Abstract
Background
Cyclosporin A (CsA) and tacrolimus block T cell activation by inhibiting the phosphatase calcineurin and preventing translocation from the cytoplasm to the nucleus of the transcription factor Nuclear Factor of Activated T cells (NFAT). NFAT compose a family of transcription factors that are turned on during T cell activation.
Aims
To study the expression of NFAT-5 mRNA and protein in normal human keratinocytes and to investigate the cellular and subcellular pattern of expression of NFAT-5 in normal human skin and psoriasis, and analyze effects of different agonists and ultraviolet radiation on NFAT-5 in normal human skin.
Methods
Tissue cultures, Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), Western analysis, immunostaining, confocal microscopy.
Results
Sequencing of RT-PCR products confirmed the identity of the product that showed 100 % homology with the predicted NFAT-5 sequence. anti-NFAT-5 mainly detected a single band in cultured keratinocytes and dermal fibroblasts using Western analysis. Immunohistochemistry showed that epidermal keratinocytes and dermal fibroblasts in normal human and psoriatic skin express NFAT-5. NFAT-5 showed predominantly nuclear localization in epidermal keratinocytes and dermal fibroblasts within five normal adult skin biopsies. Our data also suggest that UV irradiation reduces NFAT-5 nuclear localization within the epidermis. Unlike NFAT 1-4, NFAT-5/TonEBP was localized to both nucleus and cytoplasm of cultured keratinocytes. Cyclosporin A induces nuclear membrane translocation of NFAT-5 in cultured keratinocytes and raffinose (a hypertonicity inducing agent) induces more nuclear localization of NFAT-5 compared to untreated cells. In addition, differentiation-promoting agonists that induce sustained rise in intracellular calcium did not result in changes in NFAT-5 localization in cultured keratinocytes.
Conclusion
These studies provide the first observation of expression of NFAT-5/TonEBP mRNA protein in cultured keratinocytes and dermal fibroblasts and possible functional regulation in cultured keratinocytes. CsA and raffinose effects on NFAT-5/TonEBP in cultured keratinocytes suggest diverse intracellular signaling pathways for NFAT-5/TonEBP in these cells, and that NFAT-5/TonEBP might function to translate different extracellular stimuli into appropriate functional responses.
Keywords: Cyclosporin A (CsA), Human keratinocytes, Hypertonicity, NFAT-5 (TonEBP), psoriasis, UVR
Introduction
Cyclosporin A (CsA) is widely utilized for the treatment of inflammatory skin diseases such as psoriasis. The therapeutic effects of CsA are thought to be mediated via its immunosuppressive action on infiltrating lymphocytes in skin lesions. CsA and tacrolimus block T cell activation by inhibiting the phosphatase calcineurin and preventing translocation from the cytoplasm to the nucleus of the transcription factor Nuclear Factor of Activated T cells (NFAT). NFAT compose a family of transcription factors that are turned on during T cell activation (Figure 1). Five different members of the NFAT family of transcription factors have been identified so far. The NFAT family is composed of five members: NFAT 1 to 5 [1]. NFAT-5, the most recent addition to the NFAT/Rel family of transcription factors, was isolated by five independent laboratories, on the basis of its high degree of sequence similarity (41%-45%) to the DNA-binding domain (Rel homology region of NFAT), who named it NFAT-5 [2], NFATz [3], Osmotic Response Element Binding Protein (OREBP) [4] and NFATL1 [5] respectively. In addition, NFAT-5 was independently cloned in a yeast one-hybrid assay as Tonicity-responsive Enhancer Binding Protein (TonEBP), a Rel-like protein that stimulates transcription in response to hypertonicity [6].
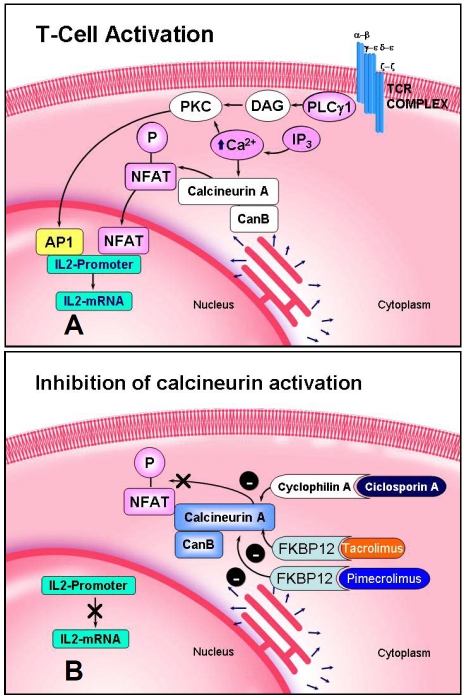
Figure 1.
Schematic representation of T cell activation (A) and mechanism of action of cyclosporin A (CsA), tacrolimus and pimecrolimus (B). Inhibition of the phosphatase calcineurin blocks nuclear translocation of NFAT.
Isolation of full-length cDNA encoding NFAT-5 showed that this new Rel-like protein possesses distinct features from those of NFAT 1-4. First, it lacks the majority of Fos and Jun contact residues and does not bind co-operatively with Fos/Jun to DNA. Second, NFAT-5 lacks the conserved regulatory domains of the calcineurin-regulated NFATs. Furthermore, immunocytochemical and biochemical analysis showed that NFAT-5 is localized to the nucleus and the cytoplasm in different cells and neither its subcellular distribution nor its phosphorylation state are affected by calcineurin [2]. In addition, NFAT-5 resembles an NFκB/Rel family stable dimer in solution in the absence of DNA, and dimerization is obligatory for DNA binding and transcriptional activity. Lopez-Rodriguez et al. showed that NFAT-5/TonEBP uniquely links the NFAT and NFκB/Rel families and regulates the production of specific cytokines in lymphocytes stimulated by osmotic stress [7]. Thus, NFAT-5 is the only member of the Rel/NFAT family that is activated by hypertonic stimulation, regulating not only the expression of osmoregulatory genes but also TNFα and lymphotoxin-β gene transcription in osmotically stressed cells [7]. These features support the hypothesis that NFAT-5 is an outlying member of the NFAT family. However, Trama et al. have demonstrated that NFAT-5 can be induced in both primary T lymphocytes and differentiated Th1 and Th2 cells upon mitogen or antigen receptor-dependent activation and this induction was inhibited by CsA and tacrolimus [5]. In addition, a reporter gene analysis showed that T-cell receptor cross-linking resulted in the induction of NFAT-5 in human Jurkat cells [5]. This study demonstrated that the induction of NFAT-5/TonEBP, in response to receptor-mediated mitogenic signals is dependent upon calcineurin activation, as this induction was inhibited by CsA and tacrolimus, while the induction by hyperosmotic stimuli was not [5].
Hypertonicity is stressful to the cells of virtually all organisms [8–11]. As cells are permeable to water, exposure to a hypertonic environment causes the cells to shrink and elevates the concentration of intracellular ions. Mammalian cells respond to hypertonic conditions by accumulating small organic molecules known as compatible osmolytes [8]. Compatible osmolytes are small organic solutes compromising five members: sorbitol, myo-inositol, betaine, taurine and glycerophos-phocholine [11]. Myo-inositol, betaine, and taurine are taken up by sodium and sodium/chloride-dependent transporters. Sorbitol is synthesised from glucose catalyzed by aldose reductase. Glycerophosphocholine is made from phosphatidylcholine [12, 13]. All compatible osmolytes are transcriptionally regulated [11, 14]. Unlike with bacteria and yeast, little is known how mammalian cells recognise hypertonicity and how the signal is conveyed to the regulatory sequence element named TonEBP [6, 15]. It has been suggested that the presence of these compounds balances increased extracellular tonicity and thus protects the cell from the damaging effects of elevated intracellular electrolyte concentrations [6, 9, 10, 15]. Miyakawa et al. reported the cloning and characterisation of the first animal transcription factor (NFAT-5/TonEBP) responsible for regulating osmolyte transporter genes during osmotic stress [6, 15]. Transcription of genes stimulated by hypertonicity plays a critical role in adaptation of mammalian cells to hypertonicity [9–11]. NFAT-5/TonEBP is known to mediate cellular responses to osmotic stress [2, 6, 7, 16]. For example, studies in Madin-Darby canine Kidney (MDCK) cells have shown that NFAT-5/TonEBP localizes to the cytoplasm and nucleus in isotonic conditions and gradually shifts to a predominantly nuclear location when cells are cultured in hypertonic medium [6]. This redistribution occurs over the course of hours [6].
NFAT-5/TonEBP is evolutionary the oldest member of the NFAT/Rel family of transcription factors. The single NFAT-like protein encoded in the Drosophila genome is closely related to NFAT-5 [17]. Recently, human NFAT-5 gene has been mapped to chromosome 16 by PCR using DNA from hybrid cell lines. The exact position of the human gene was reported to be between D16S496 and WI5254 within the 16q22.1 sub-band. The murine gene has been localized to chromosome 8D [18, 19].
The first aim of this study was to investigate the expression of NFAT-5 mRNA in cultured keratinocytes and cultured dermal fibroblasts. As a step towards elucidating the function of NFAT-5/TonEBP in skin, the subcellular localization of NFAT-5/TonEBP in response to selected agonists in cultured human keratinocytes was investigated. Recently, the expression of different genes induced by osmotic stress in human oral keratinocytes has been described [20]. Therefore, the activation of NFAT-5/TonEBP pathways in cultured human keratinocytes by hypertonicity was examined. Human keratinocytes were shown to respond to osmotic stress by the induction of heat shock proteins through a p38 MAPK regulated mechanism [21]. UV-induced phosphorylation of p38 in cultured keratinocytes has been described [22]. In addition, UV light induces phosphorylation of c-Jun through JNK [23] and JNK bound to c-Jun may phosphorylate NFAT, the binding site of which resides next to AP-1 sites [24]. Furthermore, hypertonicity also induces the expression of tumor suppressor p53 and activates p53 by phosphorylation of serine 15 [25]. Forced downregulation of p53 results in apoptosis suggesting that p53 activation in response to hypertonicity are very similar to those of ionizing radiation that causes DNA double-strand breaks [25]. Therefore, the effects of both UVA and UVB irradiation on NFAT-5/TonEBP localization in normal adult human skin were also studied using immunohistochemical techniques.
At least two signals are required for T cell activation. Phorbol ester and calcium ionophore (ionomycin) or co-stimulation of both TCR and CD28 generates these two signals. The activation of JNK was shown to be dependent on both signals [26]. These results are consistent with previous reports showing that combination of calcineurin activation and protein kinase C (PKC) resulted in JNK activation [27]. Recently, the immunosuppressive effects of CsA and tacrolimus were also shown to be mediated through inhibition of both JNK and p38 pathways activation [26, 28–30]. These inhibitory effects are mediated through the CsA-cyclophilin and tacrolimus-FKBP12 complexes [28, 29]. These results may indicate that the immunosuppressive effects on both CsA and tacrolimus are attributed, at least in part, to inhibition of JNK and p38 pathways. In addition, CsA and tacrolimus do not block stress-induced activation of JNK/p38 pathways [28, 29]. Matsuda et al. also reported that dominant negative mutants that block JNK/p38 pathways abolish NFAT transcriptional activity [28]. Therefore, two distinct pathways, the calcineurin/NFAT pathway and JNK/p38 pathway, appear to be targeted by CsA and tacrolimus to inhibit T cell activation [29]. These data support previous reports that showed that CsA and tacrolimus can inhibit an antigen-specific signaling in a calcium/calcineurin independent manner in a T cell line [31, 32]. In summary, IL-2 production is inhibited by CsA and tacrolimus via inhibition of two major pathways: 1) the calcineurin/NFAT and 2) JNK/p38 pathways. CsA and tacrolimus effects on NFAT-5/TonEBP were also investigated in cultured keratinocytes (Materials and Methods).
Materials and Methods
Cyclosporin A (CsA) and tacrolimus were provided by Novartis Pharma AG, (Basil, Switzerland) and Fujisawa Pharmaceutical Co (Osaka, Japan), respectively. Tacrolimus was also obtained from Affinity Research Products Ltd (Exeter, UK). Raffinose was obtained from Sigma laboratories (Poole, UK). Keratinocytes growth medium (MCDB 153) and trypsin/ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma laboratories (Poole, UK). NFAT-5 primers were synthesised by MWG-Biotech AG (Ebersberg, Germany). Keratinocytes differentiation agents and growth factors including TPA, ionomycin, and Dimethyl Sulphoxide (DMSO) (vehicle control) were obtained from Sigma (Poole, UK). Precast polyacrylamide gels were purchased from Invitrogen (Paisley, UK). Hybond enhanced chemiluminescence (ECL) nitrocellulose membranes, ECL molecular weight markers were obtained from Amersham (Bucking-hamshire, UK). Prestained protein standards were provided by Bio-Rad Laboratories Ltd (Herts, UK). Anti-NFAT-5 was obtained from Santa Cruz Biotechnology Inc., CA, USA.
Tissue culture
The general tissue culture methods used followed those described by Freshney [33]. Keratinocytes were isolated from normal human skin obtained from plastic and surgical procedures. Keratinocytes were cultured in T75 flasks in MCDB-153 (Sigma, Poole, UK) as described before [34, 35] The culture medium used for growing keratinocytes was the serum-free medium MCDB153 described by Boyce and Ham [36], with modifications described by Wille and Pittelkow [37, 38]. Antibiotics were added to give a final concentration of penicillin G (5 IU/ml) and streptomycin (5 μg/ml) (Sigma; Poole, UK).
Immunofluorescence of cultured cells
1. Coverslip preparations: Cells were trypsin-ised from flasks and seeded onto sterile coverslips placed in twelve well plates, so that there were 3×104 cells on each coverslip. Coverslips were incubated in an incubator at 37°C in 5% CO2. Coverslips were prepared as described [39,40]. Keratinocytes or fibroblasts were treated with specific agents, DMSO (1:1000) (vehicle control), or switched to medium containing raised extracellular calcium (1.5 mM CaCl2) 15 min and 18 h. Some coverslip cultures were pre-treated with CsA or tacrolimus for 1 h. After the time of incubations, the medium was aspirated and the cells were washed three times in Ca2+ and Mg2+ -free ice cold PBS before being fixed.
2. Fixation method: The effects of permeabilisation and fixation conditions on the subcellular localization of antigens [41] was examined carefully. Fixation methods fall generally into two categories, organic solvents and cross-linking reagents. The optimal fixation method was chosen empirically [42].
3. Cell staining for immunofluorescence microscopy: Non-specific binding was blocked by incubating coverslips in blocking serum (diluted 1:60 in PBS) by using serum from the species in which the secondary antibody was raised [43, 44] for 10 min. 100 μl of primary antibodies against NFAT-5was added to each coverslip and incubated at room temperature for 45 min. Cells were washed three times in PBS. Cells were then incubated with 100 μl of FITC-conjugated anti-rabbit and FITC-conjugated anti-goat secondary antibody for 45 min at room temperature. Cells were washed three times with Ca2+ and Mg2+-free PBS. Cells were then incubated with 50 μg/ml propidium iodide (PI) (Sigma Laboratories; Poole, UK) for 1 h at room temperature. Finally, cells were washed three times with Ca2+ and Mg2+-free PBS. Coverslips were mounted onto slides using vectorshield fluorescence mounting medium (Vector Laboratories Ltd; Peterborough, UK) and the edges sealed with clear nail varnish. Cells were stained with 2 μg/ml goat polyclonal anti-NFAT-5/TonEBP antibody. For negative control studies, goat IgG (2 μg/ml) was used. Cells were visualised using a Biorad confocal microscope.
4. Confocal microscopy: Cells were analyzed using a Bio-Rad MRC 600 confocal laser scanning microscope (BioRad; Herts, UK), mounted on a Nikon Optiphot II (Nikon UK Ltd; Surrey, UK) upright stand with a Krypton/argon laser giving 448 nm, 568 nm, and 647 nm excitation lines. Briefly, cells were fixed in 4% paraformaldehyde, permeabilised with 0.2% Triton X-100, incubated sequentially with goat-polyclonal anti-NFAT-5 antibody (2 μg/ml) or goat IgG (2 μg/ml), rabbit anti-goat FITC, propidium iodide (50 μg/ml) and visualized using a Biorad confocal microscope.
Reverse transcrcription-polymerase chain reaction
NFAT-5 cDNA sequences were obtained from GenBank at http://www.ncbi.nln.nih and complementary primers were designed to amplify target sequence specific for NFAT-5. Primers sequences were confirmed using the blast analysis at http://www.ncbi.nlm.nih.gov/blast. Coding sequence for NFAT-5 was aligned using Lasergene software (DNA Star Inc., Madison; USA) and primers were designed for each calcineurin subtype or NFAT isoform in areas of low homology. Primer set for for human NFAT 2 was forward: 5′CCACTCATACCAAGCAGTATG 3′ and backward: 5′CCTGCTGCAATAGTGCATC 3′, resulting in amplification of 340 bp.
1. Prevention of ribonuclease (RNases) contamination: RNases are particularly stable and thus difficult to destroy. A number of precautions were taken to avoid RNase contamination [45].
2. Isolation of RNA: Cultured keratinocytes and fibroblasts at approximately 70% confluence were washed twice with sterile Ca2+ and Mg2+-free PBS. Keratinocytes and fibroblasts were removed from flasks by treatment with 0.05% trypsin and 0.02% EDTA. Jurkat T cells (used as a positive control) grow in suspension and can be aspirated from flasks. Total RNA was isolated using RNeasy Mini Kit (QIAGEN; West Sussex, UK) according to manufacturer's instruction.
3. Polymerase Chain Reaction (PCR): 3–5 μl of cDNA was amplified in 50μl PCR reaction which consisted of 1.5 μl of 50 mM MgCl2 (Bioline; London, UK), 5 μl 10× NH4 buffer (Bioline; London, UK), 5 μl DMSO, 1.25 μl of 25 pmol forward primer, 1.25 μl of 25 pmol reverse primer and 4 μl of dNTP's (2.5 mM each dNTP). Distilled water was added to make the total reaction volume equal 50 μl. Negative controls were included in each reaction by replacing the cDNA with water. 0.2 μl of 0.625 U BioTaqTM DNA polymerase (Bioline; London, UK) was added to the reaction after heating to 94°C for 5 min, followed by 34 cycles of denaturation at 94°C for 1 min, re-annealing at 55–57°C for 1 min and elongation at 72°C for 2 min. A final cycle of 72°C for 15 min was used. Similar cycle conditions were used for each set of primers.
4. Agarose Gel Electrophoresis: PCR products were electrophoresed through 1.5% agarose gels to determine product size.Loaded samples were visualised on a UVP transilluminator and photographed (Mitsubishi camera/Polaroid black and white film type 667).
5. Gel extraction: PCR products were gel purified using a QIAGEN kit (QIAGEN; West Sussex, UK) to obtain single fragments for sequencing. DNA was separated using agarose gel electrophoresis. The appropriate band was excised, weighed and sent for sequencing.
6. Sequencing of PCR products: Automated sequencing was carried out by MWG-Biotech AG (Ebersberg, Germany).
Western Blotting
Cells were lysed in 2 X Sodium Dodecyl Sulphate (SDS), sample buffer (125 mM Tris-HCl, pH 6.8, 0.05% bromophenyl blue, 4% SDS, 20% glycerol and 10% β-mercaptoethanol). Equal amounts of samples and enhanced chemiluminescence molecular weight markers (Amersham, Bucks, UK) were electophoresed through 10% polyacrylamide gels, and Western Blotting were performed as described [35], using goat polyclonal anti-NFAT-5 antibody (0.4 μg/ml).
Subjects and immunohistochemical analysis of skin biopsies
Five normal volunteers (3 male, 2 females, mean age 37 years) and 5 patients with stable plaque psoriasis (3 male, 2 females, mean age 50.5 years) were recruited for study as described [39]. Five μm frozen sections were stained with goat polyclonal anti-NFAT-5 (2 μg/ml) (Santa Cruz Biotechnology Inc., CA, USA). Equal concentration of goat IgG was used as a negative control. Sections were developed and assessed as described [39].
Subjects and administration of UVR
Investigations were carried out on the healthy skin of the lower back of four adult volunteers (three males, one female; mean age 36 years). Exclusion criteria included a past history of photosensitivity and any regular oral medication. Seven doses of UVB (10–80 mJ/cm2, √2 increments) and five doses of UVA (10–40 J/cm2, √2 increments) were administered separately to the lower back of 4 volunteers. At 24 h post-irradiation, erythema intensities were recorded in triplicates using a reflectance instrument (Diastorn; Andover, UK) and a visual assessment of the minimal erythema dose (MED) was made [46]. Under local anaesthesia (intradermal lignocaine), 4 mm punch biopsies were taken from UVA and UVB irradiated sites of equal erythema that approximated to moderate erythema together with unirradiated control skin and subsequently used for immunostaining of skin biopsies. This work was carried out with Dr. M. Murphy and Dr. P. Farr.
1. Sources of UV irradiation: For irradiation of human skin, the source of both UVA and UVB was an irradiation monochromator (Applied Photophysics Limited, Surrey, UK) [Model UV90] fitted with a 10 mm diameter liquid-filled light guide, optically coupled to a light exposure Xenon arc lamp. The central wavelength and band was 300 ± 5 nm for UVB and 350 ± 30 nm for UVA in conjunction with a Schott WG355 filter for UVA. This work was carried out with Dr. M. Murphy and Dr. P. Farr.
2. Semi-quantitative assessment of UV-irradiated skin: An observer who was unaware of treatment conditions (as all slides were coded by another observer) assessed the number of positively stained epidermal nuclei. Cells showing nuclear staining were assessed in an ascending horizontal layer in basal layer, suprabasal and high suprabasal layers (in practice it was often impossible to count more than 4 to 5 consecutive layers). Cells displaying only marginal staining indistinguishable from the background were not counted. UVR effect on NFAT-5/TonEBP localization was measured by counting the number of NFAT-5 -positive nuclei per 300μm length of basement membrane.
Statistical analysis
To compare the effect of CsA on the number of cells showing positive nuclear immune-staining, Chi squared analysis was used. Data were analyzed using Arcus Quickstat (biomedical version 1.0). Two-way analysis of variance (ANOVA) test was used to compare the UVA and UVB effects on the number of cells showing positive NFAT-5 nuclear staining. MINITABTM statistical software (release 13) (MINITAB Ltd, UK) was used to analyze UV effects on NFAT-5 nuclear localization.
Results
Expression of NFAT-5 mRNA in cultured keratinocytes and cultured fibroblasts
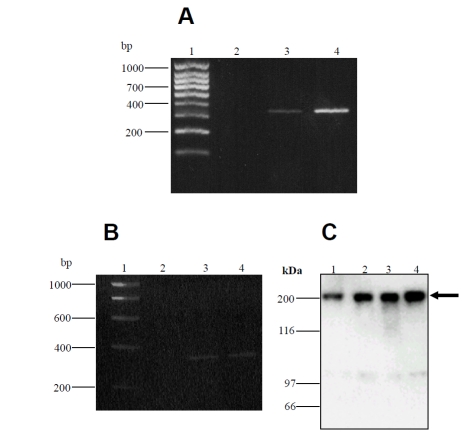
RT-PCR of keratocyte and fibroblast cDNA, using NFAT-5 specific primers, produced a 340bp fragment as predicted, demonstrating the presence of NFAT-5 in human epidermal keratinocytes and cultured dermal fibroblasts (Figure 2A and B). Sequencing of RT-PCR products followed by BLAST analysis confirmed the identity of the product that showed 100 % homology with the predicted NFAT-5 sequence (Accession#NM_006599). cDNA from Jurkat T cell mRNA was amplified as a positive control in this experiment.
Figure 2.
NFAT-5 mRNA and protein expression in cultured human keratinocytes and dermal fibroblasts. Total RNA was extracted from cultured cells, reverse transcribed and PCR performed with NFAT-5-specific primers. Reaction products were separated by electrophoresis in 1.5% agarose gels. (A), lane 1 hyperladder IV; lane 2, negative control (water); lane 3, Jurkat T cells; lane 4, cultured keratinocytes. (B), lane 1, hyperladder I; lane 2, negative control (water); lane 3, cultured keratinocytes; lane 4, cultured fibroblasts. Sequencing studies confirmed the expression of NFAT-5 in cultured cells. The predicted size of NFAT-5 is 340 bp. (C), cell lysates were prepared from cultured keratinocytes and dermal fibroblasts, separated by SDS-PAGE and immunoblotted with anti-NFAT-5 antibody. This experiment confirmed that the antibody used in immunostaining detects the appropriate molecular weight of NFAT-5 (202 kDa) (arrow). Lane 1, medium control (keratinocytes) (donor 1); lane 2, medium control (keratinocytes) (donor 2); lane 3, medium control (fibroblasts) (donor 1); lane 4, medium control (fibroblasts) (donor 2).
Western analysis confirms expression of NFAT-5 in cultured human keratinocytes and dermal fibroblasts
Western blotting showed that cultured human keratinocytes express NFAT-5/TonEBP protein. These experiments also demonstrated that the antibody used in immunohistochemistry and immunofluorescence/confocal microscopy staining detected the appropriate molecular weight protein in skin cells (202 kDa). In addition, anti-NFAT-5 mainly detected a single band in cultured keratinocytes and dermal fibroblasts (Figure 2C).
Expression of NFAT-5/TonEBP in normal and psoriatic skin
Immunohistochemistry showed that epidermal keratinocytes and dermal fibroblasts in normal human and psoriatic skin express NFAT-5 (Figure 3 and Figure 4). NFAT-5 showed predominantly nuclear localization in epidermal keratinocytes and dermal fibroblasts within five normal adult skin biopsies. In normal skin, nuclear localization of NFAT-5/TonEBP was observed throughout the whole epidermis. In lesional psoriatic skin and to a lesser extent in non-lesional psoriatic skin, the intensity of the nuclear staining diminished within the lower spinous cell layers. Nuclear staining increased in high spinous and granular layers (Table 1). These results were observed in four of five involved psoriatic skin sections (Table 1). Interestingly, expression of NFAT-5/TonEBP by Langerhans cells was observed in lesional psoriatic skin (Figure 4D), whereas no clear immunostaining of Langerhans cells was seen in normal skin.
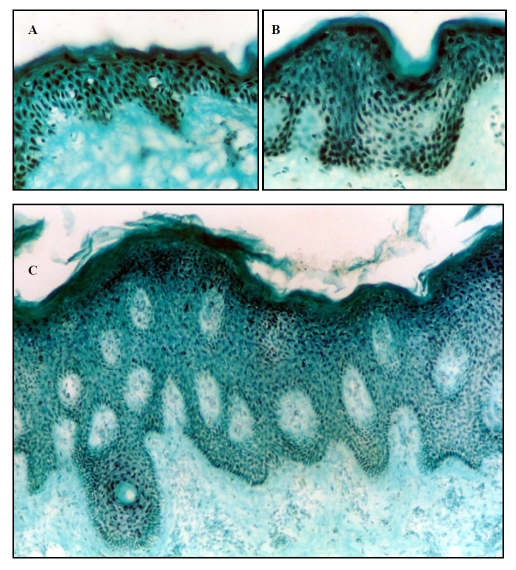
Figure 3.
Immunostaining of normal skin, lesional (plaque) and non-lesional psoriatic skin (subject 1) with an anti-NFAT-5 antibody. Frozen sections of normal human skin (A) (original magnification X25), lesional (C) (plaque) (original magnification X10) psoriatic skin and non-lesional (B) (uninvolved) (original magnification X25) psoriatic skin were stained with anti-NFAT-5 antibody. NFAT-5 shows predominantly nuclear localization in normal and psoriatic skin, but there is reduced expression in suprabasal spinous layer in psoriatic skin compared to normal skin.
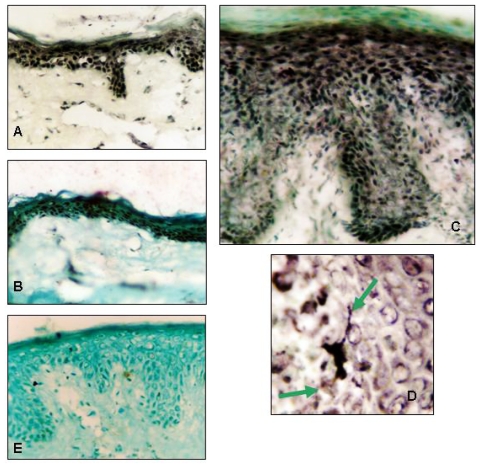
Figure 4.
Immunostaining of normal skin, lesional (plaque) and non-lesional psoriatic skin (Subject 2) with an anti-NFAT-5 antibody and expression of NFAT-5/TonEBP by Langerhans cells in psoriatic skin. Frozen sections of normal human skin (A), non-lesional (uninvolved) (B) psoriatic skin and lesional (plaque) psoriatic skin (C) were stained with anti-NFAT-5 antibody. NFAT-5 shows predominantly nuclear localization in normal and psoriatic skin. Langerhans cells express NFAT-5 in the suprabasal layer of lesional psoriatic skin (D). Arrowheads indicate dendritic processes of the Langerhans cell. (E) frozen sections of normal skin stained with goat IgG as negative control. (Original magnification x25).
Table 1.
Distribution of NFA 5/TonEBP in normal (A) and psoriatic skin (B)
| (A) Normal skin. | |||
|---|---|---|---|
| Basal | Suprabasal | High suprabasal | |
| Subject 1 | 4 N | 3 N | 4 N |
| Subject 2 | 4 N | 4 N | 4 N |
| Subject 3 | 4 N | 2 N/C | 2 N/C |
| Subject 4 | 4 N | 4 N | 4 N |
| Subject 5 | 4 N | 4 N | 4 N |
| (B) Psoriatic skin | ||||
|---|---|---|---|---|
| Skin type | Basal | Suprabasal | High suprabasal | |
| Subject 1 | Lesional | 3 N | 2 N/C | 4 N |
| Non-lesional | 4 N | 2 N/C | 4 N | |
| Subject 2 | Lesional | 3 N | 2 N | 4 N |
| Non-lesional | 3 N | 2 N/C | 3 N | |
| Subject 3 | Lesional | 4 N | 3 N/C | 4 N |
| Non-lesional | 4 N | 2 N/C | 2 N | |
| Subject 4 | Lesional | 3 N | 2 C | 3 N |
| Non-lesional | 4 N | 3 N | 2 N | |
| Subject 5 | Lesional | 4 N | 2 N/C | 4 N |
| Non-lesional | 3 N | 3 N | 3 N | |
Optimisation of fixation method for immunofluorescence studies of NFAT-5/TonEBP and negative controls
Different fixatives did not result in any significant differences in the distribution of NFAT5 in epidermal human keratinocytes (data not shown), although fixation with methanol/acetone showed more intense staining on the cytoplasm and nuclear membrane. 0.4% paraformaldehyde then 0.2% Triton X-100 was used in subsequent experiments. Goat IgG was included at equivalent concentrations as the primary antibody in immunohistochemical (Figure 3) and immunofluorescence (Figure 4A) studies as a negative control.
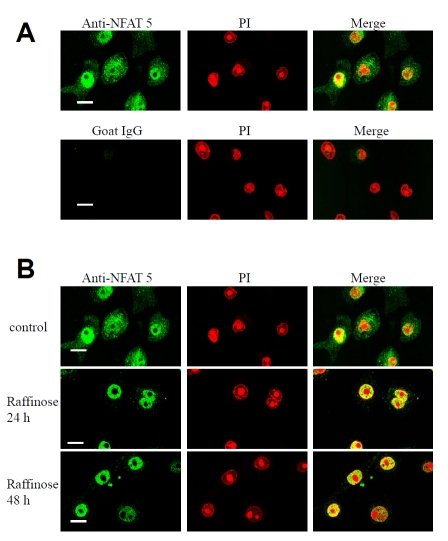
Hypertonicity induces nuclear localization of the transcription factor NFAT-5/TonEBP
NFAT-5 activation by hypertonicity was investigated in cultured keratinocytes. Immunocytochemical analysis of untreated cultured keratinocytes showed that NFAT-5/TonEBP is present in both cytoplasm and nucleus. In keratinocytes cultured in hypertonic medium using 200 mM raffinose, the nuclear staining increased while the cytoplasmic staining decreased. The overall appearance of the nuclear/cytoplasmic boundary sharpened after 24 h of switching to a hypertonic medium (Figure 5). Redistribution occurred over a time course of 24–48 h. Absent nucleolar staining was also observed in these experiments.
Figure 5.
Anti-NFAT-5 staining specificity in cultured human keratinocytes (A). Hypertonicity induces nuclear localization of NFAT-5/TonEBP in human keratinocytes (B). (A), human keratinocytes were cultured on coverslips in low calcium MCDB 153 medium. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, incubated sequentially with goat-polyclonal anti-NFAT-5 antibody (2 μg/ml) or goat IgG (2 μg/ml), rabbit anti-goat FITC, propidium iodide (50 μg/ml) and visualized using a Biorad confocal microscope. The images shown are mid-cell sections. Negative control coverslip (goat IgG) was scanned using the same settings (gain, black level and confocal aperture) as the positive control coverslip (anti-NFAT-5), thus ensuring that the pixel brightness values were due to antibody labelling rather than other factors such as autofluorescence or non-specific binding. Pixel brightness data were analyzed using COMOS software. (B), human keratinocytes were cultured on coverslips in low calcium MCDB 153 medium (control) and then switched to medium containing 200nM of raffinose for 24 h and 48 h as indicated. Cells were fixed and incubated sequentially with goat-polyclonal anti-NFAT-5 antibody, rabbit anti-goat FITC, propidium iodide (50 μg/ml) and visualized using a Biorad confocal microscope. The images shown are mid-cell sections. These results are representative of 3 experiments on keratinocytes derived from 3 independent donors. Scale bar 25 μM.
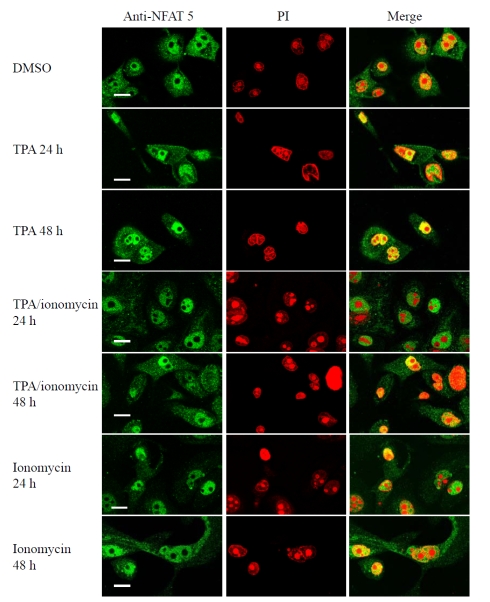
Differentiation promoting agents do not cause any change in the subcellular localization of NFAT-5 in cultured human keratinocytes
Human keratinocytes cultured on coverslips in low calcium MCDB 153 medium and treated with either DMSO (vehicle control) or agonists that induce keratocyte differentiation. The addition of ionomycin (1 μM), TPA (50 nM) or TPA (50 nM) plus ionomycin (1 μM) for 24 h and 48 h did not result in changes in the subcellular localization of NFAT-5 in human keratinocytes (Figure 6).
Figure 6.
Differentiation promoting agents do not cause any change in the subcellular localization of NFAT-5/TonEBP in human keratinocytes . Human keratinocytes were cultured on coverslips in low calcium MCDB 153 medium (control) and then then treated with DMSO (vehicle control), ionomycin (1 μM), TPA (50 nM) plus ionomycin (1 μM) or TPA (50 nM) for 24 h and 48 h as indicated. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, incubated sequentially with goat-polyclonal anti-NFAT-5 antibody, rabbit anti-goat FITC, propidium iodide (50 μg/ml) and visualized using a Biorad confocal microscope. The images shown are mid-cell sections. These results are representative of 3 experiments on keratinocytes derived from 3 independent donors. Scale bar 25 μM.
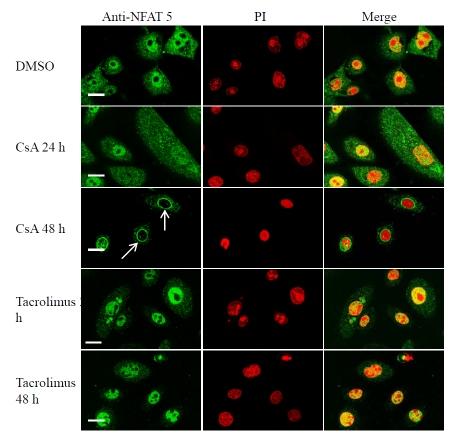
Cyclosporin A induces nuclear membrane translocation of NFAT-5/TonEBP in cultured keratinocytes
There is controversy regarding the role of calcineurin in regulating NFAT-5/TonEBP in T cells and kidney cell line [2, 5, 6, 47]. CsA and tacrolimus have been shown to inhibit activation of both p38 and JNK pathways in T cells in a calcineurin-independent way, suggesting that p38/JNK signaling pathways are activated in a CsA sensitive manner and contribute to IL-2 gene expression in T lymphocytes [28, 29]. Therefore, CsA and tacrolimus effects on NFAT-5/TonEBP might be mediated via either JNK/p38 pathways or the calcineurin pathways. In untreated cultured keratinocytes or cells treated with a vehicle, NFAT-5 was found in both the nucleus and the cytoplasm (100% nuclear positivity). Treatment of cultured keratinocytes with CsA (1 μM) for 48 h significantly induced nuclear membrane translocation of NFAT-5/TonEBP and resulted in a decrease in the number of cells showing nuclear positivity (17.3% nuclear positivity, P< 0.0001). Furthermore, a decrease in the cytoplasmic staining was also observed. These results were observed in three independent experiments (Figure 7 and Table 2).
Figure 7.
Cyclosporin A, but not tacrolimus, induces nuclear membrane translocation of NFAT-5/TonEBP in human keratinocytes . Human keratinocytes were cultured on coverslips in low calcium MCDB 153 medium (control) and then then treated with DMSO (vehicle control), CsA (1 μM) or tacrolimus (1 μM) for 24 h and 48 h as indicated. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, incubated sequentially with goat-polyclonal anti-NFAT-5 antibody, rabbit anti-goat FITC, propidium iodide (50 μg/ml) and visualized using a Biorad confocal microscope. The images shown are mid-cell sections. These results are representative of 3 experiments on keratinocytes derived from 3 independent donors. Arrowheads indicate nuclear membrane translocation of NFAT-5/TonEBP in response to CsA. Scale bar 25 μM.
Table 2.
Alteration of NFAT5 nuclear localization in human keratinocytes
| Treatment | ||
|---|---|---|
| % cells showing NFAT5 nuclear positivity | Number of cells counted | |
| Medium control* | 100 | 150 |
| DMSO control† | 100 | 150 |
| Raffinose 24h | 100 | 150 |
| Raffinose 48h | 100 | 150 |
| TPA/ionomycin‡ 24h | 100 | 150 |
| TPA/ionomycin‡ 48h | 100 | 150 |
| Ionomycin 24h | 100 | 150 |
| Ionomycin 48h | 100 | 150 |
| TPA 50 nM 24h | 100 | 150 |
| TPA 50 nM 48h | 100 | 150 |
| CsA 1 μM 24h | 100 | 150 |
| CsA 1 μM 48h | 17.3§ | 150 |
| Tacrolimus 1 μM 24h | 100 | 150 |
| Tacrolimus 1 μM 48h | 100 | 150 |
Low calcium medium;
Vehicle control;
TPA (50 nM) plus ionomycin (1 μM),
P<0.0001 compared to DMSO control.
Tacrolimus (FK506) does not cause significant changes in the subcellular localization of NFAT-5/TonEBP in cultured keratinocytes
Human keratinocytes were treated with tacrolimus (1 μM) for 24 and 48 h, then immunostained with antibody against NFAT5/TonEBP. Tacrolimus did not result in significant changes in the nuclear localization of NFAT-5/TonEBP in cultured keratinocytes (Figure 7 and Table 2). However, a decrease in the cytoplasmic staining was observed.
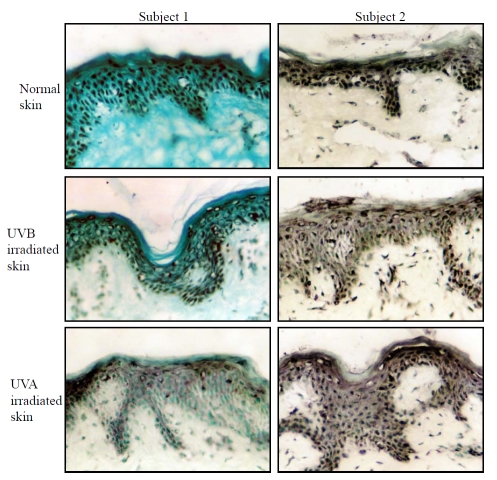
Effects of UVA and UVB on NFAT-5/TonEBP localization in human epidermis in vivo
Skin biopsies were taken from areas of approximately equal (moderate) erythema following irradiation with UVA and UVB. Biopsies were also taken from un-irradiated (control) skin. The mean (±SD) irradiation doses at the biopsy sites were 44 ± 8 mJ/cm2 and 37 ± 6 J/cm2 for UVB and UVA respectively. The mean (±SD) erythema readings from the biopsy sites were 223 ± 34 and 208.7 ± 28 (arbitary units) for UVB and UVA irradiation sites respectively. NFAT5/TonEBP protein expression was assessed by immunocytochemistry. NFAT-5 staining was mainly nuclear throughout the epidermis (Figure 8). The mean number of cells showing nuclear positivity in both UVA and UVB-irradiated skin was reduced compared to un-irradiated skin (Table 3 and Figure 8). Two-way ANOVA was used to make individual comparisons between different factors that may affect the results (e.g. subjects and UV irradiation). The variance between subjects was not significant (P=0.46). When UVA and UVB were combined, the variance in the numbers of cells showing NFAT-5 positivity between UV-irradiated and un-irradiated skin approached significance (P=0.06). Further analysis indicated that there were no significant differences in the variance of NFAT-5 nuclear positive cells between UVA and UVB irradiated epidermis. These data suggest that UV irradiation (UVA and UVB) reduces NFAT-5 nuclear localization within the epidermis.
Figure 8.
Effects of UVA and UVB on NFAT-5/TonEBP localization in human epidermis in vivo. 4 mm punch biopsies were taken from un-irradiated, UVA and UVB-irradiated sites of equal erythema as described in section 5.2.2. Sections were stained with goat polyclonal anti-NFAT-5 and the number of positively stained epidermal nuclei was counted as described. (Original magnification x25).
Table 3.
Summary of NFAT-5 cells showing nuclear positive cells in normal (un-irradiated), UVB-irradiated and UVA irradiated epidermis. The number of NFAT-5-positive nuclei per 300 μm length of basement membrane is shown here.
| Normal (un-irradiated skin) | UVB-irradiated skin | UVA-irradiated skin | |
|---|---|---|---|
| Subject 1 | 110 | 34 | 7 |
| Subject 2 | 101 | 51 | 26 |
| Subject 3 | 105 | 47 | 108 |
| Subject 4 | 45 | 63 | 29 |
| Total | 361 | 195 | 170 |
| Mean | 90.3 | 48.8 | 42.5 |
| Standard Deviation | 30.4 | 12 | 44.7 |
Discussion
Although a northern blot analysis detected NFAT-5/TonEBP transcripts in many human tissues, normal human skin was not included in that study [5]. Therefore, our studies provide the first observation of expression of NFAT-5/TonEBP mRNA protein in cultured keratinocytes and dermal fibroblasts and possible functional regulation in cultured keratinocytes.
Changes in humidity have profound effects on skin barrier function by creating an osmotic gradient across the stratum corneum [48]. These effects are more severe when the barrier function of the stratum corneum is disrupted in skin disorders such as psoriasis [48]. Our study has also shown that normal human skin and psoriatic skin express the transcription factor NFAT-5/TonEBP that regulates tonicity-responsive expression of proteins that catalyze cellular accumulation of compatible osmolytes. By immunostaining of normal skin and uninvolved psoriatic skin, NFAT-5/TonEBP appeared predominantly nuclear in all skin layers. However, NFAT-5/TonEBP in involved psoriatic skin appeared to predominantly localize to both the basal and high suprabasal but not the low suprabasal layers. The interpretation of this data is at present unclear and requires further study.
It is well known that CsA and tacrolimus through formation of a complex with cyclophilin and FKBP12, inhibit the phosphatase activity of calcineurin, which regulates nuclear translocation and subsequent activation of NFAT transcription factors. In addition to the calcineurin/NFAT pathway, recent studies indicated that CsA and tacrolimus also block the activation of JNK and p38 signaling pathways triggered by antigen recognition [26, 28, 29]. As NFAT 4 in dermal fibroblasts, nuclear membrane translocation of NFAT-5/TonEBP in response to CsA (1 μM) provide extra evidence of CsA direct effects on keratinocytes, which may account in part for the therapeutic effect of CsA in skin diseases such as psoriasis. It would be interesting to know whether CsA induced-nuclear membrane translocation of NFAT-5/TonEBP can be blocked by pre-treating cells with another calcineurin inhibitor such as tacrolimus or inhibitors of JNK/p38 pathways such as SP600125 (JNK inhibitor) [49] or SB-203580 (p38 inhibitor) [47].
Although the main NFAT activation pathways are distinct, there is evidence that cross-talk may occur. MAP kinases are involved in regulating NFAT activation. The MAP kinase p38 has been reported to phosphorylate in vitro and interacts in vivo with NFAT 1 to prevent its nuclear translocation [50], and p38 has been suggested to phosphorylate and inhibit NFAT 2 activation [51]. Also JNK was reported to phosphorylate NFAT 4 and control its cellular localization [52]. Later, another study contradicted these findings by showing that JNK does not inhibit NFAT 4 nuclear import [53]. Recently, JNK1 was shown to phosphorylate specific residues in the PxIxIT calcineurin-targeting motif of NFAT 2, thus inhibiting the interaction between NFAT 2 and calcineurin, although the corresponding region of NFAT 1 is not phosphorylated in stimulated cells [54]. In addition, T cells from JNK1-/- mice showed increased nuclear localization of NFAT 2 but not NFAT 1, suggesting that JNK may suppress the activation of NFAT 2 [55]. Furthermore, the calcium requirement for JNK activation in T and B cells appears to be mediated by calcineurin, as it is blocked by CsA [27, 56]. However, tacrolimus effects on NFAT-5/TonEBP localization were different from that of CsA in cultured keratinocytes. This cross talk between different pathways may help to explain the effects of CsA and tacrolimus on NFAT-5/TonEBP activation in human epidermal keratinocytes. Although both CsA and tacrolimus clear psoriasis, it is also likely that there are distinct targets for CsA and tacrolimus in skin. For example, although topical tacrolimus is effective in atopic eczema [57], topical tacrolimus is only partially effective in psoriasis when applied under occlusion [58] even though the barrier in psoriasis is known to be disrupted. However, the barrier is not as disrupted in psoriasis as in eczema.
Shore reported clearing of psoriatic lesions following prolonged occlusion with application of tapes [59, 60]. Friedman showed in a prospective bilateral comparison study that hydrocolloid occlusion had a similar effect on small psoriatic lesions [61]. Another clinical and immunohistologic study has shown that prolonged occlusion is an effective treatment for psoriasis either as monotherapy or in combination with a high potency topical steroid [62]. The mechanism of action of prolonged occlusion is still unknown. Occlusion therapy comprises the delivery of mechanical pressure to the epidermis. Different mechanical stimuli lead to membrane deformation in skin cells [63, 64].
Hyperosmotic stress leads to cell shrinkage, causing a fast increase in the ratio of cell surface to volume. The cellular deflation over a rigid skeleton leads to both membrane stretching and folding of the plasma membrane in various areas, thus mimicking the mechanical stress. Hence, a mechanical load applied to cells can be mimicked by hypertonic stimuli [65]. Furthermore, another group demonstrated that osmotic stress causes structural changes at the level of macromolecules via crowding and hydration [66]. They replaced, for example, a hydrostatic pressure with osmotic stress based on maintaining a concentration gradient between a concentrated bathing solution and a water-filled channel leading to a decrease in the channel volume. Recently, a hyperosmotic stimulus was shown to result in elevation of intracellular calcium and inhibition of the proliferation of HaCaT keratocyte cell line [67]. Studies in this area have shown that switching keratinocytes to a hypertonic medium resulted in localization of NFAT-5/TonEBP to the nucleus, suggesting activation of this transcription factor in response to osmotic stress. Very recently, NFAT-5/TonEBP redistribution into the nucleus of dermal fibroblasts isolated from normal human foreskin in response to hypertonic stimulus after 90 min has been reported [68]. Thus, NFAT-5/TonEBP may be involved in mediating the action of occlusive therapy in psoriasis. It would also be interesting to investigate whether calcineurin inhibitors can inhibit the hypertonicity-induced NFAT-5/TonEBP nuclear localization. However, hyperosmolarity, the main NFAT-5/TonEBP stimulus, is not blocked by the inhibition of calcineurin in T cells [5].
Unlike NFAT 1-4, NFAT-5/TonEBP was localized to both nucleus and cytoplasm of cultured keratinocytes. In addition, differentiation-promoting agonists that induce sustained rise in intracellular calcium did not result in changes in NFAT-5/TonEBP localization in cultured keratinocytes, suggesting that the effects of raffinose and CsA are not mediated by an increase in the intracellular calcium concentrations. These distinct features of NFAT-5/TonEBP in human cultured keratinocytes support the hypothesis that NFAT-5/TonEBP is an outlying member of the NFAT family [16].
Keratinocytes are exposed to the carcinogenic effects of UVR. UV-induced non-melanoma skin cancer is thought to arise from damaged basal keratinocytes and p53, a tumor suppressor, is a potential candidate involved in protecting basal keratinocytes from UVR effects [69, 70]. Using indirect immunefluo-rescence/confocal microscopy, UV radiation was shown to induce nuclear translocation of NFAT 2 in an epidermal cell line [71]. Investigation of UV effects on NFAT-5/TonEBP nuclear localization in vivo suggested that UVR (UVA and UVB) reduce nuclear localization in human epidermis (P=0.06 compared to un-irradiated skin, n=4). However, further studies including more irradiated biopsies may prove useful. These results provide the first description of NFAT-5/TonEBP export from the nucleus. However, this nuclear export mechanism is unclear at the moment.
In summary, the CsA and raffinose effects on NFAT-5/TonEBP in cultured keratinocytes suggest diverse intracellular signaling pathways for NFAT-5/TonEBP in these cells, and that NFAT-5/TonEBP might function to translate different extracellular stimuli into appropriate functional responses. Also CsA and tacrolimus have a T cell-independent effect on epidermal keratinocytes and may have two distinct mechanisms of action. As in T cells, one is the inhibition of calcineurin phosphatase activity and the other is the calcineurin-independent inhibition of JNK/p38 activation pathways [28, 29].
We have previously shown that treatment of cultured human keratinocytes with agents that induce a sustained rise in intracellular calcium, including elevation of extracellular calcium ([Ca2+]o) leads to nuclear translocation of endogenous NFAT1, which was inhibited by pre-treatment with CsA, tacrolimus[39, 72] and recently with nifedipine[40]. These data provide the first evidence that NFAT-5 is functionally active in human keratinocytes. In contrast to other NFATs in the skin, NFAT-5 appears to be primarily located in the nucleus. These data provides the first evidence that NFAT-5 is functionally active and that CsA has a direct effect on NFAT-5 subcellular localization. Ultimately, the identification of NFAT-regulated genes in skin cells will be crucial for developing an understanding of the physiological role that these transcription factors play in skin differentiation. However, the functional significance of each NFAT member in skin remains to be fully explored.
Acknowledgments
The authors thank Dr N Reynolds for supplying the clinical material, however, Dr Wael Al-daraji solely desiggned, performed all these experiments and paid for all material used. This work is part of Dr Wael Al-Daraji, Doctor of Medicine thesis, University of Newcastle 2002.
References
- 1.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci U S A. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan S, Tsuruta R, Masuda ES, Imamura R, Bazan F, Arai K, Arai N, Miyatake S. NFATz: a novel rel similarity domain containing protein. Biochem Biophys Res Commun. 2000;272:765–776. doi: 10.1006/bbrc.2000.2831. [DOI] [PubMed] [Google Scholar]
- 4.Ko BC, Turck CW, Lee KW, Yang Y, Chung SS. Purification, identification, and characterization of an osmotic response element binding protein. Biochem Biophys Res Commun. 2000;270:52–61. doi: 10.1006/bbrc.2000.2376. [DOI] [PubMed] [Google Scholar]
- 5.Trama J, Lu Q, Hawley RG, Ho SN. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol. 2000;165:4884–4894. doi: 10.4049/jimmunol.165.9.4884. [DOI] [PubMed] [Google Scholar]
- 6.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15:47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 8.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 9.Kwon ED, Zablocki K, Jung KY, Peters EM, Garcia-Perez A, Burg MB. Osmoregulation of GPC:choline phosphodiesterase in MDCK cells: different effects of urea and NaCl. Am J Physiol. 1995;269:C35–41. doi: 10.1152/ajpcell.1995.269.1.C35. [DOI] [PubMed] [Google Scholar]
- 10.Kwon ED, Jung KY, Edsall LC, Kim HY, Garcia-Perez A, Burg MB. Osmotic regulation of synthesis of glycerophosphocholine from phosphatidylcholine in MDCK cells. Am J Physiol. 1995;268:C402–412. doi: 10.1152/ajpcell.1995.268.2.C402. [DOI] [PubMed] [Google Scholar]
- 11.Burg MB, Kwon ED, Kultz D. Osmotic regulation of gene expression. Faseb J. 1996;10:1598–1606. doi: 10.1096/fasebj.10.14.9002551. [DOI] [PubMed] [Google Scholar]
- 12.Zablocki K, Miller SP, Garcia-Perez A, Burg MB. Corrections and retraction: accumulation of glycerophosphocholine (gpc) by renal cells: osmotic regulation of gpc:choline phosphodiesterase. Proc Natl Acad Sci U S A. 1991;88:9907. doi: 10.1073/pnas.88.17.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zablocki K, Miller SP, Garcia-Perez A, Burg MB. Accumulation of glycerophosphocholine (GPC) by renal cells: osmotic regulation of GPC:choline phosphodiesterase. Proc Natl Acad Sci U S A. 1991;88:7820–7824. doi: 10.1073/pnas.88.17.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiol Rev. 1991;71:1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- 15.Miyakawa H, Rim JS, Handler JS, Kwon HM. Identification of the second tonicity-responsive enhancer for the betaine transporter (BGT1) gene. Biochim Biophys Acta. 1999;1446:359–364. doi: 10.1016/s0167-4781(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Copeland NG, Gilbert DJ, Thomas S, Disteche C, Jenkins NA, Rao A. NF-AT5: the NF-AT family of transcription factors expands in a new direction. Cold Spring Harb Symp Quant Biol. 1999;64:517–526. doi: 10.1101/sqb.1999.64.517. [DOI] [PubMed] [Google Scholar]
- 17.Graef IA, Gastier JM, Francke U, Crabtree GR. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc Natl Acad Sci U S A. 2001;98:5740–5745. doi: 10.1073/pnas.101602398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1998;5:355–364. doi: 10.1093/dnares/5.6.355. [DOI] [PubMed] [Google Scholar]
- 19.Hebinck A, Dalski A, Engel H, Mattei M, Hawken R, Schwinger E, Zuhlke C. Assignment of transcription factor NFAT5 to human chromosome 16q22.1, murine chromosome 8D and porcine chromosome 6p1.4 and comparison of the polyglutamine domains. Cytogenet Cell Genet. 2000;90:68–70. doi: 10.1159/000015665. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Hong JA, Pih KT, Hwang I. Identification and isolation of differentially expressed genes in osmotically stressed human oral keratinocytes. Arch Oral Biol. 2001;46:335–341. doi: 10.1016/s0003-9969(00)00133-3. [DOI] [PubMed] [Google Scholar]
- 21.Garmyn M, Mammone T, Pupe A, Gan D, Declercq L, Maes D. Human Keratinocytes Respond to Osmotic Stress by p38 Map Kinase Regulated Induction of HSP70 and HSP27. J Invest Dermatol. 2001;117:1290–1295. doi: 10.1046/j.0022-202x.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang QS, Maddock DA, Chen JP, Heo S, Chiu C, Lai D, Souza K, Mehta S, Wan YS. Cytokine-induced p38 activation feedback regulates the prolonged activation of AKT cell survival pathway initiated by reactive oxygen species in response to UV irradiation in human keratinocytes. Int J Oncol. 2001;19:1057–1061. doi: 10.3892/ijo.19.5.1057. [DOI] [PubMed] [Google Scholar]
- 23.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 24.Jain J, Valge-Archer VE, Sinskey AJ, Rao A. The AP-1 site at -150 bp, but not the NF-kappa B site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J Exp Med. 1992;175:853–862. doi: 10.1084/jem.175.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dmitrieva N, Kultz D, Michea L, Ferraris J, Burg M. Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J Biol Chem. 2000;275:18243–18247. doi: 10.1074/jbc.M000522200. [DOI] [PubMed] [Google Scholar]
- 26.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. Embo J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda S, Moriguchi T, Koyasu S, Nishida E. T lymphocyte activation signals for interleukin-2 production involve activation of MKK6-p38 and MKK7-SAPK/JNK signaling pathways sensitive to cyclosporin A. J Biol Chem. 1998;273:12378–12382. doi: 10.1074/jbc.273.20.12378. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda S, Shibasaki F, Takehana K, Mori H, Nishida E, Koyasu S. Two distinct action mechanisms of immunophilin-ligand complexes for the blockade of T-cell activation. EMBO Rep. 2000;1:428–434. doi: 10.1093/embo-reports/kvd090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 31.Metcalfe S, Alexander D, Turner J. FK 506 and cyclosporin each block antigen-induced T cell receptor signaling that is dependent on CD4 co-receptor and operates in the absence of detectable cytoplasmic calcium fluxes. Transpl Int. 1994;7:S549–551. doi: 10.1111/j.1432-2277.1994.tb01440.x. [DOI] [PubMed] [Google Scholar]
- 32.Metcalfe S, Alexander D, Turner J. FK506 and cyclosporin A each inhibit antigen-specific signaling in the T cell line 171 in the absence of a calcium signal. Cell Immunol. 1994;158:46–58. doi: 10.1006/cimm.1994.1255. [DOI] [PubMed] [Google Scholar]
- 33.Freshney RI. Culture of animal cells: a manual of basic techniques. New York: John Wiley and Sons Inc; 2000. [Google Scholar]
- 34.Sharpe GR, Fisher C, Gillespie JI, Greenwell JR. Growth and differentiation stimuli induce different and distinct increases in intracellular free calcium in human keratinocytes. Arch Dermatol Res. 1993;284:445–450. doi: 10.1007/BF00373354. [DOI] [PubMed] [Google Scholar]
- 35.Todd C, Reynolds NJ. Up-regulation of p21WAF1 by phorbol ester and calcium in human keratinocytes through a protein kinase C-dependent pathway. Am J Pathol. 1998;153:39–45. doi: 10.1016/S0002-9440(10)65543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 37.Wille JJ, Jr., Pittelkow MR, Shipley GD, Scott RE. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyzes, growth kinetics, and cell cycle studies. J Cell Physiol. 1984;121:31–44. doi: 10.1002/jcp.1041210106. [DOI] [PubMed] [Google Scholar]
- 38.Pittelkow MR, Scott RE. New techniques for the in vitro culture of human skin keratinocytes and perspectives on their use for grafting of patients with extensive burns. Mayo Clin Proc. 1986;61:771–777. doi: 10.1016/s0025-6196(12)64815-0. [DOI] [PubMed] [Google Scholar]
- 39.Al-Daraji WI, Grant KR, Ryan K, Saxton A, Reynolds NJ. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–788. doi: 10.1046/j.1523-1747.2002.01709.x. [DOI] [PubMed] [Google Scholar]
- 40.Al-Daraji WI, Reynolds NJ. Nifedipine inhibits nuclear translocation of NFAT1 in human keratinocytes. Clin Exp Dermatol. 2005 In press. [Google Scholar]
- 41.Melan MA, Sluder G. Redistribution and differential extraction of soluble proteins in permeabilized cultured cells. Implications for immunofluorescence microscopy. J Cell Sci. 1992;101:731–743. doi: 10.1242/jcs.101.4.731. [DOI] [PubMed] [Google Scholar]
- 42.Harlow E, Lane D. Using antibodies: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 43.Zhou FC, Lumeng L, Li TK. Quantitative immunocytochemical evaluation of serotonergic innervation in alcoholic rat brain. Neurochem Int. 1995;26:135–143. doi: 10.1016/0197-0186(94)00108-7. [DOI] [PubMed] [Google Scholar]
- 44.Vente JD, Garssen J, Tilders FJ, Steinbusch HW, Schipper J. Single cell quantitative immunocytochemistry of cyclic GMP in the superior cervical ganglion of the rat. Brain Res. 1987;411:120–128. doi: 10.1016/0006-8993(87)90688-3. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Russel D. Molecular cloning: a laboratory maual. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 46.Farr PM, Diffey BL. The erythemal response of human skin to ultraviolet radiation. Br J Dermatol. 1985;113:65–76. doi: 10.1111/j.1365-2133.1985.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 47.Dahl SC, Handler JS, Kwon HM. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol. 2001;280:C248–253. doi: 10.1152/ajpcell.2001.280.2.C248. [DOI] [PubMed] [Google Scholar]
- 48.Denda M, Sato J, Masuda Y, Tsuchiya T, Koyama J, Kuramoto M, Elias PM, Feingold KR. Exposure to a dry environment enhances epidermal permeability barrier function. J Invest Dermatol. 1998;111:858–863. doi: 10.1046/j.1523-1747.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- 49.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez del Arco P, Martinez-Martinez S, Maldonado JL, Ortega-Perez I, Redondo JM. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J Biol Chem. 2000;275:13872–13878. doi: 10.1074/jbc.275.18.13872. [DOI] [PubMed] [Google Scholar]
- 51.Porter CM, Havens MA, Clipstone NA. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J Biol Chem. 2000;275:3543–3551. doi: 10.1074/jbc.275.5.3543. [DOI] [PubMed] [Google Scholar]
- 52.Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Shibasaki F, Price R, Guillemot JC, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- 54.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 55.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 56.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 57.Ruzicka T, Bieber T, Schopf E, Rubins A, Dobozy A, Bos JD, Jablonska S, Ahmed I, Thestrup-Pedersen K, Daniel F, Finzi A, Reitamo S. A short-term trial of tacrolimus ointment for atopic dermatitis. European Tacrolimus Multicenter Atopic Dermatitis Study Group. N Engl J Med. 1997;337:816–821. doi: 10.1056/NEJM199709183371203. [DOI] [PubMed] [Google Scholar]
- 58.Remitz A, Reitamo S, Erkko P, Granlund H, Lauerma AI. Tacrolimus ointment improves psoriasis in a microplaque assay. Br J Dermatol. 1999;141:103–107. doi: 10.1046/j.1365-2133.1999.02927.x. [DOI] [PubMed] [Google Scholar]
- 59.Shore RN. Clearing of psoriatic lesions after the application of tape. N Engl J Med. 1985;312:246. [PubMed] [Google Scholar]
- 60.Shore RN. Treatment of psoriasis with prolonged application of tape. J Am Acad Dermatol. 1986;15:540–542. doi: 10.1016/s0190-9622(86)80506-0. [DOI] [PubMed] [Google Scholar]
- 61.Friedman SJ. Management of psoriasis vulgaris with a hydrocolloid occlusive dressing. Arch Dermatol. 1987;123:1046–1052. [PubMed] [Google Scholar]
- 62.Griffiths CE, Tranfaglia MG, Kang S. Prolonged occlusion in the treatment of psoriasis: a clinical and immunohistologic study. J Am Acad Dermatol. 1995;32:618–622. doi: 10.1016/0190-9622(95)90347-x. [DOI] [PubMed] [Google Scholar]
- 63.Gormar FE, Bernd A, Bereiter-Hahn J, Holzmann H. A new model of epidermal differentiation: induction by mechanical stimulation. Arch Dermatol Res. 1990;282:22–32. doi: 10.1007/BF00505641. [DOI] [PubMed] [Google Scholar]
- 64.Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- 65.Dascalu A, Nevo Z, Korenstein R. The control of intracellular pH in cultured avian chondrocytes. J Physiol. 1993;461:583–599. doi: 10.1113/jphysiol.1993.sp019530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parsegian VA, Rand RP, Rau DC. Osmotic stress, crowding, preferential hydration, and binding: A comparison of perspectives. Proc Natl Acad Sci U S A. 2000;97:3987–3992. doi: 10.1073/pnas.97.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dascalu A, Matithyou A, Oron Y, Korenstein R. A hyperosmotic stimulus elevates intracellular calcium and inhibits proliferation of a human keratinocyte cell line. J Invest Dermatol. 2000;115:714–718. doi: 10.1046/j.1523-1747.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- 68.Franchi-Gazzola R, Visigalli R, Dall'Asta V, Sala R, Woo SK, Kwon HM, Gazzola GC, Bussolati O. Amino acid depletion activates TonEBP and sodium-coupled inositol transport. Am J Physiol Cell Physiol. 2001;280:C1465–1474. doi: 10.1152/ajpcell.2001.280.6.C1465. [DOI] [PubMed] [Google Scholar]
- 69.Morris RJ, Fischer SM, Slaga TJ. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res. 1986;46:3061–3066. [PubMed] [Google Scholar]
- 70.Morris RJ, Coulter K, Tryson K, Steinberg SR. Evidence that cutaneous carcinogen-initiated epithelial cells from mice are quiescent rather than actively cycling. Cancer Res. 1997;57:3436–3443. [PubMed] [Google Scholar]
- 71.Huang C, Mattjus P, Ma WY, Rincon M, Chen NY, Brown RE, Dong Z. Involvement of nuclear factor of activated T cells activation in UV response. Evidence from cell culture and transgenic mice. J Biol Chem. 2000;275:9143–9149. doi: 10.1074/jbc.275.13.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reynolds NJ, Al-Daraji WI. Calcineurin inhibitors and sirolimus: mechanisms of action and applications in dermatology. Clin Exp Dermatol. 2002;27:555–561. doi: 10.1046/j.1365-2230.2002.01148.x. [DOI] [PubMed] [Google Scholar]