Abstract
The mitotic checkpoint has evolved to prevent chromosome mis-segregations by delaying mitosis when unattached chromosomes are present. Inducing severe chromosome segregation errors by ablating the mitotic checkpoint causes cell death. Here we have analyzed the consequences of gradual increases in chromosome segregation errors on the viability of tumor cells and normal human fibroblasts. Partial reduction of essential mitotic checkpoint components in four tumor cell lines caused mild chromosome mis-segregations, but no lethality. These cells were, however, remarkably more sensitive to low doses of taxol, which enhanced the amount and severity of chromosome segregation errors. Sensitization to taxol was achieved by reducing levels of Mps1 or BubR1, proteins having dual roles in checkpoint activation and chromosome alignment, but not by reducing Mad2, functioning solely in the mitotic checkpoint. Moreover, we find that untransformed human fibroblasts with reduced Mps1 levels could not be sensitized to sublethal doses of taxol. Thus, targeting the mitotic checkpoint and chromosome alignment simultaneously may selectively kill tumor cells by enhancing chromosome mis-segregations.
Keywords: aneuploidy, CIN, mitosis, paclitaxel
Error-free chromosome segregation is vital for embryonic development and tissue homeostasis in multicellular organisms. Whereas during development the vast majority of single mis-segregation events is not tolerated (1), aneuploidy is a common genetic alteration in solid human tumors (2). In ex vivo cultures, such aneuploid tumor cells display low but significant frequencies of chromosome mis-segregations (chromosomal instability, CIN) (3). The mitotic checkpoint is one of the cell cycle checkpoints that have evolved to safeguard cells from CIN (4, 5). This checkpoint ensures the fidelity of sister chromatid segregation over the two daughter cells by inhibiting progression to anaphase until all sister chromatids are attached to microtubules of the mitotic spindle [reviewed in (6)]. Defects that lead to reduced mitotic checkpoint signaling cause aneuploidy and may eventually contribute to tumorigenesis in humans [reviewed in (5, 7)]. Although an intriguing hypothesis and a clear causative link in experimental animal models [reviewed in (8)], it has never been shown that compromised checkpoint signaling directly underlies CIN in malignant human tissue or cultured human tumor cells (9, 10). In contrast, complete inactivation of the mitotic checkpoint results in gross chromosome mis-segregations and is not compatible with cell viability (11, 12). This has led to the suggestion that inhibition of the mitotic checkpoint could have therapeutic potential in cancer treatment (5).
A widely used anti-mitotic drug is paclitaxel (taxol), which induces a mitotic checkpoint-dependent delay at high dose by inhibiting microtubule dynamics (10, 13, 14). At low, clinically relevant concentrations, taxol treatment induces aneuploidy without severely delaying cells in mitosis (14–16). Controversial results have been reported on the effect of mitotic checkpoint inhibition on taxol-induced cell death. Incomplete functioning of the checkpoint has been suggested to induce resistance to high doses of taxol (17–20), which is in line with the observation that CIN also correlated with taxol resistance (21). However, others have shown that inhibition of the mitotic checkpoint increases the effectiveness of taxol (22).
Here, we have explored the relationship between the level of chromosome mis-segregation and cell death in two severely aneuploid tumor cell lines, two diploid tumor cell lines and one immortalized fibroblast line. We show that reducing levels of the essential mitotic regulators Mps1 and BubR1 sensitizes human tumor cells, but not normal cells, to death by clinically relevant doses of taxol. This sensitization is directly due to the ability of the combined treatments to cause severe chromosome segregation errors in tumor cells.
Results and Discussion
Partial Knockdown of Mps1 or BubR1 Weakens the Mitotic Checkpoint but Does Not Affect Tumor Cell Viability.
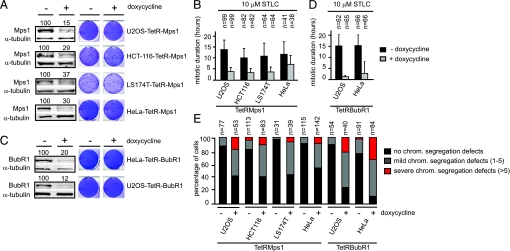
Absence of essential mitotic checkpoint components such as BubR1 or Mps1 causes cell death within six cell divisions (12, 23, 24). To further examine the therapeutic potential of inhibition of these proteins, we generated panels of cancer cells with reduced expression of Mps1 and BubR1. To test the effect on different types of cells, we created monoclonal lines of two aneuploid tumor cell lines (U2OS and HeLa) that expressed doxycycline-inducible Mps1 or BubR1 shRNA and two (near)diploid tumor cell lines (HCT-116 and LS174-T) that expressed doxycycline-inducible Mps1 shRNA. Cell lines were selected in which Mps1 or BubR1 expression was reduced between 60–88% upon doxycycline addition (Fig. 1 A and C). In contrast to cell lines that have an almost complete removal of Mps1 or BubR1 protein [Fig. S1A and (12, 24)], partial depletions of Mps1 or BubR1 had no severe effect on cell viability (Fig. 1 A and C). We only observed a moderate increase in cell death (18% vs. 4% in control) as observed by live cell imaging of propidium iodide (PI) uptake after partial depletion of Mps1 in U2OS-TetRMps1 cells (0 nM taxol) (Fig. S2 A and B). Despite the ability of the different cell lines to survive with significantly reduced levels of Mps1 or BubR1, their ability to delay in mitosis in response to spindle disruption was clearly affected. When U2OS-TetRMps1 cells were treated with 10μΜ STLC, an Eg5 inhibitor that causes a checkpoint-dependent mitotic delay by preventing bipolar spindle assembly (Fig. S1B) (24), they were delayed in mitosis for 13.2 h on average (Fig. 1B). However, upon partial depletion of Mps1 (+doxycycline) the mean duration of the mitotic arrest was reduced to 3.7 h (Fig. 1B). With the possible exception of HeLa-TetRMps1 cells, the ability of the other inducible cell lines to prolong the duration of mitosis upon treatment with STLC was similarly affected when Mps1 or BubR1 protein levels were reduced (Fig. 1 B and D).
Fig. 1.
Partial inactivation of Mps1 or BubR1 leads to chromosome segregation defects but fails to compromise tumor cell viability. (A and C) Left: Indicated cell lines, treated without (-) and with (+) doxycycline (dox) for 3 days, were immunoblotted for Mps1 or BubR1 and α-tubulin. Values above blots represent relative amount of Mps1 or BubR1 protein levels. Right: Colony formations of indicated cell lines, treated with and without dox for 11 days. (B and D) Live cell analysis (DIC) of the mean mitotic duration (+SD) in the presence of 10μΜ STLC of indicated cell lines treated with or without doxycycline for 3 days. Mitotic duration was determined as the time from nuclear envelope breakdown (NEB) to the first attempt of cytokinesis (membrane blebbing). n = amount of cells filmed per condition. (E) Quantification of time-lapse movies as performed in representative images. Dox was added 3 days before filming. n = amount of cells filmed per condition.
To examine the effect of reduced mitotic checkpoint activity in cells partially depleted of Mps1 or BubR1 on the fidelity of chromosome segregations in an unperturbed mitosis, anaphase progression was followed by time-lapse microscopy (Fig. S1C). The percentage of U2OS-TetRMps1 cells that displayed mild chromosome segregation defects in anaphase increased from 13% in control cells to 40% upon partial Mps1 depletion. The percentage of cells that severely mis-segregated their chromosomes increased from 0 to 18% (Fig. 1E). A similar increase in chromosome mis-segregations was observed in the other Mps1 and BubR1 clones, where the percentage of mild mis-segregations increased from approximately 10% to approximately 55%, while severe mis-segregations increased from approximately 4% to approximately 30% (Fig. 1E). Interestingly, as colony formation was not affected (Fig. 1 A and C), increasing the percentage of chromosome segregation errors to more than 50% (around one-third of which were severe mis-segregations), appeared to be well tolerated by the cell population as a whole in the four tumor cell lines tested. The effects on cell viability and chromosome segregation of the shRNAs used in this study were specific for knockdown of the respective proteins [Fig. S3 and (12, 25)].
Partial Depletion of Mps1 or BubR1 Sensitizes Tumor Cells to Clinically Relevant Doses of Taxol.
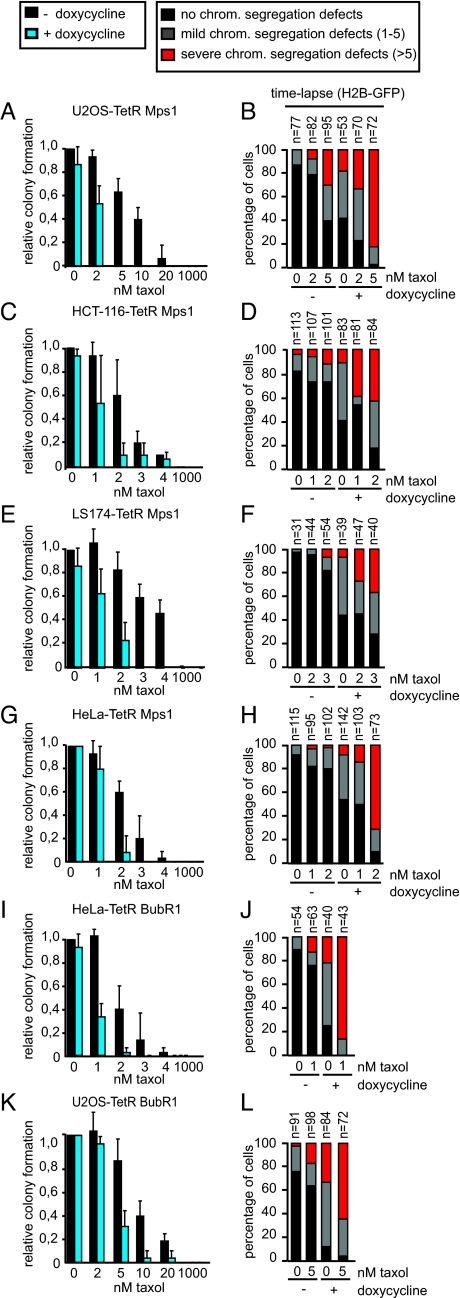
The observed correlation between the level of Mps1 or BubR1 depletion, the severity of chromosome mis-segregations and the extent of cell death pressed us to investigate if increasing the amount of segregation errors beyond that obtained by partial depletion of Mps1 or BubR1 would now impact tumor cell viability. To this end we introduced low, clinically relevant doses of taxol (1–10 nM) that are known to cause aneuploidy (15, 16) without causing the severe mitotic delay that occurs at high doses of taxol (50 nM–10 μM) (10, 14, 26). We found that in U2OS-, HCT-116-, LS174-, and HeLa-TetRMps1 cells with normal Mps1 levels, viability as well as the fidelity of chromosome segregation was only marginally compromised at 2 nM taxol (−doxycycline, 2 nM taxol) (Fig. 2 A–H). However, viability was drastically reduced by this low dose of taxol when Mps1 expression was additionally partially suppressed (+doxycycline, 2 nM taxol) (Fig. 2 A, C, E, and G): the number of colonies was reduced 2- to 10-fold when compared to doxycycline treatment alone in U2OS-, HCT-116-, LS174T, and HeLa-TetRMps1 cells (Fig. 2 A, C, E,and G). This lethality correlated strongly with an increase in the frequency and severity of segregation errors (+doxycycline, 2 nM taxol) (Fig. 2 B, D, F, and H). A slight increase in the dosage of taxol in U2OS-TetRMps1 cells (from 2 to 5 nM) or in LS174-T-TetRMps1 cells (from 2 to 3 nM) induced a moderate increase in chromosome mis-segregations when Mps1 levels were kept high, but only a partial reduction in colony formation was observed (Fig. 2 A, B, E, and F). Nonetheless, additional partial depletion of Mps1 at these slightly higher taxol concentrations completely blocked colony formation (Fig. 2 A and E and Fig. S2D), which correlated with an even further increase in the amount and severity of segregation errors (Fig. 2 B and F). We confirmed that the observed reduction in colony formation capacity was due to cell death, and not due to a postmitotic arrest, by filming U2OS-TetRMps1 cells in the presence of PI on days 4, 5, and 6 after treatment (Fig. S2 A and B).
Fig. 2.
Low doses of taxol reduce the viability of Mps1 or BubR1 depleted tumor cells. (A, C, E, G, I, and K). Quantification of colony formations of indicated cell lines treated with and without dox for 11 days. Indicated taxol concentrations were added 1 day after dox administration. Colony formation capacity of control cells (no taxol treatment) was set at 1. Average of three independent experiments is shown. (B, D, F, H, J, and L). Quantification of time-lapse movies performed as in Fig. 1E. Indicated taxol concentrations were added 1 h before filming. n = amount of cells filmed.
The frequency of severe segregation errors correlated well with increased aneuploidy after four divisions as determined by chromosome spreads (Fig. S4A). LS174T-TetRMps1 cells were (near-)diploid in the absence of any treatment. After treatment with doxycycline or 3 nM taxol the karyotype resembled that of control cells. This is in line with previous observations in which aneuploid cells in (near)diploid cell lines are removed from the population (9). Nevertheless, upon combined treatment of LS174T-TetRMps1 cells with taxol and doxycycline, the observed chromosome segregation errors (3 nM taxol) (Fig. 2F) induced a clear increase in aneuploidy and multinucleated cells (Fig. S4 A and C). Although untreated U2OS-TetRMps1 cells were already significantly aneuploid, a clear increase in the breadth of distribution in the karyotypes was observed upon combining low doses of taxol and partial Mps1 knockdown (Fig. S4A), a combination that leads to the demise of the population (Fig. 2A).
The synergistic effect with low doses of taxol was not specific for partial Mps1 depletion, since reduced BubR1 levels also sensitized HeLa and U2OS cells to low doses of taxol (Fig. 2 I and K and Figs. S2C and S3). This correlated with an increase in severe chromosome mis-segregations and enhanced aneuploidy, as revealed by the karyotypes of the HeLa-TetRBubR1 cells (Figs. 2 J and L and S4A).
To rule out the possibility that the synergy in cell killing by low taxol and reduction of Mps1 or BubR1 was due to clonal variations within one cell type, we used titration of doxycycline in the U2OS clone in which high doxycycline addition (1 μg/mL) causes almost full depletion of Mps1 (U2OS-TetRMps1-clone #2; see Fig. S1A). Such titrations enabled us to reach a reduction of Mps1 levels to 22% by adding only 2 ng/mL doxycycline (Fig. S5 A and B). In agreement with results obtained with the ‘partial reduction’ clone U2OS-TetRMps1 (Fig. 1A), the reduced levels of Mps1 achieved by 2 ng/mL doxycycline addition did not cause lethality on its own, whereas additional administration of 5 nM taxol did (Fig. S5 A and D). This again correlated with the amount of chromosome segregation errors (Fig. S5C). Unexpected side-effects of doxycycline administration on cell viability were also ruled out, as we observed no toxicity of simultaneous addition of doxycycline and low doses of taxol in the different founder cell lines (Fig. S6). Finally, the sensitization to low taxol we observed was not due to a general sensitization to cytotoxic drugs, since partial depletion of Mps1 or BubR1 did not synergize with low doses of doxorubicin, a drug resulting in induction of double-strand DNA breaks (Fig. S7).
To further address our hypothesis that cell death in our experiments was caused by inducing a certain threshold level of chromosome mis-segregations and not, for instance, by cytotoxic effects of taxol, we introduced siRNAs against CENP-E, KIF18A, or HEC-1, all known to cause chromosome congression defects upon knockdown in human cells (27, 28). Knockdown of these proteins mimicked the effects of low taxol: simultaneous reduction of Mps1 and RNAi of CENP-E, KIF18A, or HEC-1 in U2OS-TetRMps1 cells increased cell death compared to siRNA transfected controls (Fig. S8A). Importantly, cell death in CENP-E RNAi cells partially depleted of Mps1 correlated with an increase in severe segregation errors (Fig. S8 B and C).
The differential sensitivity to low taxol when reducing Mps1 or BubR1 was no longer observed when using very high doses of taxol (= 1000 nM taxol) (Fig. 2). Although this is in apparent contradiction with reports suggesting that depletion of mitotic checkpoint components can rescue the lethality induced by high doses of taxol (50 nM–10 μM) (18–20), in those studies cell death was analyzed within 48 h after taxol treatment. Indeed we have previously also observed that compromising the mitotic checkpoint causes resistance to high doses of spindle poisons in the short term (12), most likely because mitotic checkpoint inhibition prevents mitotic catastrophy in the presence of those high doses. Cell death is not induced in the short term in those analyses because the deleterious effects of severe chromosome mis-segregations are not expected to become apparent before 2–6 divisions have occurred (12). In support of this, the present study determined the effect on cell viability after 4–11 days and we find that high doses of taxol are equally toxic to parental as well as Mps1- or BubR1-depleted cells (= 1000 nM taxol) (Fig. 2).
The apparent resistance of CIN cells to high taxol treatment (21) and chromosome mis-segregations (9) has led to the hypothesis that these cells have obtained a survival mechanism that protects them against cell death induced by aneuploidy. In contrast, stable diploid (tumor) cells, which have become aneuploid, are sensitive to the genome unbalances and are removed from the cell population (9). To address the question whether such aneuploidy-tolerance mechanisms would allow CIN cells to also tolerate simultaneous Mps1 depletion and taxol treatment, we tested the effect of this combined treatment on the CIN cell line SW480. As expected (29), these cells displayed frequent chromosome mis-segregations in the absence of any treatment (Fig. S8G untreated). Similar to what was observed with the other four tumor cell lines (Fig. 2), simultaneous treatment of SW480 cells with low taxol (10 nM) and partial depletion of Mps1 (Fig. S8D) enhanced the frequency and severity of chromosome segregation errors (Fig. S8G) and caused cell death (Fig. S8 E and F). It is important to note that the level of chromosome mis-segregations induced by this combined treatment reached far beyond the level of mis-segregations found in CIN tumor cells [(29) and Fig. S8G]. In all likelihood, therefore, the tolerance to aneuploidy of CIN tumor cells is insufficient to cope with the severity of chromosome segregation errors induced by our treatments.
Together, our data show that substantial reduction of Mps1 or BubR1 leads to an increase in mild chromosome segregation errors but fails to induce massive tumor cell death. However, combining reduction in Mps1 or BubR1 with low, non-lethal doses of taxol increases the level and severity of segregation errors and severely compromises viability in all tumor cell lines tested.
Synergy in Cell Killing with Low Taxol Depends on Inefficient Chromosome Alignment in Mps1- or BubR1-Depleted Cells.
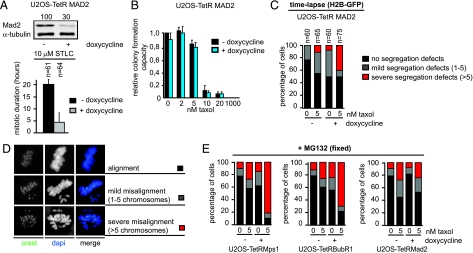
In addition to their function in the mitotic checkpoint, both Mps1 and BubR1 have been shown to control chromosome alignment by influencing the stability of the kinetochore-microtubule interaction or error-correction (24, 30, 31). In contrast, Mad2 is a protein, which is critical for the mitotic checkpoint but has no apparent role in efficient chromosome alignment (31, 32). To examine if the mitotic checkpoint deficiency caused by reduction in Mps1 or BubR1 levels is sufficient to cause cell killing with low doses of taxol, we created an inducible monoclonal U2OS-TetRMad2 cell line in which the mitotic checkpoint was compromised to a similar extent after doxycycline addition, as compared to the U2OS cells in which Mps1 or BubR1 were partially reduced (Fig. 3A and Fig. S9 A and C). Assessment of the time spent in mitosis in the presence or absence of low doses of taxol did also not reveal any difference between U2OS cells with partial Mps1, BubR1, or Mad2 knockdown (Fig. S9B). Importantly, partial Mad2 depletion did not lead to synergistic lethality with low doses of taxol (Fig. 3B), which contrasts the effects seen with partial reduction of Mps1 or BubR1 (Fig. 2 A and K). This lack of synergy in cell killing correlated with a lack of synergy in severe chromosome mis-segregations (Fig. 3C) and aneuploidy (Fig. S4B) in these cells. The rate of severe segregation errors was significantly lower than in cells with reduced Mps1 or BubR1 (40, 85, and 65% respectively) (Fig. 3C, compare with Fig. 2 B and L). In addition, in the partial Mps1- or BubR1-depleted U2OS cells almost all of the remaining cell divisions display mild mis-segregations, while 50% of all divisions in cells with partial Mad2 knockdown and 5 nM taxol show no segregation errors at all.
Fig. 3.
Chromosome alignment dysfunction in Mps1- or BubR1-depleted cells enhances cell death upon taxol [low] treatment. (A) Top: U2OS-TetRMad2 cells that were treated with and without dox were immunoblotted for Mad2 and α-tubulin. Values indicate relative percentage of Mad2 levels. Bottom: Live cell analysis of the mitotic duration of U2OS-TetRMad2 cells treated and measured as in Fig. 1B. (B) Quantification of colony formations analyzed as in Fig. 2. (C) Quantification of anaphase progression as in Fig. 1E. n = amount of cells filmed. (D) Immunofluorescence images of cells with different chromosome alignment phenotypes after 90 min addition of MG132. Centromeres (CREST) are in green and DNA (DAPI) is in blue. ‘Alignment’, ‘Mild misalignment’ or ‘Severe misalignment’ indicate cells with 0, 1–5, or more than 5 chromosomes not aligned on the metaphase plate, respectively. (E) The indicated cell lines were treated with or without dox for 3 days and 1 h before MG132 addition indicated taxol concentrations were added. n = amount of cells analyzed per condition.
The previous observations indicated that inducing chromosome missegregations to a level sufficient to cause cell killing was substantially more efficient when chromosome alignment was disturbed in addition to the mitotic checkpoint. In support of this, when cells were allowed to align their chromosomes for 90 min in the presence of the proteasome inhibitor MG132, we found that severe chromosome misalignments were more prevalent in low-taxol-treated populations of cells with diminished BubR1 or Mps1 levels than those with diminished Mad2 (Fig. 3 D and E). This suggests that reducing Mad2 levels, when weakening mitotic checkpoint activity to a similar extent as partial Mps1 or BubR1 depletion, does not cause sufficient segregation errors in the presence of low doses of taxol to compromise tumor cell viability. These results show that chromosome congression defects play an important role in the synergistic lethality with low doses of taxol.
Although congression defects per se are not expected to lead to chromosome mis-segregations when the mitotic checkpoint is working efficiently, it is formally possible that mere alignment defects are sufficient to cause lethality and that mitotic checkpoint weakening is not required. To address this, we introduced CENP-E siRNA in U2OS-TetRMad2 cells to enhance congression defects in a checkpoint-weakened cell system and thus recapitulate the effects of reducing Mps1 or BubR1 (Fig. S10 D and E). The chromosome alignment defects induced by CENP-E siRNA and 5 nM taxol treatment led to an increase in segregation errors when compared to control siRNA-treated cells (Fig. S10C -doxycycline), but caused only a minor increase in cell killing (Fig. S10 A and B). Importantly, introducing a checkpoint deficiency under these conditions, by partial knockdown of Mad2 (Fig. S10D), clearly increased the level of severe segregation errors and resulted in a significant increase in cell death (+doxycycline) (Fig. S10 A–C). These results support the hypothesis that the synergy in cell killing by low doses of taxol and partial depletion of Mps1 or BubR1 requires significant congression defects combined with a weakened mitotic checkpoint.
To rule out that the failure to compromise tumor cell viability by partial depletion of Mad2 was due to selection for cells with higher Mad2 expression during growth in low taxol, we analyzed the ability to delay mitosis in respect to spindle poison (1μΜ taxol) in U2OS-TetRMad2 cells that had survived growth for 7 days in the presence of 5 nM taxol and doxycycline (Fig. S9D). If the population would have been polyclonal, mitotic checkpoint activity is expected to be restored, at least partially, after elimination of cells with reduced Mad2 levels by synergistic lethality with low taxol. However, the checkpoint response to high taxol was weakened to the same extent in cells with reduced Mad2 levels before or after growth for 7 days in 5 nM taxol, indicating that all cells in the U2OS-TetRMad2 clone have similar Mad2 knockdown upon doxycycline addition (Fig. S9D).
Based on our studies in cancer cells, we conclude that cell death induced by massive chromosome mis-segregations can be achieved either by full ablation of the mitotic checkpoint [(11, 12) and Fig. S1A] or by simultaneously weakening the mitotic checkpoint and chromosome congression processes.
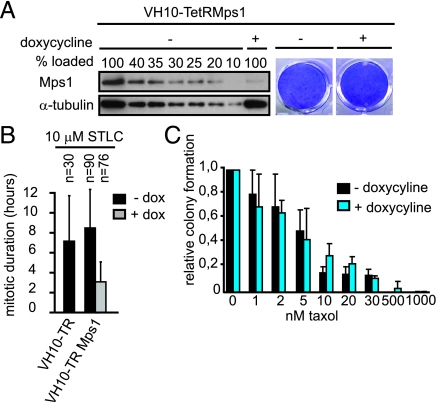
Mps1 Depleted Immortalized Fibroblasts do Not Show Increased Sensitivity Toward Taxol Treatment.
We have previously demonstrated that Mps1 inhibition efficiently abrogates the mitotic checkpoint in tumor cells, but not in immortalized fibroblasts (33). This suggested the possibility that targeting mitotic checkpoint processes required for chromosome segregation may affect tumor cells more severely than untransformed cells. To further investigate this we created an immortalized human fibroblast cell line (VH10-TetRMps1) that stably expressed inducible Mps1 shRNA. Upon doxycycline addition Mps1 levels were reduced to approximately 20% and colony formation capacity was not affected (Fig. 4A). Although the absolute checkpoint responses were incomparable (Fig. 4B), the relative reduction in the ability of the VH10-TetRMps1 cells to delay mitosis in response to STLC after reduction of Mps1 was comparable to HCT116 and LS174T-TetRMps1 cells (Fig. S9C). The checkpoint response of untreated VH10-TetRMps1 cells was also comparable to that of the parental VH10 cell line, ruling out the possibility that the relatively short mitotic delay in control cells is due to leakage of Mps1 shRNA (Fig. 4B). Despite similar checkpoint inhibition as the tumor cell lines with partial reduction in Mps1, VH10-TetRMps1 cells did not show any increase in taxol-induced cell death after Mps1 knockdown (Fig. 4C). This absence of synergy with low doses of taxol in cell death induction correlated with the level of chromosome mis-segregations observed by live cell analysis of VH10-TetRMps1 cells progressing through mitosis in the presence of 2 nM taxol: in about 50% of the cells chromosome mis-segregations were induced, however, this amount was not enhanced upon Mps1 reduction (Fig. S10F).
Fig. 4.
Taxol induced cell death is not enhanced in partial Mps1 depleted immortalized fibroblasts. (A) VH10-TetRMps1 cells that were treated with and without dox for 4 days were immunoblotted for Mps1 and α-tubulin. Indicated amounts of untreated control sample were loaded to determine knockdown of Mps1 in dox treated sample. (B) Live cell analysis of the mitotic duration of indicated cell lines treated and measured as in Fig. 1B. (C) Quantification of colony formations as in Fig. 2. Average of three independent experiments is shown. (D) Quantification of time-lapse analysis (DIC) of chromosome segregation in anaphase. n = amount of cells filmed.
To examine if the correlation between chromosome mis-segregations, synergistic toxicity with low taxol and karyotype that was observed in the cancer cell lines could be extended to the VH10 cells, we performed karyotyping on the VH10-TetRMps1 cells under the various conditions (Fig. S4B). In agreement with a lack of synergy in cell killing, VH10 cells treated with low doses of taxol did not show a clear increase in aneuploidy when partially depleted of Mps1 (Fig. S4B). Thus, in the two instances in which partial knockdown of a protein did not increase sensitivity to low taxol (VH10-TetRMps1 and U2OS-TetRMad2), no increase in severe chromosome mis-segregations and no increase in the breadth of distributions of the karyotypes of the cells was observed. Although severe chromosome mis-segregations occurred upon 2 nM taxol treatment in VH10 cells (Fig. S10F), this did not induce an increase in aneuploidy, which suggests that the population selected for (near)diploid cells. This result correlates with a previous observation showing that untransformed fibroblasts are able to maintain a diploid population of cells after induction of chromosome mis-segregations (9). In agreement with this hypothesis, a decreased growth speed of VH10 cells was observed upon 2 nM taxol treatment independent of the absence or presence of Mps1 (Fig. S10G).
Our results suggest that tumor cells, (near)diploid, aneuploid as well as CIN, are more sensitive to Mps1 or BubR1 inhibition compared to untransformed cells. As full mitotic checkpoint inhibition is unlikely to be achieved in clinical settings, simultaneous inhibition of processes required for chromosome congression and the mitotic checkpoint may be an efficient way to cause the desired level of segregation errors, especially when used in combination with low doses of taxol. Candidates for inhibition are those involved in both processes, such as Mps1, BubR1, Bub1, and TAO1 (34–36). For an increased understanding of the utility of inhibition of such proteins as an anti-cancer strategy, development of small molecule inhibitors will be extremely valuable.
Materials and Methods
Tissue Culture, Transfections, and Treatments.
All cell lines were grown in DMEM (Lonza) with 10% Tet-approved FCS (Clontech), supplemented with pen/strep (Invitrogen) and ultraglutamine (Lonza). All DNA transfections were performed using Effectene and siRNA transfections were done using HiPerfect (both from QIAGEN). Taxol, nocodazole, STLC, doxorubicin, MG132, and doxycycline (used at 1 μg/mL) were from Sigma.
Cell lines stably expressing TetR were infected with retrovirus carrying pSuperior-retro-puro-Mps1, -Mad2, or -BubR1 and selected with 2 μg/mL puromycin. Single colonies were selected after replating 1–2 cells/well. HeLa-TetRBubR1 cells were infected with retrovirus carrying pBabe Lap-BubR1 (RNAi-insensitive) and selected with blasticidine to perform the rescue experiments.
RNAi.
BubR1 (12) and Mad2 (37) shRNA sequences were used to produce pSuperior-retro-puro plasmids using standard cloning procedures (Oligo-Engine, Inc). pSuperiorMps1 (24) was used for creating inducible Mps1 RNAi cell lines and transient transfection. CENP-E siRNA (sequence AACACGGAUGCUGGUGACCUC), Hec-1 siRNA (38), and KIF18A siRNA (Dharmacon) were used.
Antibodies.
The following antibodies have been used for Western blot, Immunofluorescence, and FACS analysis: Mps1 (Upstate), BubR1 (a gift from R. Freire), Mad2 (Bethyl), α-tubulin (Sigma), α-actin (TeBu), CENP-E (39), CREST (Cortex Biochem), MPM2 (Upstate), anti-human Alexafluor647, anti-rabbit Alexafluor488, anti-mouse cy-5 (Jackson), anti-Mouse/Rabbit Alexa680/800 (Molecular Probes).
Immunoblotting.
Cells were lysed in Laemmli buffer. Samples were separated by SDS-page and transferred to PVDF (Immobilin FL, Millipore). The membranes were cut in half and blotted with anti-Mps1, Mad2, CENP-E, or BubR1, anti-α-tubulin or actin. Western blot quantification was performed on the Odyssey Infrared Imaging System (LI-COR Biosciences) and quantified using Odyssey application software version 1.2.
Immunofluorescence Microscopy.
Cells plated on 12 mm coverslips were harvested after 90 min MG treatment. Fixation was done using 4% PFA in PEM buffer. CREST was incubated O/N in PBS 3% BSA. Anti-human Alexafluor647 and DAPI were incubated in PBS 0,1% Tween. Stained coverslips were mounted with Vectashield Mounting Medium (Vector). Images were acquired on a Zeiss 510 Meta confocal laser scanning microscope with a 63X/1.4NA Plan-ApoChromat objective using the Zeiss LSM software.
Live Cell Imaging.
Cells were plated in 2- or 4-well chambered glassbottom slides (LabTek), transfected with H2B-GFP using Effectene and imaged in a heated chamber (37 °C and 5% CO2) using a 40×/1.3NA oil objective on a Zeiss Axiovert 200M microscope equipped with a 0.55NA condensor and controlled by a lambda-DG4 (Roper Scientific) and MetaMorph software. Green fluorescent (80 ms exposure) images were acquired every 3 min using a Photometrics CoolSnap HQ CCD camera (Roper Scientific). Images were processed using MetaMorph software. For cell death analysis, cells were filmed under the same conditions, Propidium Iodide (PI) was added to the medium, DIC, and red fluorescent (100 ms exposure) images were acquired every 15 min for 64 h.
Colony Formation Assays.
Cells (±50,000/well) were plated on 6-well plates (Costar). At day 11, unless stated differently, plates were washed with PBS, fixed 5 min with 96% Methanol and stained with 0.1% crystal violet dH20. Stained colony formations were scanned and quantified for intensity using Metamorph software.
Supplemental Material and Methods.
Technical procedures of experiments performed only in the supplemental section can be found in the online SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank L. Kleij for technical assistance with confocal and time lapse microscopy; all members of the S.M.A. Lens, G.J.P.L.K., and R.H.M. laboratory for discussions; M. Alvarez and L.Macurek for critically reading the manuscript; M. van de Wetering (Hubrecht Institute, Utrecht), M. Timmers (UMC Utrecht), and A. G. Jochemsen (Leiden UMC) for providing TetR expressing cell lines to us. This work was supported by Netherlands Organisation for Scientific Research Grants ZonMw 918.46.616 (to R.H.M. and A.J.) and VIDI-91776336 (to G.J.P.L.K.), Top Institute Pharma Grant T3–105 (to R.H.M. and A.J.), Dutch Cancer Society Grant UU-2006-3664 (to G.J.P.L.K.), and Netherlands Genomic Initiative of the Netherlands Organisation for Scientific Research (to R.H.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904343106/DCSupplemental.
References
- 1.Cohen J. Sorting out chromosome errors. Science. 2002;296:2164–2166. doi: 10.1126/science.296.5576.2164. [DOI] [PubMed] [Google Scholar]
- 2.Heim Sa, Mitelman F. Cancer Cytogenetics. New York: Wiley Liss Inc.; 1995. [Google Scholar]
- 3.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 4.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 6.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 7.Holland AJ, Cleveland DW. Boveri revisited: Chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foijer F, Draviam VM, Sorger PK. Studying chromosome instability in the mouse. Biochim Biophys Acta. 2008;1786:73–82. doi: 10.1016/j.bbcan.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Michel L, et al. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc Natl Acad Sci USA. 2004;101:4459–4464. doi: 10.1073/pnas.0306069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc Natl Acad Sci USA. 2004;101:8699–8704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz SB. Taxol (paclitaxel): Mechanisms of action. Ann Oncol 5 Suppl. 1994;6:S3–6. [PubMed] [Google Scholar]
- 14.Brito DA, Rieder CL. The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells. Cell Motil Cytoskeleton. 2008;66:437–447. doi: 10.1002/cm.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikui AE, Yang CP, Matsumoto T, Horwitz SB. Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle. 2005;4:1385–1388. doi: 10.4161/cc.4.10.2061. [DOI] [PubMed] [Google Scholar]
- 16.Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res. 2002;62:1935–1938. [PubMed] [Google Scholar]
- 17.Fu Y, et al. Weakened spindle checkpoint with reduced BubR1 expression in paclitaxel-resistant ovarian carcinoma cell line SKOV3-TR30. Gynecol Oncol. 2007;105:66–73. doi: 10.1016/j.ygyno.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- 19.Swanton C, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Kienitz A, Vogel C, Morales I, Muller R, Bastians H. Partial downregulation of MAD1 causes spindle checkpoint inactivation and aneuploidy, but does not confer resistance towards taxol. Oncogene. 2005;24:4301–4310. doi: 10.1038/sj.onc.1208589. [DOI] [PubMed] [Google Scholar]
- 21.Swanton C, et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci USA. 2009;106:8671–8676. doi: 10.1073/pnas.0811835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EA, et al. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther. 2004;3:661–669. [PubMed] [Google Scholar]
- 23.Jelluma N, et al. Chromosomal instability by inefficient Mps1 auto-activation due to a weakened mitotic checkpoint and lagging chromosomes. PLoS ONE. 2008;3:e2415. doi: 10.1371/journal.pone.0002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBonis S, et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol Cancer Ther. 2004;3:1079–1090. [PubMed] [Google Scholar]
- 25.Jelluma N, et al. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–246. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 26.Rieder CL, Maiato H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Mayr MI, et al. The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr Biol. 2007;17:488–498. doi: 10.1016/j.cub.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 29.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 30.Ditchfield C, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampson MA, Kapoor TM. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat Cell Biol. 2005;7:93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M, Budirahardja Y, Klompmaker R, Medema RH. Ablation of the spindle assembly checkpoint by a compound targeting Mps1. EMBO Rep. 2005;6:866–872. doi: 10.1038/sj.embor.7400483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Draviam VM, et al. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat Cell Biol. 2007;9:556–564. doi: 10.1038/ncb1569. [DOI] [PubMed] [Google Scholar]
- 35.Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 36.Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 2005;24:1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lens SM, et al. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanenbaum ME, Macurek L, Galjart N, Medema RH. Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J. 2008;27:3235–3245. doi: 10.1038/emboj.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown KD, Wood KW, Cleveland DW. The kinesin-like protein CENP-E is kinetochore-associated throughout poleward chromosome segregation during anaphase-A. J Cell Sci. 1996;109:961–969. doi: 10.1242/jcs.109.5.961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.