Abstract
Type 2 diabetes mellitus (T2DM) results from pancreatic β cell failure in the setting of insulin resistance. Heterozygous mutations in the gene encoding the β cell transcription factor pancreatic duodenal homeobox 1 (Pdx1) are associated with both T2DM and maturity onset diabetes of the young (MODY4), and low levels of Pdx1 accompany β cell dysfunction in experimental models of glucotoxicity and diabetes. Here, we find that Pdx1 is required for compensatory β cell mass expansion in response to diet-induced insulin resistance through its roles in promoting β cell survival and compensatory hypertrophy. Pdx1-deficient β cells show evidence of endoplasmic reticulum (ER) stress both in the complex metabolic milieu of high-fat feeding as well as in the setting of acutely reduced Pdx1 expression in the Min6 mouse insulinoma cell line. Further, Pdx1 deficiency enhances β cell susceptibility to ER stress-associated apoptosis. The results of high throughput expression microarray and chromatin occupancy analyses reveal that Pdx1 regulates a broad array of genes involved in diverse functions of the ER, including proper disulfide bond formation, protein folding, and the unfolded protein response. These findings suggest that Pdx1 deficiency leads to a failure of β cell compensation for insulin resistance at least in part by impairing critical functions of the ER.
Keywords: chromatin occupancy, diabetes, gene regulation, islet compensation
The pathogenesis of type 2 diabetes reflects a dynamic interaction between environmental influences and complex genetic predisposition in which the development of insulin resistance, largely associated with obesity, is initially balanced by increased insulin production by the pancreatic β cells. In some individuals, the increased demand for insulin eventually overwhelms the capacity of the β cell to respond, resulting in progressive β cell dysfunction and overt diabetes. Indeed, β cell compensation is considered the critical determinant of whether impaired glucose tolerance will advance to frank diabetes (1). The islet compensatory response consists of an increase in both insulin secretion and β cell number. Patients with type 2 diabetes have reduced β cell volume at autopsy, underscoring the importance of β cell mass in maintaining glucose homeostasis (2). Notably, β cell failure is not limited to type 2 diabetes but rather is a common feature of most forms of diabetes, including autoimmune type 1 diabetes, ketosis-prone diabetes, and the monogenic, autosomal-dominant maturity onset diabetes of the young (MODY) syndromes (3).
The β cell-enriched pancreatic duodenal homeobox 1 (Pdx1) gene, also known as Ipf1 (MODY4), encodes a transcription factor that critically regulates early pancreas formation and multiple aspects of mature β cell function, including insulin secretion, mitochondrial metabolism, and cell survival (4–6). Pdx1 directly regulates expression of the insulin gene and other components of the glucose-stimulated insulin secretion pathway (7, 8). Reduced Pdx1 expression in the β cell occurs in cellular models of glucose toxicity and accompanies the development of diabetes in complex genetic and environmental animal models of the disease, correlating low Pdx1 levels with β cell failure (9–13).
Heterozygous mutations in Pdx1 are associated with type 2 diabetes in humans, suggesting that Pdx1 plays a role in islet compensation for insulin resistance (14, 15). This hypothesis is supported by studies using genetic mouse models of insulin resistance. In mice doubly heterozygous for the insulin receptor (IR) and insulin receptor substrate 1 (IRS-1), superimposed Pdx1 haploinsufficiency impairs both insulin secretion and compensatory β cell mass expansion, leading to worsened glucose tolerance (16); however, in the insulin resistant Glut4+/− model, concurrent Pdx1 heterozygosity leads to hyperglycemia due to defects in insulin secretion with no impairment in β cell mass expansion (17). The basis for the discrepancy regarding the role of Pdx1 in compensatory expansion of β cell mass in these genetic models of insulin resistance has not been elucidated but may involve differences in the sites and severity of insulin resistance. In particular, impaired insulin responsiveness of the β cell itself in the IR+/−/IRS-1+/− model may limit Pdx1-mediated compensatory β cell mass expansion. Further, these genetic models offer limited analogy to human type 2 diabetes which is rarely caused by mutations in insulin response pathways. Therefore, we sought to define the role of Pdx1 in compensatory β cell mass expansion using a mouse model of high-fat diet (HFD)-induced insulin resistance that more closely parallels the progression to type 2 diabetes in humans.
The role of Pdx1 as a transcription factor regulating islet compensation suggests that the identification of direct Pdx1 transcriptional targets will offer insights into the molecular mechanisms of the islet response to insulin resistance that may be exploited therapeutically to prevent or delay the progression of diabetes. To this end, we performed high-throughput gene expression profiling and chromatin occupancy experiments in primary murine islets and Min6 mouse insulinoma cells. The results of these studies complemented the characterization of HFD-fed Pdx1+/− mice to reveal that Pdx1 regulates the susceptibility of pancreatic β cells to endoplasmic reticulum (ER) stress and ER stress-induced apoptosis and uncovered a broad role for Pdx1 in transcriptional regulation relevant to ER function.
Results
Pdx1+/− Mice Develop Diabetes in Response to HFD-Induced Insulin Resistance.
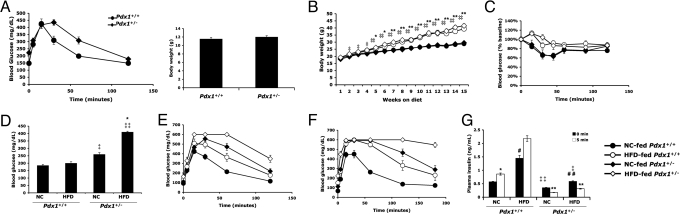
We fed Pdx1+/− male mice and their Pdx1+/+ littermates a HFD starting at 4 weeks of age and followed them for 5 months. At baseline, body weights were comparable but Pdx1+/− mice already displayed mild glucose intolerance (Fig. 1A). Although high-fat feeding induced similar weight gain (Fig. 1B) and an equivalent degree of insulin resistance (Fig. 1C, P = 0.985 by ANOVA for HFD-fed Pdx1+/+ vs. HFD-fed Pdx1+/−) in mice of both genotypes, it caused severe hyperglycemia only in Pdx1+/− mice, with blood glucose levels >400 mg/dL after a 6-h fast (Fig. 1D). Glucose tolerance tests revealed a greater impairment in glucose homeostasis in HF-fed Pdx1+/− animals relative to diet-matched Pdx1+/+ littermates evident after 1 month of HF-feeding (Fig. 1E) that progressed to overt diabetes by 4 months on the HFD (Fig. 1F).
Fig. 1.
Pdx1+/− mice develop diabetes in response to HFD-induced insulin resistance. (A) Baseline glucose tolerance test and body weights of 3.5-week-old male Pdx1+/− mice (n = 16) and their wild-type Pdx1+/+ littermates (n = 15). GTT was performed after a 6 h fast; P < 0.001 by ANOVA. (B) Weight gain profile of Pdx1+/− mice and their littermate controls after randomization into HFD or normal chow (NC) groups. *, P < 0.05, **, P < 0.005 Pdx1+/+ HFD vs. NC; ‡, P < 0.05, ‡‡, P < 0.005 Pdx1+/− HFD vs. NC. (C) Insulin tolerance test after 5 months on HFD or NC (by ANOVA, P < 0.005 Pdx1+/+ HFD vs. NC; P = 0.06 Pdx1+/− HFD vs. NC; P = 0.985 Pdx1+/+ HFD vs. Pdx1+/− HFD). (D) Six-hour fasting blood glucose levels of mice 5 months in HFD or NC group. *, P < 0.001, Pdx1+/− HFD vs. NC; ‡, P < 0.001, ‡‡, P < 0.00001 relative to diet-matched Pdx1+/+ littermates. (E and F) IP glucose tolerance tests on mice fasted overnight after 1 month (E) and 4 months (F) on HFD or NC. In (E), P < 0.005 for Pdx1+/− HFD vs. NC and P < 0.001 for all other pair-wise comparison groups by ANOVA. In (F), P < 0.0001 for all comparison groups by ANOVA. Glucose values of 600 mg/dL represent the upper limit of detection. (G) Acute insulin release in mice 3 months on HFD or NC. Plasma insulin was measured by ELISA after IP glucose injection. *, P < 0.05, **, P < 0.005 relative to time 0; ‡, P < 0.05, ‡‡, P < 0.01 relative to diet-matched Pdx1+/+ littermates; #, P < 0.05, ##, P < 0.01 relative to corresponding NC controls. For (B–G), n = 8 mice per group, and data represent the mean ± standard error.
The development of diabetes in HF-fed Pdx1+/− mice indicated a failure of Pdx1 haploinsufficient β cells to respond appropriately to diet-induced insulin resistance. As normal islet compensation involves a combination of increased insulin secretion as well as an expansion of functional β cell mass, we first examined the acute insulin secretory response in HF- or normal chow (NC)-fed Pdx1+/− and Pdx1+/+ littermates by measuring plasma insulin levels during glucose tolerance testing. Whereas Pdx1+/+ mice on either diet had a normal increase in insulin secretion 5 min after glucose injection, NC-fed and HF-fed Pdx1+/− animals both exhibited an absence of first-phase insulin release (Fig. 1G). Furthermore, while the HFD induced compensatory fasting hyperinsulinemia in all mice, fasting insulin levels in HF-fed Pdx1+/− mice were significantly lower than in HF-fed Pdx1+/+ littermates (Fig. 1G). The functional β cell defect observed in HF-fed Pdx1+/− mice is consistent with the impaired insulin secretion described in Pdx1+/− animals alone or in the context of genetic insulin resistance (4, 16, 17).
Pdx1 Is Required for Compensatory β Cell Mass Expansion in Response to HFD.
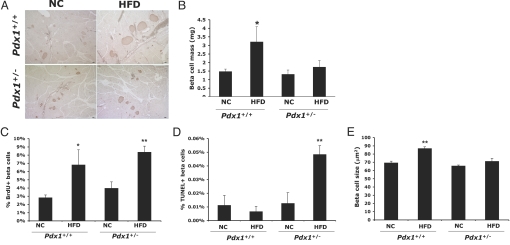
While the role of Pdx1 in functional islet compensation appears unequivocal in genetic models of insulin resistance, these models provide discrepant results regarding its role in morphological β cell compensation (16, 17). To determine whether Pdx1 is required for normal β cell mass expansion in response to HFD-induced insulin resistance, we measured pancreatic β cell mass in Pdx1+/− and Pdx1+/+ mice fed HFD or NC for 5 months. While the HFD stimulated a 2-fold increase in β cell mass in Pdx1+/+ controls, this did not occur in Pdx1+/− mice (Fig. 2 A and B). Therefore, in the context of diet-induced insulin resistance, Pdx1 is required for compensatory β cell mass expansion.
Fig. 2.
Pdx1 haploinsufficiency limits β cell mass expansion by decreasing β cell survival and blocking compensatory hypertrophy without impairing proliferation. (A) Representative images of insulin immunohistochemistry (brown) performed on pancreas sections from mice 5 months on either HFD or NC. (Scale bars, 50 μm.) (B) Quantification of β cell mass (n = 5–8 mice per group; *, P < 0.05 relative to corresponding NC controls). (C) Quantification of β cell proliferation using BrdU/insulin double immunofluorescence. n = 4–6 mice per group and >1,000 insulin-positive cells were counted per animal; *, P < 0.05, **, P < 0.005 relative to NC controls. (D) Rates of β cell apoptosis as assessed by TUNEL. For sections containing TUNEL/insulin double positive cells, cell death was quantified by normalizing to total number of insulin positive cells (≈3,000–9,000 per slide). n = 4–6 mice per group; **, P < 0.01 relative to NC control. (E) Mean β cell size was approximated by dividing total insulin-positive area per islet by number of insulin-positive nuclei per islet. n = 4–6 mice per group and 7–17 islets were analyzed per animal (**, P < 0.001 relative to NC control).
When comparing this finding with the published studies of Pdx1 haploinsufficiency in genetic models of insulin resistance, our results thus far accorded with the IR+/−/IRS-1+/− model, in which the impaired β cell mass expansion in IR+/−/IRS-1+/−/Pdx1+/− mice was attributed to reduced rates of β cell proliferation (16). However, surprisingly, the HFD induced a similar approximately 2-fold increase in β cell proliferation in both Pdx1+/+ and Pdx1+/− mice as assessed by BrdU incorporation (Fig. 2C).
As we found no significant impairment in proliferation, and recent literature suggests that neogenesis does not contribute significantly to adult β cell turnover (18), we considered whether the failure to expand β cell mass in this model might be due to decreased cell survival. Indeed, HF-feeding resulted in a 4-fold increase in β cell apoptosis only in Pdx1+/− mice (Fig. 2D). Further, although β cell hypertrophy has been suggested not to contribute to HFD-induced β cell mass expansion in mice (19), we found that Pdx1+/+ animals fed a HFD showed a significant increase in mean β cell size relative to NC-fed controls whereas HF-fed Pdx1+/− β cells failed to exhibit this hypertrophic response (Fig. 2E). Therefore, the inability of Pdx1+/− mice to expand β cell mass in response to diet-induced insulin resistance is due primarily to a decrease in β cell survival and a lack of compensatory hypertrophy rather than to an impairment in β cell replication.
Evidence of ER Stress in Pdx1-Deficient β Cells.
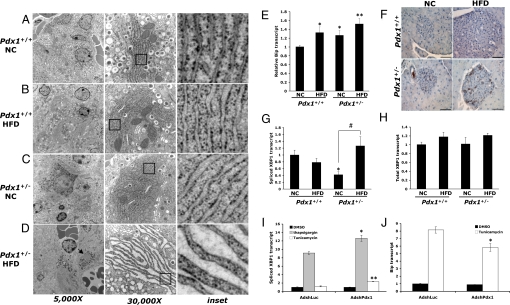
Recent literature demonstrates that β cells of T2DM patients show increased evidence of apoptosis and endoplasmic reticulum (ER) stress (2, 20, 21). In mouse models of diet-induced obesity, HF-feeding has been linked to ER stress induction in hepatocytes and adipocytes, contributing to peripheral insulin resistance (22). Moreover, multiple lines of recent evidence suggest that HFD-induced insulin resistance might impose ER stress on the β cell and that an inability to trigger an appropriate unfolded protein response (UPR) can lead to β cell death (21, 23, 24). Given that Pdx1 and ER function are both critical for insulin secretion and cell survival, we examined the ultrastructural appearance of the ER in β cells of HF- or NC-fed Pdx1+/+ and Pdx1+/− mice using electron microscopy. HF-fed Pdx1+/+ β cells showed mild ER dilation relative to NC-fed controls (Fig. 3 A and B), likely reflecting the increased demand for insulin output. The ER of NC-fed Pdx1+/− β cells was also slightly dilated compared with NC-fed Pdx1+/+ controls (Fig. 3C). However, the ER of HF-fed Pdx1+/− β cells was severely distended compared with all other groups (Fig. 3D). This marked ER distention was specific to the Pdx1-expressing insulin-producing β cells, whereas the glucagon-producing alpha cells of the islet had normal, well-organized ER (Fig. 3D, left, arrow).
Fig. 3.
β cells of HF-fed Pdx1+/− mice exhibit evidence of increased ER stress. (A–D) Electron micrographs of pancreata from Pdx1+/+ and Pdx1+/− mice fed either HFD or NC for 4 months. The β cells of both HF-fed Pdx1+/+ (B) and NC-fed Pdx1+/− mice (C) show mild ER dilation compared with NC-fed Pdx1+/+ controls (A). However, the HFD induces striking distention of the ER in β cells of Pdx1+/− mice (D). Magnification of left panels, 5,000×; magnification of middle panels, 30,000×. The arrow in (D) indicates normal ER appearance in an islet alpha cell. (E) Quantitative RT-PCR analysis of Bip mRNA levels in islets isolated from individual Pdx1+/+ or Pdx1+/− mice fed HFD or NC for 8 weeks. (F) Bip immunohistochemistry on pancreas sections from mice 5 months on either diet showing increased Bip staining in Pdx1+/− islets. (Scale bars, 50 μm.) (G and H) qPCR measurement of spliced Xbp1 transcript (G) and total Xbp1 (H) in isolated islets. For (E, G, and H) raw data are normalized to HPRT (n = 4–6 mice per group; *, P < 0.05, **, P < 0.01 relative to NC-fed Pdx1+/+; #, P < 0.05 relative to corresponding NC control). (I and J) Induction of spliced Xbp1 (I) and Bip (J) transcript in AdshPdx1- or AdshLuc-infected Min6 cells after treatment with thapsigargin, tunicamycin, or DMSO (vehicle). (n = 3 replicates per condition; *, P < 0.05; **, P < 0.001 relative to treatment-matched AdshLuc-infected cells).
To confirm that the β cells of HF-fed Pdx1+/− mice were experiencing increased ER stress, we measured expression of markers of the UPR in islets from mice of all groups. Transcript levels of the chaperone Bip after 8 weeks on either diet paralleled the pattern of ER dilation observed by electron microscopy, with higher Bip expression in HF-fed Pdx1+/+, NC-fed Pdx1+/−, and HF-fed Pdx1+/− islets relative to NC-fed Pdx1+/+ controls (Fig. 3E). Increased Bip protein was also evident by immunostaining, particularly in islets of Pdx1+/− mice fed the HFD (Fig. 3F). Further supporting the conclusion that Pdx1 haploinsufficient β cells are more susceptible to ER stress, HF-feeding induced a 2.5-fold increase in splicing of Xbp1, a marker of activation of the IRE1α arm of the UPR, only in Pdx1+/− islets (Fig. 3G), while total Xbp1 transcript levels remained unchanged (Fig. 3H). The specific reduction in spliced Xbp1 levels in NC-fed Pdx1+/− islets suggests that Pdx1 may regulate the processing of Xbp1 transcript.
HFD-induced insulin resistance is a complex model involving multiple metabolic abnormalities including hyperglycemia as well as increased levels of circulating free fatty acids, both of which are well-known triggers of ER stress in the β cell (11, 24). Therefore, we sought to determine whether Pdx1 directly regulates the ER stress response in β cells, independent of other metabolic disturbances, by examining the consequences of an acute reduction in Pdx1 expression on the UPR in the Min6 mouse insulinoma β cell line. Adenoviral shRNA-mediated knockdown of Pdx1 in Min6 cells achieved a 60–80% reduction in Pdx1 levels (Fig. S1) and increased Xbp1 splicing 2 h after pharmacological induction of ER stress with either thapsigargin, which disrupts intracellular calcium homeostasis, or tunicamycin, an inhibitor of protein glycosylation (Fig. 3I). We also noted a significant reduction in stimulated Bip expression 8 h after treatment with tunicamycin (Fig. 3J). These data indicate a direct role for Pdx1 in regulating β cell susceptibility to ER stress.
Pdx1 Deficiency Enhances Susceptibility to ER Stress-Induced Apoptosis.
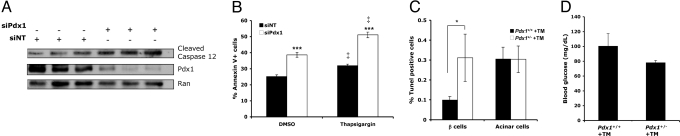
To determine whether Pdx1 deficiency promotes ER stress-induced apoptosis, we examined several measures of β cell survival in Pdx1-deficient Min6 cells and Pdx1 haploinsufficient primary β cells in vivo. First, we observed increased caspase 12 cleavage after acute reduction of Pdx1 expression in Min6 cells (Fig. 4A). Caspase 12 is localized to the ER and has been implicated as a specific mediator of ER stress-induced apoptosis (25). Next, β cell apoptosis was directly quantified by annexin V staining and flow cytometry. We found that siRNA-mediated silencing of Pdx1 increased apoptosis in Min6 cells, both at baseline and in the setting of ER stress induced by 1 μM thapsigargin (Fig. 4B). Finally, we injected NC-fed Pdx1+/− mice and their Pdx1+/+ littermates with tunicamycin to assess β cell susceptibility to ER stress-induced apoptosis in the setting of Pdx1 deficiency in vivo. As expected, tunicamycin induced ER stress in multiple tissues, including kidney (Fig. S2). Quantification of apoptosis rates using TUNEL/insulin co-staining revealed significantly increased β cell but not acinar cell death in tunicamycin-injected NC-fed Pdx1+/− mice compared with their Pdx1+/+ controls (Fig. 4C) with no evidence of hyperglycemia (Fig. 4D). Altogether, these data indicate a direct role for Pdx1 in regulating β cell susceptibility to ER stress induced apoptosis.
Fig. 4.
Pdx1 deficiency enhances β cell susceptibility to ER stress-related apoptosis. (A) Cleaved caspase 12 protein levels in Min6 cells following silencing of Pdx1 with a pool of four siRNA duplexes (siPdx1). A pool of nontargeting siRNA duplexes (siNT) was used as a control. (B) Annexin V flow cytometry analysis of Min6 cell apoptosis after nucleofection with pooled siPdx1 duplexes and subsequent ER stress induction with 1 μM thapsigargin (n = 6, ***, P < 0.0001 relative to siNT; ‡, P < 0.001 relative to DMSO). (C) β cell and acinar cell apoptosis in Pdx1+/− mice and their Pdx1+/+ littermates 96 h after IP injection of tunicamycin (TM). Cell death was quantified using TUNEL immunofluorescence. n = 3–4 mice per group; *, P < 0.05 (one-tailed t test). (D) Glucose levels in the tunicamycin-injected mice just before sacrifice.
Pdx1 Regulates Multiple Genes Involved in ER Homeostasis and the UPR.
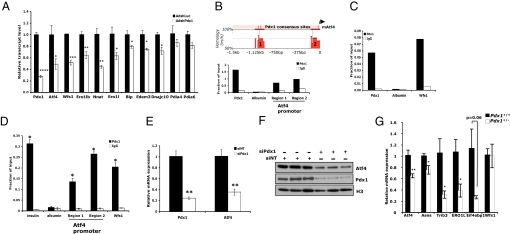
To identify Pdx1 transcriptional targets involved in ER function, we performed high-throughput expression microarray and chromatin occupancy experiments. First, we used an adenovirally-expressed shRNA to acutely silence Pdx1 expression in Min6 cells and examined global gene expression using the Mouse PancChip 6.1, which contains genes enriched in liver and pancreas (http://www.cbil.upenn.edu/epcondb3/Chips/pancChip.shtml). The entire set of genes dysregulated by acute Pdx1 deficiency in Min6 cells is presented in Table S1. Analysis of the AdshPdx1 microarray results revealed a remarkable number of ER-related genes downregulated by an approximate 50% reduction in Pdx1 expression. These include genes encoding enzymes critical for proper disulfide bond formation in the ER (Ero1l, Ero1lb, Pdia4, and Pdia6), ER chaperones (Hspa5/Bip and Dnajc10), mediators of UPR pathways (Atf4, Edem3, and Wfs1), and neuronatin (Nnat), an ER resident protein that regulates insulin secretion by currently unknown mechanisms (26). We confirmed significant downregulation of Atf4, Wfs1, Ero1l, Ero1lb, Nnat, Bip, Edem3, and Dnajc10 in AdshPdx1-infected Min6 cells by qPCR (Fig. 5A).
Fig. 5.
Pdx1 regulates multiple genes involved in maintaining ER homeostasis. (A) Quantitative RT-PCR confirmation of ER-related genes downregulated in Mouse PancChip 6.1 cDNA microarray analyses of AdshPdx1-infected Min6 cells compared with either uninfected or AdshLuc-infected controls. (n = 3; *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001 relative to AdshLuc-infected cells). (B) Upper, Schematic of the genomic sequence 1.5 kb upstream of the mouse Atf4 promoter highlighting two regions of evolutionary conservation with the human sequence and the Pdx1 consensus sites contained within (adapted from rVista 2.0). Lower, Pdx1 directly binds the Atf4 promoter as assessed by chromatin immunoprecipitation (ChIP) from Min6 cells. (C) Pdx1 also occupies the Wfs1 promoter in Min6 cells as assessed by quantitative ChIP, consistent with the high-throughput ChIP on chip data. (D) ChIPs performed on primary islets freshly isolated from 8-week-old mice demonstrate Pdx1 occupancy at the two conserved regions of the Atf4 promoter and the Wfs1 promoter in vivo. Primers designed to amplify a known Pdx1-binding region of the insulin promoter and a piece of the albumin promoter were used as positive and negative controls, respectively. n = 4 individual experiments, data are mean ± s.e.m, *, P < 0.05 relative to IgG control pulldown. (E and F) Pdx1 and Atf4 mRNA (E) and protein (F) levels in Min6 cells transfected with either a pool of four siPdx1 duplexes (siPdx1) or a nontargeting control pool (siNT). n = 3 replicates per condition; **, P < 0.01. In (F), nuclear extracts were analyzed by Western blot, using histone H3 as a loading control. (G) qPCR analysis of Atf4, known Atf4 targets and Wfs1 expression in islets isolated from individual 12-week-old Pdx1+/+ or Pdx1+/− mice. n = 4–6 mice per group; *, P < 0.05; **, P < 0.01.
We integrated these findings with the results of a high-throughput ChIP-on-chip experiment of Pdx1 occupancy, also in Min6 cells (Table S2; Mouse PromoterChip BCBC-5A; http://www.cbil.upenn.edu/epcondb3/Chips/pancChip.shtml), and found that the proximal promoters of two of these eight ER target genes, Atf4 and Wfs1, were directly occupied by Pdx1. We identified two evolutionarily-conserved genomic sequences within 1.5 kb upstream of the Atf4 transcriptional start site, each of which contain at least one conserved Pdx1 consensus binding motif T(T/A)AT (Fig. 5B, top) (27). ChIP assays demonstrated Pdx1 occupancy of both regions in Min6 cells (Fig. 5B, bottom). We also performed Pdx1 ChIPs in freshly isolated mouse islets and found strong Pdx1 enrichment at both the proximal and distal promoter regions (24-fold and 14-fold over IgG, respectively, Fig. 5D). In agreement with reduced Atf4 transcript, which we confirmed using pooled siPdx1 duplexes as an independent method of Pdx1 silencing (Fig. 5E), we also observed reduced Atf4 protein in Pdx1-deficient Min6 cells (Fig. 5F). Importantly, Atf4 transcript levels were also reduced in Pdx1+/− islets, as was the expression of several well-established Atf4 targets (Asns, Ero1l, Eif4ebp1, and Trib3) (Fig. 5G). These data demonstrate that Pdx1 binds the Atf4 promoter in vivo and, in combination with the loss-of-function results, identify Atf4 as a direct target of Pdx1.
Occupancy of the Wfs1 promoter by Pdx1 was also confirmed both in Min6 cells and in primary mouse islets (Fig. 5 C and D). Unlike Atf4 however, Wfs1 levels were not reduced in the setting of chronic Pdx1 deficiency in Pdx1+/− islets, suggesting that Wfs1 expression may be influenced by additional factors in vivo such as hyperglycemia (Fig. 5G).
Finally, we examined gene expression in islets isolated from 8- to 12-week-old Pdx1+/− and Pdx1+/+ littermates, also using the Mouse PancChip 6.1 platform (Table S3). An overlap analysis comparing the effects of acute and chronic Pdx1 deficiency on gene expression in Min6 cells and primary islets, respectively, resulted in a short list of commonly dysregulated genes (Table S4). The eight genes downregulated include the known Pdx1 targets Mafa, Slc2a2 (also known as Glut2), and Pax4. Strikingly, two of the five remaining genes, Ero1lb and Nnat, encode ER resident proteins implicated in ER stress responses whose differential expression we had already observed in AdshPdx1-infected Min6 cells (Fig. 5A) (26, 28). After confirming these results using an independent approach to silence Pdx1 (Fig. S3), we further demonstrated decreased expression of Ero1lb and Nnat in primary islets isolated from individual NC- and HF-fed Pdx1+/− mice using quantitative RT-PCR (Fig. S4). These data suggest that dysregulation of Ero1lb and Nnat may contribute to the ER dysfunction we observe in Pdx1+/− mice.
Discussion
We describe here that Pdx1 is required for both increased insulin secretion and compensatory β cell mass expansion in response to HFD-induced insulin resistance. The failure to expand β cell mass in HF-fed Pdx1+/− mice is due to a combination of decreased cell survival and cell size, with no apparent proliferative defect. These findings support the predominant role of Pdx1 in β cell survival, which was first shown in the context of age-related β cell loss (6). Our findings also represent evidence of both a contribution of β cell hypertrophy to compensation for HFD-induced insulin resistance and a role for Pdx1 in regulating β cell size. The mechanisms by which Pdx1 directly or indirectly regulates targets in cell growth pathways warrant further investigation.
Recent literature has generated an emerging appreciation for the physiological relevance of ER stress in the β cell. We find increased evidence of ER stress in the β cells of HF-fed Pdx1+/− mice, potentially underlying both the observed insulin secretion and cell survival defects. Islets of HF-fed, wild-type mice also showed signs of significant, albeit milder, ER stress. Although a link between HFD-imposed insulin resistance and β cell ER stress has been implied both in vitro using fatty acid treatment of β cell lines and in vivo by feeding a HFD to mice with an impairment in the PERK arm of the UPR (Eif2s1+/tm1Rjk), our work directly demonstrates ER stress in islets of HF-fed, wild-type animals (23, 24).
Hyperglycemia and elevated levels of circulating free fatty acids likely both contribute to β cell ER stress in the HFD-fed Pdx1+/− model. However, our findings of UPR alterations in Pdx1 deficient Min6 cells support a direct effect of Pdx1 loss-of-function on susceptibility to ER stress. Further, Pdx1 deficiency promotes ER stress-associated cell death, both in Min6 cells and in vivo after tunicamycin administration even in the absence of hyperglycemia, again implicating Pdx1 directly. Finally, reductions in Pdx1 expression in Min6 cells and in primary islets in vivo led to reductions in both insulin transcript and insulin content (Fig. S5). Even in the setting of HF-feeding, insulin content of Pdx1+/− islets did not rise above that of wild-type littermates, suggesting that the increased ER stress observed in the setting of Pdx1 deficiency occurs independently of insulin biosynthesis and supporting a model in which direct regulation of ER pathways by Pdx1 influences susceptibility to ER stress. We identify Atf4 and Wfs1 as direct transcriptional targets of Pdx1 and a remarkable number of additional genes involved in diverse aspects of ER function as downstream of Pdx1, suggesting multiple molecular mechanisms whereby Pdx1 regulates β cell susceptibility to ER stress.
Our findings uncover a broad role for Pdx1 in maintaining ER homeostasis in the β cell, which has implications both for proper folding of insulin and for β cell susceptibility to apoptosis in the setting of an increased demand for insulin production. The association of Pdx1 mutations with type 2 diabetes in humans and low levels of Pdx1 with β cell dysfunction in experimental models of diabetes points to Pdx1 regulation of ER homeostasis as a potential therapeutic target for type 2 diabetes.
Materials and Methods
Detailed methods for the use of animals and cell lines, physiological testing, morphological analyses, islet isolation, gene silencing, qPCR, chromatin immunoprecipitation, high throughput expression and promoter arrays, and statistical analyses can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank K. Kaestner, M. Lazar, and M. Birnbaum for reading the manuscript, C. Wright for providing Pdx1+/− mice, and G. Blobel for ChIP protocols. Microarrays were carried out by the Functional Genomics Core (supported by National Institutes of Health Grant DK19525). We acknowledge electron microscopy technical support from the Biomedical Imaging Core (R. Meade) and support for all other morphological analyses from G. Swain [Morphology Core (P30-DK050306)]. This work was supported by National Institutes of Health Grants P01 DK49210 and R01 DK068157 (to D.A.S.), T32-GM08216 (to M.M.S.), and R01 DK60581 (to R.G.M).
Footnotes
Conflict of interest statement: D.A.S. is a coinventor on a patent entitled “Compositions and methods for detecting pancreatic disease” which covers the detection of Pdx1/Ipf1 mutations in human disease, the royalties from which were $0 during the last 12 months.
This article is a PNAS Direct Submission.
The array data have been deposited in the Array Express database, http://www.ebi.ac.uk/microarray-as/ae/ (accession no. E-MTAB-134).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904849106/DCSupplemental.
References
- 1.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292–302. doi: 10.1210/er.2007-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brissova M, et al. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier BR, et al. Oligonucleotide microarray analysis reveals PDX1 as an essential regulator of mitochondrial metabolism in rat islets. J Biol Chem. 2004;279:31121–31130. doi: 10.1074/jbc.M405030200. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JD, et al. Increased islet apoptosis in Pdx1+/- mice. J Clin Invest. 2003;111:1147–1160. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem. 2002;277:13286–13293. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- 9.Seufert J, Weir GC, Habener JF. Differential expression of the insulin gene transcriptional repressor CCAAT/enhancer-binding protein beta and transactivator islet duodenum homeobox-1 in rat pancreatic beta cells during the development of diabetes mellitus. J Clin Invest. 1998;101:2528–2539. doi: 10.1172/JCI2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zangen DH, et al. Reduced insulin, GLUT2, and IDX-1 in beta-cells after partial pancreatectomy. Diabetes. 1997;46:258–264. doi: 10.2337/diab.46.2.258. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 12.Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem. 1997;272:30261–30269. doi: 10.1074/jbc.272.48.30261. [DOI] [PubMed] [Google Scholar]
- 13.Ahn YB, et al. Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia. 2007;50:334–342. doi: 10.1007/s00125-006-0536-5. [DOI] [PubMed] [Google Scholar]
- 14.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 15.Hani EH, et al. Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest. 1999;104:R41–48. doi: 10.1172/JCI7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni RN, et al. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brissova M, et al. Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am J Physiol Endocrinol Metab. 2005;288:E707–714. doi: 10.1152/ajpendo.00252.2004. [DOI] [PubMed] [Google Scholar]
- 18.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 19.Hull RL, et al. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: Evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- 20.Huang CJ, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 21.Laybutt DR, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 23.Scheuner D, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 24.Karaskov E, et al. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda T, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 26.Joe MK, et al. Crucial roles of neuronatin in insulin secretion and high glucose-induced apoptosis in pancreatic beta-cells. Cell Signal. 2008;20:907–915. doi: 10.1016/j.cellsig.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Loots GG, Ovcharenko I. rVISTA 2.0: Evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagani M, et al. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275:23685–23692. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- 29.Offield MF, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 30.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.