Abstract
Signal transducer and activator of transcription 3 (Stat3) plays a vital role in signal transduction pathways that mediate transformation and inhibit apoptosis. Oncogenic Stat3 is persistently activated in several human cancers and transformed cell lines. Previous studies indicate activation of Stat3 in renal cell carcinoma (RCC). However, the detailed characterization of the Stat3 expression pattern in different histologic types of RCC is lacking. We have analyzed the immunoprofile of activated or phosphorylated Stat3 (pStat3) in a tissue microarray of renal tumors of different histologic types, including 42 cases of conventional clear cell type, 24 chromophobe, and 7 papillary, 15 oncocytoma, 7 urothelial carcinoma and 21 normal kidney tissues using an anti-pStat3 antibody (recognizes only activated STAT3). pStat3 nuclear staining was observed in 25 of 42 conventional clear cell RCC (59.5 %), 8 of 24 chromophobe RCC (33.3%), 4 of 7 papillary RCC (57.1%). In the other tumor groups, 4 of 15 oncocytomas (26.7%) and 6 of 7 urothelial carcinomas (85.7%) showed positive nuclear staining. Weak nuclear immunoreactivity for pStat3 was seen in 4 of 21 cases of non-neoplastic kidney tissue (19.0%). The extent of Stat3 activation as determined by nuclear expression of its phosphorylated form is increased in histologic types of renal tumors with greater malignant potential, specifically conventional clear cell RCC, papillary RCC and urothelial carcinoma, only slightly increased in chromophobe RCC, and not increased in oncocytoma. These results suggest a role of Stat3 activation in different types of renal neoplasia, possibly serving as a prognostic marker or therapeutic target.
Keywords: Signal Transducer and activator of transcription 3 (Stat3), signal transduction, phosphorylation, renal tumors, kidney cancers
Introduction
The majority of renal tumors are malignant in nature. Malignant renal tumors account for 2.5% of all human malignancies. It is estimated that there are approximately 54,390 new cases of renal tumors and more than 13,010 deaths in the US each year [1]. Approximately 85% of malignant renal tumors are of epithelial origin and classified as renal cell carcinoma (RCC). Although remarkable efforts have been made in elucidating molecular pathogenesis of renal neoplasia, the detailed molecular mechanisms underlying the pathogenesis and progression of renal neoplasia remain largely to be elucidated. The search for key mediators in renal neoplasia not only enhances our understanding of the pathogenesis but also lays the ground for further studies of prognostic and therapeutic markers.
Recent studies have indicated that signal transducer and activator of transcription-3 (Stat3) may be a critical mediator in the oncogenesis and progression of renal tumors [2]. Stat3 is a transcriptional factor that mediates cytokine and growth factor signaling pathway [3]. In resting cells, Stat3 is predominantly latent and located in the cytoplasm. In response to extracellular stimulation, Stat3 becomes activated through tyrosine phosphorylation at Tyr705, which is regulated by growth factor receptor tyrosine kinases or cytokine receptor associated Jak kinases [4]. Phosphorylated Stat3 (pStat3) dimerizes and translocates to the nucleus where it activates target gene transcription.
pStat3 is subsequently inactivated by tyrosine dephosphorylation and returns to the cytoplasm. The activation of Stat3 depends on ligand binding to its specific receptor. The reported ligands that can activate Stat3 include EGF, IL-6 and PDEGF [5–7]. The function of Stat3 is associated with organ development and cell proliferation. Targeted disruption of the Stat3 gene leads to termination of early embryonic development [8]. In cell lines, active Stat3 is required to enhance transformation and to block apoptosis. Dysregulation of Stat3 leads to aberrant stimulation of cell growth [9].
Constitutive activation of Stat3 has been observed in a growing number of human cancers including breast cancer, multiple myeloma, head and neck tumors, ovarian and prostate cancer [10–13]. In renal tumors, over-stimulation of Stat3 was also observed and was probably responsible for the cell proliferation of tumor cells induced by cytokine interleukin-6 (IL-6) and epidermal growth factor-receptor (EGF-R) [2]. In vitro study demonstrated that inhibition of IL-6 induced phosphorylation of Stat3 by a Jak specific inhibitor (AG490) suppressed the IL-6-induced cell proliferation of renal cell carcinoma cells [2]. In vivo study in nude mice also showed that blockade of EGF-R signaling pathway by a tyrosine kinase inhibitor (PKI166) decreased phosphorylation of STAT3, which led to inhibiting the growth of human renal cell carcinoma in the kidney of nude mice [14]. All these suggest that Stat3 may be actively involved in the pathogenesis and progression of renal tumorigenesis.
It has been recently reported that a high frequency of Stat3 activation was observed in renal cell carcinoma, especially in metastatic disease [2]. But the activation of Stat3 in different histologic types of renal tumors remains unclear. Because many different categories of benign and malignant tumor are now distinguished based on histopathological appearance, it would be of clinical significance to understand the patterns of Stat3 activation in various histologic types of renal tumors. To investigate the potential of using Stat3 as a prognostic marker in renal tumors, we used immunohistochemistry to analyze the activity of Stat3 in different histologic types of renal tumors using a high-throughput tissue microarray platform.
Materials and method
Clinical Specimens
Archival formalin-fixed, paraffin-embedded renal tumor tissues were obtained from patients who underwent nephrectomy at New York University Medical Center between 1999–2001. The average age of patients was 64.6 years old with a range of 33–88. Histological subtypes (main component) were as follows: 73 cases of renal cell carcinoma (including 42 cases of conventional clear cell type, 24 chromophobe, and 7 papillary), 15 oncocytoma, and 7 urothelial carcinoma. Twenty-one non-neoplastic renal tissues from areas adjacent to renal tumors were analyzed for comparison. In the RCC group, 56% tumors were in stage pT1, 9% in pT2, 25% in pT3, and 5% in pT4. In the conventional clear cell type RCC, the nuclear grades were 12% for G1, 54% for G2, 30% for G3 and 4% for G4.
Construction of TMA
Three cylindrical core tissue-biopsies (diameter 0.6 mm) were taken from representative areas of each paraffin-embedded kidney tumor (donor blocks) and precisely arrayed into a new recipient paraffin block with 0.8 mm distance between the samples, using a Tissue Arrayer (Beecher Instruments, Silver Spring, MD). After the recipient block construction was completed, 5 μm sections were cut. The presence of tumor tissue on the arrayed samples was verified on a hematoxylin-eosin-stained section.
Immunohistochemistry
Consecutive sections from the recipient block were used for immunohistochemical staining. For immunohistochemical studies, an automated immmunostainer (DAKO-Autostainer, Universal Staining System; DAKO, Glostrup, Denmark) was used. Briefly, after microwave epitope retrieval (30 min. 160 W in tris-HCl, pH 9.5 + 5% urea) sections were incubated for 30 min with the polyclonal rabbit antibody pStat-3 (1:50; Cell Signaling Technology, Beverly, MA), which recognizes only phosphorylated form of Stat3. Binding of the primary antibodies was assessed by the DAKO LSAB2 system detection kit. Diaminobenzidine was used as a chromogen. All slides were read by two pathologists (CG, JM) independently. Nuclear pStat3 staining was analyzed and documented in the following categories: negative, no reactivity; weakly positive, less then 10% of cancer cell nuclei positive; moderately positive, 10–50% positive; strongly positive, more than 90% positive.
Statistical Methods
Contingency table analysis and Chi-square tests were used to compare the levels of pStat3 expression in renal tumors to that in adjacent non-neoplastic renal tissues.
Results
Of the 357 kidney tissue cores in the tissue microarray slide (TMA), 329 cores (92.2%) were interpretable for pStat3 staining. The remaining 28 cores were not interpretable due to either the loss of tissue or no representation of tumor tissue in the core. Anti-pStat3 antibody recognizes only the phosphorylated form of Stat3, which is typically localized in the nucleus therefore, only cells with nuclear immunoreactivity for pStat3 were considered to be positive for pStat3. The level of immunoreactivty was defined negative as no stain; weakly positive as less than 10% cells positive; moderately positive as 10–50% cells positive; strongly positive as more than 50% cells positive. The nuclear immunostaining of pStat-3 in adjacent non-neoplastic kidney tissues was used as a control for analysis of pStat-3 in renal tumors. In the non-neoplastic kidney tissue (Figure 1), weak nuclear expression of pStat3 in the tubules was observed in 19 % cases (4 of 21). As non-neoplastic kidney tissues were obtained from tumor-bearing kidneys, the weak nuclear immunoreactivity for pStat3 was possibly due to stimulation from neighboring tumor cells. In addition, occasional immunoreactivity for pStat3 was observed in endothelial cells of blood vessels regardless of the neoplastic status of cells.
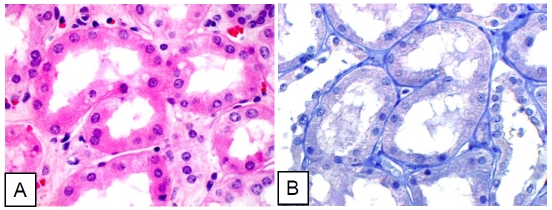
Figure 1.
Normal renal tubule cells negative for pStat3. A. Hematoxylin-eosin stain. B. Immunohistochemical stain (pStat3).
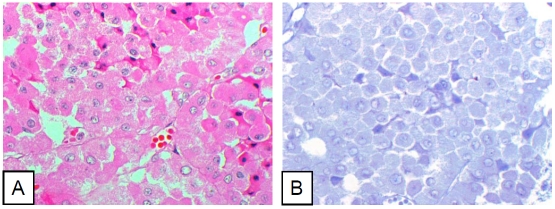
In the 73 cases of renal cell carcinomas (RCC) included in the TMA slide, 37 cases were observed with nuclear immunoreactivity for pStat3 in neoplastic cells (51%). The level of nuclear pStat3 signals in neoplastic cells was not related to tumor stage, but it was clearly related to the histologic type of tumor (Table 1). In clear cell type RCC (Figure 2), nuclear staining for pStat3 in neoplastic cells was observed in 59.5% cases (25 of 42), a significant increase compared to that in adjacent non-neoplastic kidney tissue (p<0.0001). However, this increase did not correspond to the nuclear grade in clear cell RCC. In papillary RCC (Figure 3), nuclear pStat3 signals were present in epithelial neoplastic cells but absent in the fibrovascular core and histiocytes. The positive rate, 57.1% (4 of 7 cases), was close to that in clear cell RCC, representing a significant increase over those in adjacent non-neoplastic kidney tissues. In chromophobe RCC (Figure 4), nuclear staining for pStat-3 was observed in 33% cases (8 of 24), and this moderate increase was not statistically significant probably due to the relatively small number of samples (p=0.08).
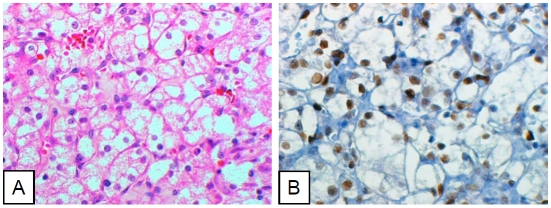
Figure 2.
Clear cell renal cell carcinoma cells diffusely positive for pStat3. A. Hematoxylin-eosin stain. B. Immunohistochemical stain (pStat3).
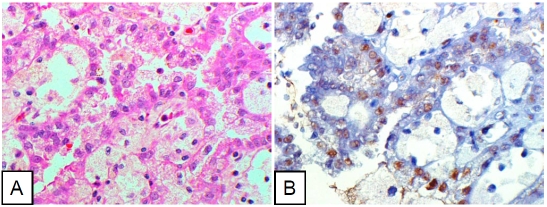
Figure 3.
Papillary cell renal cell carcinoma cells diffusely positive for pStat3. A. Hematoxylin-eosin staining. B. Immunohistochemical stain (pStat3).
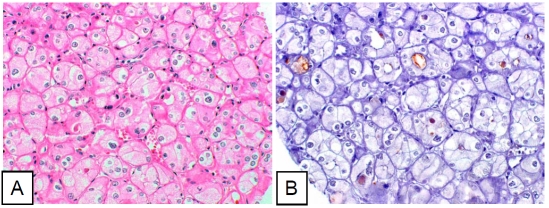
Figure 4.
Chromophobe renal cell carcinoma cells sparsely positive for pStat3. A. Hematoxylin-eosin staining. B. Immunohistochemical staining (pStat3).
Two other common types of renal tumors, oncocytoma and urothelial carcinoma were also included in the study. pStat3 nuclear reactivity was observed in 4 of 15 cases of oncocytoma (Figure 5). But this positive rate, 26.7%, was statistically insignificant when compared to the control (p=0.43). In urothelial carcinoma (Data not shown), nuclear pStat3 signal was observed in 85.7% cases (6 of 7), a significant increase over the adjacent non-neoplastic kidney tissue (p<0.001).
Figure 5.
Renal oncocytoma cells negative for pStat3. A. Hematoxylin-eosin stain. B. Immunohistochemical stain (pStat3).
Discussion
The aberrant activation of Stat3 has been observed in a number of human cancers including breast, prostate, ovarian, multiple myeloma, head and neck cancers [10–13, 15]. After examining the level of pStat3 in 48 cases of renal cell carcinoma specimens and corresponding nonneoplastic kidney tissues, Horiguchi et al found positive nuclear immunostaining signals for pStat3 in all cases [2]. While one half of the cases showed a high level of immunostaining for pStat3 (greater than 90% of nuclei), the other half showed a low level (less than 10%). A high level of pStat3 was closely associated with a poor prognosis. Although a significantly higher level of pStat3 was observed in metastatic lesions than those non-neoplastic tissues, the association of Stat3 activation with histological subtype was not significant. However, this result was limited by the composition of renal cell carcinoma in the study. The cases in their study were overwhelmingly composed of clear cell type of RCC (41/48). The remaining cases included 4 cases of granular type (an outdated classification), 1 papillary and 2 sarcomatoid (also no longer considered a separate subtype in current classification schemes). The composition of RCC in their cohort was not sufficient to draw a reliable conclusion regarding the profile of Stat3 activation in different histologic types of RCC.
In the current study we investigated the activation of Stat3 in renal neoplasia by immunohistochemistry using a tissue microarray slide, designed to include various histologic types of renal neoplasm. Studies have shown that the prognosis of renal neoplasia is closely associated with the histologic subtypes [16, 17]. A study of 405 cases of renal neoplasms by Amin et al demonstrated that the 5-year disease-specific survival for chromophobe renal cell carcinoma, papillary renal cell carcinoma, and conventional (clear cell) renal cell carcinoma was 100%, 86%, and 76% respectively; no patient with oncocytoma progressed or died of disease [16]. Another study of 2385 RCC patients by Cheville et al revealed that cancer-specific survival rates at 5 years for patients with clear cell, papillary, and chromophobe RCC were 68.9%, 87.4%, and 86.7%, respectively [17].
We found that a higher frequency of Stat3 activation in renal neoplasms that carry poor prognosis, namely clear cell type, papillary type and urothelial carcinoma. A moderate increase of Stat3 activation is also observed in chromophobe renal cell carcinoma, the RCC subtype with the best prognosis. The frequency of Stat3 activation is not increased in oncocytoma, a benign renal tumor. In our study, the follow-up periods are not long enough to permit any meaningful prognostic data, as the specimens used in this study were collected from 1999–2001. Our observations clearly demonstrate that frequent activation of Stat3 is significantly associated with these histologic types of renal tumors carrying poor outcomes.
In the current study, a significant number of RCC were negative for pStat3. The small core size on tissue microarray raises the possibility of false negative results due to non-representative sampling. Recently several studies have shown that two or more 0.6-mm cores on tissue microarrays give a better representation than do single cores [18]. In our study, three cores from each sample were used to minimize the non-representative sampling. However, it is undeniable that the area sampled using the tissue microarray 0.6 mm core is much smaller than that present in a whole tissue section (1.0 × 1.0 cm) and therefore there is always a theoretical possibility of greater sampling error compared to whole tissue sections, especially in the cases of low distribution and level of pStat3 expression. In our study, positive immunostain for pStat3 is found in 59.4% of clear cell type RCC cases. It is higher than the high-level rate (51.2%), but lower than the overall positive rate (100%) in the study by Hiroguchi et al [2]. Tissue microarray platform thus may be considered useful for establishing lower limits for the distribution of staining but may need to be supplemented with additional stained whole tissue sections, especially for the evaluation of focal staining. In the non-neoplastic kidney tissue, four samples also showed weak to moderate immunoreactivity to pStat3 in the tubules. As these non-neoplastic tissues are obtained from tumor bearing kidneys, activated stat3 may due to stimulation from neighboring tumor cells or its expression may represent a field effect. Alternatively, endogenous biotin can be significant source of false-positive staining in kidney tissues after heat-induced epitope retrieval.
The aberrant activation of Stat3 in human cancers suggests of an important role of Stat3 in initiation and progression of human cancers. In prostate cancer, the activation of Stat 3 is associated with an early disease recurrence [19]. In head and neck cancers, activated Stat3 is associated with metastasis, though it is not an independent marker [20]. .It is of great importance to determine whether the activated (phospho-) Stat3 is associated with poor outcome of renal tumors when the outcome data is available.
In addition to Stat3, other member of Stat family might also be activated, in particular Stat5 since several studies have found that Stat5 also plays an important role in human malignancies. Both Stat3 and Stat 5 are downstream targets of the Janus Kinase 2 (JAK2) [21]. In hematopoietic malignancies, the expression of pStat5 is associated with a poorer clinical outcome in diffuse large B-cell lymphomas, whereas the concomitant expression of pStat5 and pJAK2 is linked to a better prognosis in follicular lymphomas [22]. However, the expression of pStat5 is suppressed in renal cell carcinoma compared with normal renal parenchyma [23]. Interestingly, there is also an accompanying overexpression of transforming growth factor-β in renal cell carcinoma, which may interfere the interleukin-2-induced activation of Stat5. The relevance of other markers in growth factor receptor(s) (e.g. EGFR) and MEK/ERK MAP pathways will be determined in future in our cohort.
Activation of Stat3 may not only be a promising prognostic maker but also a potential effective target for the development of new anticancer treatments. Suppressing expression of Stat3 by using antisense oligomers against STAT inhibit cell growth in head and neck squamous carcinoma cell lines and in prostate cancer cells [9, 24]. Small phosphotyrosyl-peptides inhibit cell transfor-mation induced by v-Src, as these peptides bind at the STAT3 SH2 domain, thus blocking its phosphorylation, dimerization, DNA-binding, and gene regulation [25]. In ovarian tumor cells, overexpression of a dominant negative form of Stat3, which lacks the C-terminal end and therefore interfere the function of wild-type Stat3, blocks cell proliferation and consequent tumoral progression [15]. In renal tumors, indirect inhibition of Stat3 activity by inhibitors of protein tyrosine kinase also demonstrates a suppressive effect on tumor cells in vivo and vitro [6]. Therefore, further studies are needed to evaluate whether Stat3 represents a potentially prognostic marker and may provide a targeted and effective approach in the treatment of renal neoplasm.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M. Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: a study of incidence and its association with pathological features and clinical outcome. J Urol. 2002;168:762–765. [PubMed] [Google Scholar]
- 3.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal PB. Paradigm shifts in the cell biology of STAT signaling. Semin Cell Dev Biol. 2008;19:329–340. doi: 10.1016/j.semcdb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horiguchi A, Oya M, Marumo K, Murai M. STAT3, but not ERKs, mediates the IL-6-induced proliferation of renal cancer cells, ACHN and 769P. Kidney Int. 2002;61:926–938. doi: 10.1046/j.1523-1755.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 7.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells. World J Gastroenterol. 2005;11:3385–3391. doi: 10.3748/wjg.v11.i22.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci U S A. 2005;102:8585–8590. doi: 10.1073/pnas.0502343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, Lorentz H, Bommert K, Topp M, Kramer D, Muller-Hermelink HK, Einsele H, Greiner A, Bargou RC. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109:720–728. doi: 10.1182/blood-2006-05-024372. [DOI] [PubMed] [Google Scholar]
- 11.Dhir R, Ni Z, Lou W, DeMiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate. 2002;51:241–246. doi: 10.1002/pros.10079. [DOI] [PubMed] [Google Scholar]
- 12.Hambek M, Baghi M, Strebhardt K, May A, Adunka O, Gstottner W, Knecht R. STAT 3 activation in head and neck squamous cell carcinomas is controlled by the EGFR. Anticancer Res. 2004;24:3881–3886. [PubMed] [Google Scholar]
- 13.Proietti CJ, Rosemblit C, Beguelin W, Rivas MA, Diaz Flaque MC, Charreau EH, Schillaci R, Elizalde PV. Activation of Stat3 by heregulin/ErbB-2 through the co-option of progesterone receptor signaling drives breast cancer growth. Mol Cell Biol. 2009;29:1249–1265. doi: 10.1128/MCB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedar D, Baker CH, Killion JJ, Dinney CP, Fidler IJ. Blockade of the epidermal growth factor receptor signaling inhibits angiogenesis leading to regression of human renal cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 2002;8:3592–3600. [PubMed] [Google Scholar]
- 15.Huang M, Page C, Reynolds RK, Lin J. Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000;79:67–73. doi: 10.1006/gyno.2000.5931. [DOI] [PubMed] [Google Scholar]
- 16.Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, Deshpande A, Menon M. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Skacel M, Skilton B, Pettay JD, Tubbs RR. Tissue microarrays: a powerful tool for high-throughput analysis of clinical specimens: a review of the method with validation data. Appl Immunohistochem Mol Morphol. 2002;10:1–6. doi: 10.1097/00129039-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, Gelmann EP, Nevalainen MT. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 2005;11:5863–5868. doi: 10.1158/1078-0432.CCR-05-0562. [DOI] [PubMed] [Google Scholar]
- 20.Seethala RR, Gooding WE, Handler PN, Collins B, Zhang Q, Siegfried JM, Grandis JR. Immunohistochemical analysis of phosphotyrosine signal transducer and activator of transcription 3 and epidermal growth factor receptor autocrine signaling pathways in head and neck cancers and metastatic lymph nodes. Clin Cancer Res. 2008;14:1303–1309. doi: 10.1158/1078-0432.CCR-07-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Meier C, Hoeller S, Bourgau C, Hirschmann P, Schwaller J, Went P, Pileri SA, Reiter A, Dirnhofer S, Tzankov A. Recurrent numerical aberrations of JAK2 and deregulation of the JAK2-STAT cascade in lymphomas. Mod Pathol. 2009 doi: 10.1038/modpathol.2008.207. [DOI] [PubMed] [Google Scholar]
- 23.Song C, Jun SY, Hong JH, Ahn H. Transforming growth factor-beta downregulates interleukin-2-induced phosphorylation of signal transducer and activator of transcription 5 in human renal cell carcinoma. J Cancer Res Clin Oncol. 2007;133:487–492. doi: 10.1007/s00432-007-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TP., Jr Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]