Abstract
The ON pathway of the visual system, which detects increases in light intensity, is established at the first retinal synapse between photoreceptors and ON-bipolar cells. Photoreceptors hyperpolarize in response to light and reduce the rate of glutamate release, which in turn causes the depolarization of ON-bipolar cells. This ON-bipolar cell response is mediated by the metabotropic glutamate receptor, mGluR6, which controls the activity of a depolarizing current. Despite intensive research over the past two decades, the molecular identity of the channel that generates this depolarizing current has remained elusive. Here, we present evidence indicating that TRPM1 is necessary for the depolarizing light response of ON-bipolar cells, and further that TRPM1 is a component of the channel that generates this light response. Gene expression profiling revealed that TRPM1 is highly enriched in ON-bipolar cells. In situ hybridization experiments confirmed that TRPM1 mRNA is found in cells of the retinal inner nuclear layer, and immunofluorescent confocal microscopy showed that TRPM1 is localized in the dendrites of ON-bipolar cells in both mouse and macaque retina. The electroretinogram (ERG) of TRPM1-deficient (TRPM1−/−) mice had a normal a-wave, but no b-wave, indicating a loss of bipolar cell response. Finally, whole-cell patch-clamp recording from ON-bipolar cells in mouse retinal slices demonstrated that genetic deletion of TRPM1 abolished chemically simulated light responses from rod bipolar cells and dramatically altered the responses of cone ON-bipolar cells. Identification of TRPM1 as a mGluR6-coupled cation channel reveals a key step in vision, expands the role of the TRP channel family in sensory perception, and presents insights into the evolution of vertebrate vision.
Keywords: retinal neurobiology, transient receptor potential channel, visual ON-pathway
At the first retinal synapse, the postsynaptic bipolar cells respond with opposite polarities to light-induced changes in synaptic glutamate released from photoreceptor terminals (1). ON-bipolar cells depolarize and OFF-bipolar cells hyperpolarize in response to increases in light intensity. The polarity of the bipolar cell response is determined by the expression of distinct glutamate receptors. The OFF response is mediated by ionotropic glutamate receptors (iGluRs). In contrast, ON-bipolar cells express a distinctive metabotropic glutamate receptor (mGluR), mGluR6, which is localized in the tips of ON bipolar cell dendrites (2). In darkness, activation of this receptor by synaptic glutamate causes the closure of a nonselective cation channel. Deactivation of mGluR6, subsequent to the light-induced decrease in synaptic glutamate, causes these channels to open, thereby generating the depolarizing light response of ON-bipolar cells (3–5). Despite intensive research over the past two decades, the molecular identity of the channel that generates this depolarizing current has remained elusive.
Transient receptor potential (TRP) channels were first discovered in Drosophila, where they generate the photoreceptor light response (6–8). Since that time, >30 mammalian TRP channel homologs have been discovered, which serve a wide variety of sensory functions, ranging from thermosensation and nociception, to gustation and mechanosensation (9). The founding member of the TRPM family, TRPM1, was discovered by differential display as a potential suppressor of tumor metastasis [and originally named melastatin because it is down-regulated in metastatic melanoma (10)]. TRPM1 is the product of a complex gene, spanning 58 kb and 27 exons, and exists as several isoforms, produced by mRNA splice variants (11–13). Little is known about the physiological role of TRPM1, but recently it has been reported to form constitutively active cation channels in melanocytes, where it has been proposed to function in melanin trafficking (13). The basic properties of the channel, including ion selectivity and current-voltage relationship, are similar to those reported for the mGluR6-coupled ion channel in retinal ON-bipolar cells. In the Appaloosa horse, a reduction in TRPM1 has been correlated with the loss of the ERG b-wave (14), and a recent study by Shen et al. (15) showed that the ERG b-wave is lacking in the TRPM1−/− mouse implicating this channel in photoreceptor to ON-bipolar cell synaptic transmission. Here, we present molecular, immunohistochemical, and electrophysiological evidence that TRPM1 is the mGluR6-coupled cation channel in rod-bipolar cells, and provide evidence that TRPM1 is essential for the normal response of cone ON-bipolar cells.
Results
TRPM1 Is Expressed in Retinal Bipolar Cells.
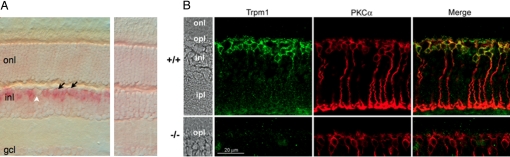
Gene expression profiling studies [SI Text and Fig. S1 (16, 32)], demonstrated that TRPM1 is enriched in ON-bipolar cells, suggesting TRPM1 as a potential candidate for the long sought after channel generating the light response in these cells. We verified that the TRPM1 mRNA was expressed in ON-bipolar cells by in situ hybridization (Fig. 1A Left). By using an anti-sense probe directed against the mouse TRPM1 mRNA (bp 2,383–3,514, GenBank accession NM_001039104), a strong signal was obtained in many cells of the distal inner nuclear layer (INL), where the majority of ON-bipolar cells are located (Fig. 1A, arrows). Unlabeled cells in this region of the INL likely correspond to horizontal cells (Fig. 1A, white arrowhead is a putative horizontal cell). A control hybridization with a sense probe directed against the same region of the channel cDNA showed no signal above background in any part of the retina (Fig. 1A Right).
Fig. 1.
TRPM1 is expressed by ON-bipolar cells in the mouse retina. (A) In situ hybridization of vertical sections of mouse retina with antisense (Left) and sense control (Right) TRPM1 probes. A hybridization signal is detected in many cell somata in the INL, where bipolar cell nuclei and somata are located (black arrows). Occasional unlabeled cell somata are likely horizontal cells (white arrowhead). (B) Vertical sections from a wild-type (Top) and TRPM1−/− (Bottom) retina were immunofluorescently labeled by antibodies directed against TRPM1 (green) and PKCα (red). Areas of colocalization appear yellow in the merged images (Right). onl, Outer nuclear layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer.
Immunofluorescence confocal microscopy demonstrated that the TRPM1 channel is localized to ON-bipolar cells. As shown in Fig. 1B, immunolabeling of vertical sections of mouse retina with an anti-TRPM1 antibody revealed punctate staining in the outer plexiform layer (OPL), as well as membrane-associated and possible intracellular staining of bipolar cell bodies. The specificity of the TRPM1 antibody was demonstrated by the absence of staining in the TRPM1−/− mouse (Fig. 1B, lower panels), as well as by labeling of HEK cells transiently transfected with TRPM1 cDNA (Fig. S2). Most of the TRPM1 labeling in the mouse retina was coincident with that for PKCα, indicating that TRPM1 is expressed in rod bipolar cells (Fig. 1B). The TRPM1-positive cells extended dendrites into the OPL, the tips of which could be labeled for mGluR6 (Fig. S3 A–C) and are apposed to labeled synaptic ribbons (Fig. S3 D–F). Immunofluorescent labeling indicated that mGluR6 is still present in the ON-bipolar cell dendrites in the TRPM1−/− retina (Fig. S4A), and that tips of TRPM1−/− rod bipolar cell dendrites are apposed to photoreceptor synaptic ribbons (Fig. S4B), suggesting that the morphology of the OPL is not grossly disrupted in the TRPM1−/− retina.
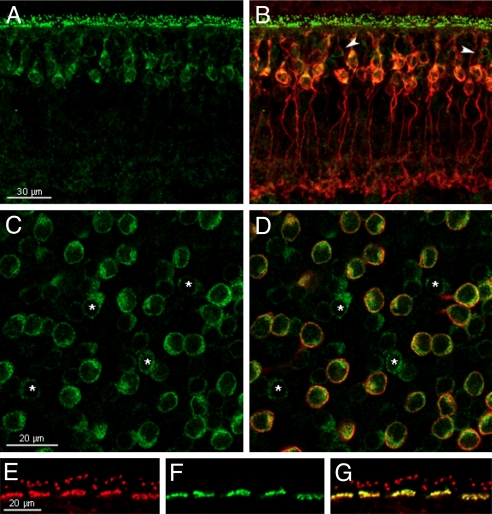
Because the TRPM1 antibody was raised against a human polypeptide, TRPM1 immunostaining was even more robust in the macaque retina (Fig. 2). In vertical sections of macaque retina, TRPM1 staining appeared as puncta in the OPL and was associated with cell plasma membranes in the distal INL. In many instances, this labeling was clearly associated with PKCα, indicating that these were rod bipolar cells (Fig. 2B). Similar to mouse, however, not all TRPM1-positive cells were immunoreactive for PKCα (arrowheads, Fig. 2B). This finding is more apparent in a horizontal optical section through the INL of a macaque retinal whole mount shown in Fig. 2 C and D (cells marked with asterisks). These cells are most likely cone ON-bipolar cells since all TRPM1 positive cells can be colabeled with Gαo (Fig. S5). If this is the case, their dendritic processes should extend to cone photoreceptor pedicles in the OPL. Indeed, immunolabeling for TRPM1 is clearly associated with cone pedicles, as well as with rod terminals, shown by double labeling with the anti-TRPM1 antiserum and the cone marker, Alexafluor peanut agglutinin (PNA) (Fig. 2 E–G). Thus, TRPM1 is localized to the dendrites of both rod and cone ON-bipolar cells, where it is optimally positioned to respond rapidly to light-induced changes in the synaptic glutamate levels.
Fig. 2.
TRPM1 is associated with both rod and cone ON-bipolar cells in the primate retina. (A and B) Vertical section of macaque retina double labeled for TRPM1 (green, A and B) and PKCα (red, B). Arrowheads indicate putative cone ON-bipolar cells (TRPM1 positive, PKCα negative cells). (C and D) Horizontal optical section in the plane of the inner nuclear layer from a whole mount macaque retina double labeled for TRPM1 (green, C and D) and PKCα (red, D). Asterisks indicate putative cone ON-bipolar cells. (E–G) Vertical section through the outer plexiform layer of the macaque retina double labeled with an antibody against TRPM1 (red, E and G) and the cone marker, peanut agglutinin-Alexafluor (green, F and G).
Vision Is Impaired in the TRPM1−/− Mouse.
The expression of TRPM1 in retinal bipolar cells implies that the TRPM1 channel is important for vision. To test this hypothesis, we measured spatial frequency and contrast sensitivity thresholds of the optokinetic response (OKR) in TRPM1−/− mice (17). The spatial frequency threshold of TRPM1−/− mice was reduced 10% compared to wild-type (0.359 ± 0.004 cycles/degree for TRPM1−/−, and 0.400 ± 0.010 cycles/degree for wild-type, P < 0.05), and contrast sensitivity was reduced 3-fold (4.61 ± 0.23 for TRPM1−/−, and 14.99 ± 3.85 for wild-type, P < 0.001, measured at a spatial frequency of 0.150 cycles/degree). The OKR measurements indicate that the TRPM1−/− mice are visually impaired, although not profoundly so, similar to the complete congenital stationary night blindness phenotype.
Electroretinogram b-Wave Is Absent in Mice Lacking TRPM1.
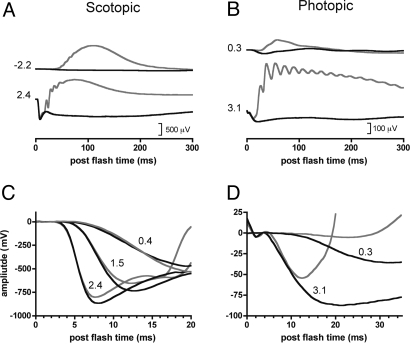
To investigate the physiological role of TRPM1 in the retina, we recorded the electroretinogram (ERG) from control and TRPM1−/− mice. The ERG responses from TRPM1+/− and wild-type mice were indistinguishable and have been combined to form the control group for analysis. Figure 3 shows representative ERGs from control (gray traces) and TRPM1−/− mice (black traces). The b-wave and oscillatory potentials, both of which are generated downstream from the photoreceptors, were absent from both rod and cone-mediated (both scotopic and photopic) ERGs of the TRPM1−/− mice (Fig. 3 A and B). Figure 3C shows an expanded view of the beginning of the rod-isolated ERGs recorded for several flash intensities. The superimposed traces show that the negative-going rod-isolated ERG a-waves were essentially identical between TRPM1-deficient and control mice. A phototransduction model was ensemble fit to the rising phases of the rod-isolated a-waves. The derived rod phototransduction parameters (mean ± SE.) were not significantly different between control {RmaxP3 = −806 ± 35 μV; S = 1,158 ± 166 [(cd-s/m2)−1 s−2]; td = 3.9 ± 0.05 ms} and TRPM1−/− mice {Rmax P3 = −877 ± 30 μV; S = 1,221 ± 120 [(cd-s/m2)−1 s−2]; td = 4.1 ± 0.1 ms}. Figure 3D shows an expanded view of the cone-isolated photopic ERGs. Rodents have little or no cone mediated photopic ERG a-wave. In our study, the initial negative going photopic response from control mice only tracked the ERG from the TRPM1−/− mice for very bright flash intensities (>3 log ph cd-s/m2) (Fig. 3D). The absence of the ERG b-wave in the TRPM1−/− mice indicates a block in signal transmission between photoreceptors and ON-bipolar cells in these animals. The ERG, however, does not indicate whether this block resides postsynaptically, in the bipolar cell dendrites, or presynaptically, in the photoreceptor terminals. The in situ hybridization and immunofluorescence results (Figs. 1 and 2) support a postsynaptic locus for the block, but to rule out any contribution from defective glutamate release by photoreceptors in the TRPM1−/− retina, we recorded directly from ON-bipolar cells using a technique that bypasses the photoreceptors entirely.
Fig. 3.
ERG b-waves and oscillatory potentials are absent in TRPM1-deficient mice. Rod isolated ERGs (A and C) and photopic cone-mediated ERGs (B and D) from combined wild-type and TRPM1+/− mice (n = 4; gray lines) compared with TRPM1−/− mice (n = 5; solid black lines). Numbers indicate scotopic (A and C) and photopic (B and D) flash intensities (log candela-seconds/meter2). Note that scales vary between scotopic and photopic graphs.
Rod Bipolar Cell Response Is Absent in TRPM1−/− Mice.
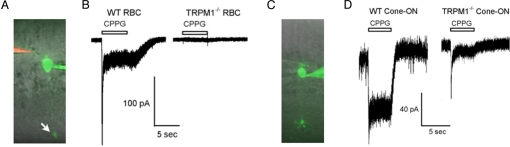
To examine the role of TRPM1 in generation of the depolarizing light response in ON-bipolar cells, we used the whole-cell patch clamp technique to record chemically simulated light responses from ON-bipolar cells in mouse retinal slices. For these experiments, the slice was bathed at all times in the mGluR6 agonist, L-2-aminophosphonobutyrate (L-AP4) to simulate darkness; pressure application of the mGluR6 antagonist, α-cyclopropyl-4-phosphonophenylglycine (CPPG), to the OPL was used to simulate a step of light. During recording, cells were filled with the dye, Alexa-488 hydrazide, which permitted morphological identification of rod and cone bipolar cells at the termination of the experiment (Fig. 4 A and C). As shown in Fig. 4B, application of CPPG to the wild-type mouse retina activated a robust current with a waveform typical of rod bipolar cells (18). These currents had a peak amplitude at approximately 120 ms of −129 ± 15 pA (SEM; n = 21), which decayed rapidly to a plateau. These currents were abolished in the TRPM1−/− retina. In the majority of morphologically identified rod bipolar cells, CPPG application activated no measurable currents (−2.7 ± 0.55 pA; SEM; n = 25); a few cells exhibited a slowly activating current of <5 pA.
Fig. 4.
Chemically simulated ON-bipolar cell light responses from wild-type and TRPM1−/− retina. Patch clamp recording of a rod (A) and a cone (C) ON-bipolar cell in a mouse retinal slice. The patch electrode was filled with internal solution containing Alexa488 hydrazide, and the puffer pipette (visible in A) was filled with Ames media containing 600 μM CPPG and Alexa-594. Rod bipolar cell synaptic terminal is indicated by an arrow in A. Current traces at −60 mV from rod (B) and cone (D) ON-bipolar cells from wild-type and TRPM1−/− retinas; CPPG was applied to the outer plexiform layer as indicated.
Cone ON-Bipolar Cell Response Is Dramatically Altered in TRPM1−/− Mice.
In wild-type retina, cone ON-bipolar cells responded to CPPG with a sustained current of −75 ± 8.6 pA (n = 13) (Fig. 4D). Approximately half of cone ON-bipolar cells in the TRPM1−/− retina showed no response to pressure application of CPPG (3/8 cells), like rod bipolar cells. The remaining cone ON-bipolar cells, however, responded to CPPG application by activating a small transient current (38 pA ± 7.6; n = 5 cells), suggesting that a subset of cone ON-bipolar cells contain additional mGluR6-coupled channels (Fig. 4D). Pressure application of a 200 μM AMPA/200 μM kainate mixture confirmed that OFF-cone bipolar cells remained responsive in TRPM1−/− mice.
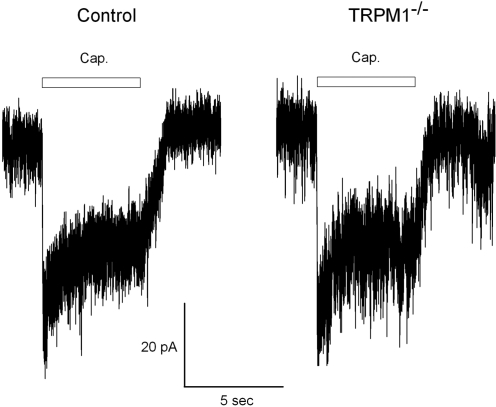
Capsaicin-Sensitive Current Persists in TRPM1−/− ON-Bipolar Cells.
The pharmacology of both TRPM1 and the endogenous mGluR6-coupled ion channel remain largely unknown. Recently a capsaicin-activated current has been discovered in mouse ON-bipolar cells with properties similar to the light-activated current (15), leading to speculation that the capsaicin-sensitive current may be mediated by TRPM1. As shown in Fig. 5, we found that the capsaicin-activated current was still present in at least some ON-bipolar cells in the TRPM1−/− retina. Overall, however, the magnitude of the capsaicin-activated current in ON-bipolar cells was somewhat reduced (t test P < 0.001) in TRPM1−/− mice (−14 ± 2 pA; n = 17) compared to wild-type (−27 ± 3 pA SEM; n = 15).
Fig. 5.
Comparison of capsaicin-activated currents in ON-bipolar cells of wild-type and TRPM1−/− mice. Patch-clamp recordings were made in the whole-cell mode from ON-bipolar cells in the retinal slice preparation at a holding potential of −60 mV. Capsaicin (100 μM) was applied to the OPL at the time indicated via a “puffer” pipet. These traces are representative of >10 recordings from each genetic background.
Discussion
The most parsimonious explanation for these data are that TRPM1 is a necessary component of the mGluR6-coupled ion channel responsible for generating the depolarizing light response in rod and cone ON-bipolar cells. Some classes of cone ON-bipolar cells, however, may express additional mGluR6-coupled ion channels, as evidenced by the residual transient current seen in some ON-cone cells from the TRPM1−/− retina. This situation is reminiscent of the light response in Drosophila photoreceptors, which consists of a sustained component mediated by trp channels and a transient component mediated by trpl channels (19). Congenital stationary night blindness (CSNB) is a group of nonprogressive retinal diseases characterized by impaired scotopic vision and a characteristic “negative” ERG, in which the b-wave is reduced or absent. In complete CSNB (CSNB1), there is a complete loss of both rod and cone mediated ERG b-waves, but the OFF pathway is preserved. CSNB1 is caused by mutations in genes expressed in ON-bipolar cells, such as nyctalopin (20, 21) and mGluR6 (22, 23). Based on the negative ERG measured in the TRPM1−/− retina, TRPM1 appears to be a candidate gene for CSNB1. In addition, the expression of TRPM1 by both bipolar cells and melanocytes, and its down regulation in metastatic melanoma raise the possibility that TRPM1 may be a target of the autoimmune response that causes visual deficits in melanoma-associated retinopathy, a paraneoplastic condition with reduced ON-bipolar cell function (24).
In a recent article, Shen et al. (15) described a capsaicin-activated current in ON-bipolar cells, and suggested that this current may arise from the same ion channels that generate the mGluR6-coupled transduction current. The authors demonstrate that the current is present in the TRPV1−/− retina, and suggest that it may be mediated by TRPM1, which they showed is necessary for the ERG b-wave. We show here, however, that a capsaicin-activated current is still present in many ON-bipolar cells in the TRPM1−/− mouse retina. Therefore, the precise relationship between the capsaicin-activated channel and the mGluR6-coupled channel remains to be established. In light of these findings, it is interesting to speculate that the endogenous mGluR6-coupled transduction channel may be comprised of TRPM1, which is essential for activation by mGluR6, as well as an additional type of channel subunit, which confers capsaicin sensitivity.
ON-bipolar cells share a number of conserved features with photoreceptors, including expression of recoverin, potassium channels, and ribbon synapse components; furthermore, both photoreceptors and ON-bipolar cells hyperpolarize in response to activation of their respective receptors (opsins and mGluR6). These observations have prompted the hypothesis that bipolar cells evolved from primordial photoreceptors (25, 26). Use of TRPM1, rather than a cyclic nucleotide-gated channel, as the mGluR6-coupled channel, however, indicates that the ON-bipolar cell signaling pathway is more analogous to phototransduction in Drosophila photoreceptors and melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs), both of which are thought to employ trp channels (19, 27).
Materials and Methods
For complete details, refer to SI Text. Mice expressing cre recombinase under the control of the mGluR6 promoter were generated and crossed with the Z/EG marker strain. GFP expressing ON-bipolar cells were purified by fluorescence activated cell sorting. Total RNA was isolated from the purified cells as well as from total retina, and gene expression was compared between the two samples. In situ hybridization methods for cryosections have been described (28). Digoxigenin-labeled probes were detected with an anti-digoxigenin antibody conjugated to alkaline phosphatase, followed by use of the Fast Red color substrate. HEK tsA-201 cells were transfected and immunostained according to standard methods. Immunofluorescent labeling of retina sections was performed as described in ref. 29. The TRPM+/− mice were obtained from Texas A&M Institute for Genomic Medicine (Houston, TX), and the TRPM1 antibody was from Sigma–Aldrich. Whole-mount retina staining was performed by successive incubations of lightly fixed macaque retina pieces in blocking/permeabilization solution (4 h), primary antibodies (72 h), then secondary antibodies (overnight). All incubations were performed at 4 °C with washing steps in between. Immunofluorescence images were obtained with an Olympus Fluoview1000. Full-field scotopic and photopic ERGs [background = 60 candella/meter2 (cd/m2)] were recorded to flashes of increasing intensity −3.9 to 2.0 and −1.0 to 3.7 log ph cd/m2, respectively. Rod-isolated ERGs were obtained by subtracting scotopic cone-ERGs, obtained with a paired flash protocol from the mixed rod/cone responses (30, 31). Phototransduction parameters were derived from the ensemble fit of a P3 model to the leading edges of rod-isolated ERG a-waves (30). Optokinetic responses were measured as described in ref. 18. Patch-clamp recordings from mouse bipolar cells were performed similarly to those described (17). The external Ames solution contained 4 μM L-AP4 to activate mGluR6, and a 5-s puff of 600 μM CPPG was applied to simulate a light flash.
Supplementary Material
Acknowledgments.
We thank Tammie Haley and Trevor McGill (Oregon Health & Science University) for help with the ERG and OKR recordings; Lane Squires (University of Washington, Seattle), Tracey Robinson (University of Wisconsin, Madison), and William Davis (Washington State University) for help with the retinal dissociation and FACS analysis; Glenn R. Wyrick (Washington State University) for performing some of the patch-clamp experiments; Robert Searles and the Oregon Health & Science University DNA Microarray Core for help with the Ilumina bead array; Shixi Zheng and Celia Gellman (Oregon Health & Science University) for assistance with immunohistochemistry; Deborah Stenkamp (University of Idaho, Moscow) for assistance with in situ hybridization experiments; Shigetada Nakanishi (Kyoto University, Japan), Rolf Sprengel (Max-Plank Institute, Heidelberg, Germany), and Craig Montell (Johns Hopkins University, Baltimore) for supplying the mGluR6 promoter sequence, iCre, and the human TRPM1 cDNA, respectively; and Peter Gillespie and Jeff Karpen (Oregon Health & Science University) for critical reading of the manuscript. This work was funded by the National Institutes of Health Grants EY09534 (to R.M.D.), EY014700 (to C.W.M.), MH067094 (to R.L.B.), and RR000163.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908711106/DCSupplemental.
References
- 1.Schiller PH, Sandell JH, Maunsell JH. Functions of the ON and OFF channels of the visual system. Nature. 1986;322:824–825. doi: 10.1038/322824a0. [DOI] [PubMed] [Google Scholar]
- 2.Nomura A, et al. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: A new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 4.Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- 5.Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- 6.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 7.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 8.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 9.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan LM, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- 11.Hunter JJ, et al. Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1) Genomics. 1998;54:116–123. doi: 10.1006/geno.1998.5549. [DOI] [PubMed] [Google Scholar]
- 12.Fang D, Setaluri V. Expression and up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun. 2000;279:53–61. doi: 10.1006/bbrc.2000.3894. [DOI] [PubMed] [Google Scholar]
- 13.Oancea E, et al. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellone RR, et al. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, et al. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DS, et al. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol. 2008;507:1795–1810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGill TJ, et al. Syngeneic Schwann cell transplantation preserves vision in RCS rat without immunosuppression. Invest Ophthalmol Vis Sci. 2007;48:1906–1912. doi: 10.1167/iovs.06-1117. [DOI] [PubMed] [Google Scholar]
- 18.Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524(Pt 3):879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 20.Bech-Hansen NT, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- 21.Pusch CM, et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26:324–327. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- 22.Dryja TP, et al. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci USA. 2005;102:4884–4889. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitz C, et al. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]
- 24.Lei B, Bush RA, Milam AH, Sieving PA. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41:262–266. [PubMed] [Google Scholar]
- 25.Reichenbach A, Robinson SR. Phylogenetic constraint on retinal organization and development. Prog Ret Eye Res. 1995;15:139–171. [Google Scholar]
- 26.Lamb TD, Collin SP, Pugh EN. Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson SM, Frey RA, Wardwell SL, Stenkamp DL. The developmental sequence of gene expression within the rod photoreceptor lineage in embryonic zebrafish. Dev Dyn. 2008;237:2903–2917. doi: 10.1002/dvdy.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur J Neurosci. 2006;23:1163–1171. doi: 10.1111/j.1460-9568.2006.04647.x. [DOI] [PubMed] [Google Scholar]
- 30.Birch DG, Hood DC, Nusinowitz S, Pepperberg DR. Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest Ophthalmol Vis Sci. 1995;36:1603–1614. [PubMed] [Google Scholar]
- 31.Pennesi ME, Howes KA, Baehr W, Wu SM. Guanylate cyclase-activating protein (GCAP) 1 rescues cone recovery kinetics in GCAP1/GCAP2 knockout mice. Proc Natl Acad Sci USA. 2003;100:6783–6788. doi: 10.1073/pnas.1130102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima Y, Moriyama M, Hattori M, Minato N, Nakanishi S. Isolation of ON bipolar cell genes via hrGFP-coupled cell enrichment using the mGluR6 promoter. J Biochem. 2009;145:811–818. doi: 10.1093/jb/mvp038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.