA | Yes | 0.01 | 2.00 (1.10–3.65) | [2] |
| Lukanova 2001 | Nested c/c | 93/186 | Serum | IRMA | No | 0.32 | 0.79 (0.29–2.19) | [4] |
| London 2002 | Nested c/c | 230/740 | Serum | RIA | No | 0.36 | 0.73 (0.43–1.24) | [4, 6, 7] |
| Spitz 2002 | Nested in RCT control | 159/297 | Serum | ELISA | No | 0.29 | 1.11 (0.64–1.93) | [4, 8–10] |
| Unsal 2005 | Hospital c/c | 24/12 | Serum | IRMA | Marginal | 0.07 | N/A | [11] |
| Ahn 2006 | Nested in RCT control | 200/400 | Serum | ELISA | No | 0.26 | 0.69 (0.41–1.15) | [12] |
| Morris 2006 | Nested c/c | 167/498 | Serum | ELISA | No | 0.45 | 1.21 (0.62–2.35) | [20] |
c/c = case/control, IGF-1 = insulin-like growth factor-1, ELISA = enzyme-linked immunosorbent assay, IRMA = immunoradiometric assay, RIA = radioimmunoassay, OR = odds ratio, CI = confidence interval, RCT = randomized controlled trial, Ref.=references
Aberrant IGF-1R activation
Although studies on EGFR indicate that increased expression of receptor tyrosine kinase might have been via mechanisms of an increase in gene copy number [40] and constitutive activation by tyrosine kinase mutations [41], these mechanisms have not been found for IGF-1R in patients with cancer. The question remains: how is IGF-1R activated in lung cancer?
Endocrine IGF ligands are produced by hepatocytes in response to growth hormone stimulation. In the blood, IGF-1 or IGF-2 binds to insulin-like growth factor binding protein-3 (IGFBP-3) and an acid-labile subunit (ALS) to form a ternary structure, which protects them from degradation; meanwhile, IGFs are prevented from displaying their insulin-like potential, because they are sequestered in the complex with IGFBP-3 [9]. Results of early investigations showed elevated levels of circulating IGF-1 and decreased levels of IGFBP-3 in the plasma of patients with lung cancer [2, 4, 8, 10]. High levels of circulating IGF-1 and low levels of IGFBP-3 may induce IGF-1R activation by increasing the bioavailability of the IGF-1 ligand, thus contributing to lung cancer risk. However, these results were not confirmed by prospective studies [4, 6, 7] or a meta-analysis [7]. The inconsistency between published papers could be a result of differences in study populations, designs, and analytical methods. It should be pointed out that IGF-1 production is also influenced by nutritional status and liver functions and therefore could be lowered in patients with lung cancer [42, 43]. Contrary to the notion that cigarette smoking may induce IGF-1 production, smoking was found to have no influence [44, 45] or even reduce the levels of circulating IGF-1 in epidemiological studies [46].
IGF ligands can be produced locally in an autocrine/paracrine manner and have been detected in serum-free or hormone-free medium of cultured lung cancer cell lines [47], implying that these ligands are secreted from lung cancer cells. Lung cancer cells of different histology may also vary in their ability to secrete different types of IGF ligand, because the IGF-1 peptide is only detected in small cell lung cancer (SCLC), whereas IGF-2 is detected in both NSCLC and SCLC [48, 49]. These results suggest that IGF-2 is the predominant local IGF ligand, which constitutes an autocrine/paracrine loop with IGF-1R and contributes to the NSCLC development [48, 50, 51]. Increased IGF-2 expression in NSCLC could be the consequence of loss of imprinting (LOI), which is characterized by aberrant activation of the normally repressed IGF-2 allele that occurs in approximately 50% of NSCLC patients [52], and, in addition to LOI, IGF-2 expression can be enhanced through tumor-stromal cell interaction. For example, integrin α11 is a commonly overexpressed gene in primary NSCLC [53]. Zhu et al. co-implanted NSCLC cell A549 in immunocompromised mice with immortalized WT or α11-deficient (knockout) mouse embryonic fibroblasts (MEFs), and they found that, compared with α11-deficient fibroblasts, α11-expressing fibroblasts increased tumorigenicity of A549 and enhanced IGF-2 gene expression by 250-fold [54]. Hypoxia also influences IGF-2 expression, and a hypoxic lung tumor environment may exist in NSCLC patients because of either insufficient angiogenesis after rapid tumor growth or primary and secondary effects of long-time cigarette smoking. Previous studies suggest that hypoxia may activate IGF-2 gene expression through up-regulating its transcriptional factors, HIF-1 alpha and Egr1 [55, 56].
Increased IGF ligand expression enhances IGF-1R activation but diminishes IGF-1R on the cell surface through the ligand binding-induced receptor internalization, thus balancing IGF signaling. In NSCLC cells, it is likely that this balancing may be weakened by the overexpression of IGF-1R. Sp1 is the major transcriptional factor of the IGF-1R gene in providing a basal level of transcription, which can be modulated by its interaction with other regulatory factors [57]. For example, several WT tumor suppressor genes (including p53, p63, p73, WT1, and BRCA1) can interact with Sp1 and sequester it from binding to the promoter site, thus downregulating IGF-1R expression (Figure 1) [58–61]. Therefore, if these genes are mutated during lung carcinogenesis, they may lose their suppression effects, and IGF-1R expression may increase. Indeed, Western blotting analysis detected substantial IGF-1R protein expression in whole-cell lysates of NSCLC cell lines [39]. High-membranous IGF-1R expression was also observed in 11 (84.6%) of 13 lung carcinoma tissues as detected by immunohistochemistry staining [62]. These results support an upregulated IGF-1R expression in tumor tissues, which may contribute to overall IGF-1R activation through interaction with increased IGF ligands. Recently, Carelli et al. [63] found that NSCLC and non-neoplastic cells could degrade IGF-1R protein through different pathways. Therefore, it is likely that NSCLC cells may degrade IGF-1R via the ubiquitin-proteosome pathway, and non-neoplastic cells may degrade IGF-1R via the lysosome pathway (Figure1). However, it is not clear whether this divergent degradation route has an effect on IGF-1 receptor signals.
Malignant transformation and lung tumor initiation
In vitro and in vivo experiments have demonstrated that IGF-1R signaling is an important factor involved in tumorigenicity. It has been shown that IGF-1R was essential for malignant transformation of mouse embryo fibroblasts by SV40 and Ras oncogenes [64, 65]. Loss of IGF-1R expression precludes the transformation and abrogates soft agar growth, which is a unique feature of malignant cells. In line with this, genetically engineered mouse models provide direct evidence that tissue-specific IGF-1R overexpression or hyperactivation is a risk factor for cancer, because it is sufficient to cause spontaneous tumor formation in mammary and skin tissues [66–68]. These findings suggest that IGF-1R can act as a driving force in tumorigenesis and therefore can be considered an“oncogene.”
Similarly, IGF-1R can influence tumorigenicity of NSCLC cells. Studies have shown that downregulating IGF-1R by ShRNA or dominant-negative IGF-1R decreased anchorage-independent colony formation ability of NSCLC cell lines in vitro [16, 69]. To confirm a causal role of IGF-1R signaling in lung cancer development, Frankel et al. developed a line of transgenic mice to assess the influence of IGF-1 on pulmonary pathology by cloning human IGF-1 cDNA into a vector under the control of surfactant protein C promoter and expressing it in alveolar type II epithelial cells [70]. They found that secreted human IGF-1 was abundantly present in bronchoalveolar lavage fluid and functionally active enough to stimulate IGF-1R and downstream signaling in lung fibroblasts; compared with WT littermates, these IGF-1 transgenic mice did show lung tumor predisposition, because there was a significant increase in premalignant epithelial adenomatous hyperplasia and a trend toward increased adenoma formation in the aged mice; however, the phenotype was relatively weak, and no malignant tumor was established in this animal model. Furthermore, it is likely that local IGF-1 secretion does not mimic natural situations in humans because IGF-2, but not IGF-1, is the predominant autocrine/paracrine ligand in NSCLC [48, 49].
The mouse mammary tumor virus (MMTV)-IGF-2 transgenic mouse model was initially designed to investigate mammary tumorigenesis and was later utilized to evaluate autocrine/paracrine IGF-2 expression and lung cancer risk; this is because IGF-2 driven by the MMTV-LTR promoter is also expressed in lung epithelial cells [71]. In this mouse model system, lung tumors were found to develop as early as 6 months of age, and the tumor incidence reached 69% at 18 months with morphological characteristics of pulmonary adenocarcinoma; therefore, this mouse model provided evidence of the role of IGF-1R signaling in lung tumorigenesis in vivo. This study suggests that first, increased IGF signaling contributes to lung tumor development in the late stage of a mouse's life; second, paracrine IGF-2 is a more important risk factor than IGF-1 is in lung tumorigenesis; and third, enhanced Akt activation may not be critical in lung adenocarcinoma development, because the phosphorylated Akt level was significantly lower in the tumor tissues of MMTV-IGF-2 transgenic mice compared with WT littermates. So far, there is not enough evidence to conclude whether IGF-1R signaling is a causative factor for lung tumor development. Since aging may also be a predisposing factor for cancer through accumulation of multiple genetic changes in senescent cells and IGF-1R signaling is a strong precipitating factor for senescence [72], the increased tumor incidence in old transgenic mice may result from precipitated senescence of lung tissues. In humans, aging is still an important risk factor for lung cancer [73], which is related with increased history of exposure to carcinogens such as tobacco, radiation, and toxic chemicals. These carcinogens cause various kinds of DNA damage and may mask the oncogenic effect of IGF-1R signaling in the etiology of lung cancer. It appears that the primary effect of increased IGF-1R signaling in human lung carcinogenesis is to provide survival signals for DNA-damaged cells, thus enabling them to progress into malignant tumors. Studies have shown that a joint effect of IGFs and genetic instability enhanced lung cancer risk by 17-fold, whereas a single risk factor increased the risk of lung cancer 1.6-fold to 2.5-fold [74]. Therefore, activated IGF-1R should be at least considered a synergistic factor in lung tumorigenesis.
Tumor progression and metastasis
IGF signaling has a strong mitogenic effect, accelerating cell division and proliferation. Elevated IGF-1R expression and activation have been observed in many NSCLC cells, which explains the phenotype of transformed bronchial epithelial cells that require increased IGF signaling to maintain a high rate of proliferation and growth [39]. Previous studies using different intervention strategies have consistently demonstrated that blocking the IGF-1R activity can dramatically inhibit cell viability through the G1 cell-cycle arrest and apoptosis, whereas activating IGF-1R has an opposite effect [75]. Cosaceanu et al. found that IGF-1R mitogenic signaling mediated NSCLC cell viability by many complex and redundant pathways [76]. For example, anti-IGF-1R antibody, tyrosine kinase inhibitor, and IGF-1R's small interfering RNA had all been shown to impair IGF-1R function at the receptor level, but they inhibited cell viability by blocking different biological pathways such as IRS-1, Shc, and 14.3.3-dependent mitochondrial translocation of Raf-1 kinase.
Early-stage NSCLC more frequently presents as a localized disease, while advanced NSCLC has a strong propensity to metastasize, which involves multiple steps that include cell detachment from the primary tumor, migration, invasion of host tissue barriers, and intra-vasculation [77]. Activated IGF-1R is capable of causing a pleiotropic effect to promote this process. One critical event of metastasis is cancer cell invasion. Extracellular matrix (ECM) proteins are a gel-like structure that consists of laminin and type IV collagen and constitutes the first barrier against tumor spread. Cancer cells form an adhesion with ECM through anchorage proteins such as integrin. Previous studies have demonstrated that IGF-1R can interact with the ß1 integrin and regulate cell proliferation and ECM adhesion [78]. It was shown that the ß1A integrin could form a complex with IGF-1R and IRS-1, contributing to IGF-IR-mediated cell proliferation and anchorage-independent growth; whereas β1C, an integrin cytoplasmic variant, increased cell adhesion to ECM in response to IGF-1, which was inhibited by an IGF-1R antagonizing antibody, alpha IR3 [79, 80]. Previous studies have demonstrated that IGF-1R can interact with integrin and regulate cell migration [81, 82]. Increased expression of IGF-1R therefore reduces cell adhesion with ECM, thus promoting cell motility. In addition, the IGF-1R signal can upregulate expression of proteolytic enzymes, such as matrix metalloproteinase-2 (MMP-2), MMP-9, and urokinase-type plasminogen activator (u-PA), which activate latent collagenases and metalloproteases to degrade ECM components [83].
One of the hallmarks of NSCLC is sustained angiogenesis, which plays an important role in the process of tumor growth and metastasis [84]. Vascular endothelial growth factor (VEGF) is one of the most potent angiogenic molecules, which regulate both angiogenesis and vascular permeability [85]. Although the connection of IGF-1R and angiogenesis in NSCLC is rarely reported, IGF-1R signaling-induced VEGF expression has been observed in breast cancer, endometrial adenocarcinoma, hepatic cancer, and colorectal cancer [29, 86–89]. The underlying mechanism involves upregulating VEGF transcriptional factor (HIF-1 alpha) expression and inducing its nuclear translocation. For example, it is estimated that IGF-1R accounts for approximately 50% constitutive HIF-1 alpha activation/expression in cancer cells [90], which suggests that IGF-1R could be a potential target for anti-angiogenetic therapy of tumors.
Targeted therapy
Because IGF-1R is frequently activated and plays a crucial role in NSCLC tumorigenesis and progression, IGF-1R signaling may be a good target for a chemopreventive/therapeutic attack. Several lines of experimental evidence support this notion. Studies have shown that tumor growth and metastasis were reduced when IGF-1R function was compromised [91–93]. In cells only carrying the extracellular domain of IGF-1R, their tumorigenicity and metastatic potential were completely lost [94]. A variety of approaches to inhibit IGF-1R signaling have been developed, including using small molecule kinase inhibitors, IGF-1R ectodomain antibodies, antisense oligonucleotides, and RNA interference; of those choices, the antagonistic/neutralizing antibodies and tyrosine kinase inhibitors are the most promising treatment options and have entered phase I clinical trials.
IGF-1R tyrosine kinase inhibitor (IGF-1R TKI)
Since Novartis reported the first IGF-1R tyrosine kinase inhibitor, NVP-AEW541, in 2004 [95], at least six different tyrosine kinase inhibitors have been produced and tested in preclinical experiments [96]. These inhibitors are structurally different but share some basic characteristics in their working mechanisms and therefore can be classified into the same category. First, these inhibitors are small molecules that compete for the ATP binding pocket of IGF-1R. Second, they display potent activities against the conformational change of tyrosine kinase domain from the basal state to the phosphorylated state. Third, they are very effective in inhibiting IGF-1R signaling with the half maximal inhibitory concentration (IC50) around the nanomolar level in vitro but display poor specificity to discriminate between IGF-1R and insulin receptors because of the high degree of structural homology. However, their ability to co-inhibit the insulin receptor function can cause metabolic disorders, which would inhibit the use of IGF-1R TKI for therapeutic purposes. The small molecule tyrosine kinase inhibitor, cis-3-[3-(4-methyl-piperazin-l-yl)-cyclobutyl]-1-(2-phenyl-quinolin-7-yl)-imidazo[1, 5-a]pyrazin-8-ylamine (PQIP), was developed by the OSI pharmaceutical company (Melville, NY) and serves as a good example for studying the antitumor activity and side effects of this category. PQIP is a 1,3-disubstituted-8-amino-imidazopyrazine derivative, which displays a cellular IC50 of 19 nmol/L for inhibition of ligand-dependent human IGF-IR activation with 14-fold selectivity over insulin receptor in kinase assays [97].
PQIP demonstrates robust antitumor activities both in vitro and in vivo, but also causes 30% blood glucose elevation in tested animals, implying that PQIP inhibits insulin receptors. It is argued that the moderate hyperglycemia induced by PQIP is well tolerable, and a therapeutic window may exist to maintain an efficacious exposure without significantly disturbing blood glucose. Additionally, overproduction and secretion of IGF ligands, particularly IGF-2 from tumors, may produce insulin-like functions and cause recurrent fasting hypoglycemia, a condition called nonislet cell tumor hypoglycemia (NICTH) [98–101]. Therefore, the hyperglycemia caused by IGFR TKI may potentially help maintain a normal glucose level in patients with lung cancer. Furthermore, insulin receptor-mediated tumor growth has long been reported and should not be ignored [102, 103]. From this perspective, IGF-1R TKI can be still considered a candidate agent in NSCLC treatment, if its use is monitored closely.
IGF-1R Monoclonal Antibody
IGF-1R monoclonal antibodies selectively bind to the extracellular domain of the IGF-IR and antagonize ligand binding and signaling. These antibodies also induce rapid receptor internalization and degradation, thus reducing IGF-1R density on the tumor cell surface to such a low level that it is insufficient to maintain tumor growth. IGF-1R monoclonal antibodies are highly selective against the IGF-IR without directly interfering with insulin receptor activity and therefore are considered relatively safe in terms of glucose metabolism compared with small molecule kinase inhibitors.
An example of this category is the fully human IgG2 monoclonal antibody, CP-751871, which was developed by Pfizer (New York, NY) from mice immunized with the human IGF-1R extracellular domain. This antibody blocks binding of IGF-1 to its receptor (IC50 at 1.8 nmol/L), inhibits IGF-1-induced receptor autophosphorylation (IC50 0.42 nmol/L), and causes downregulation of IGF-1R in vitro and in tumor xenografts [104]. Preclinical studies indicate that CP-751871 has notable activities against multiple human tumor types, including breast cancer, colon cancer, and multiple myeloma. A single dose of the CP-751871 treatment typically inhibits, but does not fully arrest, tumor growth. When used in combination with conventional chemotherapeutic agents, it displayed additive/synergistic effects. A Phase I trial of CP-751871 demonstrated that administration of 3–20 mg/kg CP-751871 in a continuous cycle of 21 days was well tolerated and stabilized tumor growth in 10 of 15 patients that were tested [105]. Major treatment-related toxicities include hyperglycemia, which is unlikely due to insulin receptor binding, but may associate with a gluconeogenetic effect of accumulated growth hormone as a result of IGF-1R inactivation. Currently, CP-751871 is being tested in a Phase II clinical trial, which investigates the efficacy of CP-751871 in combination with paclitaxel and carboplatin as a first-line treatment for advanced NSCLC. Preliminary data showed that 46% of patients in the experimental arm achieved objective responses (22/48 patients) versus 32% (8/25 patients) in the control arm [106]. Subgroup analysis by histology suggested a greater benefit in patients with squamous histology within this trial [106].
Potential biomarkers of response to IGF-1R targeted therapy
At present, there is no direct evidence from clinical trials to demonstrate which patients are likely to respond to IGF-1R targeted therapy in lung cancer treatment. Since high IGF-1/IGFBP3 ratio is associated with increased lung cancer risk [2, 4, 8, 10] and high IGF-1R expression is associated with poor prognosis in lung cancer patients [107], it is postulated that patients with IGF-1R stimulating factors may have increased dependence on IGF signaling for tumor cell proliferation and survival, and therefore, are likely to respond to IGF-1R targeted therapy. Based on this assumption, several tumor biomarkers may be used as potential predictors of response to IGF-1R targeted therapies.
Altered expression of IGF axis components
There are two methods to determine the expression of IGF axis components in vivo. Measurement of plasma concentration of IGF-1, IGF-2 and IGFBP3 is simple and straightforward to evaluate IGF signaling level in vivo, whereas investigation of human lung tissues obtained from surgery may provide more accurate and comprehensive information regarding to IGF signaling at tissue level, such as IGF-1R expression and its downstream activation. Given the importance of IGF signaling in lung cancer development, it is reasonable to speculate that patients with high levels of IGF ligand and IGF-1R expression or low IGFBP-3 expression would respond to IGF-1R targeted therapies.
Resistance to EGFR targeted therapy
Resistance to EGFR targeted therapy frequently occurs after relative long time treatment of EGFR TKI in lung cancer patients. Morgillo et al provided evidence that transactivation of IGF-1R when EGFR was inhibited could rescue NSCLC cells from apoptosis, whereas co-inhibition of IGF-1R signaling restored sensitivity of NSCLC cells to EGFR TKI [108]. Therefore, IGF-1R targeted therapy can be considered in EGFR TKI resistant NSCLC patients.
IGF-1R mutation and gene amplification
Presence of receptor tyrosine kinase mutation and increased gene copy number are common mechanisms for activation and overexpression of proto-oncogenes, which have been demon-strated to be important tumor markers of response observed in EGFR targeted therapies [109]. However, these molecular changes of IGF-1R have never been reported in lung cancer, and therefore, are not likely to be predictors of IGF-1R targeted therapies.
Ras mutation
Some IGF-1R downstream proteins may have constitutive activation as a result of protein mutation, thus abrogating their reliance on IGF signaling. Ras protein is a downstream molecule of IGF-1R, which is frequently mutated in lung cancer patients [110]. Lee et al showed that NSCLC cells might develop resistance to IGFBP-3 treatment because of oncogenic Ras-mediated signals [39]. Therefore, presence of Ras protein mutation may suggest resistance to IGF-1R targeted therapy or requirement for combined inhibition of IGF-1R and Ras signals.
Conclusions
In the past decade, treatment of advanced NSCLC has met with recurrent frustrations despite numerous research efforts. Advances in the knowledge of NSCLC genetics and biology provide a rich background for the development of molecularly targeted therapeutics. The importance of IGF-1R signaling has been demonstrated by its ability to induce broad biological effects, which are essential for tumor development, and its ability to interact with those tumor suppressor genes and oncogenes, which are frequently mutated in cancer cells [111]. Cumulative data support an association between elevated IGF-1R signaling and NSCLC initiation and progression. Lung cancer development quite likely may depend on circulating IGFs in the beginning but soon may acquire the capability of producing its own supply of IGFs. Tissue levels of IGF-1R activation have possibly been underestimated by the analysis of circulating IGF-1 concentrations due to the impact from the local IGF axis. As observed in genetically engineered mouse models, local IGF ligand expression had a pronounced effect on IGF-1R activation and enhanced lung tumor development in vivo [70, 71].
Because of technical difficulty in separating phosphorylated IGF-1Rs and insulin receptors using immunohistochemistry, the examination of IGF-1R signaling in large-scale human tissue samples has not been reported. It remains unclear whether the immunoblotting data of NSCLC cells in vitro are a true reflection of IGF-1R activation status in tissues. However, the reliance of NSCLC cells on IGF-1R signaling has been proved by disrupting the IGF-1R function, either with small tyrosine kinase inhibitors or with monoclonal antibodies, which results in lung tumor growth inhibition in several model systems and has led to the approval of human clinical trials. Some special concerns remain with regard to potential metabolic disorders that can be related with insulin receptor inhibition. Current reports suggest that the drugs described above can stabilize tumor growth and be well tolerated without significant toxicities.
Finally, as we began to understand the role of IGF-1R signaling in lung cancer development, several questions need to be addressed: Which patients are likely to have hyper-activated IGF-1R and may respond to anti-IGF-1R treatment? How is the expression of IGF-1, IGF-2, or IGF-1R regulated during lung carcinogenesis? Does IGF-1R have any function in the cytoplasm or nucleus, besides acting as a membrane receptor? Further research is needed to answer these questions.
Acknowledgments
We thank Kristi M. Speights of the Department of Scientific Publications for scientific editing. This work was supported in part by NIH grants R01 ES11740 and R01 CA131274 (Q. Wei).
References
- 1.Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist. 2008;13:16–24. doi: 10.1634/theoncologist.2007-0199. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Lukanova A, Toniolo P, Akhmedkhanov A, Biessy C, Haley NJ, Shore RE, Riboli E, Rinaldi S, Kaaks R. A prospective study of insulin-like growth factor-I, IGF-binding proteins-1, -2 and -3 and lung cancer risk in women. Int J Cancer. 2001;92:888–892. doi: 10.1002/ijc.1265. [DOI] [PubMed] [Google Scholar]
- 5.Molina JR, Adjei AA, Jett JR. Advances in chemotherapy of non-small cell lung cancer. Chest. 2006;130:1211–1219. doi: 10.1378/chest.130.4.1211. [DOI] [PubMed] [Google Scholar]
- 6.London SJ, Yuan JM, Travlos GS, Gao YT, Wilson RE, Ross RK, Yu MC. Insulin-like growth factor I, IGF-binding protein 3, and lung cancer risk in a prospective study of men in China. J Natl Cancer Inst. 2002;94:749–754. doi: 10.1093/jnci/94.10.749. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 8.Spitz MR, Barnett MJ, Goodman GE, Thornquist MD, Wu X, Pollak M. Serum insulin-like growth factor (IGF) and IGF-binding protein levels and risk of lung cancer: a case-control study nested in the beta-Carotene and Retinol Efficacy Trial Cohort. Cancer Epidemiol Biomarkers Prev. 2002;11:1413–1418. [PubMed] [Google Scholar]
- 9.Yu H, Mistry J, Nicar MJ, Khosravi MJ, Diamandis A, van Doorn J, Juul A. Insulin-like growth factors (IGF-I, free IGF-I and IGF-II) and insulin-like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J Clin Lab Anal. 1999;13:166–172. doi: 10.1002/(SICI)1098-2825(1999)13:4<166::AID-JCLA5>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Wan YX, Zhang QK. [Significance and expression of insulin-like growth factor 1 and IGF binding protein 3 in serum of patients with lung cancer] Ai Zheng. 2004;23:710–714. [PubMed] [Google Scholar]
- 11.Unsal E, Koksal D, Yurdakul AS, Atikcan S, Cinaz P. Analysis of insulin like growth factor 1 and insulin like growth factor binding protein 3 levels in bronchoalveolar lavage fluid and serum of patients with lung cancer. Respir Med. 2005;99:559–565. doi: 10.1016/j.rmed.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Ahn J, Weinstein SJ, Snyder K, Pollak MN, Virtamo J, Albanes D. No association between serum insulin-like growth factor (IGF)-I, IGF-binding protein-3, and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2010–2012. doi: 10.1158/1055-9965.EPI-06-0580. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 14.Bauman JE, Eaton KD, Martins RG. Antagonism of platelet-derived growth factor receptor in non small cell lung cancer: rationale and investigations. Clin Cancer Res. 2007;13:s4632–4636. doi: 10.1158/1078-0432.CCR-07-0212. [DOI] [PubMed] [Google Scholar]
- 15.Lee CB, Socinski MA. Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: a review of recent clinical trials. Rev Recent Clin Trials. 2007;2:117–120. doi: 10.2174/157488707780599401. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Imsumran A, Park MY, Kwon SY, Yoon HI, Lee JH, Yoo CG, Kim YW, Han SK, Shim YS, Piao W, Yamamoto H, Adachi Y, Carbone DP, Lee CT. Adenovirus expressing shRNA to IGF-1R enhances the chemosensitivity of lung cancer cell lines by blocking IGF-1 pathway. Lung Cancer. 2007;55:279–286. doi: 10.1016/j.lungcan.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Stabile LP, Lyker JS, Huang L, Siegfried JM. Inhibition of human non-small cell lung tumors by a c-Met antisense/U6 expression plasmid strategy. Gene Ther. 2004;11:325–335. doi: 10.1038/sj.gt.3302169. [DOI] [PubMed] [Google Scholar]
- 18.Giaccone G, Zucali PA. Src as a potential therapeutic target in non-small-cell lung cancer. Ann Oncol. 2008;19:1219–1223. doi: 10.1093/annonc/mdn048. [DOI] [PubMed] [Google Scholar]
- 19.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 20.Morris JK, George LM, Wu T, Wald NJ. Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. Br J Cancer. 2006;95:112–117. doi: 10.1038/sj.bjc.6603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott AM, Bueno R, Pedrini MT, Murray JM, Smith RJ. Insulin-like growth factor I receptor gene structure. J Biol Chem. 1992;267:10759–10763. [PubMed] [Google Scholar]
- 22.Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–1063. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- 23.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. Embo J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blakesley VA, Scrimgeour A, Esposito D, Le Roith D. Signaling via the insulin-like growth factor-I receptor: does it differ from insulin receptor signaling? Cytokine Growth Factor Rev. 1996;7:153–159. doi: 10.1016/1359-6101(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 25.Frattali AL, Pessin JE. Relationship between alpha subunit ligand occupancy and beta subunit autophosphorylation in insulin/insulin-like growth factor-1 hybrid receptors. J Biol Chem. 1993;268:7393–7400. [PubMed] [Google Scholar]
- 26.Condorelli G, Bueno R, Smith RJ. Two alternatively spliced forms of the human insulin-like growth factor I receptor have distinct biological activities and internalization kinetics. J Biol Chem. 1994;269:8510–8516. [PubMed] [Google Scholar]
- 27.Wu J, Li W, Craddock BP, Foreman KW, Mulvihill MJ, Ji QS, Miller WT, Hubbard SR. Small-molecule inhibition and activation-loop trans-phosphorylation of the IGF1 receptor. Embo J. 2008;27:1985–1994. doi: 10.1038/emboj.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. Embo J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B, Cheng HL, Margolis B, Feldman EL. Insulin receptor substrate 2 and Shc play different roles in insulin-like growth factor I signaling. J Biol Chem. 1998;273:34543–34550. doi: 10.1074/jbc.273.51.34543. [DOI] [PubMed] [Google Scholar]
- 30.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 31.Craparo A, O'Neill TJ, Gustafson TA. Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. J Biol Chem. 1995;270:15639–15643. doi: 10.1074/jbc.270.26.15639. [DOI] [PubMed] [Google Scholar]
- 32.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG, et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 33.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 34.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- 35.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 36.Han RN, Post M, Tanswell AK, Lye SJ. Insulin-like growth factor-I receptor-mediated vasculogenesis/angiogenesis in human lung development. Am J Respir Cell Mol Biol. 2003;28:159–169. doi: 10.1165/rcmb.4764. [DOI] [PubMed] [Google Scholar]
- 37.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 38.Ahamed K, Epaud R, Holzenberger M, Bonora M, Flejou JF, Puard J, Clement A, Henrion-Caude A. Deficiency in type 1 insulin-like growth factor receptor in mice protects against oxygen-induced lung injury. Respir Res. 2005;6:31. doi: 10.1186/1465-9921-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HY, Moon H, Chun KH, Chang YS, Hassan K, Ji L, Lotan R, Khuri FR, Hong WK. Effects of insulin-like growth factor binding protein-3 and farnesyltransferase inhibitor SCH66336 on Akt expression and apoptosis in non-small-cell lung cancer cells. J Natl Cancer Inst. 2004;96:1536–1548. doi: 10.1093/jnci/djh286. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch FR, Varella-Garcia M, Bunn PA, Jr., Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 41.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 42.Lee DY, Kim SJ, Lee YC. Serum insulin-like growth factor (IGF)-I and IGF-binding proteins in lung cancer patients. J Korean Med Sci. 1999;14:401–404. doi: 10.3346/jkms.1999.14.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]
- 45.Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosen T, Lindstedt G, Lundberg PA, Bengtsson BA. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 46.Chang S, Wu X, Yu H, Spitz MR. Plasma concentrations of insulin-like growth factors among healthy adult men and postmenopausal women: associations with body composition, lifestyle, and reproductive factors. Cancer Epidemiol Biomarkers Prev. 2002;11:758–766. [PubMed] [Google Scholar]
- 47.Havemann K, Rotsch M, Schoneberger HJ, Erbil C, Hennig C, Jaques G. Growth regulation by insulin-like growth factors in lung cancer. J Steroid Biochem Mol Biol. 1990;37:877–882. doi: 10.1016/0960-0760(90)90436-o. [DOI] [PubMed] [Google Scholar]
- 48.Reeve JG, Brinkman A, Hughes S, Mitchell J, Schwander J, Bleehen NM. Expression of insulinlike growth factor (IGF) and IGF-binding protein genes in human lung tumor cell lines. J Natl Cancer Inst. 1992;84:628–634. doi: 10.1093/jnci/84.8.628. [DOI] [PubMed] [Google Scholar]
- 49.Quinn KA, Treston AM, Unsworth EJ, Miller MJ, Vos M, Grimley C, Battey J, Mulshine JL, Cuttitta F. Insulin-like growth factor expression in human cancer cell lines. J Biol Chem. 1996;271:11477–11483. doi: 10.1074/jbc.271.19.11477. [DOI] [PubMed] [Google Scholar]
- 50.Pavelic J, Krizanac S, Kapitanovic S, Pavelic L, Samarzija M, Pavicic F, Spaventi S, Jakopovic M, Herceg-Ivanovi Z, Pavelic K. The consequences of insulin-like growth factors/receptors dysfunction in lung cancer. Am J Respir Cell Mol Biol. 2005;32:65–71. doi: 10.1165/rcmb.2004-0232OC. [DOI] [PubMed] [Google Scholar]
- 51.Pavelic J, Pavelic L, Karadza J, Krizanac S, Unesic J, Spaventi S, Pavelic K. Insulin-like growth factor family and combined antisense approach in therapy of lung carcinoma. Mol Med. 2002;8:149–157. [PMC free article] [PubMed] [Google Scholar]
- 52.Kohda M, Hoshiya H, Katoh M, Tanaka I, Masuda R, Takemura T, Fujiwara M, Oshimura M. Frequent loss of imprinting of IGF2 and MEST in lung adenocarcinoma. Mol Carcinog. 2001;31:184–191. doi: 10.1002/mc.1053. [DOI] [PubMed] [Google Scholar]
- 53.Wang KK, Liu N, Radulovich N, Wigle DA, Johnston MR, Shepherd FA, Minden MD, Tsao MS. Novel candidate tumor marker genes for lung adenocarcinoma. Oncogene. 2002;21:7598–7604. doi: 10.1038/sj.onc.1205953. [DOI] [PubMed] [Google Scholar]
- 54.Zhu CQ, Popova SN, Brown ER, Barsyte-Lovejoy D, Navab R, Shih W, Li M, Lu M, Jurisica I, Penn LZ, Gullberg D, Tsao MS. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci U S A. 2007;104:11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 56.Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–5994. [PubMed] [Google Scholar]
- 57.Beitner-Johnson D, Werner H, Roberts CT, Jr, LeRoith D. Regulation of insulin-like growth factor I receptor gene expression by Sp1: physical and functional interactions of Sp1 at GC boxes and at a CT element. Mol Endocrinol. 1995;9:1147–1156. doi: 10.1210/mend.9.9.7491107. [DOI] [PubMed] [Google Scholar]
- 58.Ohlsson C, Kley N, Werner H, LeRoith D. p53 regulates insulin-like growth factor-I (IGF-I) receptor expression and IGF-I-induced tyrosine phosphorylation in an osteosarcoma cell line: interaction between p53 and Sp1. Endocrinology. 1998;139:1101–1107. doi: 10.1210/endo.139.3.5832. [DOI] [PubMed] [Google Scholar]
- 59.Idelman G, Glaser T, Roberts CT, Jr, Werner H . WT1-p53 interactions in insulin-like growth factor-I receptor gene regulation. J Biol Chem. 2003;278:3474–3482. doi: 10.1074/jbc.M211606200. [DOI] [PubMed] [Google Scholar]
- 60.Nahor I, Abramovitch S, Engeland K, Werner H. The p53-family members p63 and p73 inhibit insulin-like growth factor-I receptor gene expression in colon cancer cells. Growth Horm IGF Res. 2005;15:388–396. doi: 10.1016/j.ghir.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Sarfstein R, Maor S, Reizner N, Abramovitch S, Werner H. Transcriptional regulation of the insulin-like growth factor-I receptor gene in breast cancer. Mol Cell Endocrinol. 2006;252:241–246. doi: 10.1016/j.mce.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 62.Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34:803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 63.Carelli S, Di Giulio AM, Paratore S, Bosari S, Gorio A. Degradation of insulin-like growth factor-I receptor occurs via ubiquitin-proteasome pathway in human lung cancer cells. J Cell Physiol. 2006;208:354–362. doi: 10.1002/jcp.20670. [DOI] [PubMed] [Google Scholar]
- 64.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valentinis B, Morrione A, Taylor SJ, Baserga R. Insulin-like growth factor I receptor signaling in transformation by src oncogenes. Mol Cell Biol. 1997;17:3744–3754. doi: 10.1128/mcb.17.7.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26:1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- 67.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DiGiovanni J, Bol DK, Wilker E, Beltran L, Carbajal S, Moats S, Ramirez A, Jorcano J, Kiguchi K. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–1570. [PubMed] [Google Scholar]
- 69.Lee CT, Wu S, Gabrilovich D, Chen H, Nadaf-Rahrov S, Ciernik IF, Carbone DP. Antitumor effects of an adenovirus expressing antisense insulin-like growth factor I receptor on human lung cancer cell lines. Cancer Res. 1996;56:3038–3041. [PubMed] [Google Scholar]
- 70.Frankel SK, Moats-Staats BM, Cool CD, Wynes MW, Stiles AD, Riches DW. Human insulin-like growth factor-IA expression in transgenic mice promotes adenomatous hyperplasia but not pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L805–812. doi: 10.1152/ajplung.00420.2004. [DOI] [PubMed] [Google Scholar]
- 71.Moorehead RA, Sanchez OH, Baldwin RM, Khokha R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene. 2003;22:853–857. doi: 10.1038/sj.onc.1206188. [DOI] [PubMed] [Google Scholar]
- 72.Dupont J, Holzenberger M. IGF type 1 receptor: a cell cycle progression factor that regulates aging. Cell Cycle. 2003;2:270–272. [PubMed] [Google Scholar]
- 73.Rainsford J, Cohen P, Dix D. On the role of aging in cancer incidence: analysis of the lung cancer data. Anticancer Res. 1985;5:427–430. [PubMed] [Google Scholar]
- 74.Wu X, Yu H, Amos CI, Hong WK, Spitz MR. Joint effect of insulin-like growth factors and mutagen sensitivity in lung cancer risk. Growth Horm IGF Res. 2000;10 Suppl A:S26–27. doi: 10.1016/s1096-6374(00)90012-1. [DOI] [PubMed] [Google Scholar]
- 75.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 76.Cosaceanu D, Carapancea M, Alexandru O, Budiu R, Martinsson HS, Starborg M, Vrabete M, Kanter L, Lewensohn R, Dricu A. Comparison of three approaches for inhibiting insulin-like growth factor I receptor and their effects on NSCLC cell lines in vitro. Growth Factors. 2007;25:1–8. doi: 10.1080/08977190600702865. [DOI] [PubMed] [Google Scholar]
- 77.Onn A, Herbst RS. Angiogenesis, metastasis, and lung cancer. An overview. Methods Mol Med. 2003;74:329–348. doi: 10.1385/1-59259-323-2:329. [DOI] [PubMed] [Google Scholar]
- 78.Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 79.Goel HL, Breen M, Zhang J, Das I, Aznavoorian-Cheshire S, Greenberg NM, Elgavish A, Languino LR. beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 80.Goel HL, Fornaro M, Moro L, Teider N, Rhim JS, King M, Languino LR. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by beta1 integrins. J Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canonici A, Steelant W, Rigot V, Khomitch-Baud A, Boutaghou-Cherid H, Bruyneel E, Van Roy F, Garrouste F, Pommier G, Andre F. Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int J Cancer. 2008;122:572–582. doi: 10.1002/ijc.23164. [DOI] [PubMed] [Google Scholar]
- 82.Kiely PA, O'Gorman D, Luong K, Ron D, O'Connor R. Insulin-like growth factor I controls a mutually exclusive association of RACK1 with protein phosphatase 2A and beta1 integrin to promote cell migration. Mol Cell Biol. 2006;26:4041–4051. doi: 10.1128/MCB.01868-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qian J, Dong A, Kong M, Ma Z, Fan J, Jiang G. Suppression of type 1 Insulin-like growth factor receptor expression by small interfering RNA inhibits A549 human lung cancer cell invasion in vitro and metastasis in xenograft nude mice. Acta Biochim Biophys Sin (Shanghai) 2007;39:137–147. doi: 10.1111/j.1745-7270.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 84.D'Amico TA. Angiogenesis in non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2004;16:13–18. doi: 10.1053/j.semtcvs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Tang HB, Ren YP, Zhang J, Ma SH, Gao F, Wu YP. [Correlation of insulin-like growth factor-1 (IGF-1) to angiogenesis of breast cancer in IGF-1-deficient mice] Ai Zheng. 2007;26:1215–1220. [PubMed] [Google Scholar]
- 87.Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–351. [PubMed] [Google Scholar]
- 88.Reinmuth N, Liu W, Fan F, Jung YD, Ahmad SA, Stoeltzing O, Bucana CD, Radinsky R, Ellis LM. Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clin Cancer Res. 2002;8:3259–3269. [PubMed] [Google Scholar]
- 89.Bermont L, Lamielle F, Fauconnet S, Esumi H, Weisz A, Adessi GL. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-I in endometrial adenocarcinoma cells. Int J Cancer. 2000;85:117–123. doi: 10.1002/(sici)1097-0215(20000101)85:1<117::aid-ijc21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 90.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003;163:1001–1011. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samani AA, Fallavollita L, Jaalouk DE, Galipeau J, Brodt P. Inhibition of carcinoma cell growth and metastasis by a vesicular stomatitis virus G-pseudotyped retrovector expressing type I insulin-like growth factor receptor antisense. Hum Gene Ther. 2001;12:1969–1977. doi: 10.1089/104303401753204544. [DOI] [PubMed] [Google Scholar]
- 92.Ma Z, Dong A, Kong M, Qian J. Silencing of the type 1 insulin-like growth factor receptor increases the sensitivity to apoptosis and inhibits invasion in human lung adenocarcinoma A549 cells. Cell Mol Biol Lett. 2007;12:556–572. doi: 10.2478/s11658-007-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Long L, Rubin R, Brodt P. Enhanced invasion and liver colonization by lung carcinoma cells overexpressing the type 1 insulin-like growth factor receptor. Exp Cell Res. 1998;238:116–121. doi: 10.1006/excr.1997.3814. [DOI] [PubMed] [Google Scholar]
- 94.Samani AA, Chevet E, Fallavollita L, Galipeau J, Brodt P. Loss of tumorigenicity and metastatic potential in carcinoma cells expressing the extracellular domain of the type 1 insulin-like growth factor receptor. Cancer Res. 2004;64:3380–3385. doi: 10.1158/0008-5472.CAN-03-3780. [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Liebetanz J, Martiny-Baron G, Ruetz S, Hofmann F. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 96.Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway–therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 97.Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, O'Connor M, Yao Y, Pirritt C, Buck E, Eyzaguirre A, Arnold LD, Gibson NW, Pachter JA. A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol Cancer Ther. 2007;6:2158–2167. doi: 10.1158/1535-7163.MCT-07-0070. [DOI] [PubMed] [Google Scholar]
- 98.Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79–91. doi: 10.1002/dmr.5610070202. [DOI] [PubMed] [Google Scholar]
- 99.Zapf J, Futo E, Peter M, Froesch ER. Can“big”insulin-like growth factor II in serum of tumor patients account for the development of extrapancreatic tumor hypoglycemia? J Clin Invest. 1992;90:2574–2584. doi: 10.1172/JCI116152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fukuda I, Hizuka N, Ishikawa Y, Yasumoto K, Murakami Y, Sata A, Morita J, Kurimoto M, Okubo Y, Takano K. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res. 2006;16:211–216. doi: 10.1016/j.ghir.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Nauck MA, Reinecke M, Perren A, Frystyk J, Berishvili G, Zwimpfer C, Figge AM, Flyvbjerg A, Lankisch PG, Blum WF, Kloppel G, Schmiegel W, Zapf J. Hypoglycemia due to paraneoplastic secretion of insulin-like growth factor-I in a patient with metastasizing large-cell carcinoma of the lung. J Clin Endocrinol Metab. 2007;92:1600–1605. doi: 10.1210/jc.2006-2573. [DOI] [PubMed] [Google Scholar]
- 102.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 103.Argiles JM, Lopez-Soriano FJ. Insulin and cancer (Review) Int J Oncol. 2001;18:683–687. [PubMed] [Google Scholar]
- 104.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, Wang F, Gualberto A, Beebe JS, Moyer JD. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 105.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 106.Karp D. D. P-ALG, Blakely L. J., Kreisman H., Eisenberg P. D., Cohen R. B., Garland L., Langer C. J., Melvin C. L., Gualberto A. Efficacy of the anti-insulin like growth factor I receptor (IGF-IR) antibody CP-751871 in combination with paclitaxel and carboplatin as first-line treatment for advanced non-small cell lung cancer (NSCLC). ASCO Annual Meeting.2007. [Google Scholar]
- 107.Lee CY, Jeon JH, Kim HJ, Shin DH, Roh TW, Ahn CM, Chang YS. Clinical significance of insulin-like growth factor-1 receptor expression in stage I non-small-cell lung cancer: immunohistochemical analysis. Korean J Intern Med. 2008;23:116–120. doi: 10.3904/kjim.2008.23.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 109.Heist RS, Christiani D. EGFR-targeted therapies in lung cancer: predictors of response and toxicity. Pharmacogenomics. 2009;10:59–68. doi: 10.2217/14622416.10.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mills NE, Fishman CL, Rom WN, Dubin N, Jacobson DR. Increased prevalence of K-ras oncogene mutations in lung adenocarcinoma. Cancer Res. 1995;55:1444–1447. [PubMed] [Google Scholar]
- 111.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]