Abstract
CAAX proteins are widely involved in global cellular functions such as proliferation, differentiation, and carcinogenesis. As an important modulator of biological activity, signal transduction via protein prenylation is a crucial step for most CAAX protein functions, particularly for anchoring these CAAX proteins to cellular membrane system. With a better understanding of the molecular mechanisms of signal transduction and intracellular messaging in this process, CAAX protein prenylation may be of particular importance for elucidating the biologic events in carcinogenesis and provide potential approaches of selectively blocking the downstream signal cascade that allows carcinogenesis. Here, we mainly focus on the prenylation process of the clinically important CAAX box proteins, and their potential as a biomarker or preventive/therapeutic target in carcinogenesis.
Keywords: CAAX box proteins, prenylation, carcinogenesis, signal transduction, biomarkers
Introduction
CAAX proteins are defined as a group of proteins with a specific amino acid sequence at C-terminal that directs their post translational modification. C is cysteine residue, AA are two aliphatic residues, and X represents any C-terminal amino acid depending on different substrate specificity. The CAAX proteins encompass a wide variety of molecules that include nuclear lamins (intermediate filaments), Ras and a multitude of GTP-binding proteins (G proteins), several protein kinases and phosphatases, etc. Most CAAX proteins are found primarily at the cytoplasmic surface of cellular membranes and are involved in a tremendous number of cellular signaling processes and regulatory events that play various roles in cell biological functions. These activity include cell proliferation, differentiation, nuclear stability, embryogenesis, spermatogenesis, metabolism, and apoptosis [1]. The proteins that have CAAX box at the end of the C-terminal always need a prenylation process before the proteins are sent to plasma membrane or nuclear membrane and exert different functions.
Protein prenylation is post-translational lipid modification process of adding of either farnesyl (15-carbon) or more commonly geranylgeranyl (20-carbon) isoprenoids to cysteine residues of the CAAX box at or near the C terminus of intracellular proteins. This process is critical for proper function of many proteins, particularly for anchoring the proteins to the plasma and nuclear membranes. The prenylation process of the CAAX proteins includes 3 steps: polyisoprenylation, proteolysis, and carboxyl methylation. The first crucial step is that an isoprenoid lipid is attached to the CAAX box by prenyltransferase, for example, farnesyltransferase (FTase) or geranylgeranyltransferase type I (GGTase-I). FTase and GGTase-I recognize CAAX box in protein, and then add the 15-carbon isoprenoid farnesyl pyrophosphate by FTase or the 20-carbon isoprenoid by GGTase-I to the cysteine residue on CAAX box. The 15-carbon isoprenoid farnesyl pyrophosphate (FPP) or the 20-carbon isoprenoid geranyl-geranyl pyrophosphate is a product of mevalonate (MVA) metabolic pathway. 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), FTase and GGTase-I are the key enzymes of the mevalonate pathway. The regulation of these key enzymes can significantly affect protein prenylation process. In this review, we mainly focus on the biologically important CAAX box proteins, the role of the prenylation process in carcinogenesis, and the key enzymes involved in the prenylation process as potential targets in cancer prevention and treatment.
Category of CAAX-box proteins
So far, more than 50 CAAX proteins from a wide range of families have been identified. The biologically important CAAX proteins include the G-protein family, nuclear lamin, protein tyrosine phosphatase, GBP Family, battenin family, heat shock protein, proteins with DEAD/H (Asp-Glu-Ala-Asp/His) box, Ser/Thr protein kinase family, GPRK subfamily, PXF/PEX19 family, paralemmin, and inositol-1,4,5 –triphosphate 5-phosphatase [1]. These proteins could be further classified into G-protein superfamily, nuclear and intracellular membrane proteins based on their final intracellular locations. A summary of the important CAAX proteins are listed in Table 1.
Table 1.
Common human proteins with Carboxyl-terminal membrane-targeting sequence elements
| G proteins | H-Ras [81], K-Ras4B [15], RhoA [15], RhoB [15], RhoC [15], Rac1/1b, Rac2, Rac3, RhoG [82], Rnd1, Rnd2, Rnd3/RhoE, Cdc42 [83], TC10/RhoQ, TCL, RhoD, Rif/RhoF, RhoH/TTF [82], Wrch-1 [83], Chp/Wrch-2 [84], RhoBTB1c, RhoB2/DB2c, Rap1b, Rap2b [85] |
| Nuclear surface membrane proteins | prelamin A [86], lamin B1, and lamin B2 [87] |
| Intracelluar membrane proteins | HsPXF [88], Cenp-F, Cenp-E [25], Pharbin [89], HDJ2 [90] |
RAS proteins and G-protein superfamily
The G-protein superfamily is the most important category of human CAAX proteins. Many G-proteins, such as Ras, Rho, Rac, and CDC42, are located at the plasma membrane and endomembranes. The G-protein super-family is actively involved in many important cellular signaling pathways and plays an important role in carcinogenesis. As one of the most important G proteins, Ras proteins have a well-established role in carcinogenesis. Ras proteins function as a signal switch that control growth signals from cell surface receptors to nuclear transcription factors. Human cancer studies show that gene mutational activation of the Ras subfamily (K-ras, N-ras and H-ras) occurs in ∼20% of human cancers. K-ras gene mutation frequently occurs in pancreatic and colorectal adenocarcinoma [2–6]. N-ras mutation has been reported in melanoma, hepatocellular cancer, myelodysplastic syndrome and acute myelogenous leukemia [7–9]. Several human cancers such as thyroid follicular and papillary carcinoma, bladder cancer and renal cell cancer harbor H-ras mutations [10, 11]. These mutations stabilize Ras in a constitutively active GTP-bound conformation, and constantly activate the downstream cellular proliferation process. In addition to the common Ras mutation, alterations in factors that lie upstream of Ras pathway such as the growth factor receptor tyrosine kinases (RTKs), or downstream of Ras pathway such as BRAF mutation, amplification of p110, AKT2 and deletion of PTEN, also contribute to the deregulated Ras signaling in 30–40% of all human malignancies including thyroid carcinoma, non-small cell lung carcinoma, ovarian and breast cancer [12–14].
Similar to Ras, the majority of the Rho GTPase family is known to undergo similar post-translational modifications on the CAAX box that directs proper subcellular localization required for GTPase function. These locations of the Rho GTPases can vary significantly; some are found predominantly at the plasma membrane (e.g. Rac1), some are associated mainly with endomembranes (e.g. RhoH), and others are associated with endosomes (e.g. RhoD) [15]. Unlike the RAS protein, constitutively active forms of the Rho GTPases as a result of mutations are very rare in tumors. Only RhoH is genetically altered in plasma cell myeloma and non-Hodgkin lymphoma [16]. It has been documented that mutations in the 5′-untranslated region of RhoH independent of chromosomal translocations affect its expression in B-cell lymphomas [17]. Over-expression of other Rho GTPases such as RhoA (in breast, colon, bladder and testicular germ cell tumors), RhoC (in melanoma, pancreatic ductal adenocarcinoma bladder and inflammatory breast cancer), RAC1 and RAC2 (in breast, colon, bladder and head and neck cancers), and CDC42 (in breast cancer) have been reported [18]. Frequently, the increased levels of these GTPases in tumor cells correlate with aggressive histologic features and clinical behavior. Although overexpression alone by no means implies a functional role in the development of the malignancy, the correlation between the increased levels of these GTPases and clinical outcome may suggest an active role of the RhoGTPase in carcinogenesis. Similar to Ras protein, the cycling of Rho protein between inactive (GDP-bound) and active (GTP-bound) conformations is also highly regulated and is necessary for biologic activities such as cytoskeletal remodeling and vesicle transport, many of these being important for malignant transformation.
Nuclear Lamins
Nuclear lamins are the major structural components of the nuclear lamina, a filamentous meshwork beneath the inner nuclear membrane. Nuclear lamin provides mechanical stability to the nuclear envelope and controls chromatin organization, DNA replication and anchoring of nuclear pore complexes during cell growth and division [19]. Association of lamins with the inner nuclear membrane is important for their physiological function. The precursor pre-lamin does not function until a series of posttranslational modifications, including isoprenylation, proteolytic trimming and carboxyl methylation process is completed. The conserved CAAX box at the C-terminal serves as the target of the posttranslational prenylation process. Similar to other prenylation modifications, the CAAX box is firstly recognized by FTase, which adds the farnesyl isoprenoid (15 carbons) to the cysteine residue. Then, farnesylated-proteins converting enzyme 1 (FACE-1), cleaves behind farnesylcysteine to release –AAX. This is followed by subsequent endoproteolytic trimming and carboxyl methylation that significantly increases the hydrophobicity of the C-termini of CAAX-modified proteins. In normal cells, essentially all prelamin A is rapidly converted to mature lamin A so prelamin A is virtually undetectable. However, using protein FTase inhibitors can block the prenylation process, and helps to evaluate the normal function of lamin A [20]. Recent studies show lamin A interacts with DNA directly through the carboxyl-terminal tail or through lamina-associated proteins, which are important for nuclear peripheral positioning and constitutive silencing of heterochromatin [21]. Lamin A also directly interacts and regulates activity of transcription factors, for example retinoblastoma protein (Rb). In addition to promoting subnuclear localization of Rb, Lamin A/C-Rb complex protects Rb from proteasomal degradation [22]. Common mutations involving the normal function of lamin are known to be related to primary laminopathies which includes at least 10 degenerative disorders affecting striated muscle, peripheral nerve, adipose tissue, and cause premature aging with multiple organ degeneration [23].
Peroxisomal farnesylated protein HsPxF
Among farnesylated proteins, the peroxisomal farnesylated protein HsPxF is the only one reported so far to be located to peroxisomes, cellular organelles performing metabolic functions. HsPxF proteins encoded by PEX genes are a family of proteins mediating peroxisome biogenesis. HsPxF contains no transmembrane domain, and is loosely associated with the peroxisome membrane rather than being an integral membrane component. For such peroxisomal membrane proteins, the presence of the CAAX box at the C terminal directs the prenylation modification and contributes to subsequent increased affinity to the cytoplasmic surface of peroxisomal membrane. Earlier research indicates mutations in the HsPxF and loss of peroxisome function may be involved in Zellweger syndrome, a human peroxisome biogenesis disorder manifested as progressive neurologic and hepatic dysfunction resulting in early death in infancy [24].
Other CAAX-box proteins
Other CAAX-box proteins are involved in diverse biological functions. For example, interferon–induced guanylate-binding protein from the GBP family mediates the anti-proliferative effect of inflammatory cytokines and inhibits endothelial cell migration in the setting of inflammation. Prostacyclin receptor (GPI receptor) is critical in controling cell proliferation by transducing extracelluar growth and proliferation signals to the nucleus. Centromere protein F (Cenp-F) is an important nuclear and kinetochore protein in normal dividing cells. Its prenylation is essential for localization to the nuclear envelope and kinetochores, and for timely progression through G2/M in cell cycle [25]. Prenylation of type I Inositol-1,4,5–triphosphate 5-phosphatase facilitates its localization to the cytosol and mitochondrial membrane, and controls the hydrolysis of Ins(1,4,5) P3 during inositol metabolism. Ins(1,4,5) P3 acts as a second messenger for cellular calcium signaling, a pathway essential for many physiological activities [26].
Prenylation process of CAAX box proteins
Prenylation is a multistep enzymatic process of adding hydrophobic prenyl moieties to proteins. Prenylation facilitates the attachment of these proteins to the cell membrane. Most of CAAX box proteins do not have a transmembrane domain, thus, the prenylation process is crucial for the function of many signal transduction proteins. The prenylated CAAX box proteins are first anchored to the plasma membrane, and are then transported to the nuclear membrane, mitochondrial membrane or remain as plasma membrane proteins depending on their different structures and cellular functions. The post-translational prenylation process starts a series of molecular signals that control different functions involved in proliferation, differentiation, and oncogenesis.
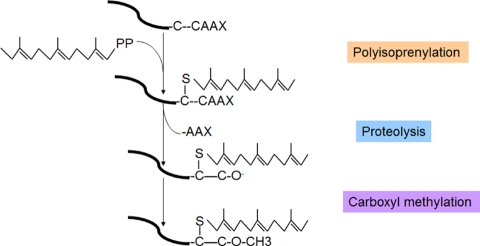
The prenylation process of the CAAX proteins includes 3 steps: polyisoprenylation, proteolysis, and carboxyl methylation (Figure 1). The prenylation process starts with the attachment of an isoprenoid lipid by protein farnesyltransferase (FTase) or geranylgeranyl-transferase type I (GGTase-I) to CAAX box.
Figure 1.
Prenylation process of the CAAX proteins. The protein prenylation process includes 3 steps: polyisoprenylation, proteolysis, and carboxyl methylation. Polyisoprenylation is the attachment of an isoprenoid lipid by protein farnesyltransferase (FTase) or geranylgeranyltransferase type I (GGTase-I) to CAAX box. In the second step, the CAAX residues are proteolysed by prenyl protein peptidase RCE1 family to release –AAX. This is followed by subsequent endoproteolytic trimming and carboxyl methylation significantly increases the hydrophobicity of the C-termini of CAAX-modified proteins.
FTase and GGTase-I (also called the CAAX prenyltransferases) recognize CAAX box protein, then adding the 15-carbon isoprenoid farnesyl pyrophosphate (FTase) or the 20-carbon isoprenoid geranyl-geranyl pyrophosphate (GGTase-I) to the Cysteine residue on the CAAX box. In the second step, the CAAX residues are proteolyzed by prenyl protein peptidase RCE1 family on the surface of the ER. The third step adds methyl esterified (-OMe) by isoprenyl-cysteine carboxyl methyl-transferase (Icmt) (Figure 2).
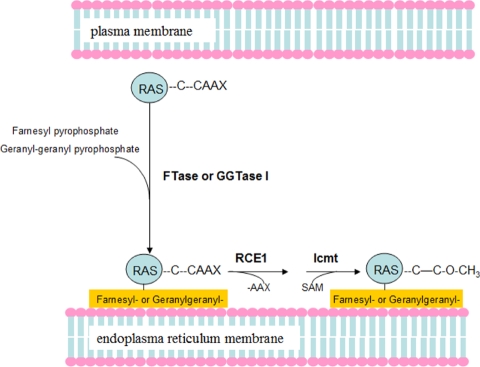
Figure 2.
Protein prenylation process is necessary for subcellular localization of mammalian Ras proteins. FTase and GGTase-I recognize CAAX box in protein, and then add the 15-carbon isoprenoid farnesyl pyrophosphate by FTase or the 20-carbon isoprenoid by GGTase-I to the Cysteine residue on CAAX box. The Prenylation modified Ras proteins are anchored to the endoplasmic reticulum (ER). Rce1 (Ras and a-factor-converting enzyme) and isoprenylcysteine carboxyl methyltransferase (Icmt) remove the AAX and methylate the farnesylcysteine residue. The post-translational modification processes are necessary for downstream effect of Ras proteins.
The enzymes responsible for isoprenoid addition to proteins are conservative and have been identified in both mammalian systems and lower eukaryotes. Based on the different lipid substrate, protein prenyltransferases can be classified into two functional classes: CAAX prenyltransferases such as farnesyltransferase (FTase) and protein geranylgeranyltransferase type I (GGTase-I); and the Rab geranylgeranyl-transferase or protein geranylgeranyltrans-ferase type II (GGTase-II). Here we mainly focus on the CAAX prenyltransferases.
Steady-state kinetic studies indicate that FTase and GGTase-I have different affinity towards their respective CAAX substrates. FTase recognize substrates better with a Ser, Gln, Met or Ala in the X position, whereas GGTase-I prefers Leu. However, there is evidence that some could be substrates for both enzymes, such as proteins with a Phe at the X residue position. It is common when the activity of FTase is compromised by treatment with FTase inhibitors, that the CAAX protein substrate can be geranylgeranylated by GGTase-I. This may explain why the clinical response is far less than expected in some clinical trials that use FTase inhibitors alone.
CAAX box proteins as useful biomarkers
Ras proteins of the G-protein superfamily are constitutionally active in most of malignancy and are known to play a significant oncogenic role in tumorigenesis, thus commonly are used as diagnostic markers. Other CAAX box proteins have also been demonstrated to be associated with cancer. Here, we mainly focus on the nuclear CAAX box proteins and their clinical utility as potential diagnostic or prognostic markers and as the biomarkers for assessing the effects of CAAX prenyltrans-ferase inhibitors.
Nuclear Lamin
The expression of nuclear lamin A appears to be linked to cell differentiation in many tumors. It is speculated that lamin A may have a higher affinity for chromatin than type B lamin, thus it may facilitate differential gene expression. Nuclear lamins are differentially expressed in tissues with a variable degree of differentiation and maturation. For example, absence of expression of nuclear lamin A reportedly is often associated with embryonal carcinoma, while the expression of lamin A more common in differentiated non-seminomas. The different staining pattern may help with evaluating the percentage of embryonal carcinoma in non-seminomatous testicular germ cell tumors as a prognostic marker [27]. Nuclear lamin expression pattern was also investigated in normal skin and basal cell carcinoma. Absence of lamin A was correlated with rapid tumor growth, while absence of lamin C was associated with slow growth within the tumor [28]. Similar observations in the expression of lamin A have also been reported in lung cancer. Lamin A expression has been reported in the fast growing small cell lung carcinomas but not in non-small cell lung carcinomas [29].
Nuclear centomere proteins
Nuclear centromere protein F (Cenp-F) has a CAAX box and the farnesylation process is necessary for CenpF function at the G2/M transition. Cenp-F gene amplification and overexpression is observed in many malignancies including head and neck squamous cell carcinoma, primary breast cancer, astrocytic gliomas and malignant salivary gland tumors [30–32]. CenpF expression correlates with telomerase activity, cyclin E over-expression, c-Myc amplification, and is associated with poor prognosis and chromosomal instability in patients with primary breast cancer. Cenp-F protein expression evaluated by immunohisto-chemistry shows correlation with aggressive tumor behavior, and high tumor grade and is an independent predictor of worse breast cancer-specific survival [32]. CenpF expression has significant correlation with Ki-67 labeling index in primary malignant salivary gland tumor by immnuohistochemical staining and has been suggested as a candidate proliferation marker [31].
CAAX proteins as surrogate biomarkers for Assessment of the effect of CAAX prenyl-transferase inhibitors
FTase and GGPTase-I inhibitors have been developed as useful anticancer agents. For clinical utility, a correlation between the inhibited farnesylation process and cellular growth, survival and transforming activity or clinical response needs to be established in preclinical studies and clinical trials. This is further complicated by the fact the farnesylated proteins can be alternatively geranyl-geranylated when treated with FTase inhibitors. Some farnesylated proteins including H-Ras, prelamin A, lamin B and HDJ2 do not have an alternative prenylation process and thus become more achievable targets to evaluate in clinical settings. The co-chaperone protein HDJ-2 and intranuclear protein lamin A are known to have mobility shifts when FTase is inhibited, therefore are appropriate pharmacologic indices after FTase inhibitor treatment in clinical trials [33]. A mobility shift of HDJ-2 and accumulation of prelamin A after FTase inhibitor treatment could be detected by immunoblotting or immunohistochemistry. Immunoassay has been used for monitoring FTase inhibition in clinical settings [33]. The measurements of FTase activity through HDJ-2 or prelamin A prenylation may not always be adequate surrogates for antitumor activity, especially when the degree of FTase inhibition is not sufficient to produce clinical antitumor effects. However, lack of farnesylation inhibition has been observed in a subset of patients following drug therapy in clinical trials, which usually indicates poor clinical response.
Therapeutic potentials of CAAX prenyl-transferase inhibitors in inhibiting carcinogenesis
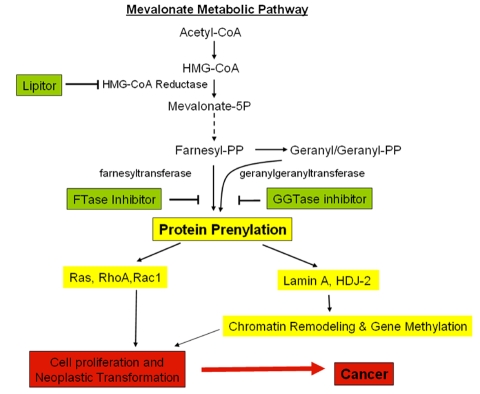
It is crucial in the first step of the prenylation process that FTase and GGTase-I recognize the CAAX box in the protein and add either the 15-carbon isoprenoid farnesyl pyrophosphate (FPP) or the 20-carbon isoprenoid by GGTase-I to the Cysteine residue. The 15-carbon isoprenoid farnesyl pyrophosphate or the 20-carbon isoprenoid geranyl-geranyl pyrophosphate are products of the Mevalonate (MVA) metabolic pathway. HMG-CoA reductase is the first rate-limiting enzyme of the mevalonate pathway. Thus, HMG-CoA reductase, FTase and GGTase-I could be potential targets for modulation of the protein prenylation process as a therapeutic approach, as proposed in Figure 3.
Figure 3.
Molecular events and mechanisms involved in mevalonate metabolic pathways and potential targets for cancer. Shown are the potential agents/inbitors (in green boxes) that may impair downstream carcinogenesis (yellow boxes). 1) Lipitor blocks protein prenylation through inhibiting HMG-CoA reductase and suppresses the formation of downstream isoprenoids FPP and GGPP, which are used as substrates for prenylation; 2) Farnesyltransferase (FTase) inhibitors and Geranylgeranyltransferase (GGTase) inhibitors inhibit a spectrum of protein prenylation (including k-ras, nuclear lamin A and HDJ-2) lead to inhibit mutant k-ras and to modulate chromatin and de-methylate key tumor suppressor genes.
Farnesyltransferase inhibitors (FTI)
Farnesyltransferase inhibitors are recently discovered novel peptide analogs capable for inhibiting FTase, thus can influence the protein anchorage to the cell membrane and subsequent signal transduction. FTase inhibitors have therapeutic potentials in inhibiting oncogenesis, suppressing unwanted cell proliferation and aberrant high signal transduction. During the last decade, several new FTase inhibitors emerged as a new generation of signal transduction inhibitor drugs targeted against the molecular abnormalities in oncogenesis. Small GTPase H, K- and N-Ras proteins are the most frequently mutated oncogenes in human cancer. Over the past decades, strategies targeting interfering Ras function have always been the subjects of searching therapeutic interventions for human cancer. Since identification of the prenylation process for the Ras pathway, prenyl-transferase inhibitors have become a logical approach in therapeutic intervention. The enzyme FTase that is required in the first and essential step of the three Ras processing steps has emerged as the most promising target for anticancer drug development. In preclinical studies, FTase inhibitors show ability in inhibiting tumorigenesis in the majority of cell lines derived from human cancer [34]. Evidence supports that inhibition of FTase of Ras proteins can lead to significant experimental antitumor effects in animal models of colon, pancreatic, lung, prostate, bladder, and breast carcinomas and melanoma [35–37].
In addition to directly interfering with signal transduction in tumors with ras mutations, FTase inhibitors also help indirectly by inhibiting angiogenesis [38], inducing apoptosis and repressing cell division by altering microtubule centromere interactions [39, 40]. In addition, the synergistic effectiveness of combination therapy of FTase inhibitors with traditional cytotoxic chemotherapeutic agents has been proven in cell lines and in experimental animals [41]. Furthermore, recent studies evaluating the effects of FTase inhibitors as a radiosensitizer show these agents can act in synergy or additively with conventional chemotherapy and sensitize tumors in vivo, or tumor cells in vitro, to radiation therapy. The mechanisms by which FTase inhibitors enhance radio-sensitization remain elusive. It is known that hypoxic cells are more resistant to radiation. Tumor oxygenation is improved after FTase inhibitor treatment in vivo. It has been postulated the increased radiation-induced apoptosis may be related to increased oxygenation of the tumor cells' surrounding tissue [42]. These observations indicate that utility of FTase inhibitors may not be limited to tumors with mutated ras, and expand their potential clinical utility as a radiosensitizer in a wide spectum of human malignancy.
In the past years, great progress has been made in the use of FTase inhibitors. Several small molecules designed as analogs of the CAAX sequence and peptide mimics have been shown to have efficacy in vitro, as well as in mouse models bearing rasdependent tumors or human xenografts with H-, N-, or K-ras mutations. These molecules are designed to compete mainly with Ras substrate, not FPP substrate. Thus Ras-dependent tumor inhibition could be achieved without interfering with normal signaling [37]. Evidence supports significant experimental antitumor effects of FTase inhibitors in animal models without significant cytotoxicity in normal cells [35–37]. FTase inhibitors have also demonstrated promising activity in preclinical studies [43]. However in clinical trials, the efficacy of FTase inhibitors is far less than expected [1]. One explanation is that inhibiting farnesylation alone is not sufficient; geranylgeranylation might activate Ras and suppress the effect of FTase inhibitors. Combined farnesyl/geranyl transferase inhibitors might achieve better clinical outcomes.
Geranylgeranyltransferase inhibitor (GGTI)
In the first step of the prenylation process of CAAX proteins, geranylgeranyltransferase type I (GGTase-I) modifies Ras-related GTPases with a 20-carbon geranylgeranyl lipid. The potential of GGTase-I as a target for anti-cancer drugs has been investigated, especially considering that partial rescue of cell proliferation could be accomplished through geranylgeranylation in farnesyltransferase inhibitor treatment. Similar to FTase inhibitors, GGTase inhibitors have been shown to arrest human tumor cell proliferation and reduce tumor growth in animals when used in combination with chemotherapeutic agents [44]. Combined farnesyl/geranyl transferase inhibitors have also demonstrated markedly higher levels of apoptosis than achievable by either farnesyltransferase inhibitors or GGTase inhibitors alone [1, 45]. However, in some preclinical studies, GGTase inhibitors have the ability to impair transformation in vitro and tumor growth in vivo when used alone [45]. Other investigators report that doses of GGTase inhibitors sufficient for inhibition of K-Ras prenylation are lethal to mice when continuously infused [46]. One explanation is that GGTase inhibitors target many proteins that are important for cell viability. These observations indicate that the clinical benefit may be limited by the toxicity associated with GGTase inhibitor treatment.
Natural inhibitors of protein prenyltransferases
Several research groups have screened compounds with inhibitory activity against protein prenyltransferases originating from natural sources. Recently, several natural inhibitory compounds have been isolated and identified from fungal (Gliocladium fimbriatum) metabolite gliotoxin and an organosulfur compound from garlic (diallyl disulfide). Gliotoxin is a sulfur-containing compound that inhibits both FTase and GGTase, and has low toxicity and pronounced anti-tumor activity against tumor cell lines derived from lymphoma and breast cancer [47, 48]. Although the mechanism of their action is unclear, it appears the molecular structures of these compounds are important. Their biologic activity is based on a negative interaction with the zinc ion in the active site of prenylating enzymes.
HMG-CoA reductase inhibitor
The mevalonate pathway has also become an important target for anti-cancer therapy. Covalent attachment of these Mevalonate (MVA) derived isoprenoid groups, such as 15-carbon isoprenoid farnesyl pyrophosphate (FPP) or the 20-carbon isoprenoid geranyl-geranyl pyrophosphate, is a required step of the prenylation process [49, 50]. There are several important enzymes that convert mevalonate to isopentenyl diphosphate (IPP): mevalonate kinase (MK), phosphomevalonate kinase (PMK), mevalonate 5-diphosphate decarboxylase (MDD), and farnesyl pyrophosphate synthase (FPPS). The enzymes in the mevalonate pathway have become targets for a post-translational modification of proteins that contain C-terminal CAAX.
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA reductase) is the first rate-limiting enzyme of the mevalonate pathway.
HMG-CoA reductase, as the main regulatory enzyme in the mevalonate metabolic pathway, is involved in the synthesis of cholesterol and the isoprenoid precursors, farnesylpyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). HMG-CoA reductase inhibitors (statins such as Lipitor) are widely used drugs for the treatment of hypercholesterolemia and their potential role as chemopreventive drugs has been investigated [41, 51–56]. Through the mevalonate pathway, HMG-CoA reductase inhibitors such as statins inhibit the formation of downstream isoprenoids FPP and GGPP, which are used as substrates for prenylation, then inhibit the membrane targeting and signal transduction of CAAX protein. Increased HMG-CoA reductase activity and elevated mevalonate synthesis have been found in various malignant tumors, including pancreatic carcinoma [57, 58], colorectal carcinoma, lung adenocarcinoma, hepatocellular carcinoma, and leukemia [52, 59–63]. It has been reported that statins such as Lipitor can inhibit cell proliferation and trigger apoptosis of cultured tumor cells derived from many cancers such as acute myelogenous leukemia cells, juvenile myelomonocytic leukemia, breast cancer, pancreatic tumor, renal carcinoma, astrocytoma, neuroblastoma and several other tumors [53, 64–70]. Dimitroulakos et al used cDNA microarray to identify the differentially expressed genes following treatment of squamous cell carcinoma cell lines with Lipitor. Their results suggest that the pronounced Lipitor-induced apoptotic response is mediated through inhibiting mevalonate synthesis and further depletion of the mevalonate metabolites [71]. In addition, Lipitor significantly increases the apoptosis rate induced by chemotherapeutic agents such as 5-fluorouracil (5-FU) or cisplatin in the colon cancer cell lines [65]. Several animal model studies using statins have exhibited actions against chemical carcinogen-induced carcinogenesis in the colon, mammary gland, liver, and lung [72–74].
HMG-CoA reductase inhibitors have also been shown to inhibit the invasive and metastatic properties of cancer cells [53, 75]. Statin-induced reduction of cell migration and invasion is believed to be independent of apoptosis and is more likely to be associated with GGPP-dependent reduction of matrix metalloproteinases (MMPs) activity and disruption the organization of the actin fibers [76]. Abnormal expression of MMPs is believed to play an important role in tumor cell invasion and progression in several cancers [77]. These findings suggest the HMG-CoA reductase inhibitors could be used to prevent and reduce tumor invasion.
In recent years, there has been growing interest in using natural or laboratory synthesized substances of low toxicity to prevent cancer or reduce cancer risk. HMG-CoA reductase inhibitor is one such chemo-preventive agent. A promising approach to enhance the chemopreventive efficacy of statins and reduce the potential toxicity is to use them in combination with other agent having different modes of action [78, 79]. It has been shown that administration of Lipitor (atorvastatin) in combination with aspirin or celecoxib (COX2 inhibitor) displays a significant synergistic effect on the inhibition of azoxymethane (AOM)-induced rat colon carcinogenesis [78–80].
Conclusion
CAAX proteins are widely involved in global cellular functions such as growth, differentiation, and carcinogenesis. As an important modulator of biologic activity, signal transduction via protein farnesylation or prenylation is a crucial step for most CAAX protein functions. With better understanding of the molecular mechanisms of signal transduction and intracellular messaging in this process, CAAX protein prenylation may be of particular importance for elucidating the biologic events in carcinogenesis and provide potential approaches of selectively blocking the downstream signal cascade that is important for tumorigenesis.
To prevent the prenylation process of the oncogenic forms of many proteins with CAAX motif has emerged as a promising strategy. Over the past decade, pharmaceutical companies have developed several prenyltransferase inhibitors with impressive antitumor effect in cancer cell lines as well as in animal models. Several of the compounds have reached phase III clinical trials. Unfortunately, the efficacy of these agents as single agents against tumors in clinical trials has been much less than expected, especially in solid tumors, though these agents show promising potential in combination with other chemotherapeutic agents. Another promising compound, HMG-CoA reductase inhibitor, has demonstrated pronounced anti-inflammatory and cancer preventive effects in the laboratory as a single agent or in combination with nonsteroidal anti-inflammatory drugs. The most important question is whether the results could be translated into clinical utility especially in terms of improved overall survival and quality of life. The clinical data so far are limited. These results need to be confirmed with ongoing randomized double-blinded clinical trials. These approaches will no doubt provide a solid foundation for defining the roles of targeted treatment and chemo-prevention that could benefit patients.
Acknowledgments
This study was supported by NIH R21 CA-122514.
References
- 1.Appels NM, Beijnen JH, Schellens JH. Development of farnesyl transferase inhibitors: a review. Oncologist. 2005;10:565–578. doi: 10.1634/theoncologist.10-8-565. [DOI] [PubMed] [Google Scholar]
- 2.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 3.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 4.Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez C, Chetaille B, Tasei AM, Sastre B, Sahel J, Payan-Defais MJ. From pancreatic intraepithelial neoplasia to cancer: a dramatic progression with K-ras status analysis. Gastroenterol Clin Biol. 2005;29:465–468. doi: 10.1016/s0399-8320(05)80818-8. [DOI] [PubMed] [Google Scholar]
- 6.Lohr M, Kloppel G, Maisonneuve P, Lowenfels AB, Luttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takada S, Koike K. Activated N-ras gene was found in human hepatoma tissue but only in a small fraction of the tumor cells. Oncogene. 1989;4:189–193. [PubMed] [Google Scholar]
- 8.Auewarakul CU, Lauhakirti D, Tocharoentanaphol C. Frequency of RAS gene mutation and its cooperative genetic events in Southeast Asian adult acute myeloid leukemia. Eur J Haematol. 2006;77:51–56. doi: 10.1111/j.1600-0609.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. Am J Surg Pathol. 2005;29:1145–1151. doi: 10.1097/01.pas.0000157749.18591.9e. [DOI] [PubMed] [Google Scholar]
- 10.Vasko V, Ferrand M, Di Cristofaro J, Carayon P, Henry JF, de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88:2745–2752. doi: 10.1210/jc.2002-021186. [DOI] [PubMed] [Google Scholar]
- 11.Boulalas I, Zaravinos A, Karyotis I, Delakas D, Spandidos DA. Activation of RAS family genes in urothelial carcinoma. J Urol. 2009;181:2312–2319. doi: 10.1016/j.juro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol 2005. 18 Suppl 2:S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 15.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preudhomme C, Roumier C, Hildebrand MP, Dallery-Prudhomme E, Lantoine D, Lai JL, Daudignon A, Adenis C, Bauters F, Fenaux P, Kerckaert JP, Galiegue-Zouitina S. Nonrandom 4p13 rearrangements of the RhoH/TTF gene, encoding a GTP-binding protein, in non-Hodgkin's lymphoma and multiple myeloma. Oncogene. 2000;19:2023–2032. doi: 10.1038/sj.onc.1203521. [DOI] [PubMed] [Google Scholar]
- 17.Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R, Dalla-Favera R. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759–776. doi: 10.2741/2718. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KL, Zastrow MS, Lee KK. Lamins and disease: insights into nuclear infrastructure. Cell. 2001;104:647–650. [PubMed] [Google Scholar]
- 20.Beck LA, Hosick TJ, Sinensky M. Isoprenylation is required for the processing of the lamin A precursor. J Cell Biol. 1990;110:1489–1499. doi: 10.1083/jcb.110.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen M, Lee KK, Wilson KL, Gruenbaum Y. Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem Sci. 2001;26:41–47. doi: 10.1016/s0968-0004(00)01727-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci U S A. 2004;101:9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B, Zhou Z. Lamin A/C, laminopathies and premature ageing. Histol Histopathol. 2008;23:747–763. doi: 10.14670/HH-23.747. [DOI] [PubMed] [Google Scholar]
- 24.Kammerer S, Arnold N, Gutensohn W, Mewes HW, Kunau WH, Hofler G, Roscher AA, Braun A. Genomic organization and molecular characterization of a gene encoding HsPXF, a human peroxisomal farnesylated protein. Genomics. 1997;45:200–210. doi: 10.1006/geno.1997.4914. [DOI] [PubMed] [Google Scholar]
- 25.Hussein D, Taylor SS. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci. 2002;115:3403–3414. doi: 10.1242/jcs.115.17.3403. [DOI] [PubMed] [Google Scholar]
- 26.Matzaris M, O'Malley CJ, Badger A, Speed CJ, Bird PI, Mitchell CA. Distinct membrane and cytosolic forms of inositol polyphosphate 5-phosphatase II. Efficient membrane localization requires two discrete domains. J Biol Chem. 1998;273:8256–8267. doi: 10.1074/jbc.273.14.8256. [DOI] [PubMed] [Google Scholar]
- 27.Machiels BM, Ramaekers FC, Kuijpers HJ, Groenewoud JS, Oosterhuis JW, Looijenga LH. Nuclear lamin expression in normal testis and testicular germ cell tumours of adolescents and adults. J Pathol. 1997;182:197–204. doi: 10.1002/(SICI)1096-9896(199706)182:2<197::AID-PATH823>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Venables RS, McLean S, Luny D, Moteleb E, Morley S, Quinlan RA, Lane EB, Hutchison CJ. Expression of individual lamins in basal cell carcinomas of the skin. Br J Cancer. 2001;84:512–519. doi: 10.1054/bjoc.2000.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broers JL, Ramaekers FC. Differentiation markers for lung-cancer sub-types. A comparative study of their expression in vivo and in vitro. Int J Cancer Suppl. 1994;8:134–137. doi: 10.1002/ijc.2910570730. [DOI] [PubMed] [Google Scholar]
- 30.de la Guardia C, Casiano CA, Trinidad-Pinedo J, Baez A. CENP-F gene amplification and overexpression in head and neck squamous cell carcinomas. Head Neck. 2001;23:104–112. doi: 10.1002/1097-0347(200102)23:2<104::aid-hed1005>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Shigeishi H, Mizuta K, Higashikawa K, Yoneda S, Ono S, Kamata N. Correlation of CENP-F gene expression with tumor-proliferating activity in human salivary gland tumors. Oral Oncol. 2005;41:716–722. doi: 10.1016/j.oraloncology.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien SL, Fagan A, Fox EJ, Millikan RC, Culhane AC, Brennan DJ, McCann AH, Hegarty S, Moyna S, Duffy MJ, Higgins DG, Jirstrom K, Landberg G, Gallagher WM. CENP-F expression is associated with poor prognosis and chromosomal instability in patients with primary breast cancer. Int J Cancer. 2007;120:1434–1443. doi: 10.1002/ijc.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adjei AA, Davis JN, Erlichman C, Svingen PA, Kaufmann SH. Comparison of potential markers of farnesyltransferase inhibition. Clin Cancer Res. 2000;6:2318–2325. [PubMed] [Google Scholar]
- 34.Sepp-Lorenzino L, Ma Z, Rands E, Kohl NE, Gibbs JB, Oliff A, Rosen N. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 35.Kohl NE, Wilson FR, Mosser SD, Giuliani E, deSolms SJ, Conner MW, Anthony NJ, Holtz WJ, Gomez RP, Lee TJ, et al. Protein farnesyltransferase inhibitors block the growth of rasdependent tumors in nude mice. Proc Natl Acad Sci U S A. 1994;91:9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Lipari P, Malkowski M, Ferrari E, Nielsen L, Prioli N, Dell J, Sinha D, Syed J, Korfmacher WA, Nomeir AA, Lin CC, Wang L, Taveras AG, Doll RJ, Njoroge FG, Mallams AK, Remiszewski S, Catino JJ, Girijavallabhan VM, Bishop WR, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer Res. 1998;58:4947–4956. [PubMed] [Google Scholar]
- 37.Ayral-Kaloustian S, Salaski EJ. Protein farnesyltransferase inhibitors. Curr Med Chem. 2002;9:1003–1032. doi: 10.2174/0929867024606687. [DOI] [PubMed] [Google Scholar]
- 38.Charvat S, Duchesne M, Parvaz P, Chignol MC, Schmitt D, Serres M. The up-regulation of vascular endothelial growth factor in mutated Ha-ras HaCaT cell lines is reduced by a farnesyl transferase inhibitor. Anticancer Res. 1999;19:557–561. [PubMed] [Google Scholar]
- 39.Ferrante K, Winograd B, Canetta R. Promising new developments in cancer chemotherapy. Cancer Chemother Pharmacol. 1999;43 Suppl:S61–68. doi: 10.1007/s002800051100. [DOI] [PubMed] [Google Scholar]
- 40.Crespo NC, Ohkanda J, Yen TJ, Hamilton AD, Sebti SM. The farnesyltransferase inhibitor, FTI-2153, blocks bipolar spindle formation and chromosome alignment and causes prometaphase accumulation during mitosis of human lung cancer cells. J Biol Chem. 2001;276:16161–16167. doi: 10.1074/jbc.M006213200. [DOI] [PubMed] [Google Scholar]
- 41.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Cohen-Jonathan E, Evans SM, Koch CJ, Muschel RJ, McKenna WG, Wu J, Bernhard EJ. The farnesyltransferase inhibitor L744,832 reduces hypoxia in tumors expressing activated H-ras. Cancer Res. 2001;61:2289–2293. [PubMed] [Google Scholar]
- 43.Eskens FA, Awada A, Cutler DL, de Jonge MJ, Luyten GP, Faber MN, Statkevich P, Sparreboom A, Verweij J, Hanauske AR, Piccart M. Phase I and pharmacokinetic study of the oral farnesyl transferase inhibitor SCH 66336 given twice daily to patients with advanced solid tumors. J Clin Oncol. 2001;19:1167–1175. doi: 10.1200/JCO.2001.19.4.1167. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Blaskovich MA, Knowles D, Qian Y, Ohkanda J, Bailey RD, Hamilton AD, Sebti SM. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 1999;59:4919–4926. [PubMed] [Google Scholar]
- 45.Peterson YK, Kelly P, Weinbaum CA, Casey PJ. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity, and cellular activity. J Biol Chem. 2006;281:12445–12450. doi: 10.1074/jbc.M600168200. [DOI] [PubMed] [Google Scholar]
- 46.Lobell RB, Omer CA, Abrams MT, Bhimnathwala HG, Brucker MJ, Buser CA, Davide JP, deSolms SJ, Dinsmore CJ, Ellis-Hutchings MS, Kral AM, Liu D, Lumma WC, Machotka SV, Rands E, Williams TM, Graham SL, Hartman GD, Oliff AI, Heimbrook DC, Kohl NE. Evaluation of farnesyl:protein transferase and geranylgeranyl:protein transferase inhibitor combinations in preclinical models. Cancer Res. 2001;61:8758–8768. [PubMed] [Google Scholar]
- 47.Singh SV, Mohan RR, Agarwal R, Benson PJ, Hu X, Rudy MA, Xia H, Katoh A, Srivastava SK, Mukhtar H, Gupta V, Zaren HA. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun. 1996;225:660–665. doi: 10.1006/bbrc.1996.1226. [DOI] [PubMed] [Google Scholar]
- 48.Vigushin DM, Mirsaidi N, Brooke G, Sun C, Pace P, Inman L, Moody CJ, Coombes RC. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med Oncol. 2004;21:21–30. doi: 10.1385/MO:21:1:21. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 50.Duncan RE, El-Sohemy A, Archer MC. Dietary factors and the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase: implications for breast cancer and development. Mol Nutr Food Res. 2005;49:93–100. doi: 10.1002/mnfr.200400053. [DOI] [PubMed] [Google Scholar]
- 51.Kim KP, Whitehead C, Piazza G, Wargovich MJ. Combinatorial chemoprevention: efficacy of lovostatin and exisulind on the formation and progression of aberrant crypt foci. Anticancer Res. 2004;24:1805–1811. [PubMed] [Google Scholar]
- 52.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 53.Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002;122:308–317. doi: 10.1053/gast.2002.31093. [DOI] [PubMed] [Google Scholar]
- 54.Narisawa T, Fukaura Y, Tanida N, Hasebe M, Ito M, Aizawa R. Chemopreventive efficacy of low dose of pravastatin, an HMG-CoA reductase inhibitor, on 1,2-dimethylhydrazine-induced colon carcinogenesis in ICR mice. Tohoku J Exp Med. 1996;180:131–138. doi: 10.1620/tjem.180.131. [DOI] [PubMed] [Google Scholar]
- 55.Elson CE, Yu SG. The chemoprevention of cancer by mevalonate-derived constituents of fruits and vegetables. J Nutr. 1994;124:607–614. doi: 10.1093/jn/124.5.607. [DOI] [PubMed] [Google Scholar]
- 56.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 57.Rao KN, Kottapally S, Shinozuka H. Lipid composition and 3-hydroxy-3-methylglutaryl-CoA reductase activity of acinar cell carcinoma of rat pancreas. Biochim Biophys Acta. 1983;759:74–80. doi: 10.1016/0304-4165(83)90191-5. [DOI] [PubMed] [Google Scholar]
- 58.L Kim JL, Yan L, Zhang M, Rao S, Yang GY. Overexpression of HMG-CoA Reductase, a Potential Therapeutic Target for Pancreatic Adenocarcinoma. Mod Pathol. 2007;20:284A. [Google Scholar]
- 59.Kawata S, Takaishi K, Nagase T, Ito N, Matsuda Y, Tamura S, Matsuzawa Y, Tarui S. Increase in the active form of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human hepatocellular carcinoma: possible mechanism for alteration of cholesterol biosynthesis. Cancer Res. 1990;50:3270–3273. [PubMed] [Google Scholar]
- 60.Caruso MG, Notarnicola M, Santillo M, Cavallini A, Di Leo A. Enhanced 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity in human colorectal cancer not expressing low density lipoprotein receptor. Anticancer Res. 1999;19:451–454. [PubMed] [Google Scholar]
- 61.Cohen LH, Griffioen M. Regulation of 3-hydroxy-3-methylglutaryl-CoA reductase mRNA contents in human hepatoma cell line Hep G2 by distinct classes of mevalonate-derived metabolites. Biochem J. 1988;255:61–67. doi: 10.1042/bj2550061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchwald H. Cholesterol inhibition, cancer, and chemotherapy. Lancet. 1992;339:1154–1156. doi: 10.1016/0140-6736(92)90744-n. [DOI] [PubMed] [Google Scholar]
- 63.Larsson O. HMG-CoA reductase inhibitors: role in normal and malignant cells. Crit Rev Oncol Hematol. 1996;22:197–212. doi: 10.1016/1040-8428(96)00193-x. [DOI] [PubMed] [Google Scholar]
- 64.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Hayakawa M. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin Cancer Res. 2004;10:8648–8655. doi: 10.1158/1078-0432.CCR-04-1568. [DOI] [PubMed] [Google Scholar]
- 65.Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22:1061–1067. doi: 10.1093/carcin/22.7.1061. [DOI] [PubMed] [Google Scholar]
- 66.Dimitroulakos J, Thai S, Wasfy GH, Hedley DW, Minden MD, Penn LZ. Lovastatin induces a pronounced differentiation response in acute myeloid leukemias. Leuk Lymphoma. 2000;40:167–178. doi: 10.3109/10428190009054894. [DOI] [PubMed] [Google Scholar]
- 67.Jones KD, Couldwell WT, Hinton DR, Su Y, He S, Anker L, Law RE. Lovastatin induces growth inhibition and apoptosis in human malignant glioma cells. Biochem Biophys Res Commun. 1994;205:1681–1687. doi: 10.1006/bbrc.1994.2861. [DOI] [PubMed] [Google Scholar]
- 68.Kawata S, Nagase T, Yamasaki E, Ishiguro H, Matsuzawa Y. Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2) Br J Cancer. 1994;69:1015–1020. doi: 10.1038/bjc.1994.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jani JP, Specht S, Stemmler N, Blanock K, Singh SV, Gupta V, Katoh A. Metastasis of B16F10 mouse melanoma inhibited by lovastatin, an inhibitor of cholesterol biosynthesis. Invasion Metastasis. 1993;13:314–324. [PubMed] [Google Scholar]
- 70.Wejde J, Blegen H, Larsson O. Requirement for mevalonate in the control of proliferation of human breast cancer cells. Anticancer Res. 1992;12:317–324. [PubMed] [Google Scholar]
- 71.Dimitroulakos J, Marhin WH, Tokunaga J, Irish J, Gullane P, Penn LZ, Kamel-Reid S. Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia. 2002;4:337–346. doi: 10.1038/sj.neo.7900247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maltese WA, Defendini R, Green RA, Sheridan KM, Donley DK. Suppression of murine neuroblastoma growth in vivo by mevinolin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Clin Invest. 1985;76:1748–1754. doi: 10.1172/JCI112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawk MA, Cesen KT, Siglin JC, Stoner GD, Ruch RJ. Inhibition of lung tumor cell growth in vitro and mouse lung tumor formation by lovastatin. Cancer Lett. 1996;109:217–222. doi: 10.1016/s0304-3835(96)04465-5. [DOI] [PubMed] [Google Scholar]
- 74.Shibata MA, Kavanaugh C, Shibata E, Abe H, Nguyen P, Otsuki Y, Trepel JB, Green JE. Comparative effects of lovastatin on mammary and prostate oncogenesis in transgenic mouse models. Carcinogenesis. 2003;24:453–459. doi: 10.1093/carcin/24.3.453. [DOI] [PubMed] [Google Scholar]
- 75.Sumi S, Beauchamp RD, Townsend CM, Jr, Uchida T, Murakami M, Rajaraman S, Ishizuka J., Thompson JC. Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology. 1992;103:982–989. doi: 10.1016/0016-5085(92)90032-t. [DOI] [PubMed] [Google Scholar]
- 76.Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, Soria J, Soria C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 77.Fromigue O, Hamidouche Z, Marie PJ. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem. 2008;283:30549–30556. doi: 10.1074/jbc.M801436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reddy BS. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- 79.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66:4542–4546. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 80.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7377. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 81.Philips MR. Compartmentalized signalling of Ras. Biochem Soc Trans. 2005;33:657–661. doi: 10.1042/BST0330657. [DOI] [PubMed] [Google Scholar]
- 82.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–337. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berzat AC, Buss JE, Chenette EJ, Weinbaum CA, Shutes A, Der CJ, Minden A, Cox AD. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J Biol Chem. 2005;280:33055–33065. doi: 10.1074/jbc.M507362200. [DOI] [PubMed] [Google Scholar]
- 84.Chenette EJ, Abo A, Der CJ. Critical and distinct roles of amino- and carboxyl-terminal sequences in regulation of the biological activity of the Chp atypical Rho GTPase. J Biol Chem. 2005;280:13784–13792. doi: 10.1074/jbc.M411300200. [DOI] [PubMed] [Google Scholar]
- 85.Canobbio I, Trionfini P, Guidetti GF, Balduini C, Torti M. Targeting of the small GTPase Rap2b, but not Rap1b, to lipid rafts is promoted by palmitoylation at Cys176 and Cys177 and is required for efficient protein activation in human platelets. Cell Signal. 2008;20:1662–1670. doi: 10.1016/j.cellsig.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 86.Barrowman J, Hamblet C, George CM, Michaelis S. Analysis of prelamin A biogenesis reveals the nucleus to be a CaaX processing compartment. Mol Biol Cell. 2008;19:5398–5408. doi: 10.1091/mbc.E08-07-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maske CP, Hollinshead MS, Higbee NC, Bergo MO, Young SG, Vaux DJ. A carboxyl-terminal interaction of lamin B1 is dependent on the CAAX endoprotease Rce1 and carboxymethylation. J Cell Biol. 2003;162:1223–1232. doi: 10.1083/jcb.200303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gotte K, Girzalsky W, Linkert M, Baumgart E, Kammerer S, Kunau WH, Erdmann R. Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol Cell Biol. 1998;18:616–628. doi: 10.1128/mcb.18.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asano T, Mochizuki Y, Matsumoto K, Takenawa T, Endo T. Pharbin, a novel inositol polyphosphate 5-phosphatase, induces dendritic appearances in fibroblasts. Biochem Biophys Res Commun. 1999;261:188–195. doi: 10.1006/bbrc.1999.0998. [DOI] [PubMed] [Google Scholar]
- 90.Hata M, Ohtsuka K. Murine cDNA encoding a novel type I HSP40/DNAJ homolog, mmDjA4(1) Biochim Biophys Acta. 2000;1493:208–210. doi: 10.1016/s0167-4781(00)00136-6. [DOI] [PubMed] [Google Scholar]