Abstract
Activation of Akt (protein kinase B) and loss of phosphatase and tensin homolog (PTEN) expression have been associated with disease recurrence and reduced survival in several cancers. We evaluated the expression patterns and prognostic value of active, phosphorylated Akt (pAkt) and PTEN in gastrointestinal (GI) carcinoid tumors. Total Akt, pAkt, and PTEN expression was assessed by Western blot analysis in 14 tumor samples from patients with GI carcinoid tumors. Expression levels were quantified with volume analysis software and correlated with clinical parameters. Total Akt, pAkt, and PTEN proteins were detectable in all tumor samples. The expression of activated pAkt and pAkt:PTEN ratios were significantly associated with elevated serum chromogranin A measurements (r=0.77 and 0.78, respectively, P≤0.02 for both). In addition, pAkt:PTEN expression ratios positively correlated with older age (r=0.65, P=0.017). Increased pAkt and pAkt:PTEN expression both were associated with reduced survival (r= -0.51, P=0.06 and r= -0.50, P=0.09, respectively). Patients with pAkt:PTEN ratios greater than one also had dramatically reduced overall survival, but this finding did not achieve statistical significance (36 vs. 153 months, P=0.19). These data suggest that pAkt and PTEN expression levels may be useful tools in understanding tumor biology and perhaps predicting survival in patients with carcinoid tumors. Furthermore, cumulative mutations may lead to upregulation of pAkt and loss of PTEN expression as patients age explaining why older age is associated with a worse prognosis in patients with carcinoid tumors.
Keywords: Carcinoid tumors, Akt, PTEN, chromogranin A, survival
Introduction
Carcinoid tumors account for nearly 50 percent of all neuroendocrine cancers and have nearly doubled in incidence over the last five decades [1]. The majority of carcinoids occur in the gastrointestinal (GI) tract and are unresectable and/or metastatic at the time of presentation [1]. In neuroendocrine cancers, such as GI carcinoids, tumorigenesis is thought to occur secondary to the accumulation of genetic mutations that result in upregulation of oncogenes and downregulation of tumor suppressor genes [2]. However, the molecular mechanisms and alterations that lead to the development of GI carcinoid tumors are poorly understood.
Activation of the proto-oncogene Akt (protein kinase B) and the phosphatidylinositol 3-kinase (PI3K)-Akt pathway has been shown to play a role in the progression of several cancers [3–8]. In the neuroendocrine tumors medullary thyroid and small cell lung cancer, upregulation of Akt also has been shown to be involved in tumor growth [3, 9]. PI3K is a receptor tyrosine kinase activated by growth factors such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin growth factor (IGF) [10]. In patients with neuroendocrine tumors, expression of EGF receptor (EGFR) has previously been shown to correlate with activated Akt expression [11]. PI3K activation leads to the phosphorylation of Akt by phosphatidylinositol-3,4,5-triphosphate (PIP3) directly or through inositol phosphate-dependent dehydrogenase kinase-1 (PDK1) [10]. To generate PIP3, PI3K first phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) which then recruits the serine threonine kinase, Akt [10]. Two individual Akt residues, threonine 308 and serine 473, must be phosphorylated for activation to occur [10].
Activated Akt (pAkt) then regulates cell survival by promoting cell cycle progression and inhibiting apoptosis [10]. Akt activation facilitates cell cycle progression through its effects on glycogen synthase kinase 3 (GSK3) and mammalian target of rapamycin (mTOR) [10]. Akt also phosphorylates and deactivates pro-apoptotic proteins, such as the Bcl-2, Bad, p53, and the forkhead family transcription factors [10]. Akt activation is inhbited by phosphatase and tensin homolg (PTEN), a well-known tumor suppressor located on chromosomal band 10q23 [12]. PTEN prevents PIP2 and PIP3 phosphorylation by inhibiting PI3K [12]. Loss-of-function mutations of PTEN lead to activated Akt [12].
In various cancers, PTEN loss has been indentified as a component of tumor formation with subsequent PI3K-Akt activation [6, 13, 14]. Expression patterns of Akt and PTEN have been correlated with recurrence and survival in patients with colorectal and endometrial carcinomas [6, 14, 15]. Furthermore, in patient with breast, gastric, hepatocellular, renal cell, and prostate cancer, expression of activated Akt has been shown to predict survival and clinical outcome [4, 5, 7, 16–22]. However, the expression patterns and importance of PI3K-Akt and PTEN in GI carcinoid tumors is unclear. The aims of the current study were to establish pAkt and PTEN expression in GI carcinoid tumors, and to determine whether expression of these proteins correlated with clinicopathologic data, such as demographics, tumor variables, and outcome measures.
Materials and method
Human tissue samples
Human GI carcinoid tumor samples (n=14) were obtained during surgical resection from 13 patients who underwent surgery at the University of Wisconsin Hospitals and Clinics, Madison, WI, from 2002 to 2007. A specimen from normal ileal tissue was obtained for control. Tumor histology was verified by pathologic review. The benign and malignant tissues were snap-frozen in liquid nitrogen and stored at -80°C until protein isolation was performed. Tumor cell lysates were prepared from the frozen samples by pulverizing in liquid nitrogen with a mortar and pestle. The resultant powder was then lysed with lysis buffer and proteins isolated as previously described [23]. Informed consent was given in all cases. Institutional Review Board approval was obtained, and research was conducted in accord with the ethical standards established by our institution.
For all patients, data recorded included demographics and tumor information such as age, sex, preoperative diagnosis, and tumor pathology. Additional laboratory, pathologic, and follow-up data were collected: serum chromogranin A, urinary 5-hydoxyindoleacetic acids (5-HIAA), tumor size and histological grade, presence of lymph node invasion and distant metastases, margin status, liver tumor burden, residual or recurrent disease, and endocrinopathy (ie, carcinoid syndrome) presence. The mean age of the patients was 57 years, and 7 (54%) were female (Table 1). The mean tumor size was 2.3 centimeters (cm). Table 1 summarizes the locations and additional demographic data obtained on these patients. One patient had tumor specimens collected from both a small bowel primary and liver metastasis. The patients were analyzed according to disease spread: localized, regional spread, and distant metastasis. The majority of the patients in this study had distant liver metastases and were alive at the time of last follow-up (85%). The mean follow-up time was 46.9 months.
Table 1.
Patient Demographics and Tumor Characteristics
| Patients | |
|---|---|
| Number | 13 |
| Age, years (range) | 57 ± 3 (30–77) |
| Sex, M:F ratio | 6:7 |
| Tumor size, cm | 2.3 ± 0.6 |
| Location of tumor | |
| Small Bowel (%) | 4 (29) |
| Appendix (%) | 1 (7) |
| Stomach (%) | 2 (14) |
| Liver metastases (%) | 6 (43) |
| Other (%) | 1 (7) |
| Disease spread | |
| Localized | 2 (15) |
| Regional | 2 (15) |
| Distant metastases | 9 (70) |
| Synchronous liver metastases (%) | 10 (77) |
| Carcinoid syndrome present (%) | 7 (54) |
| Serum Chromogranin A, ng/mL (nl 0–51) | 198 ± 89 |
| Urine 5-HIAA, mg/L (nl 0–15) | 42 ± 15 |
| Residual/recurrent disease (%) | 7 (58) |
| Survival, Dead:Alive | 2:11 |
Results reported as mean ± SE where appropriate.
Cell culture
The human GI carcinoid cell line, BON, was obtained from Drs. B. Mark Evers and Courtney M. Townsend, Jr. (University of Texas Medical Branch, Galveston, TX) and maintained in Dulbecco's modified Eagle medium-nutrient mixture Ham's F-12K (DMEM/F12K, 1:1, Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Sigma, St. Louis, MO), 100 IU/ml penicillin and 100μg/ml streptomycin (Invitrogen) in a humidified atmosphere of 5% CO2 at 37°C as previously described [23]. Proteins were isolated from BON cells as described previously and used as a positive control on all gels [23].
Western blot analysis
Total cellular protein concentrations were measured by BCA (bicinchoninic acid) assay (Pierce Biotechnology, Rockford, IL). Gel electrophoresis on NuPAGE® Novex® Bis-Tris 10% Mini Gels (Invitrogen) was performed on denatured cellular extracts (30 - 40 μg) according to the manufacturer's instructions, and then transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) by electroblotting. The membranes were blocked in milk (5% non-fat dry milk and 0.05% Tween 20 in phosphate buffered saline), and incubated with the appropriate primary antibody overnight at 4°C. The antibody dilutions were: 1:1000 for total Akt, pAkt, PTEN (Cell Signaling Technology, Beverly, MA), neuron specific enolase (NSE) and synaptophysin (Research Diagnostics Inc., Flanders, NJ); 1:2,000 for chromogranin A (CgA, Zymed Laboratories, San Francisco, CA), and 1:10,000 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Trevigen, Gaithersburg, MD). After primary antibody incubation, the membranes were washed and horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Pierce) were utilized depending upon the primary antibody source. Immunstar (Bio-Rad) or SuperSignal West Femto (Pierce) kits were used for visualization of the protein signal per the manufacturer's specifications. Quantification of protein bands was performed with the Quantity One program version 4.6.3 (Bio-Rad). Expression levels were calculated as a ratio of the index protein signal to GAPDH to control for protein loading and repeated to confirm results.
Statistical analysis
All data are reported as mean ± standard error (SE). Categorical variables were analyzed by chi-square test, and continuous variables assessed by unpaired t tests where appropriate. Pearson's correlation test was used to examine the relation between the expression of pAkt, PTEN, and pAkt:PTEN ratio and various clinicopathologic variables. Overall survival was analyzed by the Kaplan-Meier actuarial method from the time of diagnosis to the date of last follow-up or death with statistical significance ascertained by log-rank analysis. All statistical calculations were performed using SPSS statistical software (version 10.0, SPSS Inc, Chicago, IL). A P value of less than 0.05 was considered statistically significant.
Results
pAkt and PTEN expression in GI carcinoid tumors
To address the role of pAkt and PTEN in GI carcinoid tumors, the expression of total Akt, pAkt (at serine 473), and PTEN were examined by Western blot analysis. Active, pAkt and total Akt proteins were detectable by Western blot analysis in all 14 (100%) GI carcinoid tumor samples (Figure 1A). PTEN expression also was observed in all samples (n=13) examined (Figure 1A). Specimen #7 was not analyzed for PTEN expression due to lack of sufficient tissue and protein. The neuroendocrine origin of the samples was confirmed by assessing levels of CgA (Figure 1B). In tumor samples that expressed minimal to no CgA (# 7–9, 11), both NSE and synaptohpysin levels were examined since these markers are highly expressed in neuroendocrine tissues. Tumors that were weakly CgA positive (# 6, 14) underwent examination of only one additional neuroendocrine marker, NSE. The normal tissue which was obtained from patient #1 showed decreased pAkt, CgA, and NSE expression compared to the tumor sample from the same patient (Figures 1A and B). In GI carcinoid patients, despite an expected negative relationship, pAkt and PTEN expression showed no significant correlation to each other (r = -0.12, P = 0.71). In addition to assessing pAkt and PTEN expression separately, we examined the ratio of pAkt to PTEN (pAkt:PTEN) because this measure has previously been associated with survival [7]. Four tumor samples (31%) had pAkt:PTEN ratios ratios greater than 1. GAPDH was used as a loading control in all experiments, and BON cells were a positive control.
Figure 1.

Expression of active, phosphorylated Akt (pAkt), total Akt, and phosphatase and tensin homolog (PTEN) was analyzed by Western blotting in 14 gastrointestinal (GI) carcinoid tumor samples (A). Samples #1–7 were intestinal or gastric in origin, while #8–13 were from hepatic metastases, and #14 was isolated from a peri-aortic lymph node. Normal ileal tissue (far left) and BON cells (far right), a GI carcinoid tumor cell line, were also examined. Chromogranin A (CgA), neuron specific enolase (NSE), and synaptophysin expression were used to confirm the neuroendocrine origin of the tissues (B). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. For densitometry analysis, a ratio of pAkt or PTEN to GAPDH was used. A histogram of the intensity of each band is shown (C) with pAkt in black and PTEN in stripes.
Relationship of pAkt and PTEN expression to clinicopathologic measures
The demographics and tumor characteristics of the 13 patients in this study are summarized in Table 1. The correlation of the various clinicopathologic features of our GI carcinoid patients to pAkt and PTEN expression are delineated in Table 2. In addition, Table 2 examines these features in relation to the pAkt:PTEN expression ratio. A strong positive correlation was observed between the pAkt:PTEN ratio and older age (r = 0.65, P = 0.017) (Table 2). In addition, pAkt expression exhibited a moderately positive correlation with older age (r = 0.52, P = 0.06), while PTEN expression showed a weak negative correlation with older age (r = -0.47, P = 0.10) (Table 2). These observations suggest that pAkt levels may increase and PTEN levels decrease with age in patients with GI carcinoid tumors. pAkt and PTEN expression did not have any significant relation to sex, primary tumor size, tumor location, disease spread, or liver metastasis (Table 2).
Table 2.
Relationship between clinicopathologic variables and pAkt and PTEN expression levels
| P value | ||||
|---|---|---|---|---|
| Variable | Number | pAkt | PTEN | pAkt:PTEN |
| Age | 14 | 0.056 | 0.10 | 0.017 |
| Sex | 14 | 0.99 | 0.80 | 0.84 |
| Tumor size | 14 | 0.58 | 0.89 | 0.59 |
| Tumor location | 14 | 0.50 | 0.99 | 0.51 |
| Disease spread | 14 | 0.44 | 0.25 | 0.83 |
| Liver metastasis | 14 | 0.39 | 0.32 | 0.75 |
| Carcinoid syndrome | 14 | 0.24 | 0.51 | 0.19 |
| Serum Chromogranin A level | 9 | 0.016 | 0.48 | 0.021 |
| Urinary 5-HIAA level | 8 | 0.67 | 0.80 | 0.74 |
| Residual/recurrent disease | 13 | 0.21 | 0.43 | 0.18 |
| Survival | 14 | 0.063 | 0.61 | 0.085 |
Significant values in bold type.
Pearson's correlation used for all analyses
An additional significant positive correlation was seen between both the expression of pAkt and the pAkt:PTEN ratio and serum chromogranin A levels (r = 0.78, P = 0.016 and r = 0.77, P = 0.021, respectively) (Table 2). pAkt expression and the pAkt:PTEN ratio also showed weak positive correlations with the presence of carcinoid syndrome and residual or recurrent disease, but these findings did not reach statistical significance (Table 2). Furthermore, pAkt, PTEN, and pAkt:PTEN expression did not have any association with urine 5-HIAA levels (Table 2). The expression of PTEN was not correlated significantly to any of the variables examined, except a weak association to older age.
Association of pAkt and PTEN expression to survival
Several previous studies have correlated pAkt and PTEN expression to survival. Therefore, we wanted to determine if pAkt, PTEN, or pAkt:PTEN were related to survival in patients with GI carcinoid tumors. Table 2 shows that moderately negative correlations were observed for pAkt expression and the pAkt:PTEN ratio and survival (alive vs. dead) (r = -0.51, P = 0.063 and r = -0.50, P = 0.085, respectively). We then divided these markers into 2 groups based on expression patterns. For pAkt, the patients were divided (by an independent, blinded researcher) into high pAkt and low pAkt expression. Using Kaplan-Meier survival analysis and log-rank comparison, patients with high pAkt expression trended toward shorter overall mean survival compared to patients with low pAkt expression, though this finding was not statistically significant (65± 12 mos vs. 144 ± 29 mos, P = 0.56). Meanwhile, patients with low PTEN expression also had a shorter overall mean survival than patients with high PTEN expression, but again this result was not significant (39 ± 1 mos vs. 85 ± 24 mos, P = 0.21). The Kaplan-Meier survival curves for these patients based on PTEN expression is shown in Figure 2. In addition, the expression of these markers was combined, and patients with pAkt:PTEN ratios were analyzed in 2 groups: >1 and <1. Survival analysis of these patients also showed a trend toward shorter mean survival for patients with pAkt:PTEN ratios > 1 compared to patients with ratios < 1, but this observation also was not statistically significant (36.2 ± 0.3 mos vs. 153.3 ± 23.2 mos, P = 0.19). We were unable to determine median survival and produce Kaplan-Meier survival curves for all protein expression variables because the majority of patients were alive at last follow-up.
Figure 2.
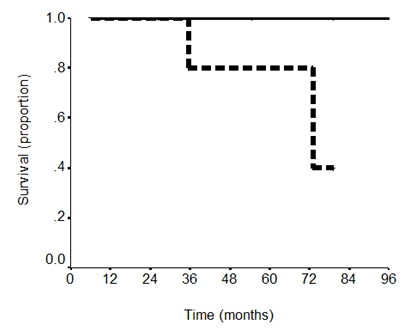
Kaplan-Meier survival curve for PTEN expression. Patients with low PTEN expression (dotted line) trended toward decreased overall mean survival compared to patients with high PTEN expression (solid black line), but this result was not significant (39 ± 1 mos vs. 85 ± 24 mos, P = 0.21).
Discussion
In the present study, Western blot analysis showed that active, pAkt, total Akt, and PTEN were expressed in all GI carcinoid tissue samples analyzed. Our study also demonstrated that pAkt levels and the pAkt:PTEN ratio significantly correlated with serum CgA levels and older age. In addition, we report that increased pAkt expression, low PTEN expression and pAkt:PTEN ratios greater than 1 trended toward an association with decreased survival. A significant amount of evidence points to the role of PI3K-Akt pathway activation and PTEN loss in the tumorigensis of several cancers [10]. PI3K activity leads to the phosphorylation and activation of Akt creating pAkt. Upregulated pAkt compared to surrounding benign tissue has been demonstrated in prostate cancer [5]. We observed pAkt expression in 100% of the GI carcinoid tumor samples analyzed. In the one patient for whom normal tissue was available, pAkt expression was higher in the GI carcinoid tumor sample than the normal tissue.
PTEN is a phosphatase that inhibits PI3K activation and, thus, Akt phosphorylation. Previous reports in sporadic colorectal, endometrial, and prostate cancer patients have correlated loss of PTEN expression with Akt upregulation [5, 14]. We found no significant relationship between pAkt and PTEN expression in our cohort of GI carcinoid patients, though 100% of the tumor samples analyzed expressed PTEN by Western blotting. In an examination of 9 carcinoid tumor samples by immunohistochemistry, Wang and colleagues also showed moderate or strong PTEN expression [13]. However, 54% of poorly differentiated neuroendocrine carcinomas were negative for PTEN suggesting that loss of PTEN expression is associated with advanced tumor progression and aggressive biologic behavior [13]. Furthermore, in carcinoids, loss of PTEN may not be an initiating event, but occur after tumor formation. One drawback of our assessment of PTEN protein levels by antibody detection is that some PTEN mutations that lead to tumor formation may be hidden by this method. Furthermore, this technique does not asses whether or not the PTEN protein is functional. Further investigation is needed to determine the exact role of PTEN in GI carcinoids.
In this investigation, our data demonstrated that increased pAkt expression and pAkt:PTEN ratios are significantly associated with higher serum CgA levels (Table 2). While pAkt expression and pAkt:PTEN ratios also positively correlated with the presence of carcinoid syndrome and residual or recurrent disease, these observations were not statistically significant. Furthermore, no relationship was demonstrated between any of the expression levels and urine 5-HIAA measurements. Few studies have examined serum tumor markers in relation to pAkt or PTEN expression. However, in patients with sporadic colorectal cancer, preoperative serum CEA levels have been correlated with pAkt expression [14]. Our data support this relationship between pAkt and tumor markers. We found no relationship between sex, tumor stage, size, location, or metastasis presence and pAkt, PTEN, or pAkt:PTEN.
Additional findings in this study were significant positive correlations between both pAkt expression and the pAkt:PTEN ratio and older age (Table 2). Conversely, we also show a trend toward reduced PTEN expression and an association with younger age (Table 2). To the best of our knowledge, pAkt expression has not previously been documented as having a relationship to age in human cancers. However, in a study by Colakoglu et al, PTEN expression was negatively associated with younger age [14]. According to these data, increased pAkt and decreased PTEN expression may be related to age in patients with GI carcinoid tumors.
In several reports, pAkt overexpression and loss of PTEN have been shown to predict survival [4, 7, 15–19, 21]. However, the relationship of pAkt and PTEN expression to survival in GI carcinoids is unknown. In this study, we were unable to demonstrate a statistically significant association between pAkt, PTEN, or pAkt:PTEN and survival. Nonetheless, pAkt expression and the pAkt:PTEN ratio both showed moderate correlations to reduced overall survival. Prior studies have shown that older age is a poor prognostic variable and is connected with decreased survival in patients with GI carcinoid tumors [24, 25]. The connection seen here between pAkt and both age and survival may provide an explanation for the association between age and survival in this patient population.
The results of this study are certainly limited by the retrospective design and small sample size. The tumor specimens also were from a variety of GI locations and were overall relatively clinically benign despite 77% of patients having metastatic disease. This observation may explain why all samples expressed PTEN. In addition, biochemical markers such as serum CgA and urine 5-HIAA, were not measured in all participants.
Reliable data on extrahepatic metastases, a variable known to influence survival, were not available.
In summary, our data indicate that Akt is activated and PTEN is present in differentiated GI carcinoid tumors. Furthermore, expression of these molecules may be useful prognostic parameters in this population. Further investigation in a larger sample is certainly warranted. Additionally, direct inhibition of pAkt may be a therapeutic option in the treatment of patients with GI carcinoid tumors.
Acknowledgments
These studies were funded in part by the American College of Surgeons Resident Research Scholarship; American College of Surgeons George H.A. Clowes Jr. Memorial Research Career Development Award; NIH grants T32 CA009614 Physician Scientist Training in Cancer Medicine, R21 CA117117, and R01 CA109053; American Cancer Society Research Scholars Grant 05-08301TBE; Carcinoid Cancer Foundation Research Grant; and the Society of Surgical Oncology Clinical Investigator Award.
References
- 1.Modlin I, Lye K, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Zikusoka M, Kidd M, Eick G, Latich I, Modlin I. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer. 2005;104:2292–309. doi: 10.1002/cncr.21451. [DOI] [PubMed] [Google Scholar]
- 3.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–22. [PubMed] [Google Scholar]
- 4.Vestey SB, Sen C, Calder CJ, Perks CM, Pignatelli M, Winters ZE. Activated Akt expression in breast cancer: correlation with p53, Hdm2 and patient outcome. Eur J Cancer. 2005;41:1017–25. doi: 10.1016/j.ejca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Jendrossek V, Henkel M, Hennenlotter J, Vogel U, Ganswindt U, Müller I, Handrick R, Anastasiadis AG, Kuczyk M, Stenzl A, Belka C. Analysis of complex protein kinase B signalling pathways in human prostate cancer samples. BJU Int. 2008;102:371–82. doi: 10.1111/j.1464-410X.2008.07703.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanamori Y, Kigawa J, Itamochi H, Shimada M, Takahashi M, Kamazawa S, Sato S, Akeshima R, Terakawa N. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001;7:892–5. [PubMed] [Google Scholar]
- 7.Merseburger AS, Hennenlotter J, Kuehs U, Simon P, Kruck S, Koch E, Stenzl A, Kuczyk MA. Activation of PI3K is associated with reduced survival in renal cell carcinoma. Urol Int. 2008;80:372–7. doi: 10.1159/000132694. [DOI] [PubMed] [Google Scholar]
- 8.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–14. doi: 10.1016/j.surg.2006.06.040. discussion 14–5. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 11.Shah T, Hochhauser D, Frow R, Quaglia A, Dhillon AP, Caplin ME. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J Neuroendocrinol. 2006;18:355–60. doi: 10.1111/j.1365-2826.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Ignat A, Axiotis CA. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:139–46. doi: 10.1097/00129039-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Colakoglu T, Yildirim S, Kayaselcuk F, Nursal TZ, Ezer A, Noyan T, Karakayali H, Haberal M. Clinicopathological significance of PTEN loss and the phosphoinositide 3-kinase/Akt pathway in sporadic colorectal neoplasms: is PTEN loss predictor of local recurrence? Am J Surg. 2008;195:719–25. doi: 10.1016/j.amjsurg.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 15.Uegaki K, Kanamori Y, Kigawa J, Kawaguchi W, Kaneko R, Naniwa J, Takahashi M, Shimida M, Oishi T, Itamochi H, Terakawa N. PTEN-positive and phosphorylated-Akt-negative expression is a predictor of survival for patients with advanced endometrial carcinoma. Oncol Rep. 2005;14:389–92. [PubMed] [Google Scholar]
- 16.Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR, Schmid KW, Baba HA. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008;48:83–90. doi: 10.1016/j.jhep.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JMS. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–46. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Tenorio G, Stål O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–5. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cinti C, Vindigni C, Zamparelli A, La Sala D, Epistolato MC, Marrelli D, Cevenini G, Tosi P. Activated Akt as an indicator of prognosis in gastric cancer. Virchows Arch. 2008;453:449–55. doi: 10.1007/s00428-008-0676-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee BL, Lee HS, Jung J, Cho SJ, Chung HY, Kim WH, Jin YW, Kim CS, Nam SY. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 200;11:2518–25. doi: 10.1158/1078-0432.CCR-04-1282. [DOI] [PubMed] [Google Scholar]
- 21.Murakami D, Tsujitani S, Osaki T, Saito H, Katano K, Tatebe S, Ikeguchi M. Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer. 2007;10:45–51. doi: 10.1007/s10120-006-0410-7. [DOI] [PubMed] [Google Scholar]
- 22.Nam SY, Lee HS, Jung G, Choi J, Cho SJ, Kim MK, Kim WH, Lee BL. Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS. 2003;111:1105–13. doi: 10.1111/j.1600-0463.2003.apm1111205.x. [DOI] [PubMed] [Google Scholar]
- 23.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–54. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 24.Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815–21. doi: 10.1097/00000658-199906000-00008. discussion 22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janson ET, Holmberg L, Stridsberg M, Eriksson B, Theodorsson E, Wilander E, Oberg K. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–90. doi: 10.1023/a:1008215730767. [DOI] [PubMed] [Google Scholar]


