Abstract
p21CIP1/WAF1 is a downstream effector of tumor suppressors and functions as a cyclin-dependent kinase inhibitor to block cellular proliferation. Breast tumors may derive from self-renewing tumor-initiating cells (BT-ICs), which contribute to tumor progression, recurrence, and therapy resistance. The role of p21CIP1 in regulating features of tumor stem cells in vivo is unknown. Herein, deletion of p21CIP1, which enhanced the rate of tumorigenesis induced by mammary-targeted Ha-Ras or c-Myc, enhanced gene expression profiles and immunohistochemical features of epithelial mesenchymal transition (EMT) and putative cancer stem cells in vivo. Silencing of p21CIP1 enhanced, and expression of p21CIP1 repressed, features of EMT in transformed immortal human MEC lines. p21CIP1 attenuated oncogene-induced BT-IC and mammosphere formation. Thus, the in vitro cell culture assays reflect the changes observed in vivo in transgenic mice. These findings establish a link between the loss of p21CIP1 and the acquisition of breast cancer EMT and stem cell properties in vivo.
Keywords: cancer stem cells, oncogene, EMT
The onset and progression of human and murine mammary tumorigenesis is thought to involve the acquisition of new cellular capabilities (1), with aberrant proliferation characterized by alteration in cell cycle control protein function (2). The cyclin/CDK (CDK) inhibitor p21CIP1 functions as a downstream effector of tumor suppressors including p53, BRCA1, WT1, and TGFβ (3). In the absence of p21CIP1 hematopoietic stem cells (HSCs) (4), proliferation and absolute number increased, suggesting p21CIP1 governs HSC cycle entry. p21CIP1 inhibits cellular growth in tissue culture and tumor xenograft formation (3, 5). PDGF-induced gliomagenesis, and ATM−/− and p53−/−-mediated tumorigenesis was reduced in the p21CIP1-deficient background, suggesting a tumor promoting function of p21CIP1 (6). In contrast, APC mutation and p18INKC-deficiency-dependent tumorigenesis (7), and mammary gland targeted Ha-Ras or c-Myc-induced tumorigenesis, were each enhanced by p21CIP1 deficiency (8).
In addition to these previously described hallmarks, recent studies implicate epithelial mesenchymal transition (EMT) and tumor cancer stem cells (or tumor-initiating cells) in tumor progression (2). Recent studies have suggested self-renewing stem-like cells, exist within tumors known as cancer stem cells or tumor initiating cells (TICs) (9–11). Epithelial cells from murine or human mammary glands or tumors contain stem-like cells that express EMT markers, suggesting a link between EMT and the gain of epithelial stem-like properties (12).
The current studies were conducted to address the following important questions. First, although p21CIP1 is known to function as a tumor suppressor, the role of p21CIP1 in regulating mammary tumor-associated EMT in vivo is unknown. Second, although p21CIP1 was shown to regulate HSCs, the role in cancer stem cells was unknown. Third, although features of stem cells have been identified by epitope markers in human cancers and cell lines, genome-wide analysis of cancer stem cell signatures induced by specific oncogenes in vivo and the role of the tumor suppressor p21CIP1 in regulating features of stem cells have not been conducted.
Results
Myc and Ras Induce EMT Gene Expression Signatures in Breast Tumors Which Are Inhibited by p21CIP1.
In our previous studies, deletion of p21CIP1 in transgenic mice accelerated mammary oncogenesis induced by MMTV-Ras and MMTV-c-Myc (8). A genome-wide analysis of Ras and c-Myc targets was conducted using mammary tumors derived from transgenic mice (MMTV-Ras, MMTV-c-Myc, MMTV-Ras/p21CIP−/−, and MMTV-c-Myc/p21CIP−/−) derived at a similar size. Compared with normal mammary gland, c-Myc regulated 761 genes and Ras regulated 539, with 344 genes common to Ras and c-Myc-induced tumors (Fig. S1A). Comparison between the MMTV-c-Myc and MMTV-Ras tumors and normal mammary epithelial glands demonstrated altered gene expression profiles reflecting the induction of features of EMT (13) (Fig. S1B). Gene expression that is induced with EMT, was induced in both Ha-Ras- and c-Myc-induced tumors. Tumors analysis showed 236 genes were differentially regulated by p21CIP1 in the context of c-Myc, and 323 genes were differentially regulated by p21CIP1 in the Ha-Ras tumors (Fig. S1C). Of these p21CIP1 regulated genes, 20 genes were common to Ha-Ras and c-Myc.
Genetic Pathways Governing EMT and Stem Cell Quiescence and Self-Renewal Are Repressed by Endogenous p21CIP1 in Oncogene-Induced Mammary Tumors of Transgenic Mice.
We used ASSESS for the molecular classification of p21CIP1-dependant gene expression sets (14). In the context of Ras, deletion of p21CIP1-induced EMT pathways and pathways regulating the stem cell population [(HSC-LTHSCs) (HSC-long-term HSCs)] (Fig. S1D). In the context of c-Myc, p21CIP1 inhibited features of the known EMT-inducing pathways TGFβ and NF-κB (15, 16) (Fig. S1E). As with the Ras tumors, p21CIP1 deletion in c-Myc mammary tumors correlated with gene expression seen as an enrichment of LTHSCs and depletion of early progenitor HSCs.
Immunohistochemical Features of EMT in Mammary Oncogene-Induced Tumors Are Repressed by p21CIP1 in Transgenic Mice.
Immunohistochemical staining for E-cadherin was reduced upon deletion of p21CIP1 in the context of either the Ras or c-Myc oncogene (Fig. S2 A and B). Conversely, vimentin staining was increased (Fig. S2 A and C). The loss of p21CIP1 was associated with the induction of Twist abundance (Fig. S2 A and D). Twist transduced MCF10A cells formed colonies in soft agar (Fig. S3A and B) and enhanced migratory capability (Fig. S3C), which was further enhanced by serum addition (Fig. S3C).
MCF10A cells grew with a typical sheet of individual epithelium abutting each other, and on collagen matrix developed a spherical shape (Fig. S3D). MCF10A cells transduced with an expression vector encoding Twist developed the mesenchymal morphology with a more extended and elongated shape, invaded the collagen and grew as tubular structures (Fig. S3D). Twist expression reduced the epithelial cell markers E-cadherin and β-catenin. Conversely, the mesenchymal markers vimentin and N-cadherin were increased (Fig. S3E).
Activity of the E-cadherin promoter, a useful surrogate for E-cadherin expression, was repressed by Twist, and mutation of the Twist-binding site abrogated Twist-mediated repression (Fig. S3F). p21CIP1 reversed Twist-mediated E-cadherin promoter repression (Fig. S3F). Twist expression correlated with an approximate 80% reduction in p21CIP1 abundance (Fig. S3G) and Twist repressed the p21CIP1 promoter 60% (Fig. S3H).
Ha-Ras and c-Myc Induce Features of EMT in Human Breast Cancer Cell Lines.
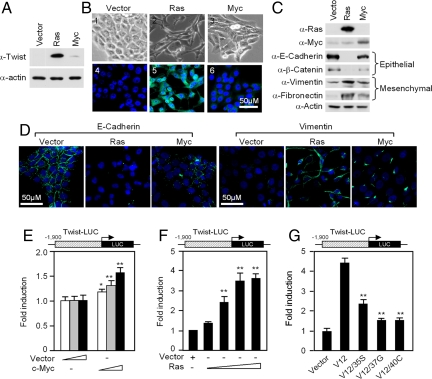
In MCF10A cells Ha-Ras strongly induced Twist expression while induction by c-Myc was modest (Fig. 1A). MCF10A-Ras cells, and to a lesser extent MCF10A-c-Myc, showed reduced cell-cell contacts with a more polarized fibroblastoid appearance representing morphological changes associated with EMT (Fig. 1B). Immunohistochemistry (Fig. 1B) for Twist reflected the Western blot finding (Fig. 1A) and showed Ha-Ras induced Twist expression with modest induction of Twist by c-Myc. Western blot analysis showed features of EMT in MCF10A-Ras cells with a reduction in E-cadherin and β-catenin, and induction of vimentin and fibronectin (Fig. 1C). c-Myc transduced cells showed some features of EMT with decreased E-cadherin and β-catenin, and increased fibronectin and vimentin (Fig. 1C). Ras-transduced MCF10A cells showed a reduction in E-cadherin and induction of vimentin (Fig. 1D). The epithelial marker ZO-1 (17) was reduced and the mesenchymal marker N-cadherin was increased by Ha-Ras expression or c-Myc transduction (Fig. S4A).
Fig. 1.
Ras and c-Myc induce epithelial to mesenchymal transition in MCF10A mammary epithelial cells. (A) Twist expression was examined by immunoblotting in MCF10A-Ras and MCF10A-c-Myc cells. (B) Representative images of cellular morphology of MCF10A control, MCF10A-Ras, and MCF10A-c-Myc (panels 1–3) and immunofluorescence-staining for Twist (panels 4–6). (C) Epithelial markers and mesenchymal markers were examined by immunoblotting in MCF10A-Ras and MCF10A-c-Myc cells. (D) Immunofluorescence staining of E-cadherin and vimentin in MCF10A-Ras and MCF10A-c-Myc cells. (E) Luciferase activity of the Twist promoter reporter in HEK293 cells cotransfected with c-Myc (n ≥ 3). (F) Luciferase activity of the Twist promoter luciferase reporter in HEK293 cells. Data are shown for the effect of 10–100 ng cotransfected RasV12 compared with vector control (n ≥ 3). (G) Luciferase activity of the Twist promoter luciferase reporter in HEK293 cells cotransfected with point mutations of Ras V12 (n ≥ 3). All luciferase reporter assays were normalized to Renilla luciferase or β-galactosidase activity and represent ± SEM and * = P < 0.05, ** = P < 0.01.
Ras increased Twist mRNA abundance approximately 2.5-fold quantitated by RT-PCR. Point mutation of S35 in Ras (V12/35S) abrogates JNK without affecting ERK signaling (18) and remained capable of inducing the Twist promoter, whereas the 37G and 40C mutations, which abolish MAPK and PI3K signaling (18) respectively, were incapable of activating Twist (Fig. 1G). These studies suggest Ha-Ras induction of Twist involves multiple pathways (PI3K, ERK, and JNK). c-Myc expression induced a modest increase in Twist abundance by Western blot and by immunohistochemical staining (Fig. 1 A and B). Twist promoter activity was only modestly induced by c-Myc (Fig. 1E and Fig. S4B). Ha-Ras-induced Twist promoter activity (Fig. 1F) and was inhibited in a dose-dependant manner by p21CIP1 (Fig. S4C).
p21CIP1 Inhibits Oncogene-Induced EMT.
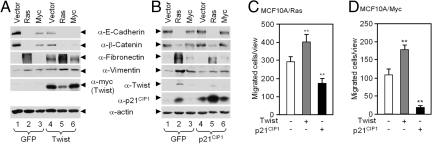
To determine the functional role of p21CIP1 in Ras-induced EMT, MCF10A cells were sequentially transduced with Ha-Ras or c-Myc and then transduced with either Twist as an inducer of EMT (Fig. 2A) or p21CIP1 (Fig. 2B). Twist (Fig. 2A, lanes 1 vs. 4), c-Myc (Fig. 2A, lanes 3 vs. 6), and Ha-Ras enhanced expression of the mesenchymal markers fibronectin and vimentin (Fig. 2A, lanes 2 vs. 5). p21CIP1-repressed Ha-Ras induced EMT, reducing expression of the mesenchymal marker fibronectin and vimentin (Fig. 2B, lanes 2 vs. 5, and Fig. 3C). p21CIP1 modestly affected features of EMT in the MCF10A-c-Myc cells (Fig. 2B, lanes 3 vs. 6). As p21CIP1 inhibited EMT, and EMT in tumor cells is tightly linked to invasive growth, we determined the role of p21CIP1 in oncogene-mediated migration. MCF10A-Ras cellular migration across a transwell membrane was significantly increased by expression of Twist and reduced by p21CIP1 (Fig. 2C). Twist transduction of MCF10A-c-Myc cells increased migration by approximately 50% (Fig. 2D). p21CIP1 inhibited the migration of MCF10A-c-Myc cells by >90%.
Fig. 2.
p21CIP1 inhibits epithelial to mesenchymal transition and cellular migration in MCF10A-Ras and MCF10A-c-Myc cells. (A) Immunoblots of epithelial markers and mesenchymal markers in MCF10A-Ras and MCF10A-c-Myc transformed with Twist compared to GFP vector control. (B) Immunoblot of epithelial markers and mesenchymal markers in MCF10A-Ras and MCF10A-c-Myc cells transduced with p21CIP1 compared to GFP control. (C) Quantitation of Western blot analysis of epithelial and mesenchymal markers in MCF10A-Ras and MCF10A-c-Myc cells transduced with p21CIP1. Data are mean ± SEM. (D and E) Transwell assay for cellular migration demonstrated p21CIP1 inhibits migration of (D) MCF10A-Ras and (E) MCF10A-c-Myc cells. Data are mean ± SEM and ** - P < 0.01.
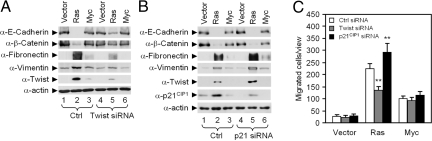
Fig. 3.
Endogenous p21CIP1 inhibits MCF10A-Ras- and MCF10A-c-Myc-induced EMT. (A) Immunoblots of epithelial markers and mesenchymal markers in MCF10A-Ras and MCF10A-c-Myc treated with siRNA to Twist compared to scrambled control siRNA. (B) Immunoblots of epithelial markers and mesenchymal markers (fibronectin and vimentin) in MCF10A-Ras and MCF10A-c-Myc treated with siRNA to p21CIP1 compared to scrambled control siRNA. Data are mean ± SEM. (C) Transwell assay for cellular migration in MCF10A-Ras and MCF10A-c-Myc cells treated with siRNA to Twist and p21CIP1 compared to scrambled control siRNA. Data are mean ± SEM of n = 3.
We next determined the role of endogenous p21CIP1 in Ras- and c-Myc-induced EMT using siRNA. Western blot analysis showed Ras- but not c-Myc-induced EMT was significantly reversed by Twist siRNA (Fig. 3A, lanes 2 vs. 5), consistent with a role for Twist in Ras-mediated EMT. Twist siRNA reversed Ras-mediated reduction in epithelial markers (E-cadherin and β-catenin) and reversed the Ras-mediated induction of the mesenchymal markers (fibronectin and vimentin). This finding is consistent with the model in which Ras and endogenous Twist function in a similar pathway to induce EMT. p21CIP1 siRNA promoted the induction of mesenchymal markers induced by Ras (fibronectin and vimentin) (Fig. 3B, lanes 2 vs. 5, S4). siRNA-mediated reduction in Twist reduced, but siRNA-mediated reduction in p21CIP1-enhanced, Ras-dependent cellular migration in transwell assays (Fig. 3C). siRNA to Twist or p21CIP1 did not significantly affect EMT associated markers (Fig. 3 A and B) or cellular migration in MCF10A-c-Myc cells (Fig. 3C).
p21CIP1 Attenuates an Embryonic Stem Cell-Like Gene Expression Pattern.
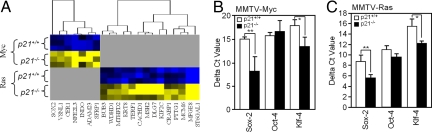
Genes that were overexpressed in ES cells (19) were increased in abundance in c-Myc-p21CIP1−/− vs. c-Myc-p21CIP1+/+. A similar pattern was observed in Ras-p21CIP1−/− vs. Ras-p21CIP1+/+ (Fig. 4A). The ES genes regulated by p21CIP1 were mainly non-overlapping in the Ras vs. c-Myc mammary tumors. The Sox2 gene was differentially regulated by p21CIP1 in the presence of c-Myc. Sox2, Oct4, Nanog, and KLF4 govern propagation of ES cells in culture (20). QT-PCR of mRNA extracted from the MMTV-Myc and MMTV-Ras tumor with either p21CIP1−/− or p21CIP1+/+ genotype confirmed the increased abundance of Sox2 and KLF4 in the Ras-p21CIP1−/− and c-Myc-p21CIP1−/− tumors (Fig. 4 B and C) (note a decrease in delta Ct levels represents an increase in mRNA abundance).
Fig. 4.
p21CIP1 inhibits ES cell gene signature in mammary tumors of transgenic mice. (A) Treeview analysis of mammary tumors of transgenic mice showing expression of ES cell marker (blue indicates decreased and yellow indicates increased expression). Data of three separate transgenic mice are shown. (B and C) Quantitative analysis (QT-PCR) of ES cell marker genes (Sox2, Oct4, and KLF4) in mammary tumors of transgenic mice, indicating increased expression in mammary tumor of p21CIP1−/− genotype. Data are mean ± SEM of n > three separate mice per genotype.
p21CIP1 Attenuates Expression of c-Myc-Induced Cancer Stem Cell Markers.
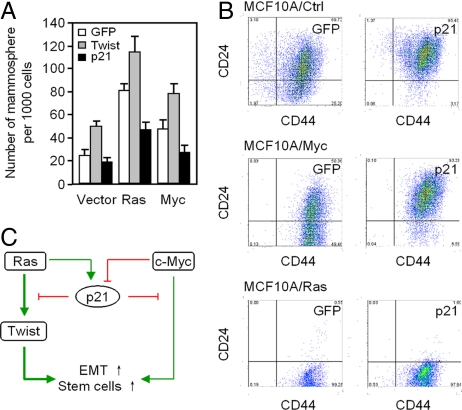
Only a small number of primary breast cancer cells, known as cancer stem cells or TICs, form secondary tumors (21). TICs form non-adherent mammospheres, similar to normal mammary gland stem cells when cultured under specific conditions in the absence of serum (22, 23). Twist enhanced mammosphere production of MCF10A cells approximately 2-fold (Fig. 5A). Ha-Ras or c-Myc transduction of MCF10A enhanced the proportion of mammospheres formed 4- and 2-fold respectively (Fig. 5A). p21CIP1 expression reduced Ha-Ras and c-Myc induced mammosphere formation by approximately 50%. Cancer stem cells can be enriched by sorting for CD44+/CD24−/low cells (11). p21CIP1 transduction of MCF10A cells reduced the proportion of CD44+/CD24− cells from 25 to 3% (Fig. 5B). The proportion of CD44+/CD24− of MCF10A-c-Myc cells were reduced approximately 7-fold by p21CIP1 expression (49.5 to 6.6%, Fig. 5B). The proportion of CD44+/CD24− MCF10A-Ras cells were unaltered by p21CIP1 which may be due to the already very high levels of p21CIP1 expression in these cells.
Fig. 5.
p21CIP1 inhibits oncogene-induced mammosphere and breast tumor stem cell marker expression. (A) Mammosphere assays were conducted of MCF10A-Ras and MCF10-Myc mammary tumor cell lines. Cells were transduced with GFP control vectors encoding Twist or p21CIP1. Assays were conducted after 10 days. (B) FACS analysis for mammary stem cell markers. MCF10A control, MCF10A-Ras, and MCF10A-c-Myc cell lines transduced with GFP or p21CIP1 vector. (C) Schematic representation of the mechanism by which p21CIP1 attenuates oncogene-induced EMT and stem cell gene expression in MCF10A-Ras and MCF10A-c-Myc cells.
Discussion
The current studies demonstrate p21CIP1 attenuates features of EMT and breast tumor stem cells in cultured cells and in transgenic mice (Fig. 5C). The gene expression profile analysis demonstrated Ras- and c-Myc-induced features of EMT within mammary tumors. These findings are consistent with findings that markers of EMT are induced in human and murine mammary tumors associated with c-Myc amplification (24). Functional pathway analysis of gene expression within the Ha-Ras and c-Myc-induced tumors using ASSESS demonstrated that p21CIP1 inhibits the gene expression profiles associated with EMT in vivo. Immunohistochemical analysis of mammary tumors provided further evidence that p21CIP1 attenuated Ha-Ras and c-Myc-induced features of EMT. p21CIP1 siRNA in MCF10A cells, demonstrated that p21CIP1 inhibited Ras-mediated EMT. Herein Twist repressed the E-cadherin promoter. p21CIP1 antagonized Twist-mediated repression of E-cadherin promoter activity, via the E-box binding site. These findings are consistent with recent findings that endogenous Twist maintains p21CIP1 gene expression and the EMT phenotype at MCF7 cells (25).
Cancer stem cells possess SC-like traits (26). The current studies provide several lines of evidence that p21CIP1 represses features of cancer stem cells in mammary tumors. p21CIP1 repressed the gene expression signature of HSCs, inhibited mammosphere production, and inhibited cell surface markers representing mammary cancer stem cells. Deletion of p21CIP1 in both Ras- and c-Myc-mediated mammary tumors increased expression of the LTHSC pathway. In prior studies, deletion of p21CIP1 increased HSC (4). Self-renewal of primitive HSCs requires p21CIP1 and was defective in p21CIP1−/− mice, suggesting p21CIP1 functions as a molecular switch governing HSC cycle entry.
Several recent studies have suggested the molecular circuitry controlling embryonic stem (ES) cells may be active in certain tumors. Some of the key regulators of ES cell identity, Oct4, Sox2, KLF4, and Nanog (27), are expressed in specific tumors (28, 29). In this regard genome scale location analysis has been used to identify Oct4, Sox2, and Nanog target genes (27). Our hierarchical clustering, comparing p21CIP1-regulated genes in the mammary tumor with gene sets associated with ES cell identity (19), demonstrated p21CIP1-repressed genes were enriched in ES cells. Gene targets of Sox2, Oct4, and Nanog were enriched among p21CIP1 target genes. Sox2 and KLF4 expression were increased by QT-PCR within Ras or c-Myc tumors upon deletion of p21CIP1 (Fig. 4 B and C). Thus the expression profiles repressed by p21CIP1 in mammary tumors in vivo overlap with repressed gene expression profiles enriched in ES cells and targets of Sox2/Oct4/Nanog.
Experimental Procedures (see SI Text)
Transgenic Animals.
The transgenic mice were described in ref. 8.
RNA Isolation, Quantitative Real-Time PCR, and Microarray Analysis.
Probe synthesis and hybridization were performed as described in ref. 30. The wild-type mammary gland data set was obtained from Gene Expression Omnibus (GSE3765). Analysis of the arrays was performed using the R statistics package (31) and the limma library (32) of the Bioconductor software package.
Cell Culture.
Mammosphere Formation Assays Were Conducted as Described in ref. 34.
Immunostaining of cell surface markers by FACS analysis for breast cancer stem cells was based on ref. 33.
Supplementary Material
Acknowledgments.
We thank Dr. S. McMahon for constructive advice and comments with the manuscript and Atenssa L. Cheek and Stefanie Pierpoint for the preparation of this manuscript. This work was supported by National Institutes of Health Grants R01CA70896, R01CA75503, and R01CA86072 (to R.G.P.); the Dr. Ralph and Marian C. Falk Medical Research Trust; and a grant from the Pennsylvania Department of Health (to R.G.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910009106/DCSupplemental.
References
- 1.Hanahan D, Weinberg R. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Zucchi I, et al. The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci USA. 2007;104:10476–10481. doi: 10.1073/pnas.0703071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.el-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 5.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, et al. Somatic cell type specific gene transfer reveals a tumor-promoting function for p21(Waf1/Cip1) EMBO J. 2007;26:4683–4693. doi: 10.1038/sj.emboj.7601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: Cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Bearss DJ, Lee RJ, Troyer DA, Pestell RG, Windle JJ. Differential effects of p21(WAF1/CIP1) deficiency on MMTV-ras and MMTV-myc mammary tumor properties. Cancer Res. 2002;62:2077–2084. [PubMed] [Google Scholar]
- 9.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Singh SK, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jechlinger M, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 14.Edelman E, et al. Analysis of sample set enrichment scores: Assaying the enrichment of sets of genes for individual samples in genome-wide expression profiles. Bioinformatics. 2006;22:e108–116. doi: 10.1093/bioinformatics/btl231. [DOI] [PubMed] [Google Scholar]
- 15.Hinata K, Gervin AM, Jennifer Zhang Y, Khavari PA. Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Oncogene. 2003;22:1955–1964. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
- 16.Verrecchia F, ChuML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 17.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumor progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Viciana P, et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 22.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 24.Trimboli AJ, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 25.Li, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- 26.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 27.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Pinilla SM, et al. Sox2: A possible driver of the basal-like phenotype in sporadic breast cancer. Mod Pathol. 2007;20:474–481. doi: 10.1038/modpathol.3800760. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, et al. Alternate Cyclin D1 mRNA splicing modulates p27KIP1 binding and cell migration. J Biol Chem. 2008;283:7007–7015. doi: 10.1074/jbc.M706992200. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Ratio statistics of gene expression levels and applications to microarray data analysis. Bioinformatics. 2002;18:1207–1215. doi: 10.1093/bioinformatics/18.9.1207. [DOI] [PubMed] [Google Scholar]
- 32.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay J, et al. ErbB2 induces Notch1 activity and function in breast cancer cells. Clin Transl Sci. 2008;1:107–115. doi: 10.1111/j.1752-8062.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.