Abstract
The major physiologic theory of aging, the disposable soma theory, links dietary restriction (DR), also known as calorie or food restriction, to prolonged lifespan and makes specific predictions about the effects of aging and DR on reproduction. A recent study in BMC Biology profiling the effects of aging and DR on gonadal gene expression provides novel molecular evidence that has a significant impact on this theory of aging.
In 1935, McCay et al. [1] published a landmark study describing the unexpected finding that dietary restriction (DR) increases the maximum lifespan of the white rat. Fifty years later, Weindruch et al. [2] modified and refined the experimental method to show that as little as 25% DR significantly extended the mean and maximum lifespan of mice and that more severe DR extended lifespan even further. A retrospective analysis of multiple DR studies performed in rodents showed that decreased caloric intake and increased duration of DR are each directly proportional to increased survival [3]. This tight correlation between DR and longevity, along with the observation that senescence is experienced by nearly all organisms with a distinction between germline and somatic tissues, forms the basis for a prevailing physiological theory of aging, the 'disposable soma' theory [4].
The idea that senescence is the result of natural selection rests on the following arguments. Despite the fact that lifespan extension could result in additional offspring and thus increased Darwinian fitness, extrinsic forces, such as predation, are the most important factor affecting lifespan in a natural environment. Thus, even though energetically costly somatic maintenance could potentially increase the intrinsic lifespan of an organism and therefore improve its Darwinian fitness, because of extrinsic forces, this is not a selected characteristic. Instead, increased investment in reproduction, while also energetically costly, is of greater benefit to Darwinian fitness, resulting in the side effect of senescence for the 'disposable soma'.
The disposable soma theory specifically predicts that there are energetic trade-offs between reproduction and somatic maintenance such that during times of famine, or DR, energy is allocated away from reproduction towards somatic maintenance [4-6]. This makes evolutionary sense for two main reasons. First, if there is a food shortage, then it would be advantageous for reproduction to be temporarily halted because this would result in conservation of food for existing parents and offspring until the food resources have been replenished. Second, gestation and lactation are energetically costly, so if there is not enough food to support these processes, then mother, child and future offspring would be lost. In the controlled laboratory environment, increased lifespan of food-restricted animals is possibly the result of a defense mechanism that evolved because it increased Darwinian fitness [6].
With the advent of microarray technology, investigators have been able to test some of the predictions that the disposable soma theory makes about aging and the changing physiology of food-restricted animals. It has also permitted a survey of gene-expression changes in an unbiased manner to determine whether aging causes any universal changes that could be directly opposed by DR [7]. A recent study by Sharov et al. in BMC Biology [8] characterizes gene-expression changes in aging gonads and the effect of DR on those changes. This study is important not only to the field of reproductive biology but also to aging research because, according to the disposable soma theory [9], many of the beneficial effects of DR are thought to be due to the reallocation of resources away from reproduction towards somatic maintenance. The results of Sharov and colleagues suggest that this model may need revision.
Dietary restriction and aging in reproduction
Considering the importance placed on the distinction between germline and somatic tissues in any theory of aging describing the evolution of senescence, surprisingly little work has been done to examine the changes in the reproductive system with aging and/or DR. The disposable soma theory of aging predicts that the maintenance needed to preserve the immortality of the germline may be greater than the maintenance used in somatic cells, suggesting that the energetic needs of the gonads are large [9]. One hypothesis for the mechanism of life extension in response to DR is that organisms have evolved a dynamic resource-allocation system in which energy is transferred from reproduction to somatic maintenance [9], but there is little molecular evidence to support this hypothesis.
Consider the molecular changes in both the ovary and the testis in response to DR at the peak of the reproductive time period in the mouse (6 months). Although long-term DR suspends ovulation [10] and affects sperm quality and counts [11], the microarray data of Sharov et al. [8] reveal very few gene-expression changes in the testis and modest changes in gene expression in the ovary. Also, the genes with lowered expression in the food-restricted ovary are not involved in cellular maintenance or other energetically costly functions. As the lifespan-extension benefits of DR are seen in non-mating mice of both genders, it is unlikely that the extra energy allocated to somatic maintenance is derived directly from energy savings in the gonads.
Extension of the reproductive period in a food-restricted female would also be a natural conclusion of the disposable soma theory. This is supported by the finding that long-term DR was partially protective against both lengthening of the estrus cycle and depletion of primordial follicles, thus extending the reproductive period of these female mice on return to a normal diet [10]. Future efforts to profile the ovarian, pituitary and hypothalamic molecular changes in a similar study would provide further insight into the mechanisms of the protective effects of DR that could be compared and contrasted to other tissues.
Expansion of the disposable soma theory of aging
Although it remains unclear if and how the processes of reproductive and somatic maintenance are energetically or molecularly connected, the neuroendocrine system is an interesting area for future endeavors as it represents a possible link between reproductive and somatic tissues. Surgical removal of the pituitary gland at 1 and 9 months, but not at 11 months, has been shown to significantly improve the mean and maximum lifespan of mice [12]. It should be noted that the age of 11 months is near the end of female fertility, so the fact that hypophysectomy does not result in lifespan extension after this age suggests that the longevity response to hypophysectomy is evolutionarily conserved. A variety of mouse mutants involving the pituitary – including growth hormone (GH) receptor knockout, Ames dwarf and Snell dwarf mice – exhibit both increased longevity and decreased fecundity. However, sterility in Ames and Snell dwarf mice may be reversed by prolactin treatment, and GH receptor knockout mice have fertility defects but are not sterile. In addition, female mice with a deletion of a single allele of the insulin-like growth factor (IGF)-1 receptor or with an adipose-specific knockout of the insulin receptor, both downstream targets of GH action, show significant life extension with normal fertility [13].
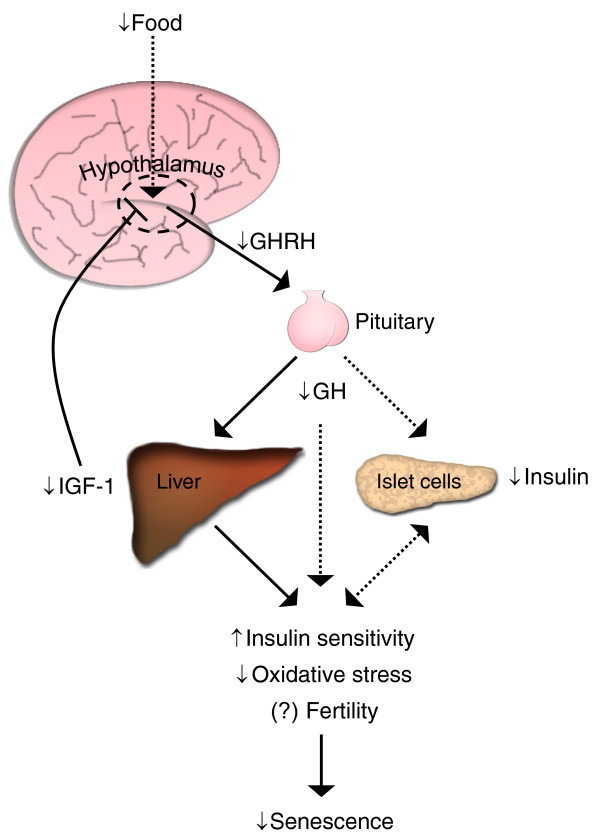
It is clear from microarray studies over multiple tissues that DR upregulates metabolic and biosynthetic genes [7], but why does this happen in an energetically restricted state? The disposable soma theory suggests that these molecular mechanisms of somatic maintenance are upregulated to prolong lifespan, thereby increasing Darwinian fitness, but gives no insight into how this upregulation occurs. Microarray studies also indicate that stress and immune/inflammatory response pathways may be downregulated over multiple tissues. Again, neuroendocrine signaling could possibly be involved, as mice with mutations in the GH/IGF-1/insulin axis that increase longevity have increased insulin sensitivity, improved stress response and decreased oxidative damage [14]. Interestingly, GH receptor knockout mice do not experience any further lifespan extension with DR, suggesting that an intact somatotropic axis is critical to DR-mediated longevity (Figure 1) [15]. We propose that diminished cellular aging induced by DR is not a cell-autonomous process induced by energy shortage as suggested by the disposable soma theory, but instead is a neuroendocrine process induced by DR-mediated changes to the hypothalamus.
Figure 1.
Alterations in somatotropic signaling caused by DR. Decreased food intake is sensed by the hypothalamus leading to diminished release of growth hormone releasing hormone (GHRH). This signals to the pituitary to secrete less growth hormone (GH). GH-responsive cells in the liver then synthesize lower amounts of insulin-like growth factor (IGF)-1, which results in lower negative feedback to the hypothalamus to regulate GHRH release. Perhaps in an indirect manner, GH causes increased insulin sensitivity and decreased insulin production from pancreatic islet cells. Although the direct and/or indirect effects on reproduction remain unclear, the changes in somatotropic signaling from DR leading to increased insulin sensitivity and reduced oxidative stress might be the mechanism whereby DR delays senescence [14,16].
There is much debate as to whether a magic potion can be discovered to slow or reverse the signs of aging in humans. It is also unclear whether the 'elixir of life' will come at the expense of reproduction since most experimental manipulations that extend lifespan also reduce fecundity. Perhaps, however, this is a misperception. Maybe these processes can be separated, and aging can be staved off without a concomitant loss in fertility – one can hope.
Acknowledgments
Acknowledgements
RLN has been supported by the MSTP and Hudson Scholar Fund. Research on aging and reproductive biology in the Pletcher and Matzuk laboratories is supported by the National Institutes of Health.
Contributor Information
Roopa L Nalam, Email: rn140107@bcm.edu.
Scott D Pletcher, Email: pletcher@bcm.edu.
Martin M Matzuk, Email: mmatzuk@bcm.edu.
References
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34:1340–1354. doi: 10.1016/S1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? BioEssays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TB. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev. 2008;129:138–153. doi: 10.1016/j.mad.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB, Longo DL, Schlessinger D, Ko MSH. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol. 2008;6:24. doi: 10.1186/1741-7007-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32:515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Brinkworth MH, Anderson D, McLean AE. Effects of dietary imbalances on spermatogenesis in CD-1 mice and CD rats. Food Chem Toxicol. 1992;30:29–35. doi: 10.1016/0278-6915(92)90133-6. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Harrison DE, Flurkey K. Pituitary removal in adult mice increases life span. Mech Ageing Dev. 2006;127:658–659. doi: 10.1016/j.mad.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Hormonal regulation of longevity in mammals. Ageing Res Rev. 2007;6:28–45. doi: 10.1016/j.arr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]