Abstract
Background
Existing mathematical models for scrapie dynamics in sheep populations assume that the PrP gene is only associated with scrapie susceptibility and with no other fitness related traits. This assumption contrasts recent findings of PrP gene associations with post-natal lamb survival in scrapie free Scottish Blackface populations. Lambs with scrapie resistant genotypes were found to have significantly lower survival rates than those with susceptible genotypes. The present study aimed to investigate how these conflicting PrP gene associations may affect the dynamic patterns of PrP haplotype frequencies and disease prevalence.
Methodology/Principal Findings
A deterministic mathematical model was developed to explore how the associations between PrP genotype and both scrapie susceptibility and postnatal lamb mortality affect the prevalence of scrapie and the associated change in PrP gene frequencies in a closed flock of sheep. The model incorporates empirical evidence on epidemiological and biological characteristics of scrapie and on mortality rates induced by causes other than scrapie. The model results indicate that unfavorable associations of the scrapie resistant PrP haplotypes with post-natal lamb mortality, if sufficiently strong, can increase scrapie prevalence during an epidemic, and result in scrapie persisting in the population. The range of model parameters, for which such effects were observed, is realistic but relatively narrow.
Conclusions/Significance
The results of the present model suggest that for most parameter combinations an unfavourable association between PrP genotype and post-natal lamb mortality does not greatly alter the dynamics of scrapie and, hence, would not have an adverse impact on a breeding programme. There were, however, a range of scenarios, narrow, but realistic, in which such an unfavourable association resulted in an increased prevalence and in the persistence of infection. Consequently, associations between PrP genotypes and fitness traits should be taken into account when designing future models and breeding programmes.
Introduction
Incidences of scrapie, a fatal transmissible spongiform encephalopathy (TSE) of sheep and goats, have been reported in European countries for several centuries [1], but progress on the control of this disease was long inhibited by the restricted understanding of the causes of infection and modes of transmission. The discovery that polymorphisms at codons 136, 154 and 171 of the prion protein (PrP) gene determine susceptibility to classical scrapie [2]–[4], constituted a major break-through for scrapie control. Since then national breeding programmes aimed to reduce and eventually eradicate small ruminant TSEs have been established in several European countries.
The scrapie eradication policies were strongly influenced by the predictions of mathematical models, which incorporated emerging information on host genetics and scrapie epidemiology and predicted the impact of selection on changes in PrP gene frequencies and scrapie prevalence over time (e.g. [5], [6]). Although the numerous mathematical models and policies vary widely in their approach and underlying assumptions, they unanimously build upon the assumption that polymorphisms of the PrP gene are associated only with scrapie susceptibility and not with any other trait such as fitness or performance. Under this assumption, models predict that scrapie will eventually disappear from a closed population as a result of natural selection (e.g. [7], [8]). The apparent conflict of these predictions with the fact that scrapie has been persistent over centuries has been explained by the exceptionally long time scales inherent in scrapie epidemiology [9].
Existing scrapie models may be challenged by recent findings in scrapie free flocks of Scottish Blackface sheep, for which an association of the PrP genotype, defined by polymorphisms at codons 154 and 171, with postnatal lamb survival has been identified [10]. The presence of the ARQ (scrapie susceptible) haplotype was generally associated with lower postnatal lamb mortality, while the presence of two ARR (scrapie resistant) haplotypes was mostly associated with increased lamb mortality rate. An association of susceptible haplotypes to lower mortality rates, may influence not only the changes of haplotype frequencies over time, but also the severity and duration of scrapie epidemics. In particular, it may have consequences on the long-term persistence of scrapie.
In this study a deterministic partial differential equations model was developed to explore how the associations between PrP genotype and both scrapie susceptibility and postnatal lamb mortality affect the prevalence of scrapie and the associated change in PrP gene frequencies in a closed flock of sheep. It will be demonstrated that this association can have strong implications for the persistence of scrapie.
Materials and Methods
Assumptions
Sheep are characterised by their scrapie infection status (infected or not infected), age and PrP genotype. It is assumed that the PrP gene is associated with susceptibility to scrapie and with lamb mortality from birth to 180 days of age caused by events other than scrapie (hereafter denoted non-scrapie lamb mortality). It is further assumed that the association between PrP genotype and lamb mortality is due to pleiotropy rather than linkage, implying that the associations remains constant over successive generations. For simplicity, it is further assumed that there are only two haplotype variants at the PrP locus: SL (associated with scrapie susceptibility and low non-scrapie lamb mortality) and RH (associated with scrapie resistance and high non-scrapie lamb mortality). Further, given the evidence that the risk of infection is greatest during the perinatal period and decreases with age [11], [12], it is assumed that scrapie infection occurs exclusively at or close to birth and that scrapie infected animals remain in the population until clinical signs appear. After the onset of clinical disease animals no longer remain in the flock (through death or removal). The model represents one closed flock with an initial frequency of the SL haplotype of p0. It is assumed that the flock size remains constant, i.e. animals that disappear from the population, whether due to scrapie or other causes, are replaced immediately by newborn animals.
Population dynamics
Let Ni(a, t) and Xi(a, t) denote, respectively, the total number of animals and the number of infected animals of genotype i, of age a at time t. The change of N and X with respect to age and time is described by:
where mi(a) is the non-scrapie mortality rate for genotype i (SL/SL, SL/RH or RH/RH) at age a, and hi(a) is the hazard function for the log-normal age-at-onset of scrapie distribution with genotype specific parameters μi and σi [6], representing death due to scrapie.
The PrP genotype specific non-scrapie mortality rate is:
where m(a) is the age-dependent baseline non-scrapie mortality rate, which is equal for all PrP genotypes, and ε
i is the factor by which mortality increases for genotype i for ages below a certain threshold aL. The baseline rate m(a) was determined using a Weibull distributed survivorship curve for the average mortality rate of the three PrP genotypes, i.e. 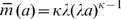 [7].
[7].
The prevalence of infection at time t, P(t), is then calculated as the proportion of infected animals in the population, i.e.
 |
where a max refers to the maximum age in the population, which is defined by the survivorship curve described above.
Boundary conditions and initial conditions (a = 0 and t = 0)
The number of infected newborns of genotype i is calculated following the approach of Gubbins and Roden [6], relating the proportion of newly infecteds to the current population prevalence and assuming that all animals of a given genotype experience the same force of infection, yielding
| (1) |
where βi is a genotype specific transmission parameter. The number of newborns of genotype i at time t is given by
where νi(t) denotes the proportion of genotype i animals in the newly born population of size N(0,t) at time t. N(0,t) is calculated such that the overall population size remains steady by setting the birth rate equal to the total death rate and νi(t) is calculated from the haplotype frequency of the breeding parents, which comprise all animals within the fertile age range [aF1, aF2]. Assuming random mating and constant prolificacy across the fertile period, the frequencies of SL/SL, SL/RH and RH/RH are p 2, 2p(1−p) and (1−p)2, respectively, where p is the frequency of haplotype SL in the breeding population at time t.
The initial genotype and age distribution Ni(a,0) is determined by the initial frequency of the SL haplotype p0 and the Weibull-distributed survivorship curve. Scrapie is introduced by Xi(a0,0) infected individuals of genotype i with age a0.
Parameter values and sensitivity analysis
Table 1 lists the model parameters and their value ranges, together with the corresponding empirical estimates, considered in this study. Baseline values for the genotype specific model parameters μi, σi, βi and εi, were derived from existing empirical estimates for genotypes ARQ/ARQ, ARQ/ARR and ARR/ARR, which represent respectively genotypes SL/SL, SL/RH and RH/RH in our model. Based on the empirical evidence and to facilitate the understanding of the key factors behind a specific model behaviour, the transmission parameter β 2 and the hazard rate parameters (μ 2, σ 2) corresponding to the heterozygote were initially set to zero, implying that only the homozygote SL/SL is susceptible to scrapie. Likewise, the non-scrapie lamb mortality coefficient ε 2 was initially set to zero implying that only the homozygote RH/RH has increased lamb mortality compared to the other PrP genotypes.
Table 1. Values for model parameters and empirical estimates.
| Parameter | Description | Value (range) used(a) | Source/empirical estimate(b) |
| βi, i = 1,2,3 | Scrapie transmission parameter for genotype i | Gubbins & Roden [6]: | |
| β 1 = 2.0–10.0 | β 1 = 2.8–4.93 | ||
| β 2 = 0(c) | β 2 = 0.02–0.04 | ||
| β 3 = 0 | β 3 = 0 | ||
| εi, i = 1,2,3 | Discrepancy in non-scrapie lamb mortality rate for genotype i from average age specific mortality rate | Sawalha et al. [10] | |
| ε 1 = 0 | ε 1 = 0.0065 | ||
| ε 2 = 0.0(c) | ε 2 = 0.0 | ||
| ε 3 = 0.0–0.1 | ε 3 = 0.0225 | ||
| (μi, σi) | Mean and standard deviation for the age-at-onset distribution hi(a) | Gubbins & Roden [6] | |
| (μ1, σ1) = (1.20, 0.43) | (μ 1, σ 1) = (1.20, 0.43) | ||
| (μ2, σ2) = (0,0)(c) | (μ 2, σ 2) = (1.54, 0.38) | ||
| (μ3, σ3) = (0,0) | (μ 3, σ 3) = (0,0) | ||
| p 0 | Initial frequency of the susceptible haplotype SL | p 0 = 0.0–1.0 | Arbitrary |
| (κ, λ) | Parameters for Weibull-survivorship function | κ = 2, λ = 0.2216(d) | Stringer et al. [7] |
| aL | Max. age with genotype dependent mortality rate | aL = 180 days | Sawalha et al. [6] |
| [aF1, aF2] | Fertile age range | aF1 = 6 months, aF2 = 6 years | Lewis & Simm [13] |
| a0 | Age at first infection | a0 = 0.5–4 years | Arbitrary |
| X 1(a 0, 0)/N 1(a 0, 0) | Proportion of initially infected animals | 0.0001 | Arbitrary |
Subscripts i = 1,2,3 refer to the genotypes SL/SL, SL/RH and RH/RH, respectively.
Subscripts i = 1,2,3 refer to the genotypes ARQ/ARQ, ARQ/ARR and ARR/ARR, respectively.
The values in the table refer to the results presented in this paper. Model results were also generated for (μ 2, σ 2) = (1.54, 0.38), β 2 = 2.0–10.0 (β 2≤β 1), ε 2 = 0.0–1.0 (ε2≤ε3).
Corresponds to an average life expectancy of 4 years.
Empirical estimates exist also for the parameters specifying the average non-scrapie mortality rate (κ, λ), the fertile age range [aF1, aF2], and the maximum lamb age aL for which different PrP genotypes have different mortality rates (Table 1). The remaining model parameters (p0 and a0) were assigned arbitrary values.
The influence of each model parameter was investigated using sensitivity analysis, in which model predictions were obtained by varying either one parameter at a time or a combination of parameters. Parameter ranges used in the simulations were either 95% upper and lower confidence limits of the estimates obtained in the original studies (Table 1) if those were available, or parameter ranges that were biologically realistic. Identified critical parameters that significantly altered the trends of scrapie prevalence and haplotype frequencies were β i, ε i, and p0. The duration of the incubation period, as specified by the hazard function parameters (μ i, σ i) was also found to have a significant influence on the model predictions. However, similar patterns for changes in scrapie prevalence and haplotype frequencies as those observed for different values μ i and σ i were obtained by modifying the transmission rates. Therefore, the hazard function was kept fixed, and the results presented show the model behaviour for a biologically realistic range of values of the parameters βi, εi and p0 (Table 1).
Prevalence threshold
Since the model is continuous, the predicted scrapie prevalence can take values that, although numerically different to zero, correspond to less than one infected individual. For the results presented here it was assumed that outbreaks resulting in prevalence values corresponding to less than one infected sheep would result in a stochastic fade-out of the disease. The population size simulated was N = 100,000 and therefore the prevalence threshold below which scrapie was considered eradicated was 0.001%.
Results
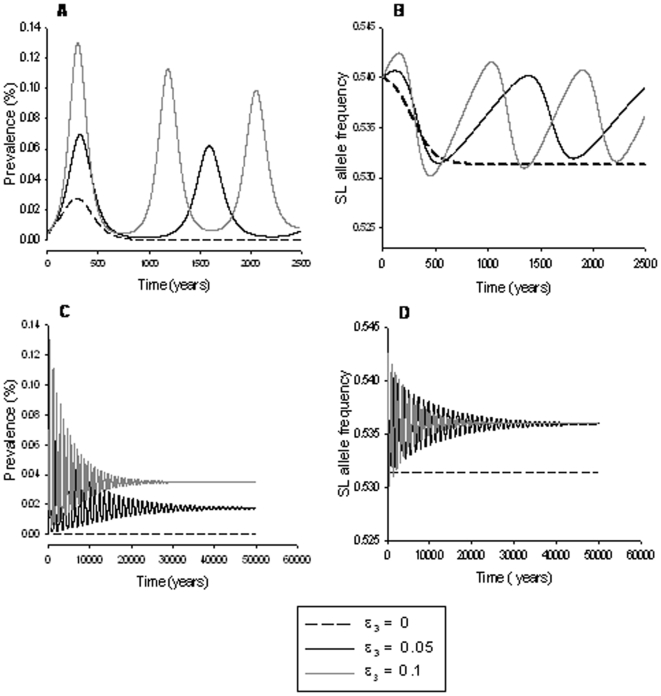
Assuming first that the PrP genotype is only associated with scrapie susceptibility (i.e. ε i = 0 for all i), so that changes in PrP haplotype frequencies are exclusively caused by scrapie related mortality, our model predicts that scrapie eventually disappears from the population within a few decades or centuries (Figures 1a, 1c). The frequency of the susceptible haplotype declines towards a non-zero steady state value (Figure 1d). This behaviour was observed for all parameter combinations within the admissible range shown in Table 1. Inclusion of unfavourable associations between the scrapie resistant PrP haplotype and non-scrapie lamb mortality (i.e. ε 3>0) does not drastically alter this behaviour for the majority of model parameter values. The lower average life expectancy and prolificacy of scrapie susceptible genotypes remain the main drivers for frequency changes throughout the epidemic phase of the infection, and lead to the gradual decrease of susceptible genotypes until the disease can no longer be sustained. The additional boost in the replenishment of susceptible genotypes due to their lower mortality rates is too weak to prevent scrapie from gradually dying out.
Figure 1. Predicted scrapie prevalence and frequency of the susceptible SL haplotype for different values of the parameter ε3.
Top graphs show predictions for 2500 years, whereas the bottom graphs illustrate the behavior up to the endemic equilibrium. Other parameter choices are ε 1 = ε 2 = 0, p 0 = 0.54, β 1 = 4.93 and β 2 = β 3 = 0. For description of the parameters and other parameter values see table 1.
However, for some parameter values within the admissible range, the association of PrP genotype with non-scrapie lamb mortality causes scrapie to prevail in the population over much longer timescales (Figures 1, 2, 3). Whether scrapie is expected to persist depends mainly on the combination of the strength of association of PrP genotype with non-scrapie lamb mortality (ε i), the disease transmission rate (β i) and the initial proportion of susceptible haplotypes (p0). Sufficiently high values for ε 3 are a prerequisite for the long-term persistence of scrapie. Extensive search through the model parameter space produced only long-term scrapie persistence for ε 3≥0.01. It was also found that long-term persistence is only associated with combinations of p 0 and β 1 that correspond to weak prevalence levels at all times (generally less than 0.2% for 0≤ε 3≤0.1). Combinations of p 0 and β 1 resulting in more severe initial outbreaks also produce a rapid decline in the proportion of susceptible sheep to a level that is insufficient for sustaining the disease.
Figure 2. Predicted scrapie prevalence (left) and frequency of the SL haplotype (right) for different values of the parameter β 1.
Top graphs show predictions for 2500 years, whereas the bottom graphs illustrate the behavior up to the endemic equilibrium. Other parameter choices are ε 1 = ε 2 = 0, ε 3 = 0.05, p 0 = 0.54, and β 2 = β 3 = 0. For description of the parameters and other parameter values see table 1. Long-term persistence was only observed for 4.80≤β 1≤4.93.
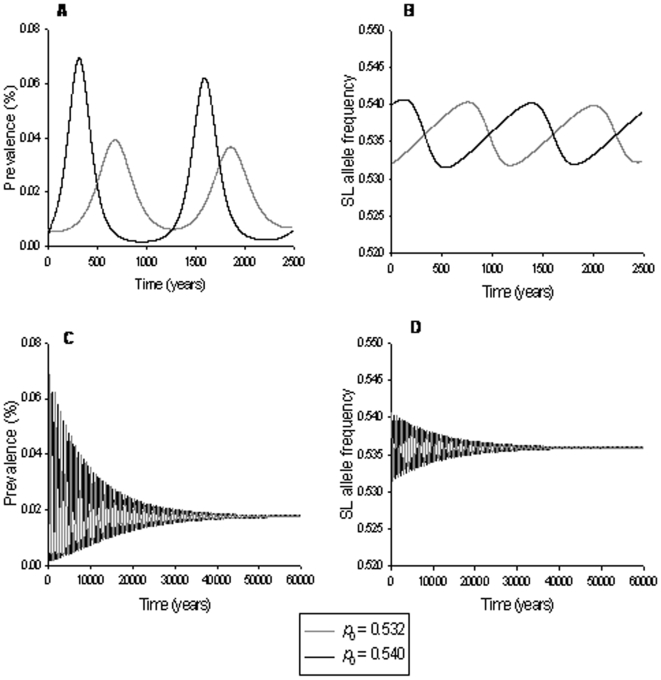
Figure 3. Predicted scrapie prevalence (left) and frequency of the SL haplotype (right) for different values of the parameter p 0.
Top graphs show predictions for 2500 years, whereas the bottom graphs illustrate the behavior up to the endemic equilibrium. Other parameter choices are ε 1 = ε 2 = 0, ε 3 = 0.05, β 1 = 4.93 and β 2 = β 3 = 0. For description of the parameters and other parameter values see table 1. Long-term persistence was only observed for 0.532≤p0≤0.540.
Table 2 shows, for given values of β 1 and ε 3, the range of p 0 that lead to persistent scrapie prevalence above the threshold 0.001%. When β 1 is low (i.e. 2. 0), scrapie persists only when p0 is relatively high (∼84.5%). In contrast, higher β 1 requires a lower p0 for preventing scrapie to disappear. The stronger the association of PrP genotype with non-scrapie lamb mortality (i.e. the higher ε 3) the wider the range of p 0 values corresponding to long-term persistence of the disease.
Table 2. Range of the initial frequency of the susceptible SL haplotype (p 0) corresponding to long-term persistence of scrapie for different values of the scrapie transmission parameter β 1 and the non-scrapie mortality rate coefficient ε 3.
| ε 3 (a) | range of p0 | |||
| β 1 = 2.00 | β 1 = 2.80(b) | β 1 = 4.93(b) | β 1 = 10.00 | |
| 0.01 | 0.845–0.845 | 0.712–0.712 | 0.535–0.536 | 0.376–0.377 |
| 0.02 | 0.844–0.845 | 0.710–0.714 | 0.533–0.539 | 0.375–0.379 |
| 0.05 | 0.843–0.846 | 0.709–0.716 | 0.532–0.540 | 0.373–0.381 |
| 0.08 | 0.842–0.847 | 0.708–0.717 | 0.531–0.542 | 0.372–0.383 |
| 0.10 | 0.842–0.848 | 0.707–0.719 | 0.530–0.544 | 0.371–0.384 |
As illustrated in Figures 1, 2 and 3, long-term persistence of scrapie is characterised by a damped oscillatory behaviour of both scrapie prevalence and SL haplotype frequency, which converge eventually to endemic equilibrium states. All parameter combinations that do not result in scrapie fading out immediately after the introduction of an infected sheep or after the first outbreak produce similar long-term persistence patterns.
Figures 1, 2 and 3 also depict the influence of the key parameters ε 3, β 1 and p 0, on the amplitudes and frequencies of the oscillations and on the equilibrium states. Stronger associations between PrP genotype and non-scrapie lamb mortality (i.e. higher values of ε 3) generate oscillations in both scrapie prevalence and SL haplotype frequency of higher amplitudes and higher frequency (Figures 1a, 1b). They also generate stronger damping and lead to higher predicted disease prevalence at the endemic equilibrium (Figure 1c). The steady state SL frequency corresponding to ε 3>0 is higher than that corresponding to ε 3 = 0, but is the same for different non-zero ε 3 values (Figure 1d).
The range of values for the transmission parameter β 1 leading to scrapie persistence for fixed values of the other model parameters is narrow. For example, as shown in Figure 2 for p 0 = 0.54 and ε 3 = 0.05, scrapie is only predicted to persist when 4.80≤β 1≤4.93. Values of β 1 outside this range result in scrapie fading out either immediately after the introduction of the disease (β 1<4.80) or in a too high mortality of susceptible genotypes to maintain a scrapie prevalence above the required threshold after the first outbreak (β 1>4.93). The persistence patterns of scrapie prevalence and SL frequency are also sensitive to changes in β 1. For example, decreasing the transmission rate may cause a delay in the initial outbreak (scrapie prevalence only starts to rise substantially after sufficient SL haplotypes are available in the population, Figures 2a, 2b) and result in a higher steady state SL haplotype frequency (Figure 2d). The steady state prevalence is however the same for different values of β 1 corresponding to long-term scrapie persistence (Figure 2c).
The amplitudes of the oscillations in scrapie prevalence and haplotype frequency were also found highly sensitive to changes in the initial SL frequency p 0 (Figure 3). Similar to the effect of high transmission parameter values β 1, higher proportions of scrapie susceptible SL haplotypes in the population lead to faster and more severe outbreaks with consecutively stronger decline in disease prevalence (Figure 3a). Slight increases in the proportion of susceptible haplotypes were found to rapidly cause the prevalence to fall below the assumed persistence threshold of 0.001% after the first scrapie outbreak. The long-term patterns of scrapie prevalence and SL haplotype frequency, including the corresponding equilibrium states were however found to be independent of p 0 (Figures 3c, 3d).
Discussion
In this study a model was developed to determine the influence of conflicting associations of the PrP genotype with scrapie susceptibility and lamb mortality on PrP haplotype frequencies and scrapie prevalence patterns. The potential impact of such associations on scrapie epidemiology and PrP haplotype frequencies had been drawn to attention in previous studies [9], [14], but was dismissed due to lack of empirical evidence. Recent findings of a positive association of the scrapie susceptible ARQ haplotype with post-natal lamb survival rate in scrapie free Scottish Blackface flocks [10] however suggest that the earlier speculations merit further consideration. Based on a quantitative genetics approach, the conflicting PrP genotype associations with respect to scrapie susceptibility and fitness imply that the scrapie susceptible haplotype will forever prevail in the population at high frequency (approx. 0.9, see Figure S1 in the supplementary material). These results may warrant speculations that scrapie is also likely to persist in the population without human intervention [10]. Quantitative predictions on the long-term persistence of scrapie require however also the consideration of epidemiological disease characteristics.
The model presented here captures relevant genetic and epidemiological aspects of scrapie and implements estimates derived from real data as baseline values for the model parameters. Results show that for the majority of model parameter combinations, associations of the PrP gene with lamb mortality in addition to scrapie susceptibility have little impact on the persistence patterns of scrapie. Only under very specific conditions, the associations between PrP genotype and lamb mortality may lead to scrapie persisting in sheep populations in the absence of human intervention. The range of model parameters leading to persistence of scrapie is narrow, but realistic. Prevalence patterns have similar characteristics to those observed in real populations, manifesting themselves in prolonged, but relatively mild recurring outbreaks, with a small percentage of sheep infected at any time. They eventually settle into an endemic equilibrium of low prevalence.
These results, although in line with empirical observations, contrast those of previous modelling studies that assume no PrP gene association with other traits besides scrapie. Using a genetic-epidemiological model that model that relates PrP genotypes only to scrapie susceptibility, Woolhouse et al. [8] predicted that scrapie will be eliminated within decades or centuries in closed flocks without human intervention. The same behaviour was observed in our model when PrP genotypes were assumed only to be related to scrapie susceptibility (i.e. setting ε3 to zero). However, our model shows that the predicted scrapie dynamics can be altered when considering conflicting associations of the PrP genotype with fitness traits other than scrapie susceptibility, even if they are weak. In particular, favourable associations of the PrP susceptible genotype with fitness can stimulate the replenishment of susceptible animals to a level that is sufficient to prevent scrapie from disappearing.
The conditions for long-term persistence of scrapie in our model are determined by specific combinations of the disease transmission parameter, the PrP genotype specific increase in non-scrapie mortality rate and the PrP haplotype frequencies at the onset of the outbreak (Table 2). Parameter combinations that cause scrapie outbreaks with peak prevalences exceeding approximately 0.2% in our model also wipe out the required proportion of susceptible animals for maintaining the disease without re-introduction of the infection. A more accurate representation of flock structure and transmission patterns would likely increase this threshold.
There are several reasons that explain why the range of model parameters leading to long-term persistence of scrapie is narrow. First, the transmission parameter in this model occurs in the exponent of an exponential function (equation 1), and model outputs are highly sensitive to changes of the exponent. Representing the transmission of scrapie in this way had the advantage that only one parameter (i.e. β) was needed to describe the currently still largely unknown transmission process. However, if disease incidence was assumed to depend linearly on disease transmission rates, as in standard SIR models, a lower sensitivity of the model results with respect to changes in β would be expected and similar prevalence patterns would be observed over a wider parameter range. Second, the threshold for scrapie prevalence corresponding to one infected individual implies that the range of model parameters corresponding to long-term persistence of scrapie in this model is partly dependent on the population size. The simulated population comprised 100,000 animals. A larger population with the same initial prevalence at the onset of infection would result in identical predictions for PrP haplotype frequencies and scrapie prevalence, but would also impose a lower prevalence threshold corresponding to one infected individual. Consequently the range of model parameters producing prevalence consistently above the imposed threshold would be wider for larger population sizes.
In this study only two haplotype variants (SL and RH) were assumed to exist at the PrP locus. The haplotypes may represent the ARQ and ARR haplotypes, which are the most frequent haplotypes in UK Scottish Blackface sheep, and which were shown to be linked to lamb mortality in the study of Sawalha et al. [10]. Results presented here are also based on the assumption that only SL/SL genotypes are susceptible to scrapie and that only RH/RH genotypes have higher lamb mortality rates not related to scrapie infections. However, to test the broader implications of our results, simulations carried out with different dominance assumptions (i.e. SL/RH genotypes are also susceptible to scrapie and have different lamb mortality rates than SL/SL genotypes) resulted in similar long-term scrapie persistence patterns, albeit for different ranges of model parameters.
Numerous recent studies have investigated the relationship between PrP genotype and performance traits (e.g. [15]–[17]). Whereas accumulative evidence suggests that there is no consistent association between PrP genotype and performance, evidence for the association with fitness traits is still sparse (as reviewed by [18]). In a recent study Gubbins et al. [19] investigated associations between PrP genotype and post-natal lamb mortality for ten sheep breeds in the UK, including the Scottish Blackface. They found no significant associations for a majority of breeds, although the observed trend for Scottish Blackface was similar to that found by Sawalha et al. [10]. The discrepancies in the results of both studies could be due to differences in the datasets – the analysis of Gubbins et al. [19] was based on commercial data in contrast to the higher number and more reliable research data used by Sawalha et al. [10]. The discrepancies could however also point to breed-specific associations between the PrP gene and genes controlling survival due to linkage disequilibrium. The consequence of this for our model is that the linkage could break down over generations, weakening or eliminating the associations between PrP genotype and lamb survival. This would result in a reduced risk of scrapie persisting in the population. Our model simulates the worst case scenario, where associations are assumed to be due to pleiotropy rather than linkage or where the linkage disequilibrium is maintained over many generations.
The PrP genotype was assumed to be only associated with scrapie susceptibility and post-natal lamb mortality in our model. However, given that a number of genes in close proximity to the PrP gene on chromosome 13 are involved in immune response functions, it is possible that additional associations of the PrP genotype with other health or fitness traits will be identified in the future [18]. Selective advantage for genotypes that are susceptible to classical scrapie may also occur as a result of different strains of scrapie in the population affecting different PrP genotypes, as outlined by Slate [14] and supported by recently emerging evidence for atypical scrapie that occurs more frequently in sheep with haplotypes resistant to classical scrapie (e.g. ARR) [20], [21]. The results of the present model support the arguments of Slate [14] that these associations should be taken into account when designing future models and breeding programmes.
For the design and evaluation of breeding programs more accurate representations of the structure of existing sheep populations and of scrapie biology would be required. For example, as demonstrated in various previous modelling studies, prevalence patterns are strongly influenced by (i) the representation of the transmission processes in the model (e.g. distinction between horizontal and vertical disease transmission scenarios) (e.g. [7], [22], [23]), (ii) the stratification of the sheep population in individual flocks of different sizes with sex specific reproduction rates, and transmission occurring both within and between flocks [24]–[26], (iii) the pathogenesis of scrapie [8], [23], (iv) seasonal patterns in the population structure [23], as well as (v) the distribution of breeds and PrP genotypes across the region under consideration [5], [27]. The model presented here could be extended to account for all these factors.
Supporting Information
A quantitative geneticist's approach for calculating change in allele frequency
(0.03 MB DOC)
Expected change of the SL allele frequency over successive generations for different initial allele frequencies (p0) assuming values for coefficients of selection s1 (associated with scrapie susceptibility) and s2 (associated with PrP specific increase in lamb mortality) of 0.0022 and 0.02, respectively.
(0.03 MB TIF)
Acknowledgments
The authors would like to thank the editor M. Baylis for very helpful comments to this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the Scottish Government. SG also acknowledges funding from the Biotechnology and Biological Sciences Research Council (BBSRC) [grant code IAH1320].The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Detwiler LA, Baylis M. The epidemiology of scrapie. Rev Sci Tech Off Int Epiz. 2003;22:121–143. doi: 10.20506/rst.22.1.1386. [DOI] [PubMed] [Google Scholar]
- 2.Goldmann W, Hunter N, Smith G, Foster JD, Hope J. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J GenVirol. 1994;75:989–995. doi: 10.1099/0022-1317-75-5-989. [DOI] [PubMed] [Google Scholar]
- 3.Hunter N, Goldman W, Foster JD, Cairns D, Smith G. Natural scrapie and PrP genotype: case-control studies in British sheep. Vet Rec. 1997a;141:137–140. doi: 10.1136/vr.141.6.137. [DOI] [PubMed] [Google Scholar]
- 4.Hunter N, Cairns D, Foster JD, Smith G, Goldman W, et al. Is scrapie solely a genetic disease? Nature. 1997b;386:137. doi: 10.1038/386137a0. [DOI] [PubMed] [Google Scholar]
- 5.Roden JA, Nieuwhof GJ, Bishop SC, Jones DA, Haresign W, et al. Breeding programmes for TSE resistance in British sheep -I. Assessing the impact on PrP genotype frequencies. Prev Vet Med. 2006;73:1–16. doi: 10.1016/j.prevetmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Gubbins S, Roden JA. Breeding programmes for TSE resistance in British sheep - II. Assessing the impact on the prevalence and incidence of scrapie. Prev Vet Med. 2006;73:17–31. doi: 10.1016/j.prevetmed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Stringer SM, Hunter N, Woolhouse MEJ. A mathematical model of the dynamics of scrapie in a sheep flock. Math Biosc. 1998;153:79–98. doi: 10.1016/s0025-5564(98)10036-6. [DOI] [PubMed] [Google Scholar]
- 8.Woolhouse MEJ, Stringer SM, Matthews L, Hunter N, Anderson RM. Epidemiology and control of scrapie within a flock. Proc R Soc Lond B. 1998;265:1205–1210. doi: 10.1098/rspb.1998.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolhouse MEJ, Coen P, Matthews L, Foster J, Elsen JM, et al. A centuries-long epidemic of scrapie in British sheep? Trends Micro Biol. 2001;9(2):67–70. doi: 10.1016/s0966-842x(00)01912-0. [DOI] [PubMed] [Google Scholar]
- 10.Sawalha RM, Brotherstone S, Conington J, Villanueva B. Lambs with scrapie susceptible genotypes have higher postnatal survival. PLoS ONE. 2007;2(11):e1236. doi: 10.1371/journal.pone.0001236. doi: 10.1371/journal.pone.0001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster JD, Dickinson AG. Age at death from natural scrapie in a flock of Suffolk sheep. Vet Rec. 1989;125:415–417. doi: 10.1136/vr.125.16.415. [DOI] [PubMed] [Google Scholar]
- 12.Matthews L, Coen PG, Foster JD, Hunter N, Woolhouse MEJ. Population dynamics of a scrapie outbreak. Arch Virol. 2001;146:1173–1168. doi: 10.1007/s007050170113. [DOI] [PubMed] [Google Scholar]
- 13.Lewis RM, Simm G. Selection strategies in sire referencing schemes in sheep. Livest Prod Sci. 2000;67:129–141. [Google Scholar]
- 14.Slate J. Molecular evolution of the sheep prion protein gene. Proc R Soc B. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawalha RM, Brotherstone S, Man WYN, Conington J, Bunger L, et al. Associations of polymorphisms of the ovine prion protein gene with growth, carcass, and computerized tomography traits in Scottish Blackface lambs. JAS. 2007;85(3):632–640. doi: 10.2527/jas.2006-372. [DOI] [PubMed] [Google Scholar]
- 16.Moore RC, Boulton K, Bishop SC. Associations of PrP genotype with lamb production traits in three commercial breeds of British hill sheep. Animal. 2009;3:336–346. doi: 10.1017/S1751731108003637. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard TC, Cahalan CM, Ap Dewi I. Association between PrP gnotypes and performance traits in Welsh Maountain flock. Animal. 2008;2:1421–1426. doi: 10.1017/S1751731108002747. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney T, Hanrahan JP. The evidence of associations between prion protein genotype and production, reproduction, and health traits in sheep. Vet Res. 2008;39(4):28. doi: 10.1051/vetres:2008004. DOI: 10.1051/vetres:2008004. [DOI] [PubMed] [Google Scholar]
- 19.Gubbins S, Cook CJ, Hyder K, Boulton K, Davis C, et al. Associations between lamb survival and prion protein genotype: analysis of data for ten sheep breeds in Great Britain. BMC Vet Res. 2009;5:3. doi: 10.1186/1746-6148-5-3. doi: 10.1186/1746-6148/5/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lühken G, Buschmann A, Brandt H, Eiden M, Groschup MH, et al. Epidemiological and genetical differences between classical and atypical scrapie cases. Vet Res. 2007;38:65–80. doi: 10.1051/vetres:2006046. [DOI] [PubMed] [Google Scholar]
- 21.Moum T, Olsaker I, Hopp P, Moldal T, Valheim M, et al. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol. 2005;86:231–235. doi: 10.1099/vir.0.80437-0. [DOI] [PubMed] [Google Scholar]
- 22.Hagenaars TJ, Ferguson NM, Donnelly CA, Anderson RM. Persistence patterns of scrapie in a sheep flock. Epidemiol Infect. 2001;127:157–167. doi: 10.1017/s0950268801005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagenaars TJ, Donnelly CA, Ferguson NM, Anderson RM. The transmission dynamics of the aetiological agent of scrapie in a sheep flock. Math Biosc. 2000;168:117–135. doi: 10.1016/s0025-5564(00)00048-1. [DOI] [PubMed] [Google Scholar]
- 24.Kao RR, Gravenor MB, McLean AR. Modelling the national scrapie eradication programme in the UK. Math Biosc. 2001;174:61–76. doi: 10.1016/s0025-5564(01)00082-7. [DOI] [PubMed] [Google Scholar]
- 25.Gubbins S. A modelling framework to describe the spread of scrapie between flocks in Great Britain. Prev Vet Med. 2005;67:143–156. doi: 10.1016/j.prevetmed.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Woolhouse MEJ, Matthews L, Coen P, Stringer SM, Foster JD, et al. Population dynamics of scrapie in a sheep flock. Proc R Soc Lond B. 1999;354:751–756. doi: 10.1098/rstb.1999.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gubbins S, Webb CR. Simulation of the options for a national control programme to eradicate scrapie from Great Britain. Prev Vet Med. 2005;69:175–187. doi: 10.1016/j.prevetmed.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th edn . Longman, UK: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A quantitative geneticist's approach for calculating change in allele frequency
(0.03 MB DOC)
Expected change of the SL allele frequency over successive generations for different initial allele frequencies (p0) assuming values for coefficients of selection s1 (associated with scrapie susceptibility) and s2 (associated with PrP specific increase in lamb mortality) of 0.0022 and 0.02, respectively.
(0.03 MB TIF)