Abstract
Regulatory T cells (Tregs) can suppress a wide range of immune cells, making them an ideal candidate for the treatment of autoimmunity. The potential clinical translation of targeted therapy with antigen-specific Tregs is hampered by the difficulties of isolating rare specificities from the natural polyclonal T cell repertoire. Moreover, the initiating antigen is often unknown in autoimmune disease. Here we tested the ability of antigen-specific Tregs generated by retroviral gene transfer to ameliorate arthritis through linked suppression and therefore without cognate recognition of the disease-initiating antigen. We explored two distinct strategies: T cell receptor (TCR) gene transfer into purified CD4+CD25+ T cells was used to redirect the specificity of naturally occurring Tregs; and co-transfer of FoxP3 and TCR genes served to convert conventional CD4+ T cells into antigen-specific regulators. Following adoptive transfer into recipient mice, the gene-modified T cells engrafted efficiently and retained TCR and FoxP3 expression. Using an established arthritis model, we demonstrate antigen-driven accumulation of the gene modified T cells at the site of joint inflammation, which resulted in a local reduction in the number of inflammatory Th17 cells and a significant decrease in arthritic bone destruction. Together, we describe a robust strategy to rapidly generate antigen-specific regulatory T cells capable of highly targeted inhibition of tissue damage in the absence of systemic immune suppression. This opens the possibility to target Tregs to tissue-specific antigens for the treatment of autoimmune tissue damage without the knowledge of the disease-causing autoantigens recognized by pathogenic T cells.
Keywords: autoimmunity, gene therapy, T-cell receptor
To exert their suppressive activity, Tregs require TCR ligation. While the exact nature of Treg TCR recognition remains unknown, the importance of antigen-specificity in successful disease treatment by Treg adoptive transfer is becoming increasingly clear. For example, while polyclonal Tregs can prevent the onset of diabetes in NOD mice (1), reversion of disease can only be demonstrated when using Tregs specific for pancreatic antigens (2, 3). In the preventative setting, the antigen-specific Tregs were 100-fold more efficacious in controlling diabetes when compared to polyclonal Tregs. Similarly, antigen-specific Tregs derived from TCR-transgenic mice were more effective than polyclonal Tregs in controlling murine models of autoimmune gastritis (4) and multiple sclerosis (5). In the transplantation setting, Tregs with specificity for indirectly presented alloantigens were effective in suppressing chronic graft rejection, while Tregs with direct alloantigen specificity were required for control of acute transplant rejection (6). Thus, Treg specificity can be exploited to selectively suppress a particular component of an ongoing immune response.
Ectopic expression of the regulatory transcription factor, FoxP3, has been used to convert conventional CD4+ T cells into T cells with regulatory function. The importance of antigen-specificity of converted CD4+ T cells has been demonstrated using the NOD diabetes model in a similar manner to that described above (7). At present, it is unclear whether the ability of antigen-specific converted CD4+ T cells to suppress immune responses in vivo is equivalent to the suppressive activity of antigen-specific natural Tregs.
In this study, we tested two TCR gene transfer strategies to generate primary T cells with defined antigen-specific regulatory activity: the first involved retroviral TCR gene transfer into a primary Treg population; while the second explored the possibility of converting primary CD4+ T cells into antigen-specific regulators by retroviral transfer of TCR and FoxP3 genes. We designed in vivo experiments to test whether antigen-specificity could be used to direct regulatory T cell function to selective sites in vivo, and whether this would result in local suppression of tissue damage caused by pathogenic effector T cells of unrelated specificity.
Results
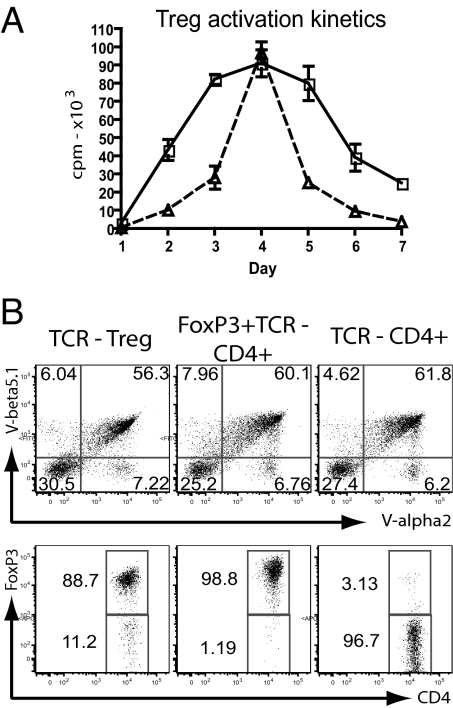
We used the OTII-TCR, which is specific for a peptide derived from ovalbumin (Ova) presented by the Ab MHC class II molecules. We generated retroviral vectors containing only the TCR α and β genes, or the TCR genes plus the gene encoding FoxP3 (Fig. S1). In an attempt to maximize gene expression and TCR assembly, all gene sequences were codon optimized and an additional disulfide bond was introduced into the OTII-TCR α and β constant domain to reduce mispairing with endogenous TCR chains (8). As previous attempts at TCR transduction into primary Tregs had been unsuccessful (9) and T cell proliferation is essential for retroviral infection, we established that stimulation of purified primary murine Tregs with anti-CD3/CD28 antibody and IL-2 triggered robust proliferation comparable to that seen with conventional CD4+ T cells (Fig. 1A). Thus, purified Tregs and CD4+ T cells were transduced with retroviral vectors 1–2 days after polyclonal stimulation, followed by phenotypic and functional analyses after an additional 1–3 days. The majority of freshly transduced Tregs and CD4+ T cells co-expressed the Vα2 and Vβ5 chains of the introduced OTII-TCR (Fig. 1B). Further analysis revealed high levels of FoxP3 in the TCR-transduced Tregs and in the FoxP3+TCR-transduced CD4+ T cells, while control CD4+ T cells that were transduced with only the OTII-TCR were largely negative for FoxP3 expression (Fig. 1B). We analyzed the expression of CD25, CTLA-4 and GITR markers that are typically expressed at elevated levels in natural Tregs (10–12) and in T cells expressing FoxP3 ectopically (13, 14). Both TCR-transduced Tregs and FoxP3+TCR-transduced CD4+ T cells expressed higher levels of these markers compared to the control transduced CD4+ T cells (Fig. S2A).
Fig. 1.
Generation of Ag-specific regulatory T cells by TCR transduction. (A) CD4 sorted T cells (open squares) and CD4+CD25+ sorted Tregs (open triangles) were stimulated with antiCD3/CD28 activation beads and IL-2, proliferation was monitored for 24 h each day over a 7-day period. (B) Purified Tregs and CD4+ T cells were transduced with the OTII-TCR (Vα2, Vβ5) or FoxP3+OTII-TCR, respectively. Control CD4+ T cells were transduced with OTII-TCR only. Transduced cells were stained for TCR α and β expression (top, gated on lymphocytes) and FoxP3 expression (bottom, gated on TCR positive cells; representative of eight separate experiments).
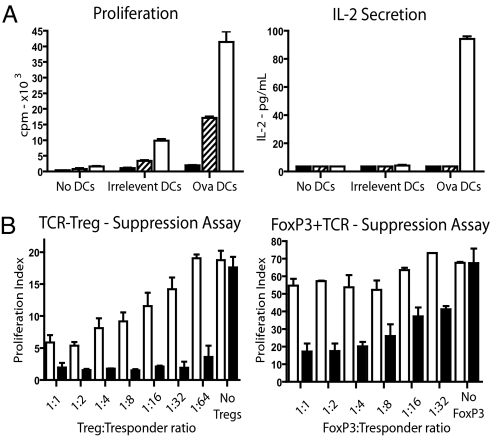
We tested whether the gene engineered T cells were antigen-responsive or whether they displayed the anergic phenotype that is associated with Tregs in vitro (15). Stimulation of the engineered T cells with dendritic cells loaded with Ova peptide revealed that OTII-TCR-transduced Tregs did not proliferate (Fig. 2A). In contrast FoxP3+OTII-TCR-transduced CD4+ T cells displayed low levels of Ova-specific proliferation. Neither TCR-transduced Tregs nor TCR+FoxP3-transduced T cells produced detectable levels of IL-2. Finally, we assessed the ability of the gene-modified T cells to suppress antigen-specific proliferation of third-party responder T cells. Responder T cells, specific for the nucleoprotein (NP) of the influenza virus, were stimulated with dendritic cells that were coated with NP peptide only, or with NP as well as Ova peptide. Fixed numbers of NP-specific responder T cells were stimulated along with increasing numbers of Tregs transduced with the OTII-TCR, or CD4+ T cells transduced with FoxP3+OTII-TCR. Both TCR-transduced Tregs and FoxP3+TCR-transduced CD4+ T cells displayed Ova-dependent suppression of the proliferative response by the NP-specific T cells. As expected some Ova-independent suppression of proliferation was observed at high ratios of regulatory:responder T cells. However, the suppressive activity was clearly enhanced in the presence of the Ova peptide that is recognized by the TCR expressed by the gene-modified T cells (Fig. 2B). Consistently the antigen-dependent suppression mediated by the TCR-transduced Tregs was more profound than that mediated by the FoxP3+TCR-transduced CD4+ T cells. Together these in vitro experiments indicated that retroviral gene transfer was effective in redirecting the specificity of Tregs, and converting conventional CD4+ T cells into FoxP3-expressing cells with phenotypic and functional properties similar to that of Tregs.
Fig. 2.
TCR transduced Tregs and FoxP3-converted T cells are anergic and can suppress a by-stander response in vitro. (A) OTII-TCR transduced CD4+CD25+ Tregs (black), FoxP3+OTII-TCR transduced CD4+ T cells (hatched) and OTII-TCR only transduced CD4+ T cells (white) were generated by retroviral transduction. Transduced cells were stimulated with irrelevant or Ova peptide pulsed DCs and proliferation and IL-2 secretion was measured (presented as mean + SEM of triplicates, representative of four independent experiments). (B) In in vitro suppression assays, fixed numbers of responder Flu-NP-specific CD8+ T cells were mixed with titrated amounts of OTII-TCR-transduced Tregs (left) or FoxP3+OTII-TCR-transduced CD4+ T cells (right). Cells were stimulated with DC pulsed with Flu-NP peptide plus irrelevant peptide (white bars) or Flu-NP plus Ova peptide (black bars). Data presented as proliferation index calculated based upon responder T cells stimulated with irrelevant peptide pulsed DCs (left plot, TCR transduced Tregs P = 0.04 at ratio 1:1, P = 0.001 at ratio 64:1; right plot, FoxP3+OTII-TCR-transduced T cells P = 0.024 at ratio 1:1 and P = 0.0036 at ratio 32:1 with n = 3 for both).
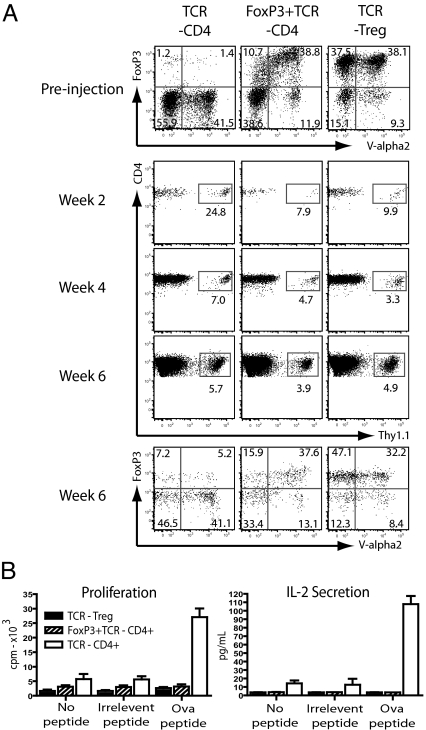
Next we explored the in vivo properties of redirected Tregs and FoxP3-converted CD4+ T cells in adoptive transfer experiments. C57BL/6 mice (Thy1.2) were partially lymphodepleted using 6 Gy total body irradiation, followed by i.v injection of 1.5 × 106 redirected Tregs, FoxP3-converted CD4+ T cells or control CD4+ T cells transduced with only the OTII-TCR. The lymphodepletion regime was utilized to facilitate engraftment and hence detection of the relatively small number of transferred cells. The transduced T cells were from congenic Thy1.1 mice, enabling tracking of the injected cells. Blood samples were taken at weeks 2 and 4, and after 6 weeks, animals were killed for detailed phenotypic and functional analysis. Flow cytometric analysis at weeks 2 and 4 demonstrated efficient engraftment of Thy1.1-positive donor T cells in the blood of recipient mice (Fig. 3A). In all animals the relative proportion of Thy1.1 cells was highest at week 2, which is most likely related to low numbers of endogenous T cells at this early time point after the lymphodepleting conditioning. At week 6, the transferred Thy1.1 T cells were readily detectable in the spleen of recipient mice, and the phenotypic profile of the engrafted cells was similar to that of the injected T cell populations (Fig. 3A). For example, the analysis of mice that received transduced Tregs or FoxP3-converted CD4+ T cells showed that the percentage of FoxP3+TCR+ cells amongst the engrafted Thy1.1 was similar to the percentage present in the populations used for injection. This indicated that both TCR and FoxP3 expression was stable, and that T cells expressing both molecules engrafted at similar efficacy as T cells lacking TCR or FoxP3 expression. Further analysis of the engrafted TCR+ Tregs and TCR+FoxP3+ T cells revealed that they retained increased expression of CD25, CTLA4 and GITR, while the level of CD127 expression was reduced, a phenotype that is characteristic of Tregs (16) (Fig. S2B). Finally, ex vivo functional analysis of the engrafted T cells revealed that the TCR-transduced Tregs and the FoxP3+TCR-transduced CD4+ T cells were anergic in response to Ova peptide stimulation, while TCR-transduced control CD4+ T cells showed Ova-specific proliferation and IL-2 production (Fig. 3B). Together, these experiments showed that the anergic phenotype observed in vitro was stably maintained upon adoptive transfer into lymphopenic hosts.
Fig. 3.
TCR-transduced Tregs and FoxP3+TCR-transduced T cells retain TCR/FoxP3 expression and Treg phenotype in vivo. TCR-transduced Tregs, FoxP3+TCR-transduced CD4+ T cells and TCR only transduced CD4+ T cells were transferred i.v into 6Gy-lymphodepleted C57BL/6-Thy1.2 mice. (A) Before injection transduced cells were analyzed for TCR-Vα2 and FoxP3 expression (top row). Transferred cells (Thy1.1) were detected in tail blood samples at 2 and 4 weeks posttransfer and in the spleen after sacrifice at week 6 (gated on CD4+, middle panels). The TCR-Vα2 and FoxP3 staining profile of the gated Thy1.1+ T cells was assessed a week 6 (bottom row). FACS plots are representative of four separate mice in each group. (B) Splenocytes from mice (6 weeks post-T-cell transfer) were stimulated ex vivo with Ova or control peptides and prolifertion and IL-2 secretion was measured. Chart represents measurements in triplicate from three mice presented as mean + SEM.
Next we explored whether TCR-transduced Tregs and FoxP3+TCR-transduced CD4+ T cells were capable of preventing T cell mediated tissue damage in vivo. To address this we used a well-established antigen-induced arthritis (AIA) model in which mice are immunized with methylated BSA (mBSA) followed by intra-articular knee rechallenge with mBSA to induce T cell-mediated tissue damage. Engineered Tregs or FoxP3-converted CD4+ T cells (1.5 × 106) were adoptively transferred before mBSA rechallenge. Recipient mice did not receive any lymphodepleting conditioning to avoid possible damage to the previously primed mBSA-specific pathogenic T cells. To test whether the transferred T cells were able to home to the appropriate site, suppress in an antigen-specific fashion, and consequently control joint inflammation locally, each animal was challenged in one knee with mBSA and Ova, and in the contralateral control knee with mBSA alone. Thus, only one knee contained the Ova antigen recognized by the gene-modified Tregs, while both knees contained the mBSA antigen recognized by the arthritis-inducing T cells.
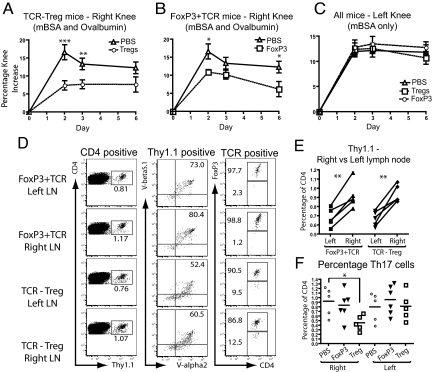
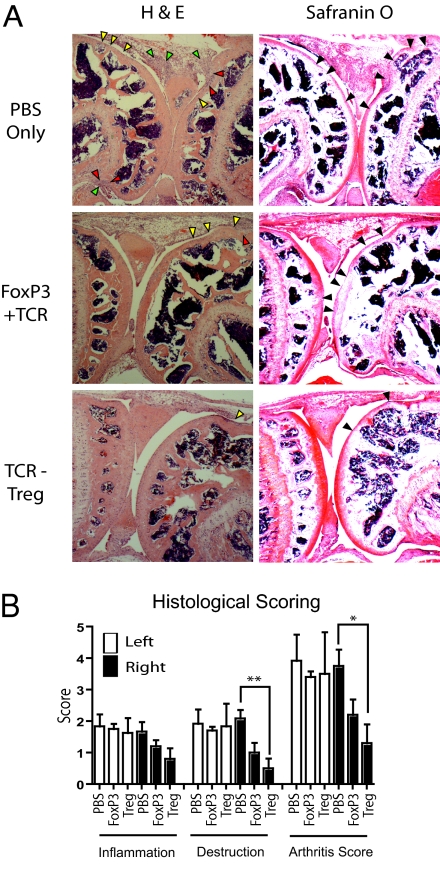
The results showed that the adoptively transferred engineered T cells substantially decreased inflammatory knee swelling when Ova was present, but had no effect in the control knee that was injected with mBSA only (Fig. 4 A–C). Using the congenic Thy1.1 marker the TCR-transduced Tregs and FoxP3+TCR-transduced T cells harvested from left and right lymph nodes were analyzed by flow cytometry. The transferred cells in both groups of mice retained high levels of the introduced TCR and FoxP3 (Fig. 4D). A relative accumulation of TCR-transduced T cells was detectable in the lymph node draining the Ova-injected knee compared with the contralateral control knee (Fig. 4E). To explore a possible mechanism for the therapeutic effect of engineered T cells we monitored the number of Th17 cells, which have been shown to play a pathogenic role in this and other arthritis models (17, 18) as well as in human rheumatoid arthritis (19). Compared to PBS-treated controls, mice treated with TCR-transduced Tregs showed a significant reduction of Th17 cells in the lymph nodes draining the Ova challenged knee (Fig. 4F). In contrast, no significant reduction in Th17 cells was observed in animals that were treated with FoxP3+TCR-transduced CD4+ T cells. All mice showed similar numbers of Th17 cells in the lymph nodes draining the control knee, indicating that suppression of Th17 numbers required the presence of the Ova antigen recognized by the engineered regulatory T cells (Fig. 4F). It remains unclear whether the reduction in Th17 cells within the draining lymph node is a consequence of Treg mediated suppression of Th17 differentiation/function or a function of reduced inflammatory cell accumulation in the lymph node. A number of studies have indicated that Tregs are inefficient suppressors of Th17 cells (20, 21), although they are capable of limiting the detrimental effects of Th17 cells by reducing accumulation at the site of inflammation and in the draining lymph nodes (22). In support of this model, we did note a reduction in absolute numbers of Th17 cells in the lymph node draining the Ova challenged knee of Treg treated mice. The ability to suppress the numbers of Th17 cells and swelling correlated with the ability to reduce joint inflammation and bone destruction as judged by histopathology of tissue sections (Fig. 5A). We observed significant reductions in the scores for tissue destruction and arthritis with engineered Tregs in the knee containing the Ova antigen but not in the contralateral control knee (Fig. 5B). Together, these experiments clearly demonstrate that adoptive therapy with gene modified primary T cells can suppress immunopathology in vivo in an antigen-dependent fashion. The results also indicate that engineered primary Tregs are more effective than converted CD4+ T cells in suppressing pathology in vivo.
Fig. 4.
TCR-transduced Tregs and FoxP3+TCR-transduced CD4+ T cells ameliorate antigen-induced arthritis. BSA-immunized mice were treated with Ova-specific TCR-transduced Tregs or FoxP3+TCR-transduced T cells before intra-articular re-challenge (BSA+Ova = right knee, BSA only = left knee). Swelling was compared in right knees of mice treated with (A) TCR-transduced Tregs (day 2 P = 0.0009 and day 3 P = 0.0097 n = 10) and (B) FoxP3+TCR-transduced T cells (day 2 P = 0.015 and day 6 P = 0.046 n = 10) with PBS-only transferred mice. (C) No difference in swelling between groups was seen in the left (control) knee (all charts presented as mean ± SEM). (D) Cells from the draining lymph node were stained for TCR (V-α2 and V-β5) and FoxP3 expression ex vivo (gating indicated above plot–representative of 5 mice). (E) Proportion of transferred cells within the CD4+ T cell compartment of the draining lymph nodes was determined by Thy1.1 staining (P = 0.0039 and P = 0.0034, n = 5). (F) Proportion of Th17 cells was determined by IL-17 staining, presented as percentage of the CD4+ gated T cells isolated from the draining lymph node (P = 0.017, n = 5).
Fig. 5.
Treatment with TCR-transduced Tregs ameliorates joint destruction. On day 7 following arthritis induction knees were removed, sectioned, stained with H&E and Safranin O, and scored blind for lymphocyte infiltration and joint destruction. (A) Representative photomicrographs (40×) of right knee joints show areas of lymphocyte infiltration and bone erosion. H and E staining of sections of the right knee from a PBS-treated mouse (top row) show considerable cellular infiltration (yellow arrows) over large proportions of the joint surface including two areas of loss of surface integrity (red arrows) and cellular accumulation in the joint space (green arrows). Safranin O staining reveals loss of cartilage (black arrows) over large areas of joint surface of both bones. H&E staining of sections from the right knee of a FoxP3 treated mouse (middle row) show a moderate degree of cellular infiltration but no loss of bone integrity. Safranin O staining shows loss of cartilage, mainly restricted to one bone-surface of the joint. H&E and Safranin O staining of sections of the knee from a TCR-transduced Treg treated mouse (bottom row) shows normal knee architecture with a small-contained area of cellular infiltration and an area of limited cartilage thinning. (B) Mean scoring for both knees from all mice is presented (white bars = left, black bars = right–P = 0.0041 and P = 0.013 respectively, n = 5, mean + SEM).
Discussion
We found that TCR-transduced Tregs as well as FoxP3+TCR-transduced CD4+ T cells selectively accumulated in the draining lymph nodes of the Ova-challenged knee, indicating that both were able to respond to Ova antigen in vivo. Compared to PBS-treated control mice, there was no increase in Th17 cells in the T cell-treated mice, indicating that in the in vivo environment of inflammatory arthritis neither the engineered Tregs nor the engineered CD4+ T cells converted into Th17 cells. Conversion of Tregs into pro-inflammatory Th17 cells has been described in certain experimental settings (23) and could potentially enhance immunopathology if adoptively transferred regulatory T cells converted into pro-inflammatory Th17 cell in vivo.
The reason why the engineered Tregs but not the FoxP3+TCR-transduced CD4+ T cells suppressed Th17 cell in vivo is currently not known. Reduced levels of FoxP3 expression are unlikely to account for the observed difference as the expression of the introduced FoxP3 in CD4+ T cells was at least as high as the endogenous FoxP3 in Tregs. While FoxP3 is widely described as a “master-switch” for Treg development, several studies have now shown that FoxP3 is not the only factor involved in Treg phenotype and function (24). Gene array studies have shown that although ectopic FoxP3 expression upregulated a large number of Treg associated genes, substantial differences in the gene expression profile of FoxP3-converted T cells and natural Tregs were detectable (25, 26). The elucidation of the molecular mechanisms that empower antigen-specific Tregs with more efficient suppressive activity compared to FoxP3 converted T cells will require further functional analysis of candidate genes that are differentially expressed in these T cell populations. It is likely that FoxP3-converted T cells are more comparable to inducible (FoxP3+) Tregs, a population of regulatory T cells generated from conventional CD4+ T cells by stimulation in the presence of TGFβ and IL-2/retanoic acid (27, 28). In fact, recent experiments with TGFβ-induced polyclonal Tregs suggested that they were less effective than natural Tregs in ameliorating autoimmune gastritis (29). Although Ag-specific FoxP3-converted T cells failed to ameliorate arthritis to the same degree as natural Ag-specific Tregs in our hands, it is worth noting that studies carried out using a similar FoxP3-conversion technique in CIA demonstrated a significant suppression of arthritis progression (30). Furthermore, these studies identified a reduction in IL-17 transcripts within the arthritic paws of mice treated with Ag-specific FoxP3-converted T cells, however, in agreement with the data presented here, found no reduction in the draining lymph nodes. The difference in these results and ours could be attributable to differences in the disease model or the target Ag. Whereas we targeted a non-disease-inducing-Ag, the study described above utilized a TCR specific for type II collagen, the primary immunising Ag in the CIA model. While this type of targeting approach is clearly efficacious it is debatable as to how relevant it is to human autoimmune disease, where the initiating-Ag is rarely known.
In the experiments described here the same dose of TCR-transduced Tregs and FoxP3+TCR CD4+ T cells were used for the adoptive T cell therapy of arthritis. Although the Tregs were more effective, it is likely that increasing the dose of converted CD4+ T cells would improve their therapeutic efficacy. As primary CD4+ T cells are readily purified in large numbers, high-dose therapy with freshly transduced CD4+ T cells is easily achievable. Possible therapeutic application of this approach is supported by recent data indicating that human CD4+ T cells can be successfully converted into stable suppressive cells when the FoxP3 is expressed under an activation state independent promoter (31). In contrast to the abundance of conventional T cells, Tregs represent fewer than 2% of the peripheral blood lymphocytes and their isolation to high purity is challenging, as there are no markers that are strictly specific for this cell type. In vitro expansion of the purified Tregs would be an option to increase their numbers before adoptive transfer. However, a major goal of this study was to shorten the culture period of primary T cells, as extended in vitro stimulation of Tregs may allow for the out-growth of the contaminating non-Treg population (32). Furthermore, prolonged culture of ‘conventional’ T cells is associated with increased T cell differentiation resulting in decreased in vivo T cell survival following adoptive transfer (33). Thus, it is possible that extended in vitro expansion of purified Tregs may impair their in vivo survival and function.
We used a retroviral transduction protocol that allowed us to redirect the specificity of primary Tregs and CD4+ T cells without the need for lengthy in vitro culture. Within 3 days, redirected Tregs and converted CD4+ T cells were available for adoptive transfer into recipient mice, where they engrafted efficiently, maintained their phenotype, and suppressed arthritic joint inflammation in an antigen-dependent fashion. In the described experiments, previously primed T cells were responsible for the joint damage, an indication that the engineered regulatory T cells were able to suppress the pathogenic potential of existing memory/effector T cells. Furthermore, suppression of arthritis occurred when the regulatory T cells and the pathogenic T cells recognized two distinct antigens. This feature of linked suppression provides an opportunity to treat autoimmune conditions without any knowledge of the specificity of disease-causing autoreactive T cells. The presented data suggests that redirecting the specificity of Tregs to tissue-specific antigens expressed in the target organs of autoimmune disease may lead to selective Treg accumulation and activation leading to local suppression of pathogenic T cells irrespective of their antigen specificity. The combination of selective homing and local immune suppression provides opportunities to develop highly targeted Treg therapy for the effective control of autoimmune mediated tissue damage without impairment of systemic immunity.
The described adoptive therapy with engineered T cells was effective as mono-therapy and there was no requirement for lymphodepleting conditioning, which is required to achieve therapeutic efficacy after adoptive T cell therapy of malignancies (34). Previous studies with a redirected Treg cell-line showed that the in vivo suppressive activity of these cells required co-treatment with other immunosuppressive drugs (9). The observation that engineered primary T cells used here can be effective without any additional therapy is in agreement with studies using antigen-specific Treg populations isolated from TCR-transgenic mice. This suggests that the in vivo potency of engineered primary T cells is similar to that of antigen-specific Tregs isolated from transgenic mice.
In summary, we describe an effective strategy to rapidly convert primary T cells into antigen-specific regulatory cells for adoptive therapy to achieve targeted immune suppression. The adoptively transferred cells can be effective as mono-therapy, and they prevent tissue damage by previously activated T cells with unrelated specificity. Thus, engineered primary regulatory T cells are promising reagents for the treatment of autoimmunity without any knowledge of the initiating autoantigens.
Methods
Mice.
C57Bl6 mice were purchased from Harlan; Thy1.1 C57BL/6 and F5-TCR transgenic (C57BL/6) were bred in house at the animal facility at University College London. All procedures were carried out in accordance with United Kingdom Home Office regulations.
Flow Cytometry and Cell Sorting.
Further antibody information is contained within the SI Materials and Methods. CD4+CD25+ T cells were sorted using CD4+ negative selection CD25 positive selection kit (Miltenyi Biotech) as per the supplied protocol. Typical purity varied between 89–94%. CD11c+ DCs were sorted using anti-CD11c MACs beads (Miltenyi Biotech) from type IV Collaginase (Worthington–46E8824) digested spleen. Typical purity varied between 91–96%. CD4 or CD8 positive T cells were sorted using anti-CD4 or anti-CD8α MACs beads (Miltenyi Biotech) as per the supplied protocol.
Retroviral Transduction.
Retroviral vectors pMX-OTIIα.IRES.OTIIβ, pMP71-OTIIα.P2A.OTIIβ, and pMP71-FoxP3.F2A.OTIIα.P2A.OTIIβ were generated. Further information on vectors can be found within the SI Materials and Methods. Phoenix-Ecotropic packaging cells (Nolan Lab, Stanford University, CA) were transfected with either vector along with pCL-Ecotropic vector using calcium phosphate precipitation and retrovirus-containing supernatant was collected on day 1 for 24 h. Conventional T cells were activated for 24 h with Con A (2 μg/mL final concentration) and CD4+CD25+ T cells were activated for 48 h using anti-CD3/CD28 beads (1:2 cell:bead-T cell Expander, Dynal, Invitrogen) and rIL-2 (500 U/mL–Chiron). After 24 or 48 h (conventional T cells or CD4+CD25+ T cells), cells were incubated with retroviral supernatant for a further 24 h. For in vivo studies cells were adoptively transferred i.v. in PBS immediately, for in vitro studies cells were rested for a further 76 h. When required cells were maintained in complete RPMI-1640 culture media supplemented with rIL-2 (500 U/mL–Chiron) at 1 × 106 cells/mL with media replaced daily. Viable cells were obtained before in vitro assays by lympho-prep (Ficoll Axis shield).
In Vitro Functional Assays.
For stimulation assays, 0.75 × 105 transduced T cells were stimulated with 5 × 103 peptide pulsed splenic DCs for 36 h. For ex vivo stimulation, red blood cells were lysed and peptide was added to 2 × 105 whole cell suspensions and incubated for 4 days. Further information on peptides and DC pulsing are contained within the SI Materials and Methods. Proliferation was measured by 3H-thymidine (1 μCi/well) uptake over the final 24 h of stimulation. The level of IL-2 in culture supernatants at the end of culture periods was determined by ELISA using the OptEIA system as per the supplied protocol (BD).
For in vitro suppression assays, naïve F5 TCR transgenic splenocytes were CD8 bead sorted and used as a responder population. CD8+ F5-TCR transgenic T cells (0.75 × 105) and 5 × 103 peptide pulsed splenic DCs (as above) were combined with reducing titrations (as indicated) of CD4+CD25+ or FoxP3-converted T cells in a total volume of 200 μL tissue culture medium. Cells were seeded in 96-well rounded-bottom tissue-culture-treated plates and incubated for 3 days. On day 3, 1 μCi of 3H-Thymidine was added per well and cells were incubated for a further 24 h before being harvested and counted.
Adoptive Transfer into Lympho-Depleted Mice.
C57BL/6 (Thy1.2) mice were conditioned with 6 Gray of total body irradiation followed 24 h later by transfer i.v with 1.5 × 106 cells. Blood samples were taken on week 2 and 4 by superficial cut to the tail vein and on week 6, mice were killed and cells were harvested from the spleen.
Induction of AIA.
At day −10, female C57BL/6 mice were immunized s.c. at the base of the tail with 100 μg mBSA (Sigma) emulsified in complete Freund's adjuvant (Difco). On day 0 mice were transferred i.v with the stated cell type or PBS only. Arthritis was induced by intra-articular injection of 20 μg o mBSA in 10 μL PBS in the left knee and 20 μg mBSA and 100 μg whole ovalbumin in 10 μL PBS in the right knee [adapted from (35)]. Arthritis severity was monitored by measurement of knee diameter using dial gauge callipers (Kroeplin). Increase in knee diameter was calculated based on preinjection knee diameter for each mouse before injection on day 0. For histopathological examination, knees were removed, fixed in 4% formalin, decalcified in EDTA, and embedded in paraffin. Sections were stained with H&E and Safranin O. Histological sections were scored in a blinded manner on a scale of 0–3 for degree of inflammation and joint destruction. A combined score was generated from the sum of the individual scores for inflammation and destruction (maximum score of 6).
Statistical Analysis.
Comparisons between groups (normally distributed) were carried out using unpaired Student's t-test (Figs. 2B and 3 A–C and F). For comparisons between related samples (e.g., left vs. right) paired Student's t-test was used (Fig. 3E). N for each calculation is shown in parenthesis in figure legends. All statistical analysis was carried out using GraphPad Prism software.
Supplementary Material
Acknowledgments.
We thank Emma Ross for excellent technical assistance and Maggie Sinclair for critical reading of the manuscript. This work was supported by the Leukaemia Research Fund (to A.H. and H.J.S.) and the Arthritis Research Campaign 17509 and 18398 (to C.A.N. and M.R.E.). G.P.W. is supported by a Medical Research Council postgraduate studentship.
Footnotes
Conflict of interest statement: H.J.S. is a consultant for Cell Medica.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907396106/DCSupplemental.
References
- 1.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 2.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarbell KV, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiPaolo RJ, et al. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 5.Stephens LA, Malpass KH, Anderton SM. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur. J Immunol. 2009;39:1108–1117. doi: 10.1002/eji.200839073. [DOI] [PubMed] [Google Scholar]
- 6.Joffre O, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 8.Cohen CJ, et al. Enhanced antitumor activity of T cells engineered to express T cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang JY, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 12.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 15.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: Induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 18.Koenders MI, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chabaud M, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci USA. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 24.Gavin MA, et al. Foxp3-dependent programme of regulatory T cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 25.Hill JA, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto N, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 27.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 29.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: Antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181:8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujio K, et al. Gene therapy of arthritis with TCR isolated from the inflamed paw. J Immunol. 2006;177:8140–8147. doi: 10.4049/jimmunol.177.11.8140. [DOI] [PubMed] [Google Scholar]
- 31.Allan SE, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann P, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 33.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setoguchi K, et al. Antigen-specific T cells transduced with IL-10 ameliorate experimentally induced arthritis without impairing the systemic immune response to the antigen. J Immunol. 2000;165:5980–5986. doi: 10.4049/jimmunol.165.10.5980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.