In this issue of PNAS, Schoonheydt, Solomon, and coworkers (1) definitively characterize the structure of the Cu-ZSM-5 site that oxidizes methane to methanol. With the successful correlation of a specific copper-oxy structure to such reactivity, the importance of this work extends beyond hydrocarbon oxidation on zeolites. This breakthrough potentially has far-reaching implications in both the fundamental and applied sciences.
Scientific interest focuses on the oxidation of methane as a chemical feedstock and alternative energy source (2, 3, 15). Methane constitutes the bulk of abundant natural gas, but its industrial processing requires an energy- and infrastructure-intensive multistep reforming via “synthesis gas” (CO plus H2). C–H bond activation has long been a prime obsession of organometallic chemists, for example, and current efforts focus on finding highly active, robust catalysts for mild oxidation of methane and other alkanes (2–4, 15). Promising alkane-oxidizing inorganic compounds have been discovered, especially Pt complexes following the breakthrough Shilov catalytic system (Fig. 1) (3–5, 15). Many prominent experimental and computational researchers have made great strides on C–H bond breaking at metal centers, providing mechanistic insights and the identification of discrete compounds or materials for homo- or heterogeneous catalysis.
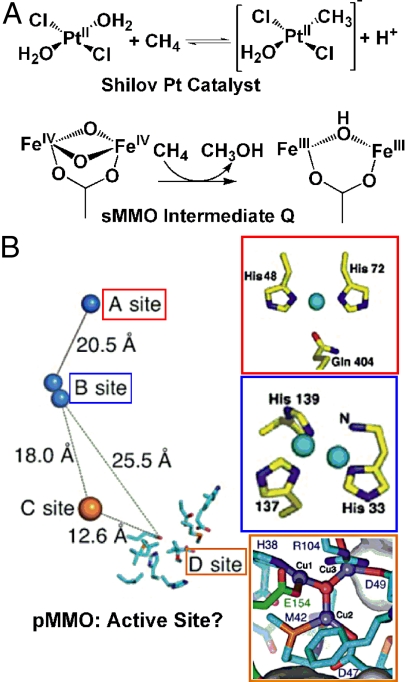
Fig. 1.
Synthetic and biological examples of methane-oxidizing metal species (A) and possible pMMO active sites, as determined by X-ray crystallography or modeling (B). sMMO, soluble methane monooxygenase; pMMO, particulate methane monooxygenase.
None of these systems has yet demonstrated catalytic utility for the industrial scale; all suffer from some combination of low selectivity, catalyst instability, and high temperature and pressure requirements (3, 4, 15). The holy grail of methane-oxidizing catalyst would be robust toward decomposition, rapid, selective for oxidizing methane to methanol, and active at low temperature and pressure. The Cu-ZSM-5 zeolite sets new standards in these regards while achieving selectivity in excess of 98% (6).
Yet Nature far outpaces the laboratory chemist and has found not one, but two solutions to harnessing methane as an energy source and for the synthesis of the molecules required for life: the soluble methane monooxygenase (sMMO), found in the cytosol of some methane-metabolizing bacteria (7), and particulate methane monooxygenase (pMMO), a methanotrophic integral membrane protein (8). The MMOs are unique not for Nature's choice of metals—Fe for sMMO, Cu for pMMO—but for their use of these “ordinary” cofactors for the extraordinary oxidation of methane C–H, at 104 kcal/mol, the strongest hydrocarbon bond (1, 9).
Inorganic chemists have attempted to understand or even reproduce the reactivity of enzymes by studying small-molecule metal complexes. Yet replication of the efficiency of Nature's exquisitely crafted “little reactors” remains a near impossibility even when an enzyme's structure and mechanism are not shrouded in mystery—the state in which current studies of pMMO are mired. sMMO appears to employ a high-valent nonheme dinuclear iron(IV) site to activate dioxygen to oxidize methane (Fig. 1) (7). For pMMO, recent crystal structures unveiled pMMO's (possible) metal sites (Fig. 1) but have also added confusion (8). That copper is the catalytic cofactor is one point of strong agreement in the contentious argument over the location, identity, and structure of the active site. But in addition to the sites found by X-ray crystallography, trinuclear Cu clusters have been proposed to lurk within pMMO, and one such site successfully modeled into the protein manifold (Fig. 1) (10).
The elucidation of the methane oxidizing site in Cu-ZSM-5, then, could potentially shed light on CH4 oxidation in pMMO. Several classes of Cux–O2 adducts demonstrate the ability to oxidize C–H bonds, but only those that are fairly weak (<90 kcal/mol) (9, 11). The few complexes capable of oxidizing stronger C–H bonds are poorly understood, although a mixed-valent CuII–O2–CuIII core was suggested to initiate certain C–H bond abstraction reactions (9).
The present contribution establishes the first specific copper-oxo structure with oxidative reactivity toward methane. With resonance-enhanced Raman (rR) spectroscopic interrogation of the Cu-ZSM-5 chromophore absorbance at 440 nm, including observation of 18O-sensitive vibrations, the researchers were able to tease out the real active site, distinguishing it from the excess of spectator copper ions present. Bolstered by density functional theory (DFT) calculations and normal coordinate analysis, the spectroscopic studies conclusively determined the geometric and electronic structure of the bent CuII–O–CuII (Fig. 2) active site. Remarkably, some half-a-dozen variants of oxidatively capable O2- or H2O2-derived complexes with the Cux–Oy(H) formula have been spectroscopically and/or structurally characterized in discrete inorganic complexes, yet none has the capacity to oxidize methane (9); and in the case of Cu-ZSM-5, the present study ruled all of them out. Complexes formulated with a CuII–O–CuII core are not entirely absent from the literature (12); however, they are not well characterized and thus far display very limited reactivity.
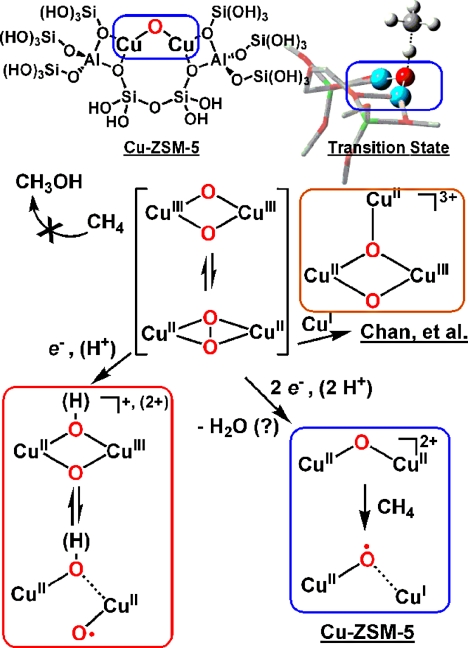
Fig. 2.
Hypothesized methane-oxidizing copper-oxy species. (Upper) The Cu-ZSM-5 active site model and C–H abstraction transition state. (Lower) Proposed evolution of methane-oxidizing Cu–O cores by reduction of species inert toward alkane C–H.
What makes the Cu-ZSM-5 site so reactive toward C–H bonds? The first copper intermediate along the calculated reaction coordinate, a CuII–OH–CuI species, possesses a strong (90 kcal/mol) O–H bond, making the prior C–H abstraction step from methane endothermic by a mere 13 kcal/mol. The active site at the transition state possesses a low-lying singly occupied molecular orbital (SOMO) centered on oxygen, perpendicular to the Cu–O–Cu plane and projecting into the zeolite cavity to abstract H···CH3 (Fig. 2). Theoretical studies probing possible pMMO mechanisms have suggested an important role for O-centered radicals (13). Intriguingly, the Cu-ZSM-5 site evolves along the reaction coordinate with a nonuniform allocation of electron density from the lattice ligands, such that the electronic structure of the core becomes asymmetric. One of the copper sites develops significant copper-oxyl (radical), i.e., “cupryl” character (1), a species described only theoretically but attributed with high reactivity toward C–H bonds (1, 13, 14).
A theoretical picture of methane oxidation at copper-oxo sites emerges in which unpaired electron density roughly localized on a Cu-bound oxo ligand may be critical for reactivity (see Fig. 2). Mononuclear cupryl or one-electron-reduced CuII–(O)(O(H))–CuIII dinuclear species [calculated to be significantly more reactive toward hydrocarbon bonds than its CuIII–CuIII congener (13)] fit the bill, and have been tantalizing if unrealized suggestions in reactivity of model complexes toward hydrocarbon substrates (Fig. 2) (9). The long-championed trinuclear cluster active site is calculated to proceed via an oxene-insertion into the methane C–H bond (10). This proposal highlights a more general point: To impart the reactivity required for methane oxidation, reduction (provided by e−, H·, or CuI) of the currently well-characterized copper-oxy cores is required (Fig. 2).
Might the CuII–O–CuII structure itself await discovery in pMMO? The X-ray dinuclear center (B site in Fig. 1) provides a possible home for such a core; but here the differences between Cu-ZSM-5 and the nature of protein ligands in pMMO may become key. The authors invoke zeolite lattice structural constraints and the effects due to the decidedly nonbiological ligands (oxides of aluminate and silicate, Fig. 2) to rationalize the unique electronic structure and oxidative power of the Cu-ZSM-5 active species. The reactivity of the core may vanish if it is removed from the zeolite environment. Future research should focus on how the CuII–O–CuII species possibly translates to the context of protein environments; the structure will likely become a focus of a bioinorganic field invigorated by the proposal. While such studies may circumscribe the relevance of Cu-ZSM-5 for understanding biological methane oxidation, the report commented on here lays crucial general groundwork for the important problem of harnessing CH4 for energy and synthesis.
Yet, how does zeolite copper reduce dioxygen? The authors conjecture that a four-electron reduction of O2 by the two active-site copper ions is followed by e−-transfer from two spectator CuI ions. What happens to the other oxide and what intermediary species form? What is the mechanism? These and other questions are important issues in terms of energy metabolism. Learning how to harness dioxygen—that abundant, mild, environmentally benign oxidant—for the catalytic transformation of chemical bonds on the industrial scale would be a giant step toward establishing cheap, sustainable, clean, large-scale synthesis as the norm, rather than the exception. The present identification of a copper-oxo species capable of oxidizing methane is a major step forward.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18908.
References
- 1.Woertink JS, et al. A [Cu2O]2+ core in Cu-ZSM-5, the active site in the oxidation of methane to methanol. Proc Natl Acad Sci USA. 2009;106:18908–18913. doi: 10.1073/pnas.0910461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa H, et al. Catalysis research of relevance to carbon management: Progress, challenges, and opportunities. Chem Rev. 2001;101:953–996. doi: 10.1021/cr000018s. [DOI] [PubMed] [Google Scholar]
- 3.Conley BL, et al. Design and study of homogeneous catalysts for the selective, low temperature oxidation of hydrocarbons. J Mol Catal A. 2006;251:8–23. [Google Scholar]
- 4.Chen GS, et al. The role of alkane coordination in C–H bond cleavage at a Pt(II) center. Proc Nat Acad Sci USA. 2007;104:6915–6920. doi: 10.1073/pnas.0610981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shilov AE, Shul'pin GB. Activation of C–H Bonds by metal complexes. Chem Rev. 1997;97:2879–2932. doi: 10.1021/cr9411886. [DOI] [PubMed] [Google Scholar]
- 6.Groothaert MH, et al. Selective oxidation of methane by the bis(mu-oxo)dicopper core stabilized on ZSM-5 and mordenite zeolites. J Am Chem Soc. 2005;127:1394–1395. doi: 10.1021/ja047158u. [DOI] [PubMed] [Google Scholar]
- 7.Kopp DA, Lippard SJ. Soluble methane monooxygenase: activation of dioxygen and methane. Curr Opin Chem Biol. 2002;6:568–576. doi: 10.1016/s1367-5931(02)00366-6. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian R, Rosenzweig A. Structural and mechanistic insights into methane oxidation by particulate methane monooxygenase. Acc Chem Res. 2007;40:573–580. doi: 10.1021/ar700004s. [DOI] [PubMed] [Google Scholar]
- 9.Himes RA, Karlin KD. Copper-dioxygen complex mediated C-H bond oxygenation: relevance for particulate methane monooxygenase (pMMO) Curr Opin Chem Biol. 2009;13:119–131. doi: 10.1016/j.cbpa.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan SI, Yu SS-F. Controlled oxidation of hydrocarbons by the membrane-bound methane monooxygenase: The case for a tricopper cluster. Acc Chem Res. 2008;41:969–979. doi: 10.1021/ar700277n. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EA, Tolman WB. Reactivity of dioxygen-copper systems. Chem Rev. 2004;104:1047–1076. doi: 10.1021/cr020633r. [DOI] [PubMed] [Google Scholar]
- 12.Karlin KD, et al. Copper(I) dioxygen reactivity, 2. Reaction of a three-coordinate Cu(I) complex with O2 with evidence for a binuclear oxo-Cu(II) species: Structural characterization of a parallel-planar di-hydroxo bridged dimer. Inorg Chem. 1984;23:519–521. [Google Scholar]
- 13.Shiota Y, Yoshizawa K. Comparison of the reactivity of bis(mu-oxo)CuIICuIII and CuIIICuIII species to methane. Inorg Chem. 2009;48:838–845. doi: 10.1021/ic8003933. [DOI] [PubMed] [Google Scholar]
- 14.Gherman BF, et al. Characterization of the structure and reactivity of monocopper-oxygen complexes supported by beta-diketiminate and anilido-imine ligands. J Comput Chem. 2006;27:1950–1961. doi: 10.1002/jcc.20502. [DOI] [PubMed] [Google Scholar]
- 15.Conley B, et al. Methane functionalization. In: Tolman WB, editor. Activation of Small Molecules: Organometallic and Bioinorganic Perspectives. New York: Wiley-VCH; 2006. pp. 235–286. [Google Scholar]