Abstract
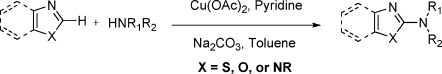
A copper-mediated aerobic coupling reaction enables direct amidation of heterocycles or aromatics having weakly acidic C−H bonds with a variety of nitrogen nucleophiles. These reactions provide efficient access to many biologically important skeletons, including ones with the potential to serve as inhibitors of HMTs.
The methylation marks on chromatin established by histone methyltransferases (HMTs) are key elements of heritable cell states and can lead to disease when disregulated.1,2 Thus, it was of interest that a compound containing a 2-amidobenzimidazole skeleton was reported to inhibit several HMTs.(3) Methods to synthesize this skeleton require multiple-step sequences that do not easily lend themselves to syntheses of many structural analogues.(4) To overcome this obstacle, we describe here the direct C−H functionalization of benzimidazoles with nitrogen-containing reagents via a copper(II)-mediated oxidative coupling that affords 2-amidobenzimidazoles.
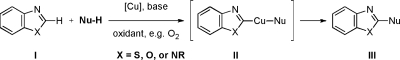
We anticipated that the aerobic cross-coupling of heterocycles (I) with nucleophiles would lead to the 2-amido-substituted heterocycles (III) through the organocopper intermediate (II) in analogy to the Chan−Lam oxidative coupling of arylboronic acids and nucleophiles (Scheme 1).5−7
Scheme 1. Copper-Mediated C−H Functionalization of Heterocyclic C−H Bonds.
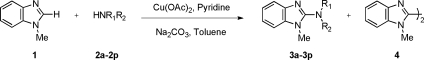
We first examined the reaction of N-methylbenzimidazole (1) with pyrrolidinone (2a) in the presence of catalytic copper salts under 1 atm of O2. In the initial screening of Cu sources, Brønsted bases, and solvents, optimal results were observed with 0.2 equiv of Cu(OAc)2 and 2 equiv of Na2CO3 with pyridine as additive in toluene.(8) The dimeric product 4 was observed in many conditions, but its formation was suppressed and the yield of 3a was increased by using 5 equiv of nucleophile (Table 1, entry 1). Among the Cu sources tested, Cu(OAc)2 generally performed better than CuCl2, CuBr2, Cu(OTf)2, and Cu(O2CCF3)2.
Table 1. Cu(II)-Mediated Oxidative Coupling of 1 with N-Nucleophilesa.
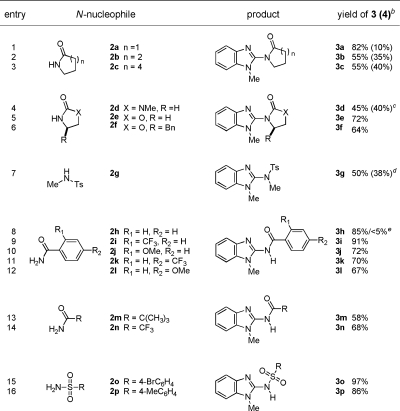 |
Reaction conditions for 2a−2g. Condition A: 1 (0.3 mmol), 2 (1.5 mmol), pyridine (6.0 mmol), Cu(OAc)2 (0.06 mmol), Na2CO3 (0.6 mmol), toluene (10 mL), O2 (balloon), 120−140 °C, 12−30 h. For 2h−2p. Condition B: 1 (0.3 mmol), 2 (1.5 mmol), pyridine (6.0 mmol), Cu(OAc)2 (0.6 mmol), Na2CO3 (0.9 mmol), toluene (10 mL), O2 (balloon), 120−140 °C, 12−30 h.
Yields correspond to isolated products.
Cu(OAc)2 (0.3 mmol).
2g (0.6 mmol), pyridine (1.5 mmol).
Yield of product 3h under condition A.
We next varied the nucleophiles in this reaction (Table 1). Cyclic amide (entries 2 and 3), urea and carbamate (entries 4−6) nucleophiles, and N-methyl benzenesulfonamide (entry 7) provided the desired products effectively, while acyclic secondary amides were not effective under similar reaction conditions. A wide range of primary amides was found to undergo the reaction (Table 1, entries 8−16). The reaction with benzamide 2h using a catalytic amount of Cu(OAc)2 formed product 3h; however, the starting material 1 was not effectively consumed. This may be due to the binding of Cu(II) to the carbonyl oxygen and imino nitrogen in the product. This problem was circumvented by using 2 equiv of Cu(OAc)2 (entry 8). Both electron-withdrawing and electron-donating functional groups are tolerated well on the aryl ring of the amides (entries 9−12). Simple alkyl primary amides are also substrates (entries 13 and 14), even ones with steric bulk on the amide. Reactions of 1 with sulfonamides resulted in excellent yields (entries 15 and 16), including one with a bromine substituent on the aryl group. Although reactions with amines under the same conditions provided the desired products, the formation of the dimeric product 4 was significant, most likely due to strong electron donation by the amine and the unfavorable deprotonation.
We also investigated the scope of this reaction with other heterocyclic and aromatic C−H bonds (Table 2). In the amidation reactions with pyrrolidinone 2a, the heterocyclic C−H bonds of benzothiazole, caffeine, and oxazole all underwent oxidative coupling (entries 1−3). Similar reactions with primary amide 2i were successful (entries 4 and 5), and intramolecular reactions delivered cyclic products with excellent yields (entries 6 and 7). The reaction condition was also effective for the direct amidation of C−H bonds in fluorinated aromatic rings, albeit in diminished yields (entries 8−10). These transformations offer successful examples of challenging intermolecular C−N bond formation using aromatic C−H and amide N−H groups. These amidation reactions (entries 8−10) indicate the potential of this method in the synthesis of fluorobenzene derivatives.
Table 2. Cu(II)-Mediated Amidations of Aromatic C−H Bondsa.
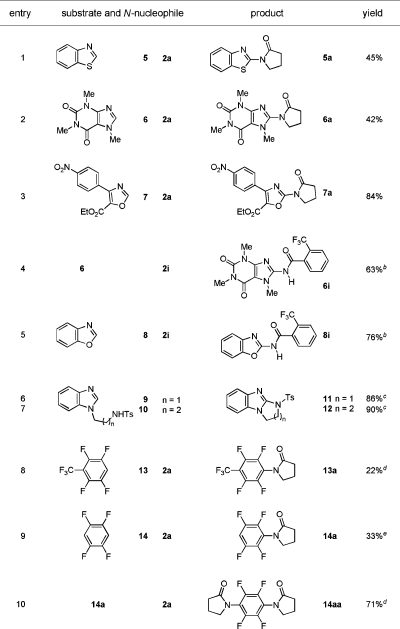 |
Standard reaction condition: heterocycle (1.0 equiv), nucleophile (5.0 equiv), Cu(OAc)2 (0.2 equiv), Na2CO3 (2.0 equiv), pyridine (20.0 equiv), toluene, O2 (balloon), 120−140 °C, 12−30 h. Yields correspond to isolated products.
Cu(OAc)2 (2.0 equiv), Na2CO3 (3.0 equiv).
Cu(OAc)2 (1.0 equiv), pyridine (5.0 equiv).
Cu(OAc)2 (1.0 equiv), K2CO3 (2.0 equiv) instead of Na2CO3.
14 (5.0 equiv), 2a (1.0 equiv), pyridine (5.0 equiv), K2CO3 (2.0 equiv).
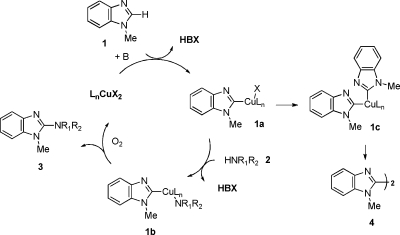
Although mechanisms of copper-catalyzed oxidative C−N couplings have been proposed,(6) the details remain uncertain. A proposed mechanism for this amidation reaction is outlined in Scheme 2. In the presence of Cu(II) and base, we imagine that organocopper intermediate 1a from the heterocycle (e.g., 1) is formed. Ligand exchange with the deprotonated nucleophile would yield intermediate 1b. C−N reductive elimination and aerobic reoxidation of the catalyst would complete the catalytic cycle.
Scheme 2. Proposed Mechanism for Copper-Mediated C−H Functionalization of Heterocyclic C−H Bonds.
Since the formation of 1b can compete with the formation of 1c, dimer 4 will form when 1b is disfavored by slow deprotonation (e.g., pKa > 25 or amine) or steric hindrance (e.g., primary vs secondary amides). Reactions with pyridone (pKa = 17) and phthalimide (pKa = 8.3) only resulted in the recovery of starting material, which suggests that the nucleophilicity of the substrates plays an important role in the reaction, presumably by facilitating the C−N reductive elimination step.
In conclusion, we have developed a copper-mediated method for aerobic coupling of 2-benzimidazoles and other heterocycles or aromatics having acidic C−H bonds with a variety of nitrogen nucleophiles. These reactions provide efficient access to many biologically important skeletons, including ones with the potential to serve as inhibitors of HMTs.(9)
Acknowledgments
We gratefully acknowledge the National Cancer Institute (NCI-PO1-CA078048) for support of this research. S.L.S. is a Howard Hughes Medical Institute Investigator.
Supporting Information Available
Experimental procedures, additional screening data, and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Schreiber S. L.; Bernstein B. E. Cell 2002, 111, 771–778. [DOI] [PubMed] [Google Scholar]; b Bernstein B. E.; Mikkelsen T. S.; Xie X.; Kamal M.; Huebert D. J.; Cuff J.; Fry B.; Meissner A.; Wernig M.; Plath K.; Jaenisch R.; Wagschal A.; Feil R.; Schreiber S. L.; Lander E. S. Cell 2006, 125, 315–326. [DOI] [PubMed] [Google Scholar]; c Kouzarides T. Cell 2007, 128, 693–705. [DOI] [PubMed] [Google Scholar]
- a Cole P. A. Nat. Chem. Biol. 2008, 4, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Marks P. A.; Breslow R. Nat. Biotechnol. 2007, 25, 84–90. [DOI] [PubMed] [Google Scholar]; c Copeland R. A.; Solomon M. E.; Richon V. M. Nat. Rev. Drug Discov. 2009, 8, 724–732. [DOI] [PubMed] [Google Scholar]
- a Kubicek S.; O’Sullivan R. J.; August E. M.; Hickey E. R.; Zhan Q.; Teodoro M. L.; Rea S.; Mechtler K.; Kowalski J. A.; Homon C. A.; Kelly T. A.; Jenuwein T. Mol. Cell 2007, 25, 473–481. [DOI] [PubMed] [Google Scholar]; Other recently reports on biological activities of 2-aminobenzimidazole cores:; b Winters M. P.; Robinson D. J.; Khine H. H.; Pullen S. S.; Woska J. R.; Raymond E. L.; Sellati R.; Cywin C. L.; Snow R. J.; Kashem M. A.; Wolak J. P.; King J.; Kaplita P. V.; Liu L. H.; Farrell T. M.; DesJarlais R.; Roth G. P.; Takahashi H.; Moriarty K. J. Bioorg. Med. Chem. Lett. 2008, 18, 5541–5544. [DOI] [PubMed] [Google Scholar]; c Powers J. P.; Li S. Y.; Jaen J. C.; Liu J. Q.; Walker N. P. C.; Wang Z. L.; Wesche H. Bioorg. Med. Chem. Lett. 2006, 16, 2842–2845. [DOI] [PubMed] [Google Scholar]; d Kim R. M.; Chang J.; Lins A. R.; Brady E.; Candelore M. R.; Dallas-Yang Q.; Ding V.; Dragovic J.; Iliff S.; Jiang G. Q.; Mock S.; Qureshi S.; Saperstein R.; Szalkowski D.; Tamvakopoulos C.; Tota L.; Wright M.; Yang X. D.; Tata J. R.; Chapman K.; Zhang B. B.; Parmee E. R. Bioorg. Med. Chem. Lett. 2008, 18, 3701–3705. [DOI] [PubMed] [Google Scholar]
- a Carpenter R. D.; DeBerdt P. B.; Lam K. S.; Kurth M. J. J. Comb. Chem. 2006, 8, 907–914. [DOI] [PubMed] [Google Scholar]; b Perkins J. J.; Zartman A. E.; Meissner R. S. Tetrahedron Lett. 1999, 40, 1103–1106. [Google Scholar]; c Seth P. P.; Robinson D. E.; Jefferson E. A.; Swayze E. E. Tetrahedron Lett. 2002, 43, 7303–7306. [Google Scholar]
- a Chan D. M. T.; Monaco K. L.; Wang R. P.; Winters M. P. Tetrahedron Lett. 1998, 39, 2933–2936. [Google Scholar]; b Lam P. Y. S.; Clark C. G.; Saubern S.; Adams J.; Winters M. P.; Chan D. M. T.; Combs A. Tetrahedron Lett. 1998, 39, 2941–2944. [Google Scholar]; c Evans D. A.; Katz J. L.; West T. R. Tetrahedron Lett. 1998, 39, 2937–2940. [Google Scholar]
- For a related reaction involving directed functionalization of C−H bond with N-nucleophiles, see:; a Hamada T.; Ye X.; Stahl S. S. J. Am. Chem. Soc. 2008, 130, 833–835. [DOI] [PubMed] [Google Scholar]; b Balsamo A.; Macchia B.; Macchia F.; Rossello A.; Domiano P. Tetrahedron Lett. 1985, 26, 4141–4144. [Google Scholar]; c King A. E.; Brunold T. C.; Stahl S. S. J. Am. Chem. Soc. 2009, 131, 5044–5045. [DOI] [PubMed] [Google Scholar]
- For examples involving transition-metal-directed functionalization of C−H bonds with heteroatoms, see:; a Chen X.; Hao X.-S.; Goodhue C. E.; Yu J.-Q. J. Am. Chem. Soc. 2006, 128, 6790–6791. [DOI] [PubMed] [Google Scholar]; b Uemura T.; Imoto S.; Chatani N. Chem. Lett. 2006, 35, 842–843. [Google Scholar]; Intramolecular:; c Brasche G.; Buchwald S. L. Angew. Chem., Int. Ed. 2008, 47, 1932–1934. [DOI] [PubMed] [Google Scholar]; d Thu H.-Y.; Yu W.-Y.; Che C.-M. J. Am. Chem. Soc. 2006, 128, 9048–9049. [DOI] [PubMed] [Google Scholar]; e Wasa M.; Yu J.-Q. J. Am. Chem. Soc. 2008, 130, 14058–14059. [DOI] [PubMed] [Google Scholar]; f Inamoto K.; Hasegawa C.; Hiroya K.; Doi T. Org. Lett. 2008, 10, 5147–5150. [DOI] [PubMed] [Google Scholar]; g Jordan-Hore J. A.; Johansson C. C. C.; Gulias M.; Beck E. M.; Gaunt M. J. J. Am. Chem. Soc. 2008, 130, 16184–16186. [DOI] [PubMed] [Google Scholar]; h Ueda S.; Nagasawa H. Angew. Chem., Int. Ed. 2008, 47, 6411–6413. [DOI] [PubMed] [Google Scholar]; i Mei T.-S.; Wang X.; Yu J.-Q. J. Am. Chem. Soc. 2009, 131, 10806–10807. [DOI] [PubMed] [Google Scholar]
- See Supporting Information.
- During the preparation of this paper, a related study of C−H amination of benzothiazoles, benzimidazoles, benzoxazoles, and thiazoles was reported:Monguchi D.; Fujiwara T.; Furukawa H.; Mori A. Org. Lett. 2009, 11, 1607–1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.