Abstract
Background and aim: The aim of our study was to assess the coagulation factors as endothelial dysfunction markers and prospectively their association with thrombotic episodes in chronic hemodialysis patients.
Patients and Methods: Fifty-four randomly selected patients on chronic hemodialysis (HD), 34 men and 20 women were included in this study. Their mean age was 56 years and the mean hemodialysis duration was 53.0861.92 months. The variations of tissue factor pathway inhibitor (TFPI), thrombomodulin (TM) and von Willebrand factor (vWF) were studied. The above-mentioned parameters were measured before and after HD session. Low molecular weight heparin (tinzaparin) was administered to all patients during hemodialysis. The results were compared with those obtained from 20 healthy volunteer-controls, age and sex matched. After the initial assessment, all patients were followed for a period of 15 months.
Results: Two patients experienced one hemorrhagic event each, regarding the upper and/or the lower gastrointestinal tract. Twenty patients showed at least one thrombotic episode. Eleven patients presented fistula thrombosis, four angina pectoris incidents and five thrombosis of the lower limbs. The rest of the patients did not experience any clinical symptomatology that could be attributed to coagulation disorders. Parameter differences between patients and controls were statistically significant (p<0.005 for TFPI and p<0.001 for TM and vWF) and were improved after hemodialysis session. The age and the elevated levels of the vWF were found to be significantly different (p<0.03 and p<0.02 respectively) between the groups of patients who experienced or not thrombotic episodes.
Conclusions: Coagulation factors TFPI, TM, and vWF are increased in hemodialysis patients and the clinical disorders are mainly thrombotic episodes. The age of patients and the elevated levels of vWF are associated with the thrombotic incidents. Hemodialysis contributes in the improvement of these coagulation factors, which could be considered as biological risk markers of endothelial dysfunction in chronic HD patients.
Keywords: coagulation factors, endothelial dysfunction, hemodialysis, thrombotic episodes
Morbidity due to cardiovascular events is very common among hemodialysis (HD) patients, being responsible for up to 50% of the all cause mortality1. Incidents attributed purely to cardiovascular problems possess a very distinct place; on the other hand cerebral vascular accidents, mainly ischemic, are also common2. While pathology of hemorrhagic disorders in end stage renal disease (ESRD) patients under hemodialysis treatment is well known, in clinical practice thromboembolic events are more common.
The pathogenesis of bleeding and thrombotic disorders in uremic patients is multifactorial3. Disturbances are caused by counteraction between platelets and vascular wall4. Reduced platelets functional activity, resulting in reduced adhesion, aggregation5–7 and reduced capacity to form clot, despite their normal count is frequently observed8 as well as quantitative and functional disorders of von Willebrand Factor (vWF), which promote platelet concentration and adhesion in subendothelial collagen9. Various studies have shown increased levels of vWF antigen with decreased activity, expressed as co-factor ristocetin10, while other studies have reported increased functional activity of vWF11–15. Reduced number of red blood cells, having as a consequence anemia, which favors bleeding tendency in uremic patients16. Correction of anemia promotes contact between platelets and vascular wall. This observation was obtained from studies using erythropoietin as treatment of renal anemia17. Prolongation of bleeding time may be noticed in uremic patients who experience hemorrhagic episodes, due to frequent administration of antiplatelet agents (acetylsalicylic acid, dipyridamole, ticlopidine, clopidogrel), administered either for thrombosis prevention, or other therapeutic indications. Frequent use of heparin alone, or in combination with antiplatelet agents can also reduce platelet count, as well as platelet adhesion7,18.
Coagulation tests represent part of the everyday practice. However, the estimation of specific coagulation factors, which are mainly released from the vascular endothelium, as vWF, TM and TFPI, could give some information about the possible endothelial dysfunction and help to differentiate candidates with altered thrombotic state. vWF is a high molecular weight glycoprotein and has been proposed as a marker of endothelial dysfunction since many years19,20. It is mainly responsible for platelet adhesion in subendothelial space and for the initiation of thrombotic procedure. Thrombomodulin is also a high molecular glycoprotein and is produced by the endothelial cells. Its role in hemostasis is very important and is expressed through the activation and transformation of thrombin from prethrombotic to antithrombotic agent. Tissue factor pathway inhibitor is a protein produced mainly by the endothelium and is used as a marker of endothelial function. Tissue factor (TF) activates clot formation by forming a complex with factor VIIa (TF/FVIIa), thus activating factor X. Tissue factor pathway inhibitor inhibits directly factor Xa and indirectly its complex TF/FVIIa, preventing the clot formation.
The above mentioned factors have not been studied sufficiently in patients with ESRD undergoing hemodialysis treatment, especially regarding their contribution to coagulation disorders and endothelial dysfunction.
Patients and Methods
Fifty-four randomly selected HD patients were included in the study. Thirty-four were men and twenty women, with a mean age of 55.94±14.58 and 56.22±14.59 years respectively. They had been on HD treatment for a mean period of 53.08±61.92 months. Primary disease was glomerulonephritis in 16(29.6%) of the patients, diabetic nephropathy in 7(13%), cystic disease in 8(14.8%), chronic interstitial nephritis in 8(14.8%), nephrosclerosis in 3(5.5%) and unknown in 12(22.3%) (Table 1).
Table 1: Patient characteristics (mean±SD).
Nr: number
Regarding the treatment with antiplatelet agents, 32 patients (59.2%) were given ticlopidine, 3 patients (5.6%) salicylates, 2 patients (3.7%) dipyridamole, and 4 patients (7.4%) a combination of salicylates and ticlopidine. The rest of the patients were not receiving any antithrombotic agent. None of the participants experienced any clinical disorder due to thrombosis or bleeding for at least one week before the initiation of the study.
All patients were on HD or hemodiafiltration treatment for 3 times a week, for a duration of 3 to 4 hours with biocompatible high flux or low flux membranes. During HD session, all participants were administered low molecular weight heparin.
Incidences of fistula thrombosis, ischemic stroke, coronary ischemic and thrombotic episodes of the lower limbs were recorded during the 15-month follow-up. The estimation of coagulation factors was performed in whole blood samples taken from all patients before and after HD session. Tubes used, contained sodium citrate, as anticoagulant, diluted in buffer solution (3.8%, Owren Koller Buffer). The first sample was drawn prior to heparin administration. Measurements were performed within the next five minutes after centrifugation at 4000 rpm for 20 minutes. For the assessment of TM and TFPI, imunoenzymatic method (ELISA) was used, while for vWF a functional method using ristocetin as a co-factor of platelet aggregation. Titration was made using plasma samples with known concentrations of vWF.
The results were compared to those of 20 healthy controls matched for age and sex. Comparisons were made using the paired samples t-test method, while x-square test was used to assess differences regarding co-morbid conditions between groups.
Results
Two patients presented hemorrhagic episodes during the 15-month follow-up period. Twenty patients (37%) experienceed one or more thrombotic episodes, while in the remaining 32 patients (59.3%) no thrombotic or bleeding event was recorded. Thrombotic incidents regarded: fistula thrombosis in ten patients, myocardial infarction in six, deep vein thrombosis in two and lower limb ischemia in two. Fourteen of the 22 patients who presented coagulation disorders, were on antiplatelet agents. The patient who experienced bleeding of the upper digestive tract was receiving combination of salicylate and ticlopidine, while the patient with bleeding of the lower digestive tract was on ticlopidine. vWF levels and age were found to be significantly higher in the patients who experienced thrombotic events in comparison to the patients who did not (Table 2). Other factors that might have influenced the incidence of thrombotic events were taken into account. None of the patients suffered from severe anemia or active infection during the study period. HD practice and efficiency was similar between patients whe experienced (Group 1) or did not (Group 2) thromboembolic episodes. Additionally the incidence of cardiovascular disease and diabetes mellitus was not found to differ significantly between groups during the 15-month follow up.
Table 2: Parameter levels before and after HD session, compared with control group.
vWF: Von Willebrand Factor; TM: Thrombomodulin; TFPI: Tissue factor pathway inhibitor
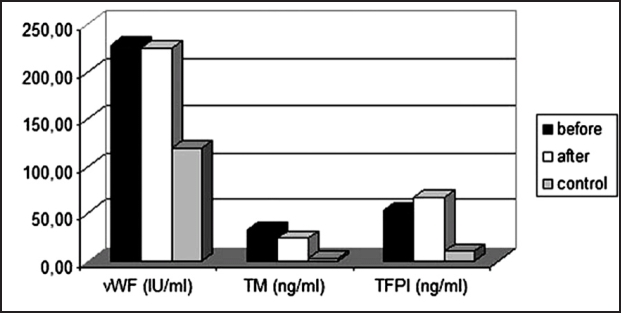
Coagulation factor levels are shown in Table 3 and Figure 1. vWF was not found different after HD, but it was significantly increased, compared to controls (p<0.005). TM was found to be significantly reduced after HD (p<0.05), but it was significantly higher than in controls (p<0.01). TFPI was found to be significantly increased (p<0.01) after HD session as well as in comparison to controls (p<0.005).
Table 3: The effect of age, HD duration and coagulation factors level in HD patients who experienced (Group 1) or did not (Group 2) thromboembolic episodes during the 15-month follow-up.
vWF: Von Willebrand Factor; TM: Thrombomodulin; TFPI: Tissue factor pathway inhibitor
Figure 1: Histogram showing the levels vWF, TM and TFPI before and after HD in comparison to control group.
TFPI levels in diabetic patients before HD (57.55±18.46 ng/ml) did not differ significantly from those in non-diabetic patients (54.05±14.97 ng/ml). After HD session, values of TFPI were both elevated (72.66±9.974 ng/ml vs 66.90 ±12.17ng/ml respectively), but also the difference between those was not significant.
Coagulation parameters were not found to be correlated with other risk factors such as hypertension, age, smoking and HD duration. No significant differences were observed among patients who received antiplatelet treatment (n=32) and those who did not (n=22): vWF 208.37±85.277 IU/ml vs. 265.27±129.592 UI/ml, TM 33.36±19.979 ng/ml vs. 33.27±26.929 ng/ml, TFPI 51.95±15.397 ng/ml vs 59.50 ±14.448ng/ml.
Discussion
ESRD is accompanied by thrombotic and hemorrhagic disorders. Additionally elevated levels of prethrombotic and antithrombotic factors as, vWF, TM, TF and TFPI have been observed in ESRD patients receiving HD treatment. In the present study, both vWF and TM levels were ESRD is accompanied by thrombotic and hemorrhagic disorders. Additionally elevated levels of prethrombotic and antithrombotic factors as, vWF, TM, TF and TFPI have been observed in ESRD patients receiving HD treatment. In the present study, both vWF and TM levels were
Despite any observed improvement in platelet function after the initiation of chronic HD treatment in uremic patients, the problem persists and will only show full remission three months after a successful renal transplantation21. Even though dialysis treatment (HD, CAPD) improves hemostatic disorders observed in uremic patients, thrombotic episodes regarding fistula, coronary or central nervous system vessels, are common. It is suggested that HD patients might undergo intermittent platelet activation22, related to dialysis membrane bio-incompatibility23–25.
Numerous coagulation and fibrinolytic disorders can appear in HD patients. Antithrombin III low levels26,27, in combination with reduced anticoagulant activity of protein S, contribute to the developement of a hypercoagulable state in uremic patients28,29. On the other hand, endothelial dysfunction, usually shown in ESRD, can alter the production of molecules with prethrombotic properties, as vWF, or molecules with anticoagulant-antithrombotic properties, as TM and TFPI. In our study we showed that all the above mentioned factors associated with functional endothelial integrity, were increased in the uremic patients in comparison to controls.
The results of a recent study concerning the concentrations of TFPI, vWF and TM in CAPD and Chronic Renal Failure (CRF) patients showed also that these factors were significantly higher in the above mentioned patients when compared to the healthy volunteers30. It is known that inflammation is common among Chronic Renal Disease (CRD) patients and accumulating evidence indicates that chronic inflammation is a major contributor to morbidity and mortality in this population31–33. Plasma levels of vWF and TM were both found to be significantly increased in patients with sepsis34. In the present study inflammatory markers were not measured. However the increased plasma levels of vWF and TM on the background of end stage of CRD shows that probably the chronic inflammation of HD patients is associated with the development of endothelial dysfunction and consequently coagulation disorders.
During the 15-month follow-up period of our patients, only two bleeding events were recorded, while the thrombotic episodes were more than twenty. From this study, it seems that in uremic patients, the thrombotic episodes are more clinically relevant than the bleeding complications.
Elevated levels of vWF found in our HD patients, are in agreement with the results reported by other investigators, who correlated the endothelial dysfunction markers with atherogenesis35. Borawski et al36 studied clotting factors in sixty three HD patients and found that TM and TF levels were higher than in healthy volunteers. Additionally, they found that the two molecules were significantly related, while the elevated vWF antigen plasma levels were only related to TF. The above-mentioned clotting factors, which are elevated in HD patients, might represent risk factors associated to vascular endothelial dysfunction. Elevated levels of vWF, in combination with other independent risk factors, as hypertension, diabetes, age, smoking, HD duration and type of heparin used, seem to contribute in the appearance of thrombotic events37. The age and the elevated levels of vWF were found to be the only factors related to the thrombotic episodes, shown in 20 patients of this study.
Elevated levels of TM before HD could possibly be attributed to the counterbalance effort of the clotting mechanism, in order to avoid thrombosis. Reduction of the levels of TM after the end of HD session is probably related to the delayed activation of fibrinolysis through the TAFI/TAFIa pathway. Inoue et al38 in a recent study concerning clotting disorders in HD patients, found TM and TFPI values to be significantly elevated both before and after HD session, in comparison with the healthy group. An important finding of the above mentioned study was that TF plasma levels were higher in HD patients who experienced thrombotic complications, than in those who did not. Continuous extracorporeal circulation seems to lead to an increased need for TFPI production due to platelet contact with filter membrane, while it is known that heparin administration leads to increased TFPI production5. Elevated levels of TFPI before and after HD are possibly related to other factors, as the continuous endothelial injury from frequent fistula paracentesis, the presence of temporary or permanent central venous catheters, the use of grafts, or atherosclerosis itself. Elevated TFPI levels found in diabetic patients of our study are in agreement with the findings of Lurs et al39.
Coagulation factors TFPI, TM, and vWF are increased in HD patients and clinical disorders are mainly the thrombotic episodes. The age of patients and the elevated levels of vWF are associated to the thrombotic incidents. HD contributes substantially in the improvement of these coagulation factors, which could be considered as biological risk factors for endothelial dysfunction in chronic HD pts.
References
- 1.Εditorial. Controlling the epidemic of cardiovascular renal disease: What do we know? What do we need to learn? Where do we go from here? Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 2.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stoke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 3.DiMinno G, Martinez J, McKean ML, DeLaRosa J, Burke JF, Murphy S. Platelet dysfunction in uremia: Multifaceted defect partially corrected by dialysis. Am J Med. 1985;79:552–559. doi: 10.1016/0002-9343(85)90051-8. [DOI] [PubMed] [Google Scholar]
- 4.Gordge MP, Neild GH. Platelet function in uremia. Platelets. 1991;2:115–123. doi: 10.3109/09537109109006021. [DOI] [PubMed] [Google Scholar]
- 5.Rabiner SF. Uremic bleeding. In: Spaet TH, editor. Progress in Hemostasis and Thrombosis. New York: Grune & Stratton; 1972. pp. 233–250. [PubMed] [Google Scholar]
- 6.Remuzzi G, Benigni A, Dodesini P, et al. Platelet function in patients on maintenance hemodialysis: Depressed or enhanced? Clin Nephrol. 1982;17:60–63. [PubMed] [Google Scholar]
- 7.Zicker MB. Biological aspects of heparin action: Heparin and platelet function. Fed Prod. 1977;36:47–49. [PubMed] [Google Scholar]
- 8.Lindsay RM, Moorthy AV, Koens F, Linton AL. Platelet function in dialyzed and non-dialyzed patients with chronic renal failure. Clin Nephrol. 1975;4:50–57. [PubMed] [Google Scholar]
- 9.Ruzzeri ZM, Zimmerman TS. Von Willebrand factor and von Willebrand disease. Blood. 1987;70:895–904. [PubMed] [Google Scholar]
- 10.Kazatchkine M, Sultan Y, Caen JP, Bariety J. Bleeding in renal failure: A possible cause. BMJ. 1976;2:612–615. doi: 10.1136/bmj.2.6036.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nermann RP, Marshall LR, Hurst PE. Bleeding in renal failure: A possible cause. BMJ. 1977;1:1601–1602. doi: 10.1136/bmj.1.6076.1601-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remuzzi G, Livio M, Roncaglioni MC, Mecca G, Donati MB, de Gaetano G. Bleeding in renal failure: Is von Willebrand factor implicated? BMJ. 1977;2:359–361. doi: 10.1136/bmj.2.6083.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrel RP, Jr, Hultin MB, Coller BS. Increased factor VIII/ von Willebrand factor antigen and von Willebrand factor activity in renal failure. Am J Med. 1979;66:226–228. doi: 10.1016/0002-9343(79)90531-x. [DOI] [PubMed] [Google Scholar]
- 14.Janson PA, Jubelirer SJ, Weinstein MJ, Deykin D. Treatment of the bleeding tendency in uremia with cryoprecipitate. N Engl J Med. 1980;303:1318–1322. doi: 10.1056/NEJM198012043032302. [DOI] [PubMed] [Google Scholar]
- 15.Mannucci PM, Remuzzi G, Pusineri F, Lombardi R, Valsecchi C, Mecca G, et al. Deamino-8-D-arginine vassopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8–12. doi: 10.1056/NEJM198301063080102. [DOI] [PubMed] [Google Scholar]
- 16.Turitto VT, Weiss HJ. Red blood cells: Their dual role in thrombus formation. Science. 1980;207:541–543. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 17.Viganò G, Benigni A, Mendogni D, Mingardi G, Mecca G, Remuzzi G. Recombinant human erythropoietin to correct uremic bleeding. Am J Kidney Dis. 1991;18:44–49. doi: 10.1016/s0272-6386(12)80289-7. [DOI] [PubMed] [Google Scholar]
- 18.Del Greco F, Soper WS, Krumlovsky FA, et al. Thrombosis of vascular access for hemodialysis. In: Remuzzi G, Rossi EC, Krumlovsky FA, et al., editors. Haemostasis and the kidney. London: Butterw; 1989. pp. 303–308. [Google Scholar]
- 19.Blann A, Tarbener D. A reliable cell marker of endothelial cell dysfunction: does it exist? Brit J Haematol. 1995;90:244–248. doi: 10.1111/j.1365-2141.1995.tb05143.x. [DOI] [PubMed] [Google Scholar]
- 20.Gaussem P, Levy C, Verny M, Angls-Cano E. Endothelial cell markers (vWF, t-PA and PAI-1) in the elderly. Thromb Haemost. 1994;72:164–165. [PubMed] [Google Scholar]
- 21.Venkateswara K, Smith EJ, Alexander JW, Fidler JP, Pemmaraju SR, Pollack VE. Thromboembolic disease in renal allograft recipients. What is its clinical significance? Arch Surg. 1976;111:1086–1092. doi: 10.1001/archsurg.1976.01360280044007. [DOI] [PubMed] [Google Scholar]
- 22.Himmelfarb J, Holbrook D, McMonagle E, Ault K. Increased reticulated platelets in dialysis patients. Kidney Int. 1997;51:834–839. doi: 10.1038/ki.1997.117. [DOI] [PubMed] [Google Scholar]
- 23.Deguchi N, Ohigashi T, Tazaki H, Handa M, Ikeda Y. Hemodialysis and platelet activation. Nephrol Dial Transplant. 1991;2:40–42. [PubMed] [Google Scholar]
- 24.Matsuda T, Takemoto Y, Kishimoto T, Maekawa M, Akutsu T. Mechanistic aspects of cellular interactions with artificial dialyzer membrane surfaces. Biomat Art Cells rt Org. 1990;18:579–584. doi: 10.3109/10731199009117324. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt GW, Moake JL, Rudy CK, Vicks SL, Hamburger RJ. Alterations in hemostatic parameters during hemodialysis with dialyzers of different membrane composition and flow design: Platelet activation and factor VIII-related von Willebrand factor during hemodialysis. Am J Med. 1987;83:411–418. doi: 10.1016/0002-9343(87)90749-2. [DOI] [PubMed] [Google Scholar]
- 26.Tomura S, Nakamura Y, Deguchi F, Ando R, Chida Y, Marumo F. Coagulation and fibrinolysis in patients with chronic renal failure undergoing conservative treatment. Thromb Res. 1991;64:81–90. doi: 10.1016/0049-3848(91)90207-d. [DOI] [PubMed] [Google Scholar]
- 27.Vaziri ND, Gonzales EC, Wang J, Said S. Blood coagulation, fibrinolytic, and inhibitory proteins in end-stage renal disease: Effect of hemodialysis. Am J Kidney Dis. 1994;23:828–835. doi: 10.1016/s0272-6386(12)80136-3. [DOI] [PubMed] [Google Scholar]
- 28.Sagripanti A, Cupisti A, Baicchi U, Ferdeghini M, Morelli E, Barsotti G. Plasma parameters of the prothrombotic state in chronic uremia. Nephron. 1993;63:273–278. doi: 10.1159/000187209. [DOI] [PubMed] [Google Scholar]
- 29.Reber G, Stoermann C, de Moerloose P, Ruedin P, Leski M. Hemostatic disturbances by two hollow-fiber hemodialysis membranes. Int J Artif Organs. 1992;15:269–276. [PubMed] [Google Scholar]
- 30.Malyszko J, Malyszko SJ, Mysliwiec M. Endothelial cell injury markers in Chronic Renal Failure on conservative treatment and continuous ambulatory peritoneal dialysis. Kidney & Blood Pressure Research. 2004;27:71–77. doi: 10.1159/000075810. [DOI] [PubMed] [Google Scholar]
- 31.den Elzen WP, van Manen JG, Boeschoten EW, Krediet RT, Dekker FW. The effect of single and repeatedly high concentrations of C-reactive protein on cardiovascular and non- cardiovascular mortality in patients starting with dialysis. Nephrol Dial Transplant. 2006;21:1558–1595. doi: 10.1093/ndt/gfk092. [DOI] [PubMed] [Google Scholar]
- 32.Racki S, Zaputovic L, Mavric Z, Vujicic B, Dvornik S. C-reactive protein is a strong predictor of mortality in hemodialysis patients. Ren Fail. 2006;28:427–433. doi: 10.1080/08860220600683581. [DOI] [PubMed] [Google Scholar]
- 33.Chawla SL, Krishnan M. Causes and consequences of inflammation on anemia management in hemodialysis patients. Hemodialysis International. 2009;13:222–234. doi: 10.1111/j.1542-4758.2009.00352.x. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Aquirre Y, Paramo JA. Endothelial cell and hemostatic activation in relation to cytokines in patients with sepsis. Thromb Res. 1999;94:95–101. doi: 10.1016/s0049-3848(98)00200-x. [DOI] [PubMed] [Google Scholar]
- 35.Borawski J, Naumnik B, Pawlak K, Mysliwiec M. Endothelial dysfunction marker von Willebrand factor antigen in haemodialysis patients: associations with pre-dialysis blood pressure and the acute phase response. Nephrol Dial Transplant. 2001;16:1442–1447. doi: 10.1093/ndt/16.7.1442. [DOI] [PubMed] [Google Scholar]
- 36.Borawski J, Naumnik B, Mysliwviec M. Tissue factor and thrombomodulin in hemodialysis patients: associations with endothelial injury, liver disease, and erythropoietin therapy. Clin Appl Thromb Hemost. 2002;8:359–367. doi: 10.1177/107602960200800408. [DOI] [PubMed] [Google Scholar]
- 37.Malyszko J, Mysliwviec M. Effects of different heparins on thrombin-activatable fibrinolysis Inhibitor-TAFI in hemodialyzed patients. J Thromb Haemost. 2003;S1 abstract no P0800. [Google Scholar]
- 38.Inoue A, Wada H, Takagi M, et al. Hemostatic abnormalities in patients with thrombotic complications on maintenance hemodialysis. Clin Appl Thromb Hemost. 2000;6:100–103. doi: 10.1177/107602960000600210. [DOI] [PubMed] [Google Scholar]
- 39.Leurs PB, van Oerle R, Hamulyak K, Wolffenbuttel BH. Tissue factor pathway inhibitor activity in patients with IDDM. Diabetes. 1995;44:80–84. doi: 10.2337/diab.44.1.80. [DOI] [PubMed] [Google Scholar]