Abstract
Transcription and metabolite analysis is a powerful way to reveal physiological shifts in response to environmental pollution. Recent studies on earthworms, including one in BMC Biology, show that the type of pollution and its availability for uptake by organisms can differentially affect transcription and metabolism.
Modern society emits and discharges many potentially toxic chemicals to the environment. If chemicals are not degraded quickly, they tend to accumulate in soils and sediments. Soil often acts as the ultimate 'sink' of environmental pollution, because clay minerals and humic materials have a large number of surfaces, chemical groups and organic particles to which pollutants can attach. Contaminated soils can pose a problem for society if agricultural functions, human health or ecological systems are adversely affected.
Soil is also a place of intense biological activity thanks to degradation of organic matter, recycling of nutrients and synthesis of humus. The greatest amount of activity is found in the upper organic layer of the soil. Culture-independent metagenomics and modeling studies have shown that biodiversity of soil organisms is much greater than previously thought, and that the soil harbors many unexplored functions and is highly sensitive to contamination [1,2].
Contaminants in soil, even if they are potentially toxic, pose no harm as long as they are firmly bound to the solid phase of the soil. Only the fraction that is mobile (bioavailable) can have an impact on organisms. This fraction, often equated with the fraction that is dissolved or found in pore water, is highly variable because it depends on many factors and on the duration of contact between pollutants and soil. Appropriate risk assessment of contaminants is therefore geared towards assessing the biological effects of a polluted soil, rather than the total concentration of contaminant it contains.
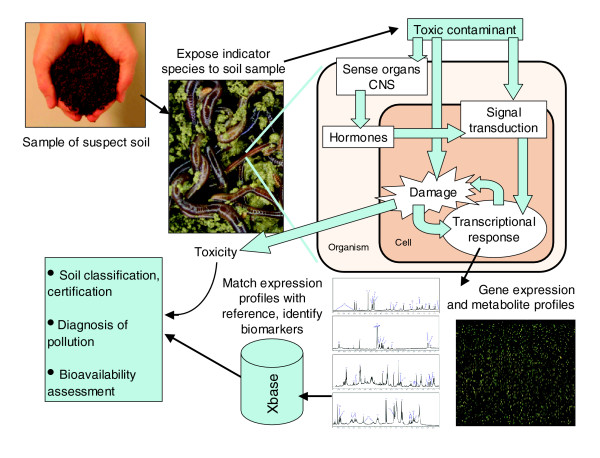
It has been suggested that genomics technology, especially transcription profiling, allows new ways of assessing the biological effects of environmental pollution [3-7]. The basic idea is that gene expression is one of the very first things that will change when an organism is exposed to a stressful condition. To maintain homeostasis of the internal environment, the metabolic machinery requires continuous adjustment to any new situation; gene expression is expected to reflect these adjustments. A rationale for the use of transcription profiling in risk assessment of contaminated soil is outlined in Figure 1. Because of the potential advantages, several regulatory authorities are now discussing how genomics tools could fit into the risk assessment process [8,9]. The US Environmental Protection Agency is developing new guidance that outlines how genomics may contribute to a weight-of-evidence approach towards assessing environmental pollution [8].
Figure 1.
How a combination of genomics and environmental toxicology can support risk assessment of soil pollution. An indicator species is exposed to a sample of soil. Traditional soil assessment evaluates soils only on the basis of whole-body endpoints, such as survival, growth and reproduction. Genomic analysis can add specificity, sensitivity and rapidity, as discussed in the text, and can give more detail of how the contaminants in the soil affect cellular processes such as signal transduction or DNA damage. In addition, consideration of metabolite patterns (such as the graphs at the bottom of the figure) can help with the sifting and interpretation of the transcriptional response, as illustrated by recent work on earthworms [10-13]. 'Xbase' refers to bioinformatic analyses.
Transcription profiling as an environmental monitoring tool seems to have some advantages over traditional bioassays that focus on survival, growth and reproduction of test animals. Three possible benefits have been outlined [4]. Firstly, specificity: gene expression will be specific to the type of stress, unlike classical endpoints such as growth and reproduction. Secondly, sensitivity: gene expression will be more sensitive, that is, effects can be recognized at lower exposure concentrations, than classical endpoints. And thirdly, rapidity: gene expression will respond quickly, in the order of hours to days, allowing tests that otherwise could take several weeks.
These claims have not yet been substantiated, certainly not for soil testing, but several pioneering studies are now beginning to be published that are creating a basis for testing these assumptions and evaluating the high expectations raised. A recent study on effects of soil pollution on earthworms in BMC Biology [10] exemplifies this, and, with other recent earthworm studies [11-13], shows that transcriptome profiles bear a signature of the type of pollution to which the animal was exposed.
Bundy et al. [10] document effects of copper on the transcriptome of the earthworm Lumbricus rubellus. In a promising new 'systems toxicology' approach, they complement their transcriptome data with metabolomics data and pay particular attention to alterations in metabolic categories that are supported by both high-throughput approaches. This avoids the problems pointed out by Feder and Walsher [14], who warned against placing too much confidence in transcriptomic data to predict effects on the phenotype, because of the long chain of biochemical steps between gene expression and a change of metabolism. In addition, there seems to be inherent noise in the transcriptome data, such that there is often a very poor correlation between transcriptome and proteome. The reason for this transcriptional noise is not clear. Spellmann and Rubin [15] have pointed out that many genes in Drosophila are expressed in transcriptional territories. Applied to environmentally induced transcriptomes, there could be many genes that do not respond to the environmental stimulus itself but are transcribed only because they happen to be in an active territory.
Interestingly, the paper by Bundy et al. [10] shows that improved understanding of the transcriptome and metabolome is reached when they are studied jointly; the most important added value of metabolomics may be to filter out the noise inherent in gene expression and to select those gene expression measurements that are consistent with the metabolome.
Considering the four earthworm papers together [10-13], there seems to be a good basis for saying that the first benefit of transcription profiling, specificity, is real. Table 1 shows the general picture emerging from the earthworm papers. Five chemicals are compared: two heavy metals (copper and cadmium), a polycyclic aromatic compound (fluoranthene), a herbicide (atrazine) and an explosive (trinitrotoluene, TNT). Such a comparison is obviously very preliminary, as the studies used two different species (L. rubellus and Eisenia fetida), different exposure conditions and different platforms (Table 1).
Table 1.
Gene expression changes seen in earthworms exposed to five different soil contaminants
| Copper | Cadmium | Fluoranthene | Atrazine | TNT | |
| Species | L. rubellus | L. rubellus | L. rubellus | L. rubellus | Eisenia fetida |
| Exposure time | 70 days | 28 days | 28 days | 28 days | 28 days |
| Exposure concentration range (mg/kg) | 10-480 | 13-500 | 14-533 | 19-59 | 2-39 |
| Metabolic category | |||||
| Detoxification | Y | Y | Y | Y | |
| Oxidative stress | Y | Y | Y | Y | |
| Mitochondrial electron transport | Y | Y | Y | Y | Y |
| Calcium binding and signaling | Y | Y | Y | ||
| Iron homeostasis and oxygen transport | Y | Y | Y | ||
| Blood coagulation and fibrinolysis | Y | ||||
| Impaired immune function | Y | Y | |||
| Protein damage repair and catabolism | Y | Y | Y | ||
| Lipid metabolism | Y | ||||
| Glycolysis and carbohydrate metabolism | Y | ||||
| Cell cycle and apoptosis | Y | Y | |||
| Muscle contraction | Y | Y | |||
| DNA damage and repair | Y | Y | |||
| Neurological dysfunction | Y | ||||
| References | [10] | [11] | [11] | [11] | [12,13] |
Y indicates the broad metabolic categories in which significant changes in gene expression were observed in response to the indicated contaminants.
A substantial fraction of an earthworm's stress-responsive transcriptome change is found to be induced by all the compounds. This is true for defense against oxidative stress and changes in the electron-transport chain (although oxidative stress seems to be less important in the case of atrazine). Effects on calcium binding and iron homeostasis also seem to be part of a general stress response, although these are less obvious for fluoranthene and atrazine. There are also transcriptome changes that are more or less specific to one chemical. For example, strong effects on lipid and carbohydrate metabolism are reported only for copper, and effects on blood coagulation, fibrinolysis and neurological dysfunction are reported only for TNT.
Comparing the compounds, it seems that the two metals, cadmium and copper, share a considerable part of the transcription profile, whereas the expression profile of TNT is more like those of the metals than those of the other organic compounds, fluoranthene and atrazine. Of course such comparisons can be done better on a gene-by-gene basis rather than in terms of broad metabolic categories, but a sufficiently large database for earthworm toxicity is not yet available.
The few studies published so far seem to support the assertion that indeed, soil contaminants induce substance-specific profiles in earthworms; this supports the specificity advantage of transcription profiling. This conclusion may well be restricted to single-chemical exposures, however. In the study on TNT, when the investigators added another explosive, 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX), this radically altered the expression profile of TNT. Although TNT alone regulated 321 genes, a mixture of TNT and RDX regulated only three genes. Thus RDX had a strong antagonistic effect on the TNT-induced expression profile, the reason for this remains unknown.
The four transcription profiling studies [10-13] were done at a range of concentrations that did not cause mortality but had sublethal effects on reproduction. However, clear evidence for effects on gene expression in the absence of effects on growth and reproduction has not yet been documented. It seems that sensitivity might not be the strongest advantage of transcription profiling.
The third issue, rapidity of testing, could well turn out to be the greatest advantage of transcription profiling. There are many situations in which a quick decision on the quality of a certain soil sample could be of great value, for example when there are large costs associated with storing or transport of soil, or when a large number of samples has to be evaluated. The earthworm studies [10-13] have all applied rather long exposure conditions (28-70 days). Gene expression patterns observed after shorter exposure periods, for example three days, will be different; some genes regulated during the early phase of an exposure might not be differentially expressed after several weeks, and vice versa. Whether short-term gene expression patterns can be predictive of phenotypic effects after longer exposure remains an issue for future research.
We have done a short survey among stakeholders in environmental risk assessment, asking them what they see as the greatest obstacle for accepting genomics tools in environmental risk assessment (R. Kloet, D. Roelofs and N.M. van Straalen, unpublished work). The obvious outcome was that new tests will always be viewed as competing with already accepted test methodologies and, to replace accepted tests, they will need to have a considerable advantage. On the basis of this result, we feel that it is advisable to focus genomics tools on test systems that have already gained international acceptance through, for example, Organization for Economic Co-operation and Development (OECD) or International Organization for Standardization (ISO) guidelines. Then, if genomics tools are predictive of the outcome in such tests but have an advantage in terms of specificity, sensitivity or rapidity, this will help them to gain acceptance in the regulatory arena.
References
- Gans J, Wolinsky M, Dunbar J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science. 2005;309:1387–1390. doi: 10.1126/science.1112665. [DOI] [PubMed] [Google Scholar]
- Daniel R. The metagenomics of soil. Nature Rev Microbiol. 2005;3:470–478. doi: 10.1038/nrmicro1160. [DOI] [PubMed] [Google Scholar]
- Snape JR, Maund SJ, Pickford DB, Hutchinson TH. Ecotoxicogenomics: the challenge of integrating genomics into aquatic and terrestrial ecotoxicology. Aquat Toxicol. 2004;67:143–154. doi: 10.1016/j.aquatox.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Van Straalen NM, Roelofs D. An Introduction to Ecological Genomics. Oxford: Oxford University Press; 2006. [Google Scholar]
- Parro V, Moreno-Paz M, González-Toril E. Analysis of environmental transcriptomes by DNA microarrays. Environ Microbiol. 2006;9:453–464. doi: 10.1111/j.1462-2920.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- Robbens J, van der Ven K Van der, Maras M, Blust R, De Coen W. Ecotoxicological risk assessment using DNA chips and cellular reporters. Trends Biotechnol. 2007;25:460–466. doi: 10.1016/j.tibtech.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Roelofs D, Aarts MGM, Schat H, Van Straalen NM. Functional ecological genomics to demonstrate general and specific responses to abiotic stress. Funct Ecol. 2008;22:8–18. [Google Scholar]
- Dix DJ, Gallagher K, Benson WH, Groskinsky BL, McClintock JT, Dearfield KL, Farland WH. A framework for the use of genomics data at the EPA. Nature Biotech. 2006;24:1108–1111. doi: 10.1038/nbt0906-1108. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Daston GP, Degitz SJ, Denslow ND, Hoke RA, Kennedy SW, Miracle AL, Perkins EJ, Snape J, Tillit DE, Tyler CR, Versteeg D. Toxicogenomics in regulatory ecotoxicology. Environ Sci Technol. 2006;40:4055–4065. doi: 10.1021/es0630184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy JG, Sidhu JK, Rana F, Spurgeon DJ, Svendsen C, Wren JF, Stürzenbaum SR, Morgan AJ, Kille P. 'Systems toxicology' approach identifies coordinated metabolic responses to copper in a terrestrial non-model invertebrate, the earthworm Lumbricus rubellus. BMC Biol. 2008;6:25. doi: 10.1186/1741-7007-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Hedley BA, Svendsen C, Wren JF, Jonker MJ, Hankard PK, Lister LJ, Stürzenbaum SR, Morgan AJ, Spurgeon DJ, Blaxter ML, Kille P. Transcriptome profiling of developmental and xenobiotic responses in a keystone animal, the oligochaete annelid Lumbricus rubellus. BMC Genomics. 2008;9:266. doi: 10.1186/1471-2164-9-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Guan X, Inouye LS, Pirooznia M, Indest KJ, Athow RS, Deng Y, Perkins EJ. Toxicogenomic analysis provides new insights into molecular mechanisms of 2,4,6-trinitrotoluene in Eisenia fetida. Environ Sci Technol. 2007;41:8195–8202. doi: 10.1021/es0716352. [DOI] [PubMed] [Google Scholar]
- Gong P, Guan X, Inouye LS, Deng Y, Pirooznia M, Perkins EJ. Transcriptomic analysis of RDX and TNT interactive sublethal effects in the earthworm Eisenia fetida. BMC Genomics. 2008;9(Suppl 1):S15. doi: 10.1186/1471-2164-9-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Walser JC. The biological limitations of transcriptomics in elucidating stress and stress responses. J Evol Biol. 2005;18:901–910. doi: 10.1111/j.1420-9101.2005.00921.x. [DOI] [PubMed] [Google Scholar]
- Spellman PT, Rubin GM. Evidence for large domains of similarly expressed genes in the Drosophila genome. J Biol. 2002;1:5. doi: 10.1186/1475-4924-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]