Abstract
Cyclic guanosine monophosphate (cGMP) is a critical second messenger that regulates cardiovascular function and vision in humans. Two recent papers, including one in BMC Structural Biology, have revealed atomic structures of the enzymes that catalyze the synthesis of cGMP providing new clues about the molecular basis of substrate specificity and allosteric regulation in nucleotide cyclases.
The cyclic mononucleotides cAMP and cGMP are potent signaling molecules that exert profound effects on cellular homeostasis. cAMP, which was originally identified by its ability to cause breakdown of glycogen in response to adrenaline, is produced by adenylyl cyclase, an enzyme first characterized in 1958. It took another decade to establish that cGMP and the enzyme responsible for its synthesis, guanylyl cyclase, are also present in mammalian tissues. In the rod and cone cells of the retina, cGMP plays a central role in the visual signal transduction cascade by opening ligand-gated ion channels in sensory neurons. cGMP is also produced in response to hormones, such as nitric oxide and atrial natriuretic peptide, which leads to the relaxation of vascular smooth muscle and is thus important for regulation of blood pressure [1].
Now, more than a decade after the first reported adenylyl cyclase structures, two atomic structures of a guanylyl cyclase catalytic core have been reported. The first describes a cyanobacterial guanylyl cyclase from Synechocystis PCC6803 [2] and the second a eukaryotic guanylyl cyclase from the green alga Chlamydomonas reinhardtii [3]. They provide a fresh look at the molecular basis for nucleotide selectivity and allosteric regulation in these important enzymes.
Domain architecture and regulation
Mammalian adenylyl and guanylyl cyclases belong to the class III nucleotide cyclase family [4,5]. Atomic structures of mammalian adenylyl cyclase [6-8] showed that the catalytic core of class III cyclases consists of either a homodimer or a heterodimer of homologous domains that associate to form a wreath-like structure (Figure 1, top left). One or two active sites are formed at the interface between the domains. In mammalian adenylyl cyclases, the catalytic core is composed of homologous domains (C1 and C2) that form only one functional active site. The non-functional active site is the binding site for the activator forskolin, a well-known hypotensive agent.
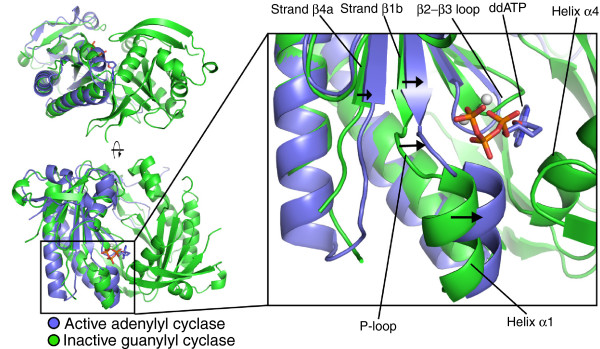
Figure 1.
The nucleotide cyclase wreath-like structure and active-site conformation. Comparison of the C. reinhardtii guanylyl cyclase catalytic core (green) with that of the C1:C2 domains of activated mammalian adenylyl cyclase (PDB ID: 1CJU; blue) bound to a nucleotide analog (shown as a stick model). The two active sites of the guanylyl cyclase are found in a cleft formed at the interface of two identical subunits (top left). The two subunits form a wreath-like structure, with active sites located at either end of the cleft (bottom left). The nucleotide analog marks the position of the single functional adenylyl cyclase active site. The guanylyl cyclase structure is presumed to be in an inactive conformation: in the active state of the nucleotide cyclases, it is expected that helix α1 (labeled in the right-hand panel) moves into the active site and toward helix α4 of the other subunit of the dimer. The arrows in the right-hand panel indicate the conformational changes required to generate the active configuration. From Winger et al. [3] Figure 4 (PDB 3ET6).
Most mammalian adenylyl cyclases are large transmembrane proteins and subject to complex regulation, most importantly through direct interactions with heterotrimeric GTP-binding (G) proteins (Figure 2, left-hand side). In contrast, mammalian guanylyl cyclases are modular receptor enzymes: they exist as homo- or heterodimers with one catalytic domain in each polypeptide chain, and their activity can be modulated directly by hormones or other factors that bind to their accessory domains (Figure 2, right-hand side) [9].
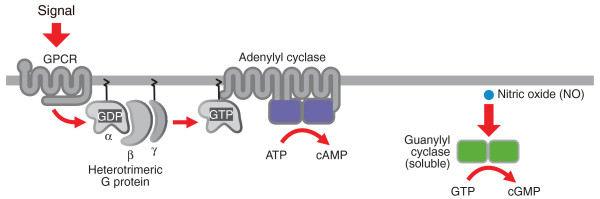
Figure 2.
Schematic diagram of adenylyl and guanylyl cyclases. Mammalian adenylyl cyclases (left-hand side) are typically transmembrane proteins activated by heterotrimeric G proteins. Mammalian guanylyl cyclases (right-hand side) are receptor enzymes directly activated by hormones, such as nitric oxide (NO), or other factors that bind to its regulatory domains (for simplicity, the regulatory domains are not shown).
Snapshots of the guanylyl cyclase catalytic core
Not surprisingly, the catalytic core of both the Synechocystis Cya2 guanylyl cyclase (which has approximately 30% sequence identity to mammalian adenylyl cyclase and guanylyl cyclase isoforms) and the C. reinhardtii enzyme (which has 40–50% sequence identity to mammalian guanylyl cyclases) adopt the same wreath-like structure that was described for adenylyl cyclase (Figure 1). What then can be learned from these structures? Discrimination between GTP and ATP among class III nucleotide cyclases is critical for the fidelity of signaling by cGMP and cAMP. This specificity is determined, at least in part, by a pair of active-site residues (Glu and Cys) in guanylyl cyclases and their counterparts (Lys and Asp) in adenylyl cyclases. For example, when the guanylyl cyclase Glu-Cys pair is replaced with a Lys-Asp pair, the guanylyl cyclase is converted into a highly specific adenylyl cyclase [10,11]. Although the adenylyl cyclase Lys-Asp pair interacts directly with the purine base [7], it is not known for certain whether their equivalents in guanylyl cyclases contribute to specificity in the same manner.
Unfortunately, neither of the guanylyl cyclase structures contains a bound ligand to help define the mechanism of specificity in the active site. The C. reinhardtii enzyme [3] contains the Glu-Cys pair typical of mammalian guanylyl cyclases at their expected topological positions, but covalent modification of the active-site cysteine (a consequence of the conditions necessary for crystallization) precludes further interpretation. Interestingly, the bacterial Cya2 guanylyl cyclase [2] has a non-canonical Glu-Gly pair, with the critical Glu side chain being supported by a third basic residue, conserved as Arg or Lys in guanylyl cyclases. Unexpectedly, guanylyl cyclase activity is reduced and adenylyl cyclase activity increased when the Glu-Gly pair of Cya2 is replaced by a Glu-Cys pair [2]. Thus, the specific features that help discriminate between ATP and GTP in class III cyclases are likely to be complex and not universally conserved. Indeed, the relatively nonspecific mycobacterial Rv1900c adenylyl cyclase contains a non-canonical Asn-Asp pair that seems to be completely dispensable for binding nucleotides [12].
Once a wreath, always a wreath?
The molecular mechanism of mammalian adenylyl cyclase activation has been elusive because there is as yet no structure representing a physiologically relevant basal, inactive state of the catalytic core (a C1:C2 heterodimer in the absence of activating ligands). The structure of the C2 homodimer of adenylyl cyclase has been used as a surrogate, but its conformation is probably influenced by the presence of two molecules of forskolin, which is used to stabilize the dimer interface, and the fact that it lacks a C1 domain, which is required for catalytic activity [6].
The guanylyl cyclase structures provide new images of basal conformations that may be more physiologically relevant. The catalytic core of the Synechocystis guanylyl cyclase enzyme [2] assumes a conformation most similar to catalytically competent states of adenylyl cyclases [8,12], although, surprisingly, it adopts a closed conformation that apparently needs to open before nucleotides can access the active site. This could represent a regulatory difference from the C. reinhardtii guanylyl cyclase and mammalian cyclases. The C. reinhardtii guanylyl cyclase [3] adopts a conformation similar to that of the adenylyl cyclase C2 homodimer structure, in that the α1 helix, which binds to the β- and γ-phosphates of the nucleotide (Figure 1), is not properly aligned with the rest of the active site. The structure may therefore serve as a useful model for the inactive state of mammalian cyclases. However, the fact that crystals could not be obtained without covalent modification of cysteines leaves open the possibility that this structure still does not represent the true ground-state conformation.
In 2005, Tews et al. [13] described the active and inactive structures of a pH-sensing mycobacterial class III adenylyl cyclase. Importantly, this structure included the regulatory domains of the enzyme. In the active state, the catalytic domains assume the familiar wreath-like dimer. In the inactive state, they adopt a dramatically different conformation in which they interact extensively with the regulatory domains. Although this enzyme is more distantly related to mammalian adenylyl cyclases than the two guanylyl cyclases described here (approximately 23% identity) [2,3], it highlights the fact that accessory domains, such as those found in the two guanylyl cyclase enzymes and in mammalian adenylyl cyclases, can have a dramatic impact on the conformation of the catalytic core in its inactive state.
There is also evidence that the ground-state structures of some class III nucleotide cyclases may consist simply of loosely associated, and therefore minimally active, catalytic domains. Although the C. reinhardtii guanylyl cyclase enzyme [3] was a homodimer under all conditions tested (J Winger, personal communication), the Cya2 catalytic core exists in an equilibrium between monomeric, dimeric and oligomeric forms [2]. Forskolin and G proteins are known to dramatically enhance the affinity of the independently expressed C1 and C2 domains of mammalian adenylyl cyclase, and the Rv1900c adenylyl cyclase forms an asymmetric homodimer in its unliganded state, with an unusually open active-site cleft [12].
Obviously, more structures of class III nucleotide cyclases are needed, especially of those that include regulatory domains. Although interpretation of the molecular mechanism of activation for nucleotide cyclases is somewhat hindered by the conditions required to bring about their crystallization, it is also possible that each class III enzyme has evolved a distinct basal conformation that can take optimal advantage of the regulatory inputs unique to that enzyme. This would probably enhance the fidelity of signaling when multiple cyclase isoforms are present.
Acknowledgments
Acknowledgements
The author is supported by NIH grants HL086865 and HL071818.
References
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Rauch A, Leipelt M, Russwurm M, Steegborn C. Crystal structure of the guanylyl cyclase Cya2. Proc Natl Acad Sci USA. 2008;105:15720–15725. doi: 10.1073/pnas.0808473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger JA, Derbyshire ER, Lamers MH, Marletta MA, Kuriyan J. The crystal structure of the catalytic domain of a eukaryotic guanylate cyclase. BMC Struct Biol. 2008;8:42. doi: 10.1186/1472-6807-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JU, Schultz JE. The class III adenylyl cyclases: multi-purpose signalling modules. Cell Signal. 2003;15:1081–1089. doi: 10.1016/S0898-6568(03)00130-X. [DOI] [PubMed] [Google Scholar]
- Sinha SC, Sprang SR. Structures, mechanism, regulation and evolution of class III nucleotidyl cyclases. Rev Physiol Biochem Pharmacol. 2006;157:105–140. doi: 10.1007/112_0603. [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu Y, Ruoho AE, Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature. 1997;386:247–253. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα·GTPγS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Johnson RA, Gosselin G, Gilman AG, Sprang SR. Two-metal-ion catalysis in adenylyl cyclase. Science. 1999;285:756–760. doi: 10.1126/science.285.5428.756. [DOI] [PubMed] [Google Scholar]
- Wedel B, Garbers D. The guanylyl cyclase family at Y2K. Annu Rev Physiol. 2001;63:215–233. doi: 10.1146/annurev.physiol.63.1.215. [DOI] [PubMed] [Google Scholar]
- Tucker CL, Hurley JH, Miller TR, Hurley JB. Two amino acid substitutions convert a guanylyl cyclase, RetGC-1, into an adenylyl cyclase. Proc Natl Acad Sci USA. 1998;95:5993–5997. doi: 10.1073/pnas.95.11.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Beuve A, Tesmer JJG, Sprang SR, Garbers DL, Gilman AG. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem. 1998;273:16332–16338. doi: 10.1074/jbc.273.26.16332. [DOI] [PubMed] [Google Scholar]
- Sinha SC, Wetterer M, Sprang SR, Schultz JE, Linder JU. Origin of asymmetry in adenylyl cyclases: structures of Mycobacterium tuberculosis Rv1900c. EMBO J. 2005;24:663–673. doi: 10.1038/sj.emboj.7600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tews I, Findeisen F, Sinning I, Schultz A, Schultz JE, Linder JU. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005;308:1020–1023. doi: 10.1126/science.1107642. [DOI] [PubMed] [Google Scholar]