Abstract
Neural activity can induce persistent strengthening or weakening of synapses, known as long-term potentiation (LTP) or long-term depression (LTD), respectively. As potential cellular mechanisms underlying learning and memory, LTP and LTD are generally regarded as synapse-specific “imprints” of activity, although there is evidence in vitro that LTP/LTD may spread to adjacent synapses. Here, we report that LTP and LTD induced in vivo at retinotectal synapses of Xenopus tadpoles undergo rapid long-range retrograde spread from the optic tectum to the retina, resulting in potentiation and depression of bipolar cell synapses on the dendrites of retinal ganglion cells, respectively. The retrograde spread of LTP and LTD required retrograde signaling initiated by brain-derived neurotrophic factor and nitric oxide in the tectum, respectively. Such bidirectional adjustment of the strength of input synapses in accordance to that of output synapses may serve to coordinate developmental refinement and learning functions of neural circuits.
Keywords: neural development, retrograde signaling, synaptic plasticity, retinal ganglion cell
Persistent modification of synaptic efficacy by neural activity represents a cellular mechanism underlying learning and memory functions of the brain (1, 2). Activity-induced long-term potentiation (LTP) and long-term depression (LTD) of synaptic transmission are found in many regions of developing and mature nervous systems (3–5). Because LTP/LTD induction often requires correlated presynaptic and postsynaptic activation, it is generally assumed that these bidirectional modifications are synapse-specific, i.e., only those synapses experiencing correlated presynaptic and postsynaptic activities are modified (2). This notion of synapse specificity has been widely used in computational analysis of activity-dependent modification of neural circuits. Experimental studies have indeed demonstrated input specificity in the induction of LTP/LTD, when activated and unactivated synaptic inputs are well separated (6–8).
However, many studies in the past two decades have suggested that this notion of input specificity needs to be modified. Induction of LTP in one pathway was found to be accompanied by heterosynaptic depression of other pathways (9). Transmitters released at activated synapses may also “spill over” and modify adjacent synaptic sites (10). By examining specifically the issue of input specificity, several in vitro studies have shown that LTP/LTD induced at one set of synapses on a neuron could spread laterally to nearby synapses (11–16). Most surprisingly, in random networks formed by dissociated hippocampal neurons in culture, there is a rapid retrograde spread of potentiation/depression from the site of LTP/LTD induction to the synaptic inputs on the dendrite of the presynaptic neuron (17, 18). Such long-range presynaptic spread of retrograde signals accompanying LTP/LTD induction is also implicated by the findings of rapid global modifications of intrinsic excitability of the presynaptic neuron after LTP/LTD induction (19, 20). However, the configuration of synapses formed in cultures between dissociated neurons are quite different from that in natural neural circuits; it is thus important to determine whether long-range retrograde spread of LTP/LTD can indeed occur in intact brain tissues. In the present study, we have chosen the retinotectal system of Xenopus tadpoles to investigate whether LTP/LTD generated at output synapses of retinal ganglion cells (RGCs) in the optic tectum can spread to the synapses on the dendrites of RGCs in the retina.
Retrograde signals received by the axonal terminals are responsible for the trophic influence of target cells on neuronal survival (21, 22), dendritic morphology (23, 24), and the integrity of synaptic connections on the dendrite (25, 26). Recently, we found a rapid form of retrograde modulation in the developing Xenopus visual system, application of exogenous brain-derived neurotrophic factor (BDNF) to the RGC axon terminals in the optic tectum triggered a retrograde signal in the RGC, resulting in the potentiation of bipolar cell (BC) synapses on RGC dendrites within 20 min (27). This study suggests that axon-to-dendrite retrograde signaling can occur rapidly and cause persistent synaptic modulation at the dendrite. Because LTP induction is known to depend on BDNF signaling at retinotectal and hippocampal CA3–CA1 synapses (28, 29), it is possible that LTP induced at retinotectal synapses may spread to the retina via the action of endogenous BDNF secreted in the tectum.
In the present study, we monitored the efficacy of BC-RGC synapses by perforated whole-cell recording from the RGC and found that light-induced excitatory synaptic currents were increased and decreased within minutes after the induction of LTP and LTD at retinotectal synapses, respectively. Measurements of the properties of BC-RGC synapses indicated that this retrograde potentiation/depression was caused by modification of the single-channel conductance and the density of postsynaptic AMPA subtype of glutamate receptors (AMPARs). Furthermore, we showed that BDNF and NO secreted at retinotectal synapses as a result of LTP and LTD induction are responsible for triggering the retrograde potentiation and depression, respectively. Together, these results provide an in vivo demonstration of long-range retrograde spread of LTP and LTD. Such retrograde spread of LTP/LTD points to the existence of coordinated changes in the strength of synaptic inputs to a neuron in accordance to that of its synaptic outputs, reminiscent of the back-propagation algorithm used for supervised learning in artificial neural networks (30).
Results
Induction of LTP and LTD at Retinotectal Synapses.
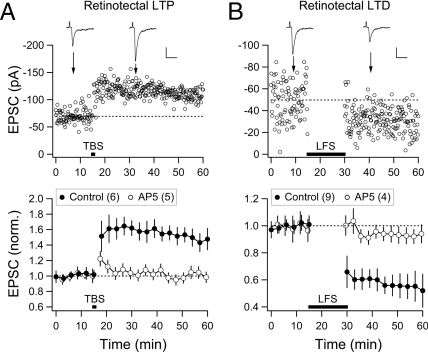
In the Xenopus retinotectal system, RGCs send out axons to establish retinotectal synapses with tectal neurons in the contralateral optic tectum and receive synaptic inputs from bipolar and amacrine cells at their dendrites in the retina. In this study, we first examined activity-induced LTP and LTD at retinotectal synapses (stages 42–44) by stimulating RGC axons at the optic nerve head and recording evoked excitatory postsynaptic currents (EPSCs) from the tectal neuron in the contralateral tectum, using perforated-patch whole-cell recording. For LTP induction, we applied five episodes (at 5-s intervals) of theta burst stimulation (TBS), with the tectal neuron held in the current clamp (c.c.) mode. We found that TBS induced an immediate increase in the amplitude of EPSCs that persisted for as long as the recording was made (up to 1 h; Fig. 1A). The mean EPSC amplitude during 5–45 min after TBS was 155 ± 12% (SEM, n = 6) of the control value obtained before TBS. There was no change in the EPSC amplitude (104 ± 7% of control, n = 5) when TBS was applied in the presence of 50 μM AP5 [D(-)-2-amino-5-phosphonovaleric acid], an antagonist of N-methyl-d-aspartate subtype of glutamate receptors (NMDARs), in the recording chamber (Fig. 1A).
Fig. 1.
Induction of LTP and LTD at retinotectal synapses. (A) LTP induced by TBS. (Upper) Data from an example experiment. Changes of the EPSC amplitude in a tectal neuron (Vc = − 65 mV) before and after five episodes (with 5-s intervals) of TBS, each consisting of 10 trains (spaced by 200 ms) of five pulses at 100 Hz. Dotted line is the mean value before TBS. Traces above show EPSCs at the time marked by the arrow. (Scales: 50 pA, 20 ms.) (Lower) Summary of data from all experiments. Filled circles indicate experiments similar to that shown above. Open circles indicate experiments with AP5 (50 μM) in the bath. Data points were normalized by the mean value before TBS and laterally displaced for clarity. (B) LTD induced by LFS. Data from an example experiment (Upper) and summary of all experiments (Lower) show changes in the EPSC amplitude before and after 15 min LFS (1 Hz). (Scales: 25 pA, 20 ms.)
In contrast to the effect of TBS, activation of RGC axons at the optic nerve head with low-frequency stimulation (LFS) (1 Hz for 15 min), which is known to induce LTD at many excitatory synapses (5), resulted in an immediate reduction in the EPSC amplitude (57 ± 7% of control at 5–30 min after LFS; n = 9; Fig. 1B) that persisted for as long the recording was made. This LTD also required NMDAR activation, because it was absent (95 ± 4%, n = 4) when AP5 was present in the recording medium (Fig. 1B). Application of LFS (1 Hz) for a shorter duration also induced LTD, but the extent of depression (5-min LFS: 70 ± 10%, n = 3; 10-min LFS: 62 ± 6%, n = 5) was smaller than that induced by LFS for 15 min.
Potentiation of BC-RGC Synapses After Retinotectal LTP.
To examine whether LTP/LTD induction at retinotectal synapses in the optic tectum affects the efficacy of excitatory synapses made by BCs on the dendrite of RGCs, we monitored light-evoked excitatory compound synaptic currents (eCSCs) in the RGC by performing perforated whole-cell recording at the reversal potential of inhibitory synaptic currents (27). Changes of eCSCs in the recorded RGC after LTP/LTD induction at retinotectal synapses by stimulating the optic nerve head would suggest retrograde spread of synaptic modification. Because simultaneous in vivo whole-cell recording from a tectal neuron and a RGC is technically not yet feasible, we examine changes in eCSCs only in RGCs that exhibited antidromic action potentials in response to stimulation of the optic nerve head during LTP/LTD induction in all experiments to ensure that retinotectal synapses made by the recorded RGC undergo LTP/LTD.
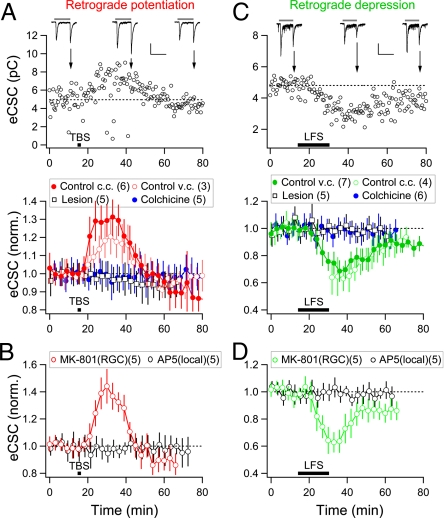
Whole-field illumination of the retina (2-s duration) elicited both “on” and “off” responses in most RGCs. The efficacy of the BC-RGC synapse was measured by the total integrated charge associated with the eCSC (Vc = −65 mV) within a 50-ms window after its onset (27). When examined at a low frequency (0.033 Hz), eCSCs remained stable for up to 2 h. However, we found that the induction of LTP at retinotectal synapses (by TBS at optic nerve head; Fig. 1A) resulted in a marked enhancement of eCSCs for both on and off responses within 5 min after TBS. This enhancement of eCSCs lasted for ≈20 min (Fig. 2A). In Fig. 2A and all subsequent figures, only off responses are presented. The mean eCSC charge at 15–30 min after TBS was 130 ± 9% of control (n = 6) for the off responses. Similar effects were observed for on responses.
Fig. 2.
Retrograde modulation of light-evoked excitatory responses in RGCs. (A) Enhancement of light-evoked eCSCs in RGCs induced by TBS. (Upper) Data from an example experiment. Data points depict the changes in the total integrated charge associated with eCSCs (at Vc = −65 mV) of the off responses within a 50-ms window after the onset of eCSCs before and after TBS (as described in Fig. 1A). The RGC was held at c.c. mode during TBS. Samples of single on and off eCSCs (arrow) are shown at the top. (Scales: 50 pA, 2 s.) (Lower) Summary of data from all experiments. Control c.c. and Control v.c.: RGCs were kept at current- or voltage-clamp mode during TBS. Lesion: The optic nerve was severed (n = 3) or the optic tectum was surgically removed (n = 2). Colchicine: The tadpole was preincubated (1 h) in colchicine (5 μM). (B) Requirement of NMDAR activation for the enhancement of eCSCs in RGCs. The enhancement was prevented by local bath application of AP5 (50 μM) [AP5 (local)] at the optic tectum but not by loading of MK-801 (500 μM) into RGCs [MK-801 (RGC)]. (C and D) Reduction of eCSCs in RGCs induced by LFS identical to that described in Fig. 1A. Data from an example experiment (C Upper) and summary data for all experiments (C and D Lower) are presented in the same manner as in A and B.
Two lines of evidence argued that the potentiation effect of TBS on eCSCs in RGCs was not caused by antidromic action potentials triggered by TBS, but required signals mediated through the optic nerve. First, the potentiation effect was absent (96 ± 7% of control, n = 5) when the optic nerve of the recorded retina was severed at the site of entry into the midbrain (at >200 μm from the stimulation site) or when the optic tectum was surgically removed before the optic nerve stimulation (Fig. 2A). Severing of the optic nerve or ablation of the optic tectum by itself without TBS did not cause any detectable effect on light-evoked eCSCs in the RGCs (103 ± 6% of control, n = 12; Fig. S1), suggesting no immediate deleterious effect of these surgical operations. Second, TBS-induced enhancement of eCSCs was absent (97 ± 5%, n = 5) when the tadpole was preincubated (for 1 h) with the microtubule-disrupting agent colchicine (5 μM; Fig. 2A), which by itself did not affect eCSCs in the RGCs (95 ± 7%, n = 13; Fig. S1). This colchicine effect suggests the potential involvement of microtubule-based axonal transport in mediating the retrograde signal.
The induction of LTP at retinotectal synapses is required for the apparent retrograde potentiation at BC-RGC synapses, because blocking TBS-induced retinotectal LTP with bath-applied AP5 (Fig. 1A) prevented the enhancement of eCSCs in RGCs (97 ± 7%, n = 4; data not shown). To exclude the possibility of direct effect of AP5 in the retina, we performed additional local perfusion of the tectum with AP5 with a pair of inlet and outlet pipette and monitored the perfusion with a blue dye in the perfusion solution. Under this local tectal AP5 perfusion, LTP induction of retinotectal synapses was prevented (102 ± 6%, n = 5), and the enhancement of eCSCs in RGCs was also absent (97 ± 7%, n = 5) (Fig. 2B). Finally, we loaded the open NMDAR channel blocker MK-801 (500 μM) into the RGC through the whole-cell recording pipette and found that TBS-induced enhancement of light responses was not affected (134 ± 10%, n = 5) (Fig. 2B). That intracellular loaded MK-801 had reached the postsynaptic site of BC-RGC synapses was confirmed by the observation that the NMDAR-mediated component of sEPSCs recorded in RGCs disappeared after MK-801 loading (Fig. S2; see also refs. 31 and 32). Thus, the potentiation of BC-RGC synapses is unlikely to be attributed to potential direct NMDAR-dependent LTP of these synapses caused by TBS at the optic nerve head.
For all experiments described above, we have examined changes at BC-RGC synapses only in those RGCs exhibiting antidromic action potentials during optic nerve stimulation. In a total of 27 antidromically activated RGCs under various experimental conditions, 21 exhibited potentiation of BC-RGC synapses after TBS. In contrast, 17 of 18 RGCs not exhibiting antidromic action potentials showed no changes at BC-RGC synapses. Thus, TBS-induced potentiation of BC-RGC synapses is a cell-autonomous retrograde effect and not shared by adjacent RGCs that did not undergo LTP in their output synapses in the optic tectum.
Depression of BC-RGC Synapses After Retinotectal LTD.
Retrograde modulation of the light-evoked responses in RGCs was also observed after the induction of LTD at retinotectal synapses by applying LFS (1 Hz, 15 min) to the optic nerve head. A reduction of the total charge associated with light-evoked eCSCs in the RGC was observed 5–10 min after the onset of LFS (the mean eCSC charge 0–15 min after the offset of LFS was 73 ± 7% of control, n = 7) and lasted for ≈20 min (Fig. 2C), indicating depression of BC-RGC synapses. Similar to the TBS-induced potentiation, LFS-induced depression of eCSCs in RGCs was absent when the optic nerve was severed or the optic tectum was removed (98 ± 5%, n = 5), or when the tadpole was pretreated with colchicine for 1 h (96 ± 5%, n = 6) (Fig. 2C). Finally, we found that blocking LTD induction in the tectum by local perfusion of the tectum with AP5 also abolished retrograde depression in the retina (99 ± 4%, n = 5) (Fig. 2D). Furthermore, intracellular loading of MK-801 into the RGC had no effect on the depression of BC-RGC synapses (69 ± 12%, n = 5) (Fig. 2D), suggesting that the latter depression was not caused by potential direct induction of NMDAR-dependent LTD at BC-RGC synapses. Together with the finding on retrograde potentiation, these results indicate that LTP/LTD induced at retinotectal synapses undergo rapid long-range retrograde spread along the optic nerve to the retina, resulting in retrograde potentiation/depression of BC-RGC synapses.
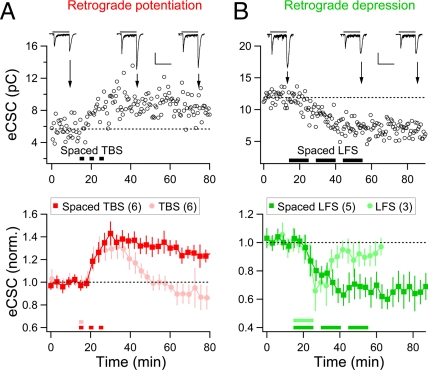
Persistent Retrograde Potentiation/Depression Caused by Spaced LTP/LTD Induction.
In the above experiments, the tectal neuron was not recorded and could fire action potentials spontaneously. Previous studies have shown that LTP/LTD of retinotectal synapses induced by one set of TBS/LFS was readily reversed by spontaneous spiking activity of tectal cells in vivo and that three repetitions of TBS (with an optimal interval of 5 min) was required to produce stable LTP of these synapses (33). Consistent with the latter report, we observed stable LTP of retinotectal synapses by three episodes of TBS. Furthermore, recording from RGCs showed that this spaced TBS protocol resulted in a persistent enhancement of eCSCs in the RGC (136 ± 7% of control, n = 6) that lasted for as long as the recording was made (up to 1 h; Fig. 3A). Similarly, three episodes of LFS (each for 10 min at 1 Hz) spaced by 5-min intervals also induced a long-lasting reduction of eCSCs in RGCs (75 ± 8% of control, n = 5; Fig. 3B). These persistent changes of light-evoked RGC responses may be attributed to the cumulated effects of retrograde signals resulting from repeated TBS or LFS (33).
Fig. 3.
Persistent potentiation/depression of light-evoked excitatory responses in RGCs induced by spaced stimulation protocols. (A) Persistent enhancement of eCSCs in RGCs after three sets of TBS (spaced by 5-min intervals), each identical to that described in Fig. 1A. Example (Upper) and summary (Lower) data presented in the same manner as that in Fig. 2, with the summary data in Fig. 2A (Control c.c.) duplicated by symbols in the light color. (Scales: 50 pA, 2 s.) (B) Persistent reduction of eCSCs in RGCs after three sets of LFS (spaced by 5-min intervals), each lasted for 10 min (at 1 Hz). (Scales: 50 pA, 2 s.) Data from experiments using a single set of LFS (10 min at 1 Hz) are also shown by symbols in light color.
Bidirectional Modulation of Synaptic AMPARs on RGC Dendrites.
Modifications of excitatory light responses in RGCs induced by TBS or LFS reflect changes in the efficacy of BC-RGC synapses. Further studies showed that spaced TBS protocol caused an increase in the amplitude but not the frequency or time course of spontaneous EPSCs (sEPSCs) in the RGC (Fig. S3A). Noise analysis of the decay phase of sEPSCs (27, 34) indicates that spaced TBS of the optic nerve increased both the weighted single-channel conductance and the mean channel number of synaptic AMPARs (Fig. S3B), whereas spaced LFS resulted in opposite changes (Fig. S4). Thus, retrograde potentiation and depression involves the elevation and reduction, respectively, of both the conductance and total number of postsynaptic AMPARs at BC-RGC synapses.
Retinotectal LTP Depends on both Presynaptic and Postsynaptic BDNF Signaling.
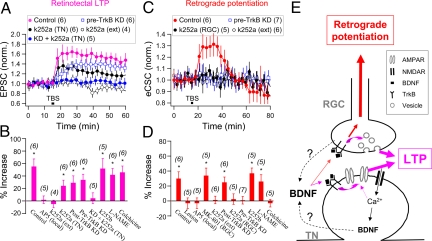
In Xenopus tadpoles, BDNF and its receptor TrkB are expressed in both tectal neurons and RGCs (35, 36). We further examined whether endogenous BDNF-TrkB signaling is responsible for the retrograde spread of LTP. Using the anti-xTrkB morpholino oligonucleotides (MO oligos) to down-regulate the xTrkB expression unilaterally in the retina contralateral to the recorded tectal cell via injection of MO oligos into eight-cell stage embryos (Fig. S5), we found that the magnitude of TBS-induced LTP of retinotectal synapses was significantly reduced, as compared with that found in control tadpoles (Fig. 4A, pre-TrkB KD; 134 ± 9% of control, n = 6). Similarly, unilateral down-regulation of TrkB in the tectum by MO oligo injection on the same side as the recorded tectal cell (post-TrkB KD) also resulted in a reduced magnitude of LTP induced by TBS (129 ± 11%, n = 6). This result is consistent with that obtained by inhibiting postsynaptic TrkB signaling with tyrosine kinase inhibitor k252a (200 nM) loaded into the tectal cell via whole-cell recording pipette [k252a (TN)]. Furthermore, blocking both presynaptic and postsynaptic TrkB activity by loading k252a into the tectal neuron in pre-TrkB KD tadpoles resulted in complete absence of LTP after TBS (104 ± 8%, n = 5). Consistently, extracellular preincubation of tadpoles with k252a (200 nM, 30 min), which would inhibit both presynaptic and postsynaptic TrkB signaling, also prevented LTP induction (95 ± 6%, n = 4), whereas preincubation with the less membrane-permeable analogue k252b (200 nM) had no effect on LTP induction (152 ± 16%, n = 5; Fig. 4A).
Fig. 4.
BDNF-TrkB signaling is required for retinotectal LTP and retrograde potentiation. (A) LTP induction at retinotectal synapses by TBS requires both presynaptic and postsynaptic BDNF-TrkB signaling. Data depict the normalized evoked EPSC amplitude of retinotectal synapses before and after TBS of the optic nerve head. Control: Standard LTP induction in control neurons, the same dataset as that shown in Fig. 1A. pre-TrkB KD: Presynaptic morpholino knockdown of TrkB in RGCs. k252a (TN): Loading of k252a (200 nM) into tectal neurons. k252a (ext): Bath application of k252a (200 nM). KD + k252a (TN): Loading of k252a into tectal neurons in pre-TrkB KD tadpoles. (B) Summary of results from experiments shown in A, together with additional experiments performed in tadpoles with postsynaptic morpholino knockdown of TrkB in tectal neurons (post-TrkB KD), using local application of AP5 (50 μM) at the optic tectum [AP5 (local)] or using bath application of k252b (200 nM), l- NAME (1 mM), or colchicine (5 μM). The data represent averaged percentage increases in the EPSC amplitude at t = 20–60 min after TBS relative to that of the baseline control values. Number in parentheses indicate the total number of experiments (*, P < 0.01, t test). (C) Retrograde potentiation of BC-RGC synapses after TBS requires BDNF-TrkB signaling within the RGC. Data depict the normalized eCSC charge of RGC light responses before and after TBS of the optic nerve head, for various treatments as those in A. Control: The same dataset as that shown in Fig. 2A (Control c.c.). k252a (RGC): Loading of k252a into the RGC. (D) Summary of all results, including those shown in Fig. 2 A and B [Lesion, Colchicine, AP5 (local), MK-801 (RGC)], together with additional experiments performed in tadpoles using postsynaptic morpholino knockdown of TrkB in tectal neurons (post-TrkB KD), or bath application of k252b, or l-NAME. The data represent percentage increases in the eCSC charge at t = 25–40 min after TBS (*, P < 0.01). (E) A proposed sequence of events for the induction of LTP at retinotectal synapses (pink) and the retrograde potentiation at excitatory BC-RGC synapses (red). Note that BDNF secreted from presynaptic and/or postsynaptic neurons may act on both cells to generate retinotectal LTP, whereas BDNF action on the RGC may initiate the signaling responsible for the retrograde potentiation. TN: tectal neuron.
The results of the above experiments are summarized in Fig. 4B as the percentage increase in the mean EPSC amplitude at 5–45 min after TBS, relative to the mean value before TBS. Furthermore, LTP induction was prevented by local infusion of AP5 in the tectum (local AP5), but unaffected by the colchicine treatment, which abolished the retrograde spread of LTP. The latter result indicates that the colchicine effect in preventing retrograde potentiation (Fig. 2A) could not be attributed to the colchicine effect on LTP induction. Finally, treatment with the NO synthase (NOS) inhibitor Nw-nitro-l-arginine methyl ester (l-NAME), which abolished LTD induction at these synapses (see below), had no effect on LTP induction. Together, these results indicate that TrkB activation by BDNF in both presynaptic RGCs and postsynaptic tectal neurons is required for the full induction of retinotectal LTP (Fig. 4E), in agreement with that found for LTP induction at CA3–CA1 synapses in the hippocampus (29).
Retrograde Potentiation Requires Presynaptic BDNF Signaling.
Is BDNF-TrkB signaling also involved in the retrograde potentiation in RGCs after retinotectal LTP? We found that TBS-induced enhancement of eCSCs in RGCs was totally absent in tadpoles that were preincubated with k252a (101 ± 5% of control, n = 6), which completely blocked retinotectal LTP, but not in those preincubated with k252b (137 ± 7%, n = 5) (Fig. 4 C and D). Importantly, the enhancement of eCSCs was also totally absent when only presynaptic TrkB signaling was blocked by using either pre-TrkB morpholino KD tadpoles (101 ± 5%, n = 7) or loading of k252a into the RGC of control tadpoles (102 ± 7%, n = 5; Fig. 4 C and D). Note that, although substantial LTP was still present in pre-TrkB KD tadpoles, the suppression of TrkB signaling in the RGC completely prevented the retrograde potentiation. In contrast, suppression of postsynaptic TrkB activity through the use of post-TrkB KD tadpoles did not significantly change the retrograde potentiation (125 ± 7%, n = 6; Fig. 4D), suggesting that the reduced LTP generated under post-TrkB KD is sufficient to generate the retrograde BDNF-TrkB signals for inducing retrograde potentiation. As shown in Fig. 4D, the retrograde potentiation was prevented by blocking LTP induction with local AP5 perfusion, severing the optic nerve, or pretreatment with colchicine, but unaffected by blocking LTD induction with l-NAME. Together, these results support the model (Fig. 4E) that LTP induction at retinotectal synapses by TBS is accompanied by BDNF release from either tectal neurons or RGC axon terminals, or both, and resultant BDNF-TrkB signaling in both cells is required for the full expression of LTP. Furthermore, presynaptic BDNF-TrkB signaling in the RGC is required for the retrograde potentiation of BC-RGC synapses.
Retinotectal LTD Requires Postsynaptic NO Signaling.
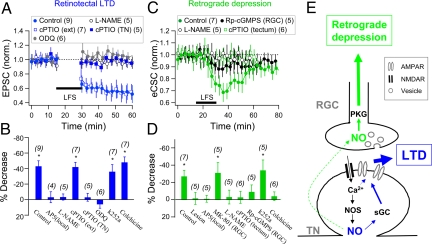
Because NO is involved in LTD induction at several central synapses (28, 37) and Xenopus tectal neurons express neuronal NO synthase (37, 38), we have examined whether endogenous NO synthesis is required for retinotectal LTD and the associated retrograde spread of depression to RGCs. Consistent with a previous report (28), bath application of NOS inhibitor l-NAME (1 mM) prevented LTD induction at retinotectal synapses (97 ± 4% of control, n = 5) (Fig. 5 A and B). In addition, postsynaptic loading of the membrane-impermeant NO scavenger 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yloxy-3-oxide (cPTIO; 30 μM) into the tectal neuron (via whole-cell recording pipette) also prevented LTD induction (97 ± 5%, n = 5) (Fig. 5 A and B). Interestingly, bath application of cPTIO (30 μM) had no effect on LTD induction (58 ± 6%, n = 7), suggesting that NO exerts its action within the cytoplasm of the tectal neuron. Furthermore, this NO-dependent LTD required cGMP-dependent signaling, because LTD induction was prevented by the bath application of 1H-[1,2,4] oxadiazolo [4,3-a]quinoxaline-1-one (ODQ; 10 μM) (106 ± 5%, n = 6), an inhibitor of NO-sensitive soluble guanylyl cyclase (sGC) (Fig. 5 A and B). Also included in Fig. 5B are results showing that LTD induction at these retinotectal synapses was blocked by inhibiting NMDARs with local perfusion of AP5, whereas extracellular infusion with k252a (which blocked LTP induction) or pretreatment with colchicine had no effect on LTD induction. Thus, LTD induction at these synapses requires NO/cGMP signaling in the postsynaptic tectal neuron, but not extracellular NO nor its retrograde action on the RGC axon terminals.
Fig. 5.
NO-PKG signaling is required for retinotectal LTD and retrograde depression. (A) LTD induction at retinotectal synapses by LFS requires postsynaptic NO-cGMP signaling. Data depict the normalized evoked EPSC amplitude of retinotectal synapses before and after LFS of the optic nerve head. Control: Standard LTD induction in control neurons, the same dataset as that in Fig. 1B. l-NAME, cPTIO (ext), and ODQ: Bath application of l-NAME (1 mM), cPTIO (30 μM), or ODQ (10 μM). cPTIO (TN): Loading of cPTIO (30 μM) into tectal neurons. (B) Summary of all results in A, together with additional experiments using local application of AP5 (50 μM) at the optic tectum [AP5 (local)] or bath application of k252a or colchicine. The data represent averaged percentage decreases in the EPSC amplitude at t = 35–60 min after LFS (*, P < 0.01). (C) Retrograde depression by LFS at excitatory input synapses on RGC dendrites requires NO-PKG signaling in the RGC. Data depict the normalized eCSC charge of RGC light responses before and after LFS of the optic nerve head. Control: The same dataset as that shown in Fig. 2C (Control v.c.). l-NAME: Bath application of l-NAME. Rp-cGMPS (RGC): Loading of Rp-8-pCPT-cGMPS (5 μM) into the RGC. cPTIO (tectum): Intraventricular application of cPTIO. (D) Summary of all results, including those shown in Fig. 2 C and D [Lesion, Colchicine, AP5 (local), and MK-801 (RGC)], together with additional experiments using bath application of k252a. The data represent percentage decreases in the eCSC charge at t = 30–45 min after LFS (*, P < 0.01). (E) A proposed sequence of events for the induction of LTD at retinotectal synapses (blue) and the retrograde potentiation at excitatory input synapses on RGC dendrites (green).
Retrograde Depression Requires NO Signaling.
Is NO signaling involved in the retrograde depression induced by retinotectal LTD? We found that LFS-induced retrograde depression of RGC light responses was prevented by either bath-applied l-NAME (97 ± 8% of control, n = 5) or intraventricular infusion of NO scavenger cPTIO near the optic tectum (97 ± 7%, n = 6) (Fig. 5 C and D). Whereas depleting NO in the tectal cell cytoplasm by intracellular loading of cPTIO was required to prevented LTD induction (Fig. 5A), extracellular cPTIO was sufficient to prevent the retrograde spread of depression to BC-RGC synapses. This result is consistent with the notion that NO synthesized in the tectal cells must diffuse through the extracellular space before its retrograde action on RGCs. Furthermore, the retrograde depression observed at BC-RGC synapses depended on the activity of protein kinase G (PKG) in the RGC, because it was largely abolished when the membrane-permeable PKG inhibitor Rp-cGMPS (Rp-8-pCPT-cGMPS; 5 μM) was loaded into the RGC via the perforated patch recording pipette (91 ± 8%, n = 5; Fig. 5 C and D). Fig. 5D summarizes these findings, together with results showing that the retrograde depression was prevented by blocking LTD induction with AP5 infusion in the tectum, severing the optic nerve, and pretreatment with colchicine, but unaffected by blocking LTP induction with extracellular perfusion of k252A. Together, these results support the notion that LFS triggers NO production in the postsynaptic cytoplasm of tectal cells, where NO activates sGC and contributes to the induction and/or expression of LTD. Through diffusion to the extracellular space and subsequent entry into RGC axon terminals NO may further activate PKG and its downstream effectors in RGCs, leading to the retrograde spread of depression to the BC inputs on the RGC dendrite (Fig. 5E).
Discussion
The present study demonstrated the existence of rapid long-range retrograde spread of LTP and LTD in the intact retinotectal system, through two distinct mechanisms involving BDNF-TrkB and NO-PKG signaling, respectively. Our findings showed that LTP/LTD is not localized only to the site of induction or neighboring synapses, but can spread retrogradely from the axon terminal to the dendrite of the presynaptic neuron, leading to a specific pattern of distributed synaptic modifications in the neural circuit. This finding of rapid retrograde modification adds another dimension to synaptic signaling in the polarized neuron; whereas membrane potential-mediated dendrite-to-axon synaptic signaling provides rapid anterograde flow of electrical signals, cytoplasmic axon-to-dendrite retrograde signaling allows functional adjustments of input synapses at the dendrite based on the status of the output synapses at the axon terminals.
Retrograde Actions of Target-Derived Factors.
More than three decades ago, Purves (25) found that axotomy of postganglionic axons of sympathetic ganglion neurons led to a severe depression of preganglionic synaptic inputs and withdrawal of synaptic contacts within 3–11 days. Interestingly, exogenously applied NGF prevented axotomy-induced synapse disintegration in sympathetic ganglia (26). Since then, it has been well established that target-derived factors can be retrogradely transported from the axonal terminal to the soma/dendrites (22, 39, 40) and are responsible for the regulation of neuronal differentiation and survival (21, 22), axon and dendrite growth (23, 24), and circuit formation (24). Our study revealed a more rapid form of retrograde signaling between axon terminals and dendrites. Based on the delay of onset (≈ a few min) of retrograde potentiation/depression at BC-RGC synapses after retinotectal LTP/LTD induction and the length of developing Xenopus RGC axons (0.2–0.5 mm), the rate of retrograde spread of potentiation/depression was ≈1 μm/s, similar to that of fast axonal transport, a microtubule-dependent and colchicine-sensitive process. That colchicine pretreatment abolished retrograde potentiation and depression is consistent with the involvement of fast axonal transport.
BDNF/NO Signaling and Retrograde Plasticity.
Activity-induced LTP at several excitatory synapses depends on BDNF-TrkB signaling (29, 39, 41). Both BDNF and TrkB are highly expressed in the retina and the optic tectum of Xenopus tadpoles (23, 35–37). Because BDNF can be rapidly endocytosed and retrogradely transported from axon terminals to the soma (22, 39, 40) and dendrites (40) in the form of “reactive endosomes,” the retrograde spread of LTP observed here may be attributed to the action of these endosomes on RGC dendrites, although their presence along the optic nerve after LTP induction remains to be demonstrated. We have shown previously that exogenously application of BDNF in the optic tectum is sufficient to induce an immediate potentiation of retinotectal synapses and a gradual potentiation of BC-RGC synapses, with a delay of ≈20 min (27). Similar to the retrograde spread of LTP shown here, BDNF-induced retrograde synaptic potentiation is also absent when the optic nerve is severed or when the tadpole is pretreated with colchicine. Because the TBS-induced LTP of retinotectal synapses also depends on BDNF-TrkB signaling in the tectum and blocking TrkB signaling in RGCs (but not in tectal neurons) prevented the retrograde spread of LTP, BDNF-TrkB signaling is the most likely mechanism for mediating the retrograde spread of LTP. The time course of retrograde potentiation induced by tectal application of BDNF is slower (>20 min) than that found for the retrograde spread of LTP shown here (5–10 min). This difference is likely to be caused by the slow penetration of BDNF into the tectal tissue and gradual potentiation of retinotectal synapses located deep within the tectum, in contrast to the immediate LTP induction triggered by TBS of the optic nerve (Fig. 1).
Our finding that NO-PKG signaling is required for the induction of retinotectal LTD is consistent with previous studies showing the NO dependence of LTD at several central synapses (28, 42). Expression of NOS has been shown in Xenopus tectal neurons but not in RGC axon terminals (37). We found that NO production in the tectal cell and the presence of extracellular NO both are required for the retrograde depression, suggesting that NO serves as a transsynaptic retrograde signal. However, given the short lifetime of NO (42), PKG or its downstream effectors rather than NO itself are likely to serve as the long-range cytoplasmic signal that induces the retrograde depression at RGC dendrites.
Functional Implication of Retrograde LTP/LTD.
In the visual system, BDNF promotes axon growth in RGCs (35, 36), dendrite arborization in cortical neurons (43), and synapse formation in the tectum (36, 44). It is also required for the development of ocular dominance columns that requires stabilization and elimination of early thalamocortical connections (45). Furthermore, BDNF can be retrogradely transported from RGC axon terminals to the soma/dendrites (23), resulting in an enhanced growth of RGC dendrites (23) and a reduced apoptosis of RGCs (21). However, NO disrupts RGC axon growth in the optic tectum (38) and acts as a retrograde signal to increase neuronal apoptosis (21). Because LTP/LTD may be causally related to the stabilization/elimination of synapse, BDNF-dependent LTP and NO-dependent LTD at retinotectal synapses may contribute to the refinement of the retinotopic map in the optic tectum (4, 37, 45). Changes in excitatory synaptic inputs to RGCs have been shown to cause dendritic remodelling (46), thus retrograde spread of LTP/LTD may lead to structural refinement of RGC dendrites (47).
Our results suggest that the retrograde potentiation and depression in the retina accompanying the induction of LTP and LTD at retinotectal synapses may allow coordinated strengthening and weakening of BC-RGC synapses, respectively. This retrograde effect may provide a positive feedback mechanism for activity-dependent refinement in the retinotectal system. If the retinotectal synapses that undergo LTP or LTD represent “correct” or “incorrect” connections, respectively, the retrograde potentiation or depression of the BC-RGC synapses will facilitate further LTP or LTD of those retinotectal synapses made by the corresponding RGC, leading to eventual stabilization or elimination of retinotectal connections, respectively. Such retrograde functional coordination between retinotectal and BC-RGC connections may contribute to the activity-dependent refinement of retinal circuits, e.g., stratification of BC axons and RGC dendrites in the inner plexiform layer (48).
In the developing retina, RGCs spontaneously fire rhythmic bursts of spikes that propagate across the retina, with the spiking frequencies in the range from a few Hz to a few hundred Hz (49). Such retinal waves can induce LTP of retinogeniculate synapses in the developing rat brain (50). The TBS and LFS used here for LTP and LTD induction may correspond to the high- and low-frequency spontaneous spiking activities in the retina, respectively. Thus, physiological spontaneous activity in the retina may cause retinotectal LTP/LTD, which in turn results in retrograde potentiation/depression of BC-RGC synapses, leading to a coordinated refinement of tectal and retinal circuits. Finally, we note that in mature neural circuits this form of “scaling” input synapses in accordance to the efficacy of output synapses may also serve for learning functions of neural circuits in a manner analogous to that of back-propagation algorithm used in supervised learning of artificial neural network (30).
Materials and Methods
Xenopus laevis tadpoles were staged by using the criteria of Nieuwkoop and Faber (51). For in vivo recording, stage 42–44 tadpoles were anesthetized with saline containing 0.02% MS222 (Sigma) and incubated in Hepes-buffered saline containing 115 mM NaCl, 2 mM KCl, 10 mM Hepes, 3 mM CaCl2, 10 mMglucose, and 1.5 mM MgCl2 (pH 7.4). For recording from tectal neurons, the skin on top of the head was removed and the brain was split open along the midline to expose the inner surface of the optic tectum. For recording from RGCs, the lens was removed to expose the surface of the retina. Whole-cell perforated patch recording were made under visual control as described (27). The micropipette solution contained amphotericin B (200 μg/mL) and 115 mM K-gluconate, 4 mM NaCl, 1.5 mM MgCl2, 20 mM Hepes, and 0.5 mM EGTA (pH 7.3). To activate retinotectal synapses, the axons of some RGCs were extracellularly stimulated at the optic nerve head. For visual stimulation, a small LCD screen (Sony PLM-A35) was used to project uniform whole-field stimulus of 2-s duration to the tadpole's eye. For more detail see SI Text.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants EY01979 and NS36999 and National Basic Research of China Grants 2006CB806605 and 2006CB943802.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910659106/DCSupplemental.
References
- 1.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Bi GQ, Poo MM. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 5.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (London) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen P, Sundberg SH, Sveen O, Wigstrom H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977;266:736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- 8.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 9.Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: A postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- 10.Kullmann DM. Spillover and synaptic cross-talk mediated by glutamate and GABA in the mammalian brain. Prog Brain Res. 2000;125:339–351. doi: 10.1016/S0079-6123(00)25023-1. [DOI] [PubMed] [Google Scholar]
- 11.Bonhoeffer T, Staiger V, Aertsen A. Synaptic plasticity in rat hippocampal slice cultures: Local “Hebbian” conjugation of presynaptic and postsynaptic stimulation leads to distributed synaptic enhancement. Proc Natl Acad Sci USA. 1989;86:8113–8117. doi: 10.1073/pnas.86.20.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cash S, Zucker RS, Poo MM. Spread of synaptic depression by presynaptic cytosolic signaling. Science. 1996;272:998–1001. doi: 10.1126/science.272.5264.998. [DOI] [PubMed] [Google Scholar]
- 13.Engert F, Bonhoeffer T. Synapse specificity of long-term potentiation breaks down at short distances. Nature. 1997;388:279–284. doi: 10.1038/40870. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- 15.Schuman EM, Madison DV. Locally distributed synaptic potentiation in the hippocampus. Science. 1994;263:532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- 16.Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzsimonds RM, Song HJ, Poo MM. Propagation of activity-dependent synaptic depression in simple neural networks. Nature. 1997;388:439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- 18.Tao HW, Zhang LI, Bi GQ, Poo M. Selective presynaptic propagation of long-term potentiation in defined neural networks. J Neurosci. 2000;20:3233–3243. doi: 10.1523/JNEUROSCI.20-09-03233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganguly K, Kiss L, Poo MM. Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat Neurosci. 2001;3:1018–1026. doi: 10.1038/79838. [DOI] [PubMed] [Google Scholar]
- 20.Li CY, Lu JT, Wu CP, Duan SM, Poo MM. Bidirectional modification of presynaptic neuronal excitability accompanying spike timing-dependent synaptic plasticity. Neuron. 2004;4:257–268. doi: 10.1016/s0896-6273(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 21.Bahr M. Live or let die: Retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- 22.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signaling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 23.Lom B, Cogen J, Sanchez AL, Vu T, Cohen-cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Purves D. Functional and structural changes in mammalian sympathetic neurons following interruption of their axons. J Physiol (London) 1975;252:429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purves D. Effects of nerve growth factor on synaptic depression after axotomy. Nature. 1976;260:535–536. doi: 10.1038/260535a0. [DOI] [PubMed] [Google Scholar]
- 27.Du JL, Poo MM. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature. 2004;429:878–883. doi: 10.1038/nature02618. [DOI] [PubMed] [Google Scholar]
- 28.Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron. 2006;50:115–125. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Gärtner A, et al. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cγ signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumelhart DE, Hinton GE, Williams RJ. Learning internal representations by error propagation. In: Rumelhart DE, McClelland JL, editors. Parallel Distributed Processing. Vol 1. Cambridge, MA: MIT Press; 1986. pp. 318–362. [Google Scholar]
- 31.Humeau Y, Shaban H, Bissieres S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 32.Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Visual stimuli-induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Tao HW, Poo MM. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–1957. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]
- 34.Benke TA, Lüthi A, Isaac JTR, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 36.Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt JT. Activity-driven sharpening of the retinotectal projection: The search for retrograde synaptic signaling pathways. J Neurobiol. 2004;59:114–133. doi: 10.1002/neu.10343. [DOI] [PubMed] [Google Scholar]
- 38.Cogen J, Cohen-Cory S. Nitric oxide modulates retinal ganglion cell axon arbor remodeling in vivo. J Neurobiol. 2000;45:120–133. doi: 10.1002/1097-4695(20001105)45:2<120::aid-neu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 40.von Bartheld CS. Axonal transport and neuronal transcytosis of trophic factors, tracers, and pathogens. J Neurobiol. 2003;58:295–314. doi: 10.1002/neu.10315. [DOI] [PubMed] [Google Scholar]
- 41.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 43.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 44.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: Dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 45.Calbelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 46.Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: Dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu XR, et al. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007;27:7256–7267. doi: 10.1523/JNEUROSCI.0779-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian N, Copenhagen DR. Visual stimulation is required for refinement of on and off pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 49.Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 50.Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:651–661. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. Amsterdam: North–Holland; 1967. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.