Abstract
Virus-resistant transgenic squash are grown throughout the United States and much of Mexico and it is likely that the virus-resistant transgene (VRT) has been introduced to wild populations repeatedly. The evolutionary fate of any resistance gene in wild populations and its environmental impacts depend upon trade-offs between the costs and benefits of the resistance gene. In a 3-year field study using a wild gourd and transgenic and nontransgenic introgressives, we measured the effects of the transgene on fitness, on herbivory by cucumber beetles, on the incidence of mosaic viruses, and on the incidence of bacterial wilt disease (a fatal disease vectored by cucumber beetles). In each year, the first incidence of zucchini yellow mosaic virus occurred in mid-July and spread rapidly through the susceptible plants. We found that the transgenic plants had greater reproduction through both male and female function than the susceptible plants, indicating that the VRT has a direct fitness benefit for wild gourds under the conditions of our study. Moreover, the VRT had no effect on resistance to cucumber beetles or the incidence of wilt disease before the spread of the virus. However, as the virus spread through the fields, the cucumber beetles became increasingly concentrated upon the healthy (mostly transgenic) plants, which increased exposure to and the incidence of wilt disease on the transgenic plants. This indirect cost of the VRT (mediated by a nontarget herbivore and pathogen) mitigated the overall beneficial effect of the VRT on fitness.
Keywords: Cucurbita pepo, Erwinia tracheiphyla, plant–herbivore–pathogen interaction, zucchini yellow mosaic virus, cucumber beetles
Gene flow between crops and their wild relatives is common and difficult to contain (1, 2) and spontaneous hybridization between transgenic cultivars and wild relatives occurs for 12 of the world's 13 most important crops (3). Consequently, there are concerns that crop transgenes conferring resistance to herbivores or pathogens could escape and enhance the fitness and weediness of wild species, impact nontarget species such as pollinators, herbivores, predators, or soil fauna, or alter the biodiversity within communities (1, 2, 4–6).
A variety of models suggest that the evolutionary fate of resistance genes (both natural and transgenic) in natural populations often depends upon trade-offs between the benefits and costs of the resistance gene in the presence and absence of the natural enemy (7–9). Fitness trade-offs can be either direct (genetic resistance causes a reduction in one of the components of fitness) or indirect (pleiotropy results in ecological trade-offs that affect growth and development or resistance to other natural enemies) (10–14). Direct trade-offs (e.g., in flower production) have been well studied and characterized for many systems (15, 16). Ecological trade-offs, however, have been frequently hypothesized (10, 16, 17) but less often demonstrated. In most cases these trade-offs and costs of resistance have been studied using relatively simple experimental settings in which plants are challenged with individual natural enemies. However, the predictions from pairwise challenges will not necessarily hold at the community scale when host plants are subject to attack from multiple enemies, and natural enemies must compete for hosts (15, 16, 18–20). As such, it is unclear how ecological trade-offs affect the spread of resistance alleles at the scale of natural communities.
For vectored pathogens, transmission depends on the exposure of the host to the pathogen, as mediated through the foraging behavior of the vector, and on the resistance of the host to the pathogen. Unlike pathogen resistance, which can often be thought of as a fixed characteristic, conditional on the genotype or phenotype of the host individual, pathogen exposure is highly plastic. Vector foraging behavior, and the resultant patterns of pathogen exposure, can be highly dependent on the spatial arrangement and distribution of plant traits within the population (21, 22) and the dynamics of the vector. Further, as vector foraging may be affected by disease or other natural enemies (e.g., refs. 19 and 20), the distribution of pathogen exposure can be dynamic in time. These complexities are difficult to capture within the framework of pairwise pathogen challenges that lack the full host–vector–pathogen community context.
Gene flow from cultivated squash (Cucurbita pepo) to free-living taxa of Cucurbita is common and well documented (23–25). In 1996, the U.S. Department of Agriculture deregulated a transgenic cultivar (CZW-3) with coat protein (CP)-based resistance to watermelon mosaic virus (WMV), zucchini yellow mosaic virus (ZYMV), and cucumber mosaic virus (CMV) (26). In the transgenic cultivar a marker gene, neomycin phosphotransferase II (NPTII), was cotransferred. By the late 1990s, several cultivars with the transgene were developed, marketed, and grown throughout the United States (5). In 2004, the growth of transgenic squash was deregulated in several Mexican states (27). Because transgenic cultivars are often grown near wild populations, it is likely that the virus-resistant transgene (VRT) has been introduced to natural populations repeatedly.
The Cucurbita pathosystem that we study consists of the interactions among Cucurbita pepo ssp. texana (a wild C. pepo, texana gourd), its primary herbivores (cucumber beetles and aphids), and the bacterial and viral pathogens these insects transmit (22, 28–30). Cucumber beetles (Diabrotica spp. and Acalymma spp.) are specialist herbivores of the Cucurbitaceae. The leaves and other organs of the Cucurbitaceae produce bitter compounds called cucurbitacins (oxygenated tetracyclic triterpenes) that are toxic to most herbivores (31). Cucumber beetles, however, are attracted to cucurbitacins in the foliage of Cucurbita (which they use both for defense and in mating) and to floral volatiles that also attract bee pollinators (32–36). Cucumber beetles transmit Erwinia tracheiphila (Enterobacteriaceae), the causative agent of bacterial wilt disease, when fecal pellets containing Erwinia fall onto the open wounds as beetles feed on the leaves and when the fecal pellets fall in the vicinity of the nectaries when the beetles aggregate in the flowers to mate (37). Cucumber beetles tend to forage selectively on large plants with many flowers, resulting in biased exposure and disease mortality in larger plants (22). A variety of generalist aphids transmit CMV, WMV, and ZYMV. Wilt disease is always fatal once visible symptoms appear whereas the mosaic viruses slow growth and reproduction but generally do not kill the plant.
In the Cucurbita pathosystem, the VRT should convey a fitness advantage in the face of a viral epidemic. However, when both pathogens are present, avoidance of the smaller, viral infected plants by cucumber beetles may make VRT plants relatively more attractive to the beetles, resulting in increased exposure to the lethal, nontarget pathogen. We examined the fitness (flower and fruit production and proportion of progeny sired) of the VRT during introgression into the texana gourd and the effects of the VRT on herbivory by cucumber beetles, the incidence of the three mosaic viruses, and the incidence of wilt disease in a 3-year field scale study within the full host–vector–pathogen community. We created F1, backcross (BC1), BC2, BC3, and BC4 by crossing a transgenic squash cultivar, Liberator III (hemizygous for the VRT with resistance to three viruses), to texana gourds with the texana gourd as the recurrent parent. In 2006, we transplanted 18 texana plants, 3 F1, 3 BC1, and 3 BC2 transgenic plants and 3 F1, 3 BC1, and 3 BC2 nontransgenic siblings from each of five families (180 total plants, 25% were transgenic) into each of four 0.4-ha fields. In 2007 we planted two fields as above and two fields with texana, transgenic BC3 (25% of total plants), and nontransgenic BC3 from each of five families. In 2008 we planted two fields using the same design as the BC3 fields except that we planted texana and BC4 (with and without the VRT) from each of five families. We monitored flower and fruit production and the incidence of viral and wilt diseases throughout the growing season and recorded beetle damage to the leaves of new growth on June 15, July 15, and August 15 of each year (see Materials and Methods). Our fields were located at the Penn State University Experimental Farms at Rock Springs, PA.
Results
In all years, the VRT effectively deterred viral infections in the introgressives [supporting information (SI) Fig. S1]. In 2006 and 2008, the first symptoms of viral disease (ZYMV) occurred in mid-July and spread rapidly through the fields so that by September most of the remaining texana and non-VRT introgressives (those that had not died of wilt disease) had become infected with ZYMV (Fig. S1). In 2007, the first incidence of a viral disease (WMV) occurred in mid-June and spread slowly until mid-July when we recorded the first incidence of ZYMV that spread rapidly through the remaining non-VRT plants (texana and introgressives). In each year, a few VRT plants became infected with ZYMV after August 15 but the symptoms were very mild (Fig. S1). There was no significant difference in the proportion of texana and non-VRT introgressives that contracted a viral disease over the 3 years of this study (χ2 = 0.36; df = 1; P > 0.10).
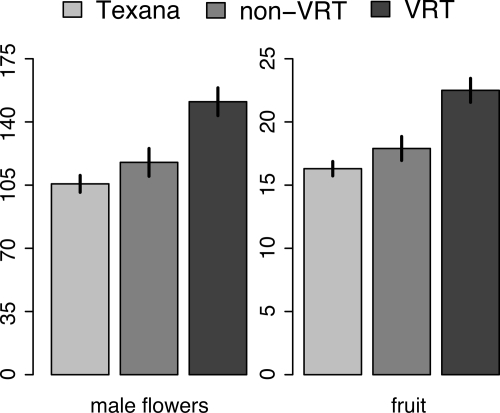
In each year, flowering commenced in late June and peak flower and fruit production occurred from late July to late August (when ZYMV was spreading through the fields). The VRT introgressives produced significantly more flowers and mature fruits than did the virus-susceptible types and there were no significant differences in flower number and fruit number between non-VRT introgressives and texana gourds (Fig. 1). If fertilization is random with respect to the presence of the VRT in the populations that we planted, we expected 12.5% of the seeds on the non-VRT introgressives and texana gourds to have the VRT (25% of the plants in each field were hemizygous for the VRT). We found that the VRT plants sired a higher proportion of the seeds on the virus-susceptible plants than is expected by chance alone (27% in 2006, 26.9% in 2007, and 29.4% in 2008; all χ2 > 175; df = 1; all P < 0.0001).
Fig. 1.
Number of male flowers and fruits produced per plant on texana gourds, non-VRT and VRT introgressives. LS means ± SE bars are shown. Model: Male flowers (or fruits) per plant = year + family(year) + plant type [texana, non-VRT, VRT]. Plant type F2,2155 = 12.15 for male flowers and F2,2155 = 15.23 for fruits; both P < 0.0001.
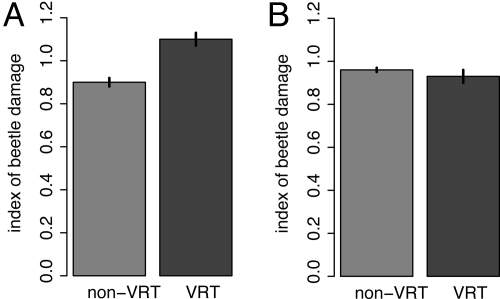
We found that the non-VRT plants and the VRT plants did not differ in the amount of beetle damage they experienced before July 15 (before viral diseases were prevalent in our fields) (F1,1821 = 0.56; P = 0.45) but that the VRT plants experienced significantly greater beetle damage during August (when viral diseases were prevalent in the non-VRT plants) (Fig. 2A). However, if the time with virus is included as a covariate in the model, there is no significant difference between the amount of damage sustained by the VRT and non-VRT plants in August (Fig. 2B), suggesting that the beetles prefer to feed on healthy plants, rather than VRT plants per se. Moreover, we found that significantly more beetles aggregated in the flowers of healthy plants (mostly VRT introgressives) than in the flowers of viral-infected plants during August [least-square (LS) means ± SE: healthy = 3.1 ± 0.1; viral infected = 2.6 ± 0.2; F1,4553 = 8.6; P < 0.005].
Fig. 2.
Amount of beetle damage on leaves of non-VRT (texana and non-VRT introgressives) and VRT plants. LS means ± SE bars are shown. Model: Beetle damage = year + family(year) + plant type [VRT vs. non-VRT]. (A) Beetle damage on August 15. Plant type F1,1821 = 5.63; P = 0.017. (B) Beetle damage on August 15 with time with viral disease in the model. Plant type F1,1820 = 0.05; P = 0.83.
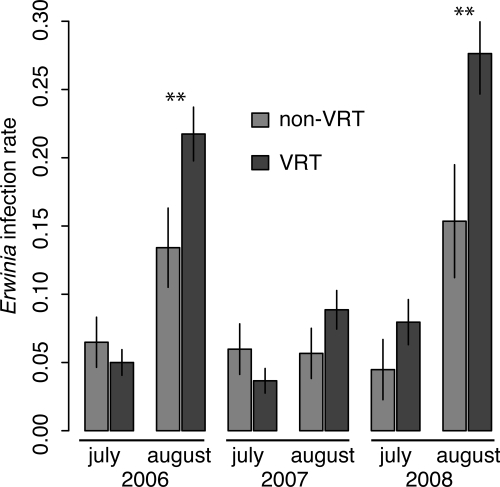
In each of the 3 years, the first symptoms of bacterial wilt disease appeared in our fields within 2 weeks after transplanting. There were, however, no differences in the incidence of wilt disease on VRT (5.6%) and non-VRT (5.8%) plants before mid-July, indicating that the VRT does not alter the resistance of plants to the nontarget pathogen, Erwinia. However, we found that the incidence of wilt disease on the transgenic plants from mid-July until September 1 was significantly higher (17.5%) than the incidence of wilt disease on the nontransgenic plants (10.9%) over the 3 years of this study (χ2 = 10.9; df = 1; P < 0.001). Moreover, a logistic regression model revealed a significant positive interaction between month and the presence of the transgene (P = 0.02), indicating that the odds of developing wilt disease increased for transgenic plants after mid-July in all 3 years (Fig. 3). In 3 years, only two viral-infected plants (0.4% of the viral-infected plants) contracted wilt disease and there was no significant difference in the percentage of texana and non-VRT introgressives that contracted wilt disease (χ2 = 2.25; df = 1; P > 0.10).
Fig. 3.
Proportion of susceptible plants (alive and not infected with virus) that became infected with Erwinia in the interval June 15 to July 15 (July) and July 15 to August 15 (August). Error bars indicate ± SE. Asterisks indicate significant differences within month (P < 0.05).
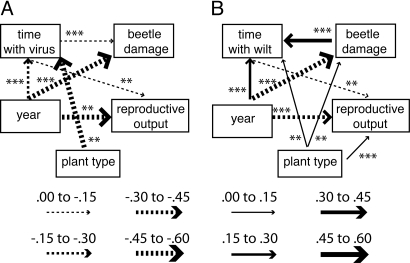
To determine both direct and indirect effects of the VRT on fitness, we performed a series of path analyses. In these analyses, non-VRT plants are coded as 0 and VRT plants as 1: Thus, a path proceeding from plant type reveals the relationship to the VRT plants. Because wilt-infected plants die and cannot later contract a viral disease, we are unable to include time with wilt disease and time with viral disease in the same model. The first analysis, using only those plants that survived to the end of August during 2006–2008 (i.e., no plants that contracted wilt disease), revealed that the VRT has no direct effect on reproductive output (see Materials and Methods for calculation) or beetle damage (Fig. 4A). Rather, the VRT has an overall positive effect on reproductive output via its strong negative relationship with time with viral disease, which, in turn, has a negative effect on reproductive output. The second analysis, using all plants but ignoring time with viral disease, reveals that the VRT has a positive and direct effect on reproductive output, beetle damage, and time with wilt disease (Fig. 4B). From Fig. 4A, we know that the positive relationship between the VRT and reproductive output is because of viral resistance and the negative effects of viral diseases on reproductive output. However, the overall positive effect of the VRT on reproductive output is reduced because the time with wilt disease is negatively related to reproductive output. Moreover, beetle damage has a positive effect on the duration of infection with wilt disease (beetles transmit wilt disease while feeding on leaves), which, in turn, is negatively related to reproductive output (Table S1).
Fig. 4.
Final path analysis including the effects of (A) time with virus and (B) time with wilt disease on reproductive output. Values of standardized regression coefficients are given by line weight and dotted vs. solid lines (see key above). Final models include only significant paths. Significance levels of correlations are denoted by **, P < 0.01 and ***, P < 0.001. (A) χ2 = 7.0, df = 3, P = 0.07, RMSEA = 0.03, AIC = 41.08. (B) χ2 = 0.03, df = 1, P = 0.85, RMSEA = 0, AIC = 38.03
Discussion
In this 3-year study, viral diseases (mostly ZYMV) colonized and spread rapidly through our fields during the period of peak gourd reproduction. The VRT plants (25% of the population) produced 30.6% of the fruits and 38% of the male flowers (but only half of the pollen grains in these flowers have the VRT). These data clearly demonstrate that the squash VRT would have a fitness advantage through both male and female functions during the initial stages of introgression into a population of wild texana gourds under the conditions of our study. Moreover, our data show that the VRT plants sired even more of the progeny than would be predicted on the basis of male flower production. Assuming all male flowers in the population produce the same number of pollen grains and that pollen removal and deposition are random with respect to the VRT, we would expect that 19% of the seeds on non-VRT plants would contain the transgene. However, 26−29% of the seeds were sired by pollen with the transgene, suggesting that the viral-free VRT plants attract more pollinators, produce and disseminate more pollen per flower, and/or the pollen from VRT plants is competitively superior to pollen from the non-VRT plants.
We have also shown that the fitness advantage of the VRT plants comes with a cost because of the interactions with the herbivore and pathogen community. This study revealed that the VRT did not affect resistance to cucumber beetles or bacterial wilt disease before the establishment and spread of viral pathogens in the susceptible plants. Infection with a viral pathogen, however, reduced the attractiveness of the non-VRT plants as both a food source and a mating location for the cucumber beetles. The VRT plants experienced higher levels of leaf herbivory in August than the non-VRT plants, viral infection reduced flower production on non-VRT plants compared to VRT plants, and the VRT plants attracted more beetles per flower. In short, the presence of viral pathogens tended to concentrate the cucumber beetles onto healthy (mostly VRT) plants, resulting in increased Erwinia exposure on VRT plants through both modes of transmission (foliar feeding and floral transmission). As viral infection spread through the susceptible plants in the population, the incidence of wilt disease increased on the VRT plants. Our path analysis indicates that the VRT has an indirect effect on the feeding preference of a nontarget herbivore (cucumber beetles) and the pathogen it transmits (Erwinia) that mitigates the overall fitness of the transgene during introgression. Thus, there is an indirect, ecological cost associated with the VRT when the nontarget pathogen and its vector are also present in the population.
Texana gourds have long been recognized as an important weed in soybean and cotton fields (38). Whether introgression of the VRT into wild populations of Cucurbita will result in a more problematic weed and pose a threat to natural biodiversity depends upon the fitness of the VRT introgressives. Our data indicate that the VRT could increase in frequency in wild Cucurbita. However, the indirect costs due to Erwinia exposure may mitigate the reproductive benefits of the VRT. The fitness of the VRT may depend upon (a) the arrival times and transmission rate of both the target and the nontarget diseases in the population, (b) the number of cucumber beetles in the population, and (c) the proportion of plants in the population with the VRT [which will determine the increase in beetle concentration onto VRT plants as non-VRT plants become infected with virus and perhaps also affect the transmission rate of the virus (39, 40)].
A large body of theory (but limited empirical data) suggests that ecological trade-offs between the costs and benefits of a resistance gene, such as the Cucurbita VRT, play important roles in determining the gene's effects on fitness (7, 8, 10–15). Most studies of the fitness of herbivore- or pathogen-resistant transgenes in crop–wild plant hybrids (and their backcross progeny) have tended to examine direct trade-offs in pairwise comparisons (e.g., with and without the target herbivore/pathogen) that lack the full host–natural enemy–community complex. Typically, these studies have found that seed production of hybrids carrying resistance to herbivores or pathogens increases in the presence of their target herbivores or pathogens (41–44). However, upon escape into natural populations, resistance transgenes face a complex suite of direct and indirect costs. As we have shown here, a full understanding of the combined effect of these forces on the fitness of an escaped transgene may not be apparent without the context of the complete ecological community.
Materials and Methods
Study System.
The texana gourd, C. pepo ssp. texana (Cucurbitaceae) is an annual monoecious vine with indeterminate growth and reproduction. It is native to Texas and states along the lower Mississippi River. It is completely interfertile with the cultivated pumpkins and squashes (C. pepo ssp. pepo and ssp. ovifera) and several annual Cucurbita taxa from Mexico (25, 45, 46). After a period of vegetative growth (five to seven nodes), texana gourds produce one large yellow flower (either male or female) in the axil of each leaf. The flowers last for only one morning and are pollinated by bees. The fruits of the wild gourd are round and typically contain 150–300 seeds (47).
Cucumber beetles (Diabrotica spp. and Acalymma spp.) are specialist herbivores on the Cucurbitaceae and are the only known vector of E. tracheiphila (Enterobacteriaceae), the causative agent of bacterial wilt disease, which overwinters in the guts of cucumber beetles. Wilt symptoms typically develop 10–15 days after infection and the disease is fatal once symptoms appear. However, fruits that are >10 days old when the first symptoms appear on the plant will mature (30).
WMV and ZYMV are the two most common viral diseases of cucurbits at our field site in central Pennsylvania. Both are single-stranded, positive-sense RNA viruses of the family Potyviridae and are transmitted via aphids. These diseases are rarely fatal but do depress reproductive output and produce symptoms that include leaf blisters, necrotic lesions, and branch deformities.
Field Experiments.
The Liberator III crookneck squash cultivar is a commercially available transgenic squash with CP-based resistance to ZYMV, WMV, and CMV. In this cultivar, the VRT is hemizygous and, importantly, the NPTII gene conferring resistance to neomycin has not been deactivated and is still tightly linked to the CP genes of the three viruses. Consequently, we have been able to introgress the transgene (CP genes and NPTII) into texana gourds (using texana gourd as the recurrent parent) because the presence of the transgene in hybrid progeny can be identified using DAS-ELISA (kit available from Agdia) to detect the NPTII protein (half of the progeny from each cross are transgenic). No permit is required as this VRT has been deregulated for all subspecies and cultivars of C. pepo.
Extensive seed germination and screening for NPTII in F1 (1,410 seeds), BC1 (1,410 seeds), BC2 (1,410 seeds), BC3 (940 seeds), and BC4 (940 seeds) as part of the field studies described below revealed no transmission bias via pollen with respect to the VRT (6,100 seeds; 50.3% transgenic). In 2006, 2007, and 2008, we germinated in a greenhouse (in mid-May) and transplanted (late May) 18 texana plants, 9 transgenic introgressives and 9 nontransgenic (full siblings) introgressives from each of five families (180 total plants per field; 25% were transgenic), into each of four (2006–2007) or two (2008) 0.4-ha fields as described in the Introduction. The fields were not sprayed with insecticide and viral and wilt diseases were allowed to occur naturally. The plants in each field during each year were monitored throughout the growing season for incidence of CMV, WMV, and ZYMV (field diagnosis confirmed by DAS-ELISA tests; Agdia) and wilt disease [field diagnosis confirmed by isolating Erwinia from diseased plants and using the isolate to infect greenhouse-grown plants (see ref. 22 for techniques)]. We counted male and female flowers weekly (an unbiased estimate of total flower production because flowers last for only one morning) and we counted total fruit production per plant at the end of the growing season. We nondestructively estimated beetle damage to leaves of new growth on June 15, July 15, and August 15 of each year, using a 0–5 index in which 0 = most leaves with no beetle damage and no leaf with 5% of the leaf area removed and 5 = all leaves damaged and at least one leaf with >50% of the leaf area removed. Three people, who were blind with respect to plant family and type of plant, simultaneously and independently evaluated damage on each plant. If two or three of the evaluators agreed on the score, we recorded that value. If the evaluators differed on their assessments, we recorded the middle score (≈5% of the cases).
To determine whether healthy and diseased plants differ in the number of beetles that aggregate in the flowers to mate, we counted the number of beetles in one randomly chosen male and one female flower on all plants that produced at least one flower on three dates in August in each of 3 years (n = 3,093 male flowers; n = 1,486 female flowers). To determine whether the frequency of the VRT changed in the progeny generation, we harvested two mature fruits from each non-VRT introgressive and texana plant at the end of each growing season, removed and pooled their seeds, and scored a random sample (475 seeds per field per year) for the presence of the VRT (NPTII protein) using DAS-ELISA.
Statistical Analysis.
We used a mixed-effects model analysis of variance (ANOVA) (48) to determine the effects of plant type (texana gourd, non-VRT introgressives, and VRT-introgressives), year, family (random) nested within year, and field nested within year (a blocking variable that was dropped from the final analysis because it was not significant) on male flower and fruit production. To determine whether the cultivar genes affect the amount of beetle damage during introgression, we examined the effects of year, generation (texana, non-VRT F1, BC1, BC2, BC3, and BC4) nested within year, family (random) nested within year, and field nested within year on the amount of beetle damage before July 15. For those plants that survived until the July 15 assessment of beetle damage, we used the mean of the June 15 and July 15 assessments. For those plants that died between June 15 and July 15 we used the June 15 assessment. Those plants that died before June 15 were excluded from the analysis. This analysis revealed that generation nested within year had no significant affect on beetle damage (Table S2 and Table S3) (i.e., the texana and non-VRT introgressives did not differ in their resistance to cucumber beetles and were subsequently pooled in our analyses). To determine the effect of the VRT on resistance to cucumber beetles, we used a mixed-effects model ANOVA to determine the effects of plant type [VRT introgressives and non-VRT plants (texana and non-VRT introgressives)], year, and family (random) nested within year on beetle damage before July 15. We again used a mixed-effects model ANOVA to determine the effects of plant type (VRT introgressives and non-VRT plants), year, and family (random) nested within year on beetle damage on August 15. In this analysis, we used the August 15 assessment of beetle damage. Plants that died before August 15 were treated as above. We then repeated the analysis of the August 15 beetle damage assessment but we included the time with virus (log days with virus + 1 from first visible symptoms until September 1) as a covariate to determine the effect of viral disease on beetle herbivory. Finally, we performed a fixed-effects model ANOVA to determine the effects of disease status (healthy or virus infected), plant type (VRT or non-VRT plants), flower type (male or female), year, family nested within year, and date nested within year on the number of beetles per flower.
We used a logistic regression to assess the effect of plant type (non-VRT or VRT), month, and year on the likelihood of infection with Erwinia. We assumed that those plants infected during June 15 to July 15 and July 15 to August 15 were conditionally independent. Thus, we analyzed the plants that were infected during July 15 to August 15 as coming from the pool of plants that remained alive and uninfected on July 15. We included an interaction between plant type and month in the model to account for changing odds of infection throughout the season.
To assess the direct and indirect effects of plant type (VRT introgressives and non-VRT plants), year, beetle damage, and viral and wilt diseases on reproductive output, we used structural equation modeling/path analysis (14, 49–51). [Reproductive output = fruit number per plant + (male flowers per plant/7) because across all plants there were seven times more male flowers than fruits; see Fig. 1.] With path analysis it is possible to examine the effect of the VRT on each character in the model while holding constant all other factors that have paths leading to that character (52). We performed the path analysis using AMOS 16 for Windows (53). We coded the VRT introgressives as 1 and non-VRT plants as 0 so that paths proceeding from plant type reveal the relationship with the VRT plants. The initial models included family nested within year but this term was dropped from the final models because no significant paths proceeded from it. The final models were assessed by (a) goodness of fit (χ2), (b) root mean square error approximation (RMSEA), and (c) Akaike's information criterion (AIC) calculated for the final model tested and a saturated model (51).
Supplementary Material
Acknowledgments.
We thank A. Guirand, J. Verbano, R. Kariyat, K. Wall, M. Bothe, C. Dryoff, S. Scanlon, and G. Stephenson for field, greenhouse, and lab assistance; T. Omeis for the use of the biology greenhouses; and R. Oberheim for use of the Horticulture Farm at the Pennsylvania State University Agriculture Experiment Station at Rock Springs, PA. This research was supported by National Science Foundation grant DEB02–35217 (to A.G.S. and J.A.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905106106/DCSupplemental.
References
- 1.Ellstrand NC. Current knowledge of gene flow in plants: Implications for transgene flow. Philos Trans R Soc Lond B. 2003;358:1163–1170. doi: 10.1098/rstb.2003.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellstrand NC. Dangerous Liaisons? When Cultivated Plants Mate with Their Wild Relatives. Baltimore: John Hopkins Univ Press; 2003. [Google Scholar]
- 3.Ellstrand NC, Prentice HC, Hancock JC. Gene flow from domesticated plants into their wild relatives. Annu Rev Ecol Syst. 1999;30:539–563. [Google Scholar]
- 4.Pilson D, Prendeville HR. Ecological effects of transgenic crops and the escape of transgenes into wild populations. Annu Rev Ecol Evol Syst. 2004;35:149–174. [Google Scholar]
- 5.Fuchs M, Gonsalves D. Safety of virus-resistant transgenic plants two decades after their introduction: Lessons from realistic field risk assessment studies. Annu Rev Phytopathol. 2007;45:173–202. doi: 10.1146/annurev.phyto.45.062806.094434. [DOI] [PubMed] [Google Scholar]
- 6.National Research Council. Environmental Effects of Transgenic Plants: The Scope and Adequacy of Regulation. Washington, DC: National Academy Press; 2002. [PubMed] [Google Scholar]
- 7.Mitchell-Olds T, Bradley D. Genetics of Brassica rapa. 3. Costs of disease resistance to three fungal pathogens. Evolution. 1996;50:1859–1865. doi: 10.1111/j.1558-5646.1996.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 8.Boots M, Bowers RG. Three mechanisms to host resistance to macroparasites—avoidance, recovery and tolerance—show different evolutionary dynamics. J Theor Biol. 1999;201:13–23. doi: 10.1006/jtbi.1999.1009. [DOI] [PubMed] [Google Scholar]
- 9.Kniskern JM, Rausher MD. Natural selection on a polymorphic disease-resistance locus in Ipomoea purpurea. Evolution. 2007;61:377–387. doi: 10.1111/j.1742-4658.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 10.Bergelson J, Purrington CB. Surveying patterns in the cost of resistance in plants. Am Nat. 1996;148:536–558. [Google Scholar]
- 11.Heil M, Baldwin IT. Fitness costs of induced resistance: Emerging experimental support for a slippery theory. Trends Plant Sci. 1997;7:61–67. doi: 10.1016/s1360-1385(01)02186-0. [DOI] [PubMed] [Google Scholar]
- 12.Purrington CB. Costs of resistance. Curr Opin Plant Biol. 2000;3:305–308. doi: 10.1016/s1369-5266(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 13.Tian DM, Traw B, Chen JQ, Kreitman M, Bergelson J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature. 2003;423:74–77. doi: 10.1038/nature01588. [DOI] [PubMed] [Google Scholar]
- 14.Gassmann AJ, Futuyma DJ. Consequences of herbivory for the fitness cost of herbicide resistance: Photosynthetic variation in the context of plant-herbivore interactions. J Evol Biol. 2005;18:447–454. doi: 10.1111/j.1420-9101.2004.00819.x. [DOI] [PubMed] [Google Scholar]
- 15.Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends Ecol Evol. 2002;17:278–285. [Google Scholar]
- 16.Leimu R, Koricheva J. A meta-analysis of genetic correlations between plant resistances to multiple enemies. Am Nat. 2006;168:E15–E37. doi: 10.1086/505766. [DOI] [PubMed] [Google Scholar]
- 17.Puustinen S, Koskela T, Mutikainen P. Direct and ecological costs of resistance and tolerance in the stinging nettle. Oecologia. 2004;139:76–82. doi: 10.1007/s00442-004-1488-4. [DOI] [PubMed] [Google Scholar]
- 18.Burdon JJ. Diseases and Plant Population Biology. Cambridge, UK: Cambridge Univ Press; 1987. [Google Scholar]
- 19.Irwin ME, Thresh JM. Epidemiology of barley yellow dwarf; a study in ecological complexity. Annu Rev Phytopathol. 1990;28:393–424. [Google Scholar]
- 20.Maddox GD, Root RB. Structure of the encounter between goldenrod (Solidago altissima) and its diverse insect fauna. Ecology. 1990;71:2115–2124. [Google Scholar]
- 21.Ferrari MJ, Bjornstad ON, Partain JL, Antonovics J. A gravity model for the spread of a pollinator-borne plant pathogen. Am Nat. 2005;168:294–303. doi: 10.1086/506917. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari MJ, Winsor JA, Du D, Stephenson AG. Inbreeding alters host plant quality and incidence of an insect borne pathogen in Cucurbita pepo ssp. texana. Int J Plant Sci. 2007;168:603–610. [Google Scholar]
- 23.Kirkpatrick KJ, Wilson HD. Interspecific gene flow in Cucurbita: C. texana vs. C pepo. Am J Bot. 1988;75:519–527. [Google Scholar]
- 24.Wilson HD, Lira R, Rodríguez I. Crop/weed gene flow: Cucurbita agyrosperma Huber and C. fraterna LH Bailey. Econ Bot. 1994;48:293–300. [Google Scholar]
- 25.Decker-Walters DS, Walters TW, Cowan CW, Smith BD. Isozymic characterization of wild populations of Cucurbita pepo. J Ethnobiol. 1993;13:55–72. [Google Scholar]
- 26.U.S. Department of Agriculture. Environmental Assessment for Upjohn Company/Asgrow Seed Company Petition for Determination of Non-Regulated Status for CZW-3 Squash. Washington, DC: U.S. Department of Agriculture; 1996. [Google Scholar]
- 27.Senasica. Regulations for Genetically Modified Organisms in Agriculture [Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food, Mexico (In Spanish)] 2004. http://www.web2.senasica.sagarpa.gob.mx/xportal/inocd/trser/Doc403/
- 28.Stephenson AG, Leyshon B, Travers SE, Hayes CN, Winsor JA. Interrelationships among inbreeding, herbivory, and disease on reproduction in a wild gourd. Ecology. 2004;85:3023–3034. [Google Scholar]
- 29.Ferrari MJ, De Moraes CM, Stephenson AG, Mescher MC. Inbreeding effects on blossom volatiles in Cucurbita pepo ssp. texana. Am J Bot. 2006;93:1768–1774. doi: 10.3732/ajb.93.12.1768. [DOI] [PubMed] [Google Scholar]
- 30.Du D, Winsor JA, Smith M, DeNicco A, Stephenson AG. Timing of herbivory affects reproductive performance and incidence of disease in a wild gourd. Am J Bot. 2008;95:84–92. doi: 10.3732/ajb.95.1.84. [DOI] [PubMed] [Google Scholar]
- 31.Tallamy DW. Squash beetle feeding behavior: An adaptation against induced cucurbit defenses. Ecology. 1985;66:1574–1579. [Google Scholar]
- 32.Metcalf RL, Rhodes AM. In: Biology and Utilization of the Cucurbitaceae. Bates DM, Robinson RW, Jeffrey C, editors. Ithaca, NY: Cornell Univ Press; 1990. pp. 167–182. [Google Scholar]
- 33.Anderson JF, Metcalf RL. Identification of a volatile attractant for Diabrotica (Coleoptera, Chrysomelidae) and Acalymma (Coleoptera, Chrysomelidae) spp. from blossoms of Cucurbita maxima duchesne. J Chem Ecol. 1986;12:687–699. doi: 10.1007/BF01012102. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JF, Metcalf RL. Factors influencing distribution of Diabrotica spp. (Coleoptera, Chrysomelidae) in blossoms of cultivated Cucurbita spp. J Chem Ecol. 1987;13:681–699. doi: 10.1007/BF01020152. [DOI] [PubMed] [Google Scholar]
- 35.Lampman RL, Metcalf RL. The comparative response of Diabrotica species (Coleoptera, Chrysomelidae) to volatile attractants. Environ Entomol. 1988;17:644–648. [Google Scholar]
- 36.Metcalf RL, Lampman RL. Evolution of diabroticite rootworm beetle (Chrysomelidae) receptors for Cucurbita blossom volatiles. Proc Natl Acad Sci USA. 1991;88:1869–1872. doi: 10.1073/pnas.88.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleischer SJ, de Mackiewicz D, Gildow FE, Lukezic FL. Serological estimates of the seasonal dynamics of Erwinia tracheiphila in Acalymma vittata (Coleoptera: Chrysomelidae) Environ Entomol. 1999;28:470–476. [Google Scholar]
- 38.Oliver L, Harrison S, McClelland M. Germination of Texas gourd (Cucurbita texana) and its control in soybeans (Glycine max) Weed Sci. 1983;31:700–706. [Google Scholar]
- 39.Garrett KA, Mundt CC. Epidemiology in mixed host populations. Phytopathology. 1999;89:984–990. doi: 10.1094/PHYTO.1999.89.11.984. [DOI] [PubMed] [Google Scholar]
- 40.Klas FE, Fuchs M, Gonsalves D. Comparative spatial spread over time of Zucchini yellow mosaic virus (ZYMV) and Watermelon mosaic virus (WMV) in fields of transgenic squash expressing the coat protein genes of ZYMV and WMV, and in fields of nontransgenic squash. Trans Res. 2006;15:527–541. doi: 10.1007/s11248-006-9001-y. [DOI] [PubMed] [Google Scholar]
- 41.Burke JM, Rieseberg LH. Fitness effects of transgenic disease resistance in sunflowers. Science. 2003;300:1250–1252. doi: 10.1126/science.1084960. [DOI] [PubMed] [Google Scholar]
- 42.Snow AA, et al. A Bt transgene reduces herbivory and enhances fecundity in wild sunflowers. Ecol Appl. 2003;13:279–286. [Google Scholar]
- 43.Fuchs M, Chirco EM, McFerson JR, Gonsalves D. Comparative fitness of a wild squash species and three generations of hybrids between wild X virus-resistant transgenic squash. Environ Biosafety Res. 2004;3:17–28. doi: 10.1051/ebr:2004004. [DOI] [PubMed] [Google Scholar]
- 44.Chen L-Y, Snow AA, Wang F, Boa-Rong L. Effects of insect-resistance transgenes on fecundity in rice (Oryza sativa, Poaceae): A test for underlying costs. Am J Bot. 2006;93:94–101. [Google Scholar]
- 45.Lira R, Andres TC, Nee M. Systematic and Ecogeographic Studies on Crop Genepools. Vol 9. Mexico City and Rome: International Plant Genetic Resources Institute; 1995. pp. 1–115. [Google Scholar]
- 46.Arriaga L, Huerta E, Lira-Saade R, Moreno E, Alarcón J. Assessing the risk of releasing transgenic Cucurbita spp. in Mexico. Agric Ecosyst Environ. 2006;112:291–299. [Google Scholar]
- 47.Avila-Sakar G, Krupnick GA, Stephenson AG. Growth and resource allocation in Cucurbita pepo ssp. texana: Effects of fruit removal. Int J Plant Sci. 2001;162:1089–1095. [Google Scholar]
- 48.SAS Institute. SAS User's Guide. v9. Cary, NC: SAS Inst; 2007. [Google Scholar]
- 49.Scheiner SM, Mitchell RJ, Callahan HS. Using path analysis to measure natural selection. J Evol Biol. 2000;13:423–433. [Google Scholar]
- 50.Shipley B. Cause and Correlation in Biology: A User's Guide to Path Analysis, Structural Equations and Causal Inference. Cambridge, UK: Cambridge Univ Press; 2000. [Google Scholar]
- 51.Pigliucci M, Kolodynska A. Phenotypic integration and response to stress in Arabidopsis thaliana: A path analytical approach. Evol Ecol Res. 2006;8:415–433. [Google Scholar]
- 52.Sokal RR, Rohlf FJ. Biometry. New York: Freeman; 1995. [Google Scholar]
- 53.AMOS. AMOS 16 for Windows. Chicago: SPSS; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.