Abstract
The creation of the pool of follicles available for selection and ovulation is a multi-faceted, tightly regulated process that spans the period from embryonic development through to the first reproductive cycle of the organism. In mice, this development can occur in mere weeks, but in humans, it is sustained for years. Embryonic germ cell development involves the migration of primordial germs cells to the genital ridge, and the mitotic division of germ cell nuclei without complete cytokinesis to form a multi-nucleated syncytia, or germ cell nest. Through combined actions of germ cell apoptosis and somatic cell migration, the germ cell nuclei are packaged, with surrounding granulosa cells, into primordial follicles to form the initial follicle pool. Though often dismissed as quiescent and possibly uninteresting, this initial follicle pool is actually quite dynamic. In a very strictly controlled mechanism, a large portion of the initial primordial follicles formed is lost by atresia before cycling even begins. Remaining follicles can undergo alternate fates of continued dormancy or selection leading to follicular growth and differentiation. Together, the processes involved in the fate decisions of atresia, sustained dormancy, or activation carve out the follicle pool of puberty, the pool of available oocytes from which all future reproductive cycles of the female can choose. The formation of the initial and pubertal follicle pools can be predictably affected by exogenous treatment with hormones or molecules such as activin, demonstrating the ways the ovary controls the quality and quantity of germ cells maintained. Here, we review the biological processes involved in the formation of the initial follicle pool and the follicle pool of puberty, address the alternate models for regulating germ cell number and outline how the ovary quality-controls the germ cells produced.
Keywords: primordial follicle, nest breakdown, pre-pubertal, ovarian reserve, multi-oocyte follicles
Introduction
The germ cell nest is an important developmental stage in the formation of the germline that is evolutionarily conserved in males and females of species ranging from higher insects to frogs, rodents and other vertebrates (Pepling et al., 1999). In each of these organisms, most of the single primordial germ cells divide synchronously with incomplete cytokinesis to form a cluster of cells connected by intercellular bridges (Gondos et al., 1971; Pepling and Spradling, 1998). Spermatogenesis occurs in nests in many organisms; because spermatogonia undergo much of the meiotic program while still in nests, interconnection is thought to increase the favoring of some genotypes over others and thereby decrease the likelihood of defective germ cells (Braun et al., 1989). This is not the case for oocyte development, where oogonia in nests are clonally derived and undergo most of their development either before nest formation or after its breakdown (Wartenberg et al., 1998). However, premeiotic female germ cells from insects, rabbits, mice, rats, hamsters and humans all show intercellular cytoplasmic bridges characteristic of nests (reviewed in Pepling et al., 1999), demonstrating that the structure is still important for oocyte development. It is believed that nests in oocyte development help to increase the store of materials and nutrients that are required for later development. This idea is supported by oocyte development in Drosophila where all but one of the cells in the nest become nurse cells that then contribute materials to the oocyte (de Cuevas et al., 1997). It is also possible that removing the metabolic and biosynthetic duties from the oocyte nucleus allows it to become inactive and possibly reduce its susceptibility to mutagenesis (de Cuevas et al., 1997).
Within the germ cell nest, the murine oogonia nuclei enter a premeiotic state around dpc 12.5 (meiosis in the human begins around embryonic week 13 (Motta et al., 1997)) that is marked by the expression of meiotic genes such as Scp3 (Di Carlo et al., 2000; Chuma and Nakatsuji, 2001). Meiotic arrest in the mouse occurs in a rostral to caudal wave that is not complete until dpc 16.5 (Menke et al., 2003; Bullejos and Koopman, 2004); in comparison to the development of other organs, the 4-day process of meiotic entry is extremely long. The developmental delay (extended meiosis) of the caudal oocytes compared with the rostral oocytes may ‘tag’ the oocytes for later exit from the primordial follicle pool, the proposed ‘production line’ hypothesis (Henderson and Edwards, 1968).
When nest formation is complete, the ovary consists of a few single mitotic germ cells and many germ cell syncytia with most oocyte nuclei arrested at diplotene stage of meiotic prophase I and remaining connected to each other through cytoplasmic bridges. These syncytia are organized into long ovigerous cords that are surrounded by somatic cells of the ovary, namely pre-granulosa cells and stromal mesenchymal cells (Rajah et al., 1992; Pepling and Spradling, 2001). Just after birth in the mouse and in the second trimester of human gestation (Konishi et al., 1986; Pepling and Spradling, 2001), these nests begin to break down to form individual follicles. Nest breakdown is a coordinated effort that involves the degeneration of many germ cell nuclei and the invasion into the germ cell nests by the pre-granulosa cells (Pepling and Spradling, 2001). During this process, many of the nuclei are lost and the cytoplasmic bridges between remaining nuclei are either retracted or cleaved, likely through protease action of the surrounding somatic cells. Granulosa cells then completely surround the remaining nuclei and a basement membrane is laid down that completely encompasses and delineates each newly formed primordial follicle (Rajah et al., 1992).
Loss of germ cells during nest breakdown
The loss of oocytes during nest breakdown is substantial, and it is possible that a quality control mechanism exists through which deficient nuclei are lost and healthy oocytes are preferentially encapsulated into primordial follicles. Using different sectioning and counting methods, the oocyte attrition has been estimated at between one-third and two-thirds of all oogonia in nests (Pepling and Spradling, 2001; Bristol-Gould et al., 2006a). Like the death of primordial germ cells during pre-meiotic migration and proliferation, oocyte attrition has been attributed to classic apoptotic mechanisms in both the mouse and human (Pepling and Spradling, 2001; Jefferson et al., 2006; Ghafari et al., 2007; Lobascio et al., 2007; Albamonte et al., 2008; De Felici et al., 2008), likely through actions of the B-cell lymphoma/leukemia-2 (Bcl-2) family of proteins (Ratts et al., 1995; Rucker et al., 2000; Flaws et al., 2001; Greenfeld et al., 2007). Several possibilities for the reason behind the selective loss during nest breakdown have been suggested, including genetic defects or failure of the germ cell to produce all the cytoplasmic organelles required, including mitochondria. Indeed, genetic defects, such as Spo11 mutations, which causes a complete absence of the normal homologous recombination during meiosis (Hunt and Hassold, 2002), lead to greatly increased germ cell loss during nest breakdown (Di Giacomo et al., 2005). In addition to mutations in the chromosomal genome, defects in the number of mitochondria or their genome could lead to apoptosis (Perez et al., 2000). In the mouse, mitochondria very quickly multiply during the day before nest breakdown begins and some mitochondria can be detected within the intracellular bridges connecting sister oocyte nuclei (Pepling and Spradling, 2001). This observation gives support to the idea that mouse germ cell nests function just as germ cell nests in insects like Drosophila in that some of the germ cells simply serve as nurse cells to help produce nutrients and even organelles like mitochondria. These ‘nurse cells’ are then sacrificed during nest breakdown while the surviving oocytes benefit from their donated supplies. As mitochondrial load has been positively correlated with the prevention of apoptosis (Perez et al., 2000), the act of transfer of one germ cell's stock of proteins, nutrients or mitochondria may itself be the trigger for apoptosis of that cell, rather than a preventative measure against apoptosis of the surviving cell. Finally, the existence of multi-oocyte follicles (MOFs) provides evidence that correct nest breakdown selectively rids the ovary of defective oocytes (Iguchi et al., 1990; Iguchi et al., 1991; Hahn et al., 2005; Kipp et al., 2007). MOFs are follicles in which two or more oocytes are contained within a single follicle, without a separating basement membrane between them. Because these follicles can be seen even at the primordial stage, it seems likely that they occur from an incomplete breakage of cytoplasmic rings connecting germ cell nuclei during breakdown. MOFs can be induced by high estrogen levels, estrogen mimetics or the peptide hormone activin during neonatal development (Kipp et al., 2007). In the mouse, oocytes from these follicles are less viable, having a 30% lower fertilization rate than their monoovular counterparts (Iguchi et al., 1991), demonstrating the link between correct nest breakdown and oocyte quality. MOFs from humans seem to have no significant decrease in in vitro fertilization rates (Dandekar et al., 1988), though it is unknown what impact a greatly increased incidence of MOFs might have on human fertility. It also still unknown how some oocytes are selected to survive while the others are designated as nurse cells destined to die, but a positive correlation between incorrect nest breakdown and low oocyte quality does exist in mice.
Molecular modifiers of nest breakdown
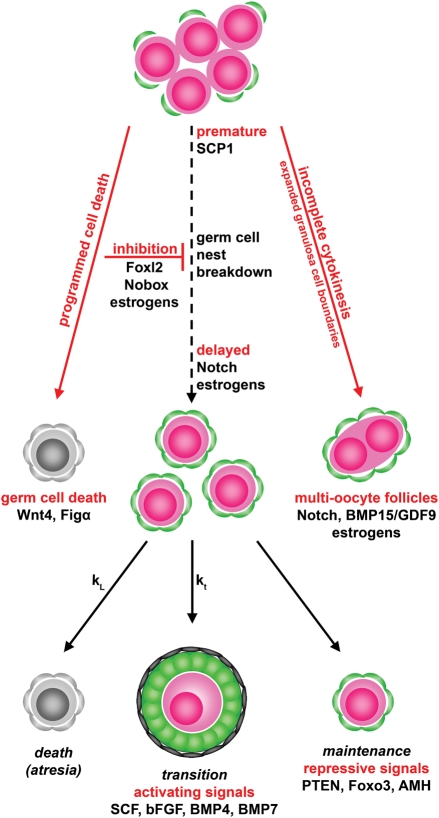
Factors that are produced by the oocytes or somatic cells themselves that are not necessarily part of the apoptotic loss of the germ cell nuclei can also affect nest breakdown (Fig. 1). Many of these factors affect the onset of nest breakdown; one of the most interesting of these is synaptonemal complex protein-1 (SCP1). This protein is expressed only in oocytes in the female and drops precipitously within 24 h after birth, when nest breakdown is known to begin in the mouse. More importantly, when SCP1 protein expression is knocked down, the oocytes reach diplotene stage prematurely and nests begin a premature breakdown to produce healthy primordial follicles (Paredes et al., 2005). An intriguing interpretation of this data is that meiotic progression is not merely concomitant with nest breakdown, but causative of it. Several other proteins, including Foxl2, Nobox and members of the Notch signaling pathway and transforming growth factor (TGF-β) superfamily, can affect the timing of nest breakdown. Mutation of the gene Foxl2, which encodes a transcription factor normally expressed in pre-granulosa cells, is associated with premature ovarian failure in both mice and humans (Crisponi et al., 2001; Uda et al., 2004). In the mouse model, the knockout of Foxl2 impairs nest breakdown by apparently affecting granulosa cell differentiation and the proper laying down of basal lamina around forming follicles (Uda et al., 2004). Nobox is an oocyte-specific homeobox gene that is expressed in germ cell nests and in oocytes of primordial and growing follicles (Suzumori et al., 2002). A knockout mouse model is normal at birth with proper formation of germ cell nests. However, nest breakdown is severely impaired and many germ cells remain in nests past the time that nest breakdown is complete in the wild type (Rajkovic et al., 2004). The development is not merely delayed, as oocytes from the mouse degenerate and never form growing follicles (Rajkovic et al., 2004). The Notch signaling pathways of transmembrane proteins have also been found to be important in nest breakdown. Components of the pathways are expressed by germ cells in the nest, as well as surrounding pre-granulosa cells (Trombly et al., 2009b). When signaling is suppressed in an ex vivo ovary culture, nest breakdown is attenuated or delayed, and a higher than normal number of germ cells remain in nests compared with untreated cultured ovaries (Trombly et al., 2009b). At a later time point, Notch signaling disruption can lead to the formation of MOFs (Hahn et al., 2005), likely from the incomplete nest breakdown examined. In addition to other fertility defects, MOFs are also formed in the mouse when bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9) are mutated together (Yan et al., 2001). In the human, mutations in both these genes have been found to be associated with premature ovarian failure (Dixit et al., 2005, 2006). While not necessarily affecting the timing of nest breakdown, Wnt-4, which is expressed in the gonad during embryonic development, seems to play a role in maintaining germ cell survival. Normal numbers of germs cells are present in Wnt-4 knockout animals at dpc 14.5, but by birth, when nest breakdown is initiating, the knockout animals have less than 10% the number of healthy germ cells as wild type animals (Vainio et al., 1999). A very similar phenotype is seen in mice where the oocyte-specific transcription factor, Figα is mutated. In these mice, morphologically healthy oocytes are present in correct numbers at dpc 18, but the oocytes all die during nest breakdown. No primordial follicles are formed, and no oocytes even mature to the stage to produce zona pellucida proteins (Soyal et al., 2000). Many of these genes known to affect nest breakdown in the mouse are also important in the human. As mentioned, Foxl2, GDF9 and BMP15 have known genetic variants that correlate with the occurrence of POF in the human. Additionally, it is known that patients with classical Turner's syndrome experience a fetal loss of germ cells around the 18th week of embryonic development that leads to chronic POF (Hjerrild et al., 2008). The timing of this loss coincides very closely with nest breakdown, which occurs at approximately embryonic weeks 17–20 in the human (Motta et al., 1997), suggesting that an increased loss of germ cells during nest breakdown could be responsible for the ensuing sterility.
Figure 1.
Molecular modifiers involved in female germ cell nest breakdown and subsequent primordial follicle fates. The breakdown of germ cell nest to form primordial follicles involves interplay of several molecular factors that control the timing and extent of the breakdown. Aberrant breakdown can lead to increased loss of germ cells or the formation of MOFs. Following their formation, primordial follicles have three separate possible fates: follicular atresia, transition to the growth phase or maintained primordial status. SCP1, synaptonemal complex protein-1; BMP, bone morphogenetic protein; GDF, growth differentiation factor; SCF, stem cell factor; bFGF, basic fibroblast growth factor; AMH, anti-Mullerian hormone; Figα, oocyte-specific transcription factor; Foxl2, forkhead box transcription factor; PTEN, phosphatase and tensin homolog, a tumor suppressor.
Finally, in addition to apoptotic pathway members and locally produced factors, circulating hormone and steroid factors can regulate nest breakdown and primordial follicle assembly. Neonatal exposure to genistein, a phytoestrogen, leads to the inhibition, or delay of nest breakdown in vivo and in ex vivo ovary cultures of neonatal mouse ovaries (Jefferson et al., 2006; Chen et al., 2007). Adult animals treated neonatally with genistein have a significantly higher incidence of MOFs, suggesting that the occurrence of these aberrant follicles results from incomplete nest breakdown (Jefferson et al., 2002). A very similar phenotype, with inhibited nest breakdown and resulting MOFs can be seen in treatments with estrogen, estradiol, progesterone and synthetic estrogens like diethylstilbestrol (Iguchi et al., 1990; Kezele and Skinner, 2003; Chen et al., 2007; Kipp et al., 2007). Progesterone's actions were independent of the estrogen receptor, suggesting that it is not simply the conversion to estrogen that creates the effect (Chen et al., 2007). Furthermore, ovaries removed at dpc 16.5 underwent premature nest breakdown that could be inhibited by addition of exogenous estrogen or progesterone (Chen et al., 2007). Together, these studies have lead to the comprehensive hypothesis that oocytes are maintained in nests by the high levels of estrogens and progesterone present in the maternal milieu of pregnancy. When pups are born, they experience a drastic drop in levels of circulating hormones, and it is hypothesized that it is this drop that initiates nest breakdown (Chen et al., 2007). In the studies performed in vivo, there was some indication that progesterone prevented cellular apoptosis, possibly giving a mechanism by which the hormones could act to prevent nest breakdown (Kezele and Skinner, 2003). However, none of the estrogenic compounds or progesterone seemed to prevent apoptosis in vitro, but nest breakdown was still inhibited. It is therefore unlikely that the hormones act by modifying levels of cellular apoptosis. A different mechanism of action has been proposed by our recent studies of the TGF-β family member, activin. It has been shown that neonatal exposure to estrogens suppresses activin expression and signaling in the neonatal ovary (Kipp et al., 2007). Further, we have shown exogenous activin treatment neonatally actually increases the number of primordial follicles formed by increasing germ cell proliferation (Bristol-Gould et al., 2006a), a result also seen in cultures of fetal human ovaries treated with activin (Martins da Silva et al., 2004). Moreover, a recent study in the human has proposed a downstream mechanism for the actions of activin in germ cell proliferation by demonstrating that exogenous activin decreases the expression of membrane-bound kit ligand (Childs and Anderson, 2009). As kit ligand can induce the expression of its receptor, c-kit, the inhibition of kit ligand expression by activin is hypothesized to down-regulate the expression of c-kit in germ cells. Lower c-kit is then proposed to lead to a block in meiotic arrest and primordial follicle activation, thereby allowing more proliferation. It seems possible therefore that the downstream target of estrogen and progesterone is the hormone activin, possibly acting through kit ligand regulation. When the steroid hormones are high, activin subunit mRNA and protein levels are suppressed and nest breakdown is inhibited (Kipp et al., 2007). Estrogen and progesterone decrease at birth in the mouse, activin signaling is freed from repression and the increase in activin initiates nest breakdown. While this model is very reasonable in the mouse, it becomes problematic when applied to the human. In the human, nest breakdown occurs during the second trimester of embryonic development, when the fetal ovary is still exposed to high levels of steroid hormones (Nagamani et al., 1979; Wu et al., 1979). It is possible, therefore, that a different method for regulating nest breakdown initiation is present in humans. However, proponents of the model also suggest that while total steroid levels remain high, the amount that is actually accessible to fetal tissues is decreased at the time of nest breakdown. A steroid binding protein such as α-fetoprotein could bind to estrogen (with a similar binding protein for progesterone) and sequester the hormone, making it unavailable to the fetal ovaries. The belief that a drop in estrogen and progesterone can regulate nest breakdown in the larger mammals as it does in the mouse is supported by a study that shows administration of an aromatase inhibitor to baboons during nest breakdown inhibits the process and causes more germ cells to remain in nests (Zachos et al., 2004).
New developments
Quorum sensing model versus stem cell supplementation
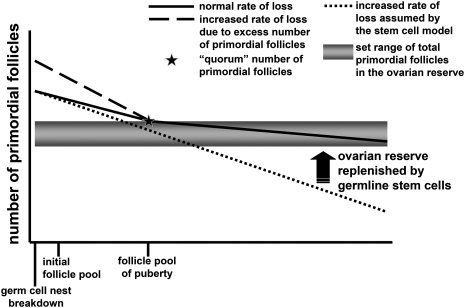
The initial follicle pool formed after nest breakdown is therefore tightly regulated through mechanisms of controlled germ cell loss, which can be modified by many different molecules. This intervention can increase or decrease the number of primordial follicles initially, but it is important to understand how these early changes might impact the long-term fertility of the animal. In our model where exogenous activin administered neonatally significantly increased the number of primordial follicles formed, the long-term persistence of these excess follicles was also examined. It was found that by dpn 19, activin-treated animals had the same number of follicles as vehicle-treated animals. Therefore, the ovary somehow rid itself of the excess follicles that had formed during nest breakdown (Bristol-Gould et al., 2006a). This phenomenon has also been seen in the mouse model where the anti-apoptotic protein Bcl-2 is overexpressed. Though the ovaries have significantly more primordial follicles at dpn 4 than wild type (approximately 43% more across all transgenic lines produced), by between dpn 27 and 60 the transgenic ovaries has the same number of follicles as wild type (Flaws et al., 2001). In the activin study, when mice were superovulated at an early age, activin-treated animals ovulated a higher number of oocytes, but the oocytes were of poor quality, as indicated by smaller size, spindle disorganization and more meiotic immaturity than the ovulated oocytes from wild type animals. By a later time point, however, the activin and wild type animals ovulated comparable numbers of oocytes; at this point oocyte health and size was also comparable between the two groups. This data have caused our group to propose a quorum-sensing model for the maintenance of the initial follicle pool (Fig. 2). By this model, the ovary has a set point for the number of follicles that it can successfully maintain. This set point is made during nest breakdown by the formation of a correct number of follicles. If this set point is altered, by increasing the number of follicles formed, then these excess follicles correspond to unhealthy follicles that should have been lost during nest breakdown. The ovary will recognize this and will eliminate excess follicles back down to the set point of follicle load. The cell death gene BAX may be involved in this process since elimination of this gene over-rides the set point at puberty and permits an excess number of follicles to enter the ovarian reserve (Perez et al., 1999).
Figure 2.
Alternative models for the creation and maintenance of the ovarian reserve. During the pre-pubertal period, primordial follicles are lost at a high rate that is slowed at the time of the FSH surge. Models where the number of initial follicles is increased show an increased rate of loss so that they reach typical numbers of follicles, indicating a quorum-sensing model with a ‘set point’ for follicle number. Stem cell supplementation models propose that the number of follicles can be increased throughout reproductive life. The Tilly stem cell model indicates a constant number of oocytes is created from stem cells. We favor a model in which the initial follicle pool is established at birth and these follicles are necessary to adult fertility (there are no additional follicles added to the pool) and sufficient to the fertility needs of the adult (follicle loss is not so much to prematurely lose all follicles.).
The obvious other side of a quorum-sensing mechanism is whether the ovary can not only decrease the number of follicles back down to a set point, but can also slow the rate of loss or retain more of the follicles formed at nest breakdown if insufficient follicles are within the initial follicle pool. Many of the models we have discussed thus far that involve a decrease in the number of primordial follicles formed would suggest that the ovary is not capable of this feat. Modifications of FIG1α, NOBOX and BMP15 that cause a complete or partial block of initial primordial follicle formation lead to long-term subfertility or premature ovarian failure of the mouse (Soyal et al., 2000; Yan et al., 2001; Rajkovic et al., 2004). Indeed, it is generally accepted in the field of ovarian development that the initial pool of follicles formed following nest breakdown is the entire complement of follicles available to the animal throughout its reproductive life.
This notion of pubertal follicle pool sufficiency has recently been challenged by the idea that there are stem cells that can form new oocytes de novo in the adult ovary (Johnson et al., 2004). The hypothesis comes from work that seems to show that rates of atresia of follicles far exceeds the amount of healthy follicle loss that they see and a transplant model that seems to show cells from a graft-populating follicles in the host ovary. In a follow-up paper, the same group suggested that the bone marrow is a source for the germline stem cells (Johnson et al., 2005). In the group's model, 77 new follicles per ovary are replenished by the germ line stem cells every day in the post-natal mouse. Since the original paper, several groups have joined the debate with data for (Bukovsky et al., 2004, 2005) and against (Eggan et al., 2006; Faddy and Gosden, 2007; Liu et al., 2007) the theory. Most of the work has focused on trying to identify the actual stem cells and their origin in different species. Our group has taken a different approach and has examined the basic tenet behind the theory: that stem cells must exist in order to reconcile the amount of follicles being lost with the amount of healthy follicles that remain.
To address the idea that follicle numbers are malleable in the adult mouse, we performed detailed follicle counts on mice aged dpn 6, 10, 19, 45 and months 4.5, 6 and 12 (Bristol-Gould et al., 2006b). Using this empirical data, which included counts of primordial, primary and secondary follicles, we developed a mathematical model using measured transition kinetics for each follicle fate and simulated either a replenishment of follicles due to stem cells, or no such replenishment. We find that that the model that incorporates 77 new follicles formed de novo fail to accurately predict the accumulation of primary follicles seen experimentally; neither does it recapitulate the steady loss of follicles after dpn 60. Allowing some leeway in the stem cell model, we varied the mathematical model to include contribution of stem cells to the follicle pool to be between 5 and 100 follicles per ovary per day. We find that the fewer follicles assumed to be generated de novo by stem cells, the closer the model is to the empirical data. Moreover, a model of a fixed follicle pool is sufficient to match empirical results. It is therefore unnecessary to invoke a stem cell model at all; a rate of loss from a fixed initial pool fits empirical observations. Our data in combination with the findings from our mathematical model suggest that the initial follicle pool is necessary (because there are no rescuing adult germline stem cells) and sufficient (because there is no need for adult germline stem cells) for adult fertility.
Several critiques of our model have recently been raised (Tilly et al., 2009) that warrant reply. The first contention is an apparent misunderstanding, with the belief that our model does not reflect very high numbers of atetric immature follicles. While true that the model assumes a negligible atresia rate for primary and small secondary follicles (an assertion supported by previous data (Hirshfield and Midgley, 1978; Faddy et al., 1983), the model clearly expects a large amount of atresia in primordial follicles (Tingen et al., 2009) and does notdismiss the loss of tertiary follicles by atresia. Secondly, the fixed mathematical model was critiqued because the stem cell model and the fixed pool model predict experimental data equally well, aside from the stem cell model's inability to accurately predict the accumulation of primary follicles. Our model was based on the original hypothesis of a constant rate of stem cell contribution to the follicle pool of 77 de novo follicles formed each day. The critiques are correct in that our model does not incorporate the revision to the hypothesis that stem cell contribution also decreases with age. Nevertheless, the aim of our work was not to demonstrate that a stem cell model is completely inconsistent with the experimental data, but to show that such a model is not necessary. If a fixed pool of follicles model can equally predict the empirical data on follicle loss, there is simply no need to invoke unknown cells into the process of folliculogenesis or follicle atresia.
The stem cell debate has emerged anew recently with the publication of a study that claims to have isolated the much sought after female germline stem cells (Zou et al., 2009). The cells were isolated from pre-pubertal and adult ovaries by virtue of being immunopositive for mouse vasa homolog (MVH) protein and being mitotically active, as indicated by BrdU incorporation. It is currently unclear how the group used an internalized protein that is not expressed on the cell surface (MVH) to column purify these cells. Nevertheless, as the MVH protein used to mark these ‘stem cells’ is expressed only in primordial germ cells after their arrival at the gonad (Toyooka et al., 2000), it seems possible that the cells isolated are mitotic oogonia. A second study, which claimed to have derived stem cells from the ovarian surface epithelium of post-menopausal women supports this idea, as the group characterized ‘stem cells’ merely by virtue of the expression of ‘pluripotency’ genes (Virant-Klun et al., 2008), which are also known to be expressed on primordial germ cells and oogonia (Kerr et al., 2008). These findings actually fit well with early observations from our group showing that the neonatal mouse ovary contains approximately 5% oogonia that have not entered meiosis and are instead still proliferative (Bristol-Gould et al., 2006a). We propose that these neonatal ‘laggard oogonia’ are the same cells isolated in the newest study, and that by their very nature can proliferate and give rise to new oocytes (Rosenwaks and Woodruff, 2009). While unnecessary to maintain follicular numbers at the observed counts, these laggard oogonia may be able to be induced into proliferation in the in vitro system. Though an in vitro system may induce proliferation of these laggard oogonia, it is still unlikely that they are induced in normal pubertal or adult animals, as indicated by the abundance of genetic models where early follicle loss causes permanent fertility decrease, without any replenishment of follicles.
Primordial follicle fates
The dynamics of the initial follicle pool are therefore still not completely known, but they involve interplay between activation, atresia or continued primordial follicle fate. Several molecular factors have been shown to act as activating cues in the mouse primordial follicles, initiating the transition into primary follicles. Kit ligand (Parrott and Skinner, 1999; Nilsson et al., 2001) and basic fibroblast growth factor (bFGF) increase the ratio of developing to primordial follicles in culture. It has been suggested that the role of oocyte-derived bFGF is to increase the production of kit ligand in granulosa cells and, through this interaction, activate the primordial follicles (Nilsson and Skinner, 2004). Several members of the TGF-β super family are also important in initiating follicle activation (Trombly et al., 2009a). Addition of exogenous BMP4 or BMP7 to ex vivo ovary cultures increases the primary to primordial follicle ratio (Lee et al., 2001, 2004; Nilsson and Skinner, 2003). In addition to activating signals, there also appear to be repressing signals within the ovary that maintain primordial follicles in their ‘resting’ state. A very impressive paper has shown that oocyte-specific ablation of the PI3K antagonist, PTEN, causes a global activation of all primordial follicles, suggesting that sustained inhibition of the PI3K pathway is required to maintain primordial follicle dormancy (Reddy et al., 2008). In these ovaries, the follicles are quickly lost after activation, leading to eventual premature ovarian failure for the mouse. With regard to the stem cell model hypothesis, it is important to note that in the tissue sections from these mice, the genetic defect was never associated with a repopulation of the adult ovary (Personal communication with Kui Liu). The genetic effect in oocyte development occurs post-meiotically and post-formation of the primordial follicle. Thus, if germline stem cells do exist, they should have the capability of repopulating the adult ovary of these animals. Addressing this question very carefully may finally provide the concrete evidence needed to accept or refute the notion of an adult ovary capable of autonomous oocyte repopulation. A follow-up study to the PTEN investigation has shown that the downstream target of PI3K signaling is likely Foxo3; Foxo3 oocyte-specific null mutants show a phenotype identical to that of the PTEN mutants (John et al., 2008). It is currently unknown what genes the Foxo3 gene may regulate to promote primordial follicle dormancy, but the Fox family of transcription factors can act as transcriptional activators or repressors and have been found to play a role in TGF-β signaling (Carlsson and Mahlapuu, 2002). Finally, while the resulting phenotype is less penetrant than the PI3K mutants, anti-mullerian hormone (AMH) deficient mice have suggested that the hormone can also act to suppress primordial follicle activation (Durlinger et al., 1999; Durlinger et al., 2002), and AMH has the same effect in the human (Carlsson et al., 2006). Primordial follicle activation therefore likely involves both the release from active repression, and the initiation of activation through the expression of a variety of activating proteins in both the oocyte and somatic cells of follicles.
While the transition to the growth phase of development is the most studied fate for the primordial follicles formed originally, the initial pool of follicles also undergoes a massive amount of atresia. In fact, our laboratory has recently demonstrated that 88 primordial follicles transition to primary stage in each ovary every day of the pre-pubertal period, while 155 follicles per ovary per day are lost to atresia (Tingen et al., 2009). Therefore, the dominant fate of the follicle formed in the initial follicle pool is to die. The precise mechanisms governing this loss are not known, but contrary to embryonic germ cell death and attrition during nest breakdown, primordial follicle death does not seem to occur through classic apoptosis (Tingen et al., 2009). Why so many primordial follicles are lost during the pre-pubertal period is also not fully understood, but is likely an extension of the quality control mechanisms seen during nest breakdown. In fact, in several models where excess germ cells are eliminated from the ovary, the process takes several weeks, demonstrating that the quality checkpoint involves primordial follicles even after nest breakdown (Flaws et al., 2001; Bristol-Gould et al., 2006a). Therefore, even if they survive the encapsulation process following nest breakdown, oocytes that did not receive a complete complement of mitochondria, or sufficient cytoplasm, or who either did not correctly dissociate from sister nuclei or form proper connections with granulosa cells can be lost as primordial follicles. This checkpoint provides a final point of possible demise for unhealthy germ cells before the follicle pool of puberty is decided. As the follicle pool of puberty will, in conventional thought at least, provide the entire set of follicles available for ovulation for the life of the animal, the death of primordial follicles may be exceptionally important in maintaining the highest possible chances of ovulation, fertilization and healthy offspring development.
Implications for future research
Genetic mouse models and in vitro cultures systems have dramatically expanded our knowledge of the initial event in oo- and folliculogenesis, but many questions remain. What regulates the set point number for the quorum-sensing mechanism of the ovary? Could it be related to sheer physical mechanics of the numbers of follicles fit into the space, or could there be an endocrine control directed by the follicles present? It seems obvious that processes of demise throughout follicle development are tightly regulated in a non-random way to produce the highest quality cohort possible, but can the same be said for follicle formation or primordial follicle activation? Are these processes random or are follicles utilized in relation to the order they were encapsulated, or in which the germ cells entered meiosis? With a mind to clinical relevance, can a synthetic rescue of germ cells at any of the physiological death decision points prolong or enrich fertility? These questions open a broad avenue of research that will undoubtedly have implications not only for our understanding of the rarest cells in the body, but also possible clinical impacts for various reproductive disorders and fertility problems.
Funding
This work is supported by NIH/NICHD Hormone Signals that Regulate Ovarian Differentiation, P01 HD021921; NIH/NICHD.
References
- Albamonte MS, Willis MA, Albamonte MI, Jensen F, Espinosa MB, Vitullo AD. The developing human ovary: immunohistochemical analysis of germ-cell-specific VASA protein, BCL-2/BAX expression balance and apoptosis. Hum Reprod. 2008;23:1895–1901. doi: 10.1093/humrep/den197. [DOI] [PubMed] [Google Scholar]
- Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;a 298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, Woodruff TK. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;b 298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Svetlikova M, Upadhyaya NB. Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod Biol Endocrinol. 2004;2:20. doi: 10.1186/1477-7827-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol. 2005;3:17. doi: 10.1186/1477-7827-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Devel. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Estradiol, progesterone and genestein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Anderson RA. Activin A selectively represses expression of the membrane-bound isoform of Kit ligand in human fetal ovary. Fertil Steril. 2009;92:1416–1419. doi: 10.1016/j.fertnstert.2009.03.095. [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Develop Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- Dandekar PV, Martin MC, Glass RH. Polyovular follicles associated with human in vitro fertilization. Fertil Steril. 1988;49:483–486. [PubMed] [Google Scholar]
- de Cuevas M, Lilly MA, Spradling AC. Germline cyst formation in Drosophila. Ann Rev Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- De Felici M, Lobascio AM, Klinger FG. Cell death in fetal oocytes: many players for multiple pathways. Autophagy. 2008;4:240–242. doi: 10.4161/auto.5410. [DOI] [PubMed] [Google Scholar]
- Di Carlo AD, Travia G, De Felici M. The meiotic specific synaptonemal complex protein SCP3 is expressed by female and male primordial germ cells of the mouse embryo. Int J Dev Biol. 2000;44:241–244. [PubMed] [Google Scholar]
- Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci USA. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause (New York N. Y.) 2005;12:749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 2006;119:408–415. doi: 10.1007/s00439-006-0150-0. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derived from non-circulating germ cells. Nature. 2006;441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- Faddy M, Gosden R. Numbers of ovarian follicles and testing germ line renewal in the postnatal ovary: facts and fallacies. Cell Cycle (Georgetown, Tex.) 2007;6:1951–1952. doi: 10.4161/cc.6.15.4517. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Edwards RG. Ovarian follicle dynamics in mice: a comparative study of three inbred strains and an F1 hybrid. J Endocrinol. 1983;96:23–33. doi: 10.1677/joe.0.0960023. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Hirshfield AN, Hewitt JA, Babus JK, Furth PA. Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol Reprod. 2001;64:1153–1159. doi: 10.1095/biolreprod64.4.1153. [DOI] [PubMed] [Google Scholar]
- Ghafari F, Gutierrez CG, Hartshorne GM. Apoptosis in mouse fetal and neonatal oocytes during meiotic prophase one. BMC Dev Biol. 2007;7:87. doi: 10.1186/1471-213X-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondos B, Bhiraleus P, Hobel CJ. Ultrastructural observations on germ cells in human fetal ovaries. Am J Obstet Gynecol. 1971;110:644–652. doi: 10.1016/0002-9378(71)90245-6. [DOI] [PubMed] [Google Scholar]
- Greenfeld CR, Pepling ME, Babus JK, Furth PA, Flaws JA. BAX regulates follicular endowment in mice. Reproduction (Cambridge, England) 2007;133:865–876. doi: 10.1530/REP-06-0270. [DOI] [PubMed] [Google Scholar]
- Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218:22–28. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN, Midgley AR., Jr Morphometric analysis of follicular development in the rat. Biol Reprod. 1978;19:597–605. doi: 10.1095/biolreprod19.3.597. [DOI] [PubMed] [Google Scholar]
- Hjerrild BE, Mortensen KH, Gravholt CH. Turner syndrome and clinical treatment. Br Med Bull. 2008;86:77–93. doi: 10.1093/bmb/ldn015. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Kamiya K, Uesugi Y, Sayama K, Takasugi N. In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In vivo (Athens, Greece) 1991;5:359–363. [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of pluripotent stem cell markers in the human fetal ovary. Hum Reprod. 2008;23:589–599. doi: 10.1093/humrep/dem411. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148:1968–1976. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- Konishi I, Fujii S, Okamura H, Parmley T, Mori T. Development of interstitial cells and ovigerous cords in the human fetal ovary: an ultrastructural study. J Anat. 1986;148:121–135. [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- Lee WS, Yoon SJ, Yoon TK, Cha KY, Lee SH, Shimasaki S, Lee S, Lee KA. Effects of bone morphogenetic protein-7 (BMP-7) on primordial follicular growth in the mouse ovary. Mol Reprod Devel. 2004;69:159–163. doi: 10.1002/mrd.20163. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, Liu L. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306:112–120. doi: 10.1016/j.ydbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Lobascio AM, Klinger FG, Scaldaferri ML, Farini D, De Felici M. Analysis of programmed cell death in mouse fetal oocytes. Reproduction (Cambridge, England) 2007;134:241–252. doi: 10.1530/REP-07-0141. [DOI] [PubMed] [Google Scholar]
- Martins da Silva SJ, Bayne RA, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation before primordial follicle formation. Dev Biol. 2004;266:334–345. doi: 10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S, Nottola SA. The ultrastructure of human reproduction. I. The natural history of the female germ cell: origin, migration and differentiation inside the developing ovary. Hum Reprod Update. 1997;3:281–295. doi: 10.1093/humupd/3.3.281. [DOI] [PubMed] [Google Scholar]
- Nagamani M, McDonough PG, Ellegood JO, Mahesh VB. Maternal and amniotic fluid steroids throughout human pregnancy. Am J Obstet Gynecol. 1979;134:674–680. doi: 10.1016/0002-9378(79)90649-5. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod. 2003;69:1265–1272. doi: 10.1095/biolreprod.103.018671. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol. 2004;214:19–25. doi: 10.1016/j.mce.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. doi: 10.1016/s0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- Paredes A, Garcia-Rudaz C, Kerr B, Tapia V, Dissen GA, Costa ME, Cornea A, Ojeda SR. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology. 2005;146:5267–5277. doi: 10.1210/en.2005-0965. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Pepling ME, de Cuevas M, Spradling AC. Germline cysts: a conserved phase of germ cell development? Trend Cell Biol. 1999;9:257–262. doi: 10.1016/s0962-8924(99)01594-9. [DOI] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403:500–501. doi: 10.1038/35000651. [DOI] [PubMed] [Google Scholar]
- Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn. 1992;194:177–192. doi: 10.1002/aja.1001940303. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–3668. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Rosenwaks Z, Woodruff TK, Brannstrom M. Follicle of youth. Nat Med. 2009;15:495. doi: 10.1038/nm0509-495. [DOI] [PubMed] [Google Scholar]
- Rucker EB, 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol (Baltimore, Md.) 2000;14:1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Suzumori N, Yan C, Matzuk MM, Rajkovic A. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev. 2002;111:137–141. doi: 10.1016/s0925-4773(01)00620-7. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol Reprod. 2009;81:16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93:139–149. doi: 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;a 27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology. 2009;b 150:1014–1024. doi: 10.1210/en.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Ruelicke T. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem cell Dev. 2009;18:137–150. doi: 10.1089/scd.2007.0238. [DOI] [PubMed] [Google Scholar]
- Wartenberg H, Hilscher B, Hilscher W. Germ cell kinetics during early ovarian differentiation: an analysis of the oogonial cell cycle and the subsequent changes in oocyte development during the onset of meiosis in the rat. Microsc Res Tech. 1998;40:377–397. doi: 10.1002/(SICI)1097-0029(19980301)40:5<377::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wu CH, Mennuti MT, Mikhail G. Free and protein-bound steroids in amniotic fluid of midpregnancy. Am J Obstet Gynecol. 1979;133:666–672. doi: 10.1016/0002-9378(79)90016-4. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol (Baltimore, Md.) 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology. 2004;145:959–966. doi: 10.1210/en.2003-1078. [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]