Abstract
The mid-cycle surge of luteinizing hormone (LH) induces ovulation, a process during which a fertilizable oocyte is released from a mature ovarian follicle. Although ovulation is a physiologically well-characterized event, the underlying molecular pathways remain poorly understood. Progesterone receptor (PGR), which mediates the biological effects of the steroid hormone progesterone, has emerged as a key regulator of ovulation in mice. The development of a progesterone-receptor-null (Pgr-null) mouse model confirmed a critical role of this hormone in ovulation because in these mutant mice, mature pre-ovulatory follicles fail to release the oocytes. This animal model has thus presented a unique opportunity to study the molecular pathways underlying ovulation. Gene-expression profiling experiments by several groups, using the ovaries of Pgr-null mice, revealed novel gene networks, which act downstream of PGR to control ovulation. These genes encode diverse molecules such as proteases, transcription factors, cell-adhesion molecules, modulators of vascular activities and regulators of inflammation. Functional analyses using gene-knockout mouse models have confirmed that some of these factors play critical roles during ovulation. The knowledge gained from these studies has helped us to understand better the molecular mechanisms that facilitate the release of oocytes from pre-ovulatory follicles. Further analysis of the role of molecular regulators of ovulation will help identify useful molecular targets that would allow the development of improved contraceptives and new therapeutics for anovulatory infertility.
Keywords: ovulation, progesterone, luteinizing hormone
Introduction
Ovulation is an essential reproductive event, which involves the breakdown of a mature ovarian follicle to release a fertilizable oocyte. In mammals, it is a highly coordinated process initiated by hormones released from the hypothalamic–pituitary–ovarian axis. In mice and rats, primary follicles are released daily from the primordial follicle pool by mechanisms that are yet to be elucidated but likely involve changes in oocytes and somatic cells. These primary follicles commence to grow in response to the pituitary gonadotrophins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). A much smaller pool is selected from these growing follicles for further maturation and eventually reaches the pre-ovulatory stage. In response to the high levels of estrogen produced from these maturing ovarian follicles, the pituitary releases LH midway through a menstrual cycle in humans or at pro-estrus of an estrous cycle in rodents. This LH surge initiates the ovulatory process that culminates in the release of fertilizable oocyte(s) (Richards, 1994).
The hormonal regulation of ovulation in the mouse is remarkably similar to that of the human (Espey, 1998). In addition, since mice are amenable to genetic manipulation, they serve as an excellent model for investigating the signaling pathways that control follicular growth and release of mature oocytes. Ovulation, controlled primarily by the cyclic changes in FSH and LH, can be experimentally induced in mice by administration of exogenous gonadotrophins in a process known as superovulation. In this procedure, mice are treated with equine chorionic gonadotrophin, which mimics FSH action, for 48 h to stimulate follicular development; subsequent administration of human chorionic gonadotrophin (hCG), which mimics LH action, induces ovulation (Espey, 1994; Robker et al., 2000). Typically, ovulation occurs 11–14 h following the hCG treatment. Researchers in many laboratories have used a superovulation protocol to analyze gene-expression patterns in ovarian follicles during the ovulatory period in mice or rats (Robker et al., 2000; Espey and Richards, 2002).
Although the physiological function of ovulation is well defined, the mechanisms controlling this process have eluded researchers for many decades. Ovulation is broadly understood to be a complex yet timely process, involving the coordinated expression of numerous genes in the ovary (Richards et al., 2002). LH, the critical trigger of ovulation, exerts its effects by acting through the LH receptor, which is a G-protein-coupled membrane-bound receptor, in granulosa cells. The LH receptor is induced in granulosa cells during the growth of pre-ovulatory follicles, thereby allowing only these follicles to respond to the LH surge (Peng et al., 1991). An increase in the LH levels terminates the program of follicular growth and sets off a cascade of gene-expression changes in the ovary that are critical for ovulation and luteinization (Couse et al., 2005). A number of genes that are induced in ovarian follicular cells in response to the LH surge have been shown to be critical for ovulation in mice. These include progesterone receptor (Pgr), prostaglandin-endoperoxide synthase 2 (Ptgs2), CCAAT/enhancer-binding protein β (Cebpb) and epidermal growth factor (EGF)-like growth factors (Lydon et al., 1995; Lim et al., 1997; Sterneck et al., 1997; Hsieh et al., 2007). In this review, we will focus on the role of PGR during ovulation in mice. We will discuss a few key PGR-regulated pathways and their potential functions during ovulation.
PGR plays an essential role in ovulation
Earlier studies indicated that the steroid hormone progesterone is a key player in ovulation. Inhibition of progesterone synthesis by epostane blocked ovulation in rats (Tanaka et al., 1991). The biological effects of progesterone are mediated by PGR, a ligand-activated transcription factor. Ovarian PGR expression is undetectable during follicular development (Park and Mayo, 1991; Robker et al., 2000). In mouse and rat ovaries subjected to gonadotrophin-induced superovulation, a rapid and robust induction of PGR expression is observed in the mural granulosa cells of pre-ovulatory follicles following hCG administration. The expression of PGR peaks at 4–8 h and then declines to undetectable levels, which is maintained throughout the rest of the ovulatory period (Robker et al., 2000). The induction of PGR, following the LH surge, is accompanied by coordinated expression of progesterone biosynthetic enzymes in granulosa cells. Several proteins essential for steroidogenesis, including steroidogenic acute regulatory protein (Star) and cholesterol side-chain cleavage enzyme (Cyp11a1; P450scc), are also induced in the ovary during gonadotrophin-induced ovulation (Ronen-Fuhrmann et al., 1998).
Several lines of evidence support a critical role of PGR in ovulation. Blockade of PGR function with a selective antagonist RU486 or CDB-2914 reduces the number of ovulated oocytes in mice (Loutradis et al., 1991; Palanisamy et al., 2006). The development of Pgr-null mice by Lydon et al. (1995) firmly established the essential role of PGR in ovulation. Pgr-null mice fail to ovulate because pre-ovulatory follicles are unable to undergo follicle-wall degradation and the oocytes remain trapped within luteinized follicles. These mutant mice exhibit, however, normal follicular development. Furthermore, the oocytes isolated from the ovaries of these mutant mice mature normally and are fertilizable (Robker et al., 2000). Interestingly, the granulosa cells of unruptured follicles in Pgr-null mice undergo luteinization, as indicated by the expression of the luteinization marker Cyp11a1 (P450scc) (Robker et al., 2000).
PGR exists in two isoforms, PGR-A and PGR-B, which are generated from a single gene via alternative usage of two distinct promoters or alternative translation initiation from two different AUG initiation codons (Kraus et al., 1993). Both PGR-A and PGR-B proteins are expressed in the mouse ovary, although PGR-A is the predominant form (Shao et al., 2003). Accordingly, when PGR-A or PGR-B was selectively ablated in mice, PGR-A-null mice exhibited impaired ovulation, resulting in unruptured pre-ovulatory follicles (Mulac-Jericevic et al., 2000). In contrast, PGR-B-null mice showed normal ovulation (Mulac-Jericevic et al., 2003). These findings established that PGR-A, but not PGR-B, is the key isoform mediating the effects of progesterone during the ovulatory process (Conneely and Lydon, 2000; Conneely et al., 2002).
The anovulatory phenotype of the Pgr-null mouse with a distinct defect in follicle-wall breakdown presents a unique opportunity to investigate the molecular pathways underlying ovulation. It is generally believed that PGR, a transcription factor, controls the expression of a number of downstream target genes whose products function in an autocrine/paracrine manner within the ovarian tissue to control the ovulatory process. To decipher the precise molecular nature of progesterone action during ovulation, it is thus imperative to understand the identities of the PGR-regulated gene networks and the mechanisms by which these molecules interact with one another to control the biological events leading to follicle-wall degradation.
Identification of PGR-regulated gene networks that control ovulation
After the LH surge, PGR expression is induced primarily in mural granulosa cells of the pre-ovulatory follicles that are destined to ovulate. As a transcription factor, PGR is presumed to regulate the expression of its primary target genes in these cells. PGR modulates the transcription of these primary target genes by binding directly to specific regulatory sequences, termed progesterone response element, or by interacting with other promoter-bound transcription factors via protein–protein interaction (Li and O'Malley, 2003; Li et al., 2004; Sriraman et al., 2008). Because PGR appears to control the breakdown of the follicle wall leading to the release of oocytes, it is important to understand how the factors produced by its action in the mural granulosa cells mediate the rupture process by helping to break down the follicular wall. It is likely that some PGR-regulated factors are secreted by the granulosa cells and reach other cell types within the ovary, such as cumulus cells, theca cells and endothelial cells of blood vessels. These factors are likely to act as paracrine effectors to induce downstream pathways within the target cells, regulating critical events that facilitate follicle-wall degradation. Research during the past years has identified several genes acting downstream of PGR during the ovulatory process. In this review, we will highlight a few key genes which mediate PGR function during ovulation.
Richards and co-workers identified a disintegrin and metalloproteinase with thrombospondin motifs (Adamts1) as the first PGR-regulated gene that is expressed during the ovulatory process (Robker et al., 2000). Although its expression is not detectable in developing follicles, Adamts1 is induced by hCG specifically in the granulosa cells of pre-ovulatory follicles prior to ovulation. This granulosa-cell-specific induction of Adamts1 expression is impaired in Pgr-null ovaries, indicating that Adamts1 acts downstream of Pgr. Mice deficient in Adamts1 are subfertile due to their inability to ovulate, confirming that ADAMTS-1 is indeed a critical factor mediating the ovulatory process of PGR (Mittaz et al., 2004; Shozu et al., 2005). Since the follicle wall needs to break down prior to oocyte release, it was proposed that ADAMTS-1, a protease, plays a key role in the degradation of the collagen layer. Amino acid sequence analysis of the ADAMTS family of proteins revealed a highly conserved metalloproteinase domain harboring a zinc-binding site, consistent with the prediction that the primary function of this protein family is to catalyze proteolysis (Andreini et al., 2005). Although it remains to be established that ADAMTS-1 is directly involved in the breakdown of a follicle wall, this protease displayed a capacity to cleave versican (chondroitin sulfate proteoglycan 2: CSPG2), a hyaluronan-binding extracellular-matrix proteoglycan, which accumulates during the expansion of a cumulus cell–oocyte complex (COC) (Russell et al., 2003). There is evidence in the literature that ADAMTS proteins are also associated with inflammatory processes and control of angiogenesis (Kuno et al., 1997; Porter et al., 2005).
A disintegrin and metalloprotease 8 (ADAM8) is another PGR-regulated gene that belongs to the ADAM family. It is primarily expressed in the granulosa cells of pre-ovulatory follicles during ovulation (Sriraman et al., 2008). Like other members of the ADAM family, it possesses an intrinsic metalloprotease and disintegrin domain, and is thought to perform proteolytic cleavage of the membrane-anchored precursors of active signaling molecules and release them in soluble forms into the intercellular space (Yamamoto et al., 1999). In the context of the paracrine mode of PGR signaling, one may envision that the proteolytic activity of ADAM8 is involved in releasing signaling molecules from mural granulosa cells, enabling them to act on other ovarian cell types to mediate the paracrine effects of PGR action. Further investigations are necessary to verify this plausible mechanism of ADAM8 action.
It is of interest to note that synaptosomal-associated protein 25 (SNAP25), another novel PGR-regulated gene, is a protein involved in membrane fusion mechanisms that control exocytosis (Shimada et al., 2007). It is a constituent of a soluble N-ethylmaleimide sensitive-factor attachment protein receptor complex, which mediates fusion of cellular transport vesicles with the cell membrane. SNAP25 plays a key role in the release of signaling molecules by facilitating the fusion of the vesicles, containing these molecules, with the plasma membrane (Sorensen, 2005). In the context of the ovary, it is proposed that SNAP25 contributes to the PGR-mediated ovulatory pathways by controlling the release of paracrine mediators from mural granulosa cells (Shimada et al., 2007). Consistent with this hypothesis, it was shown that blockade of SNAP25 function in granulosa-cell cultures decreases the secretion of the cytokine interleukin-6 (IL6), along with other cytokines, by these cells (Shimada et al., 2007).
That PGR controls a paracrine mode of signaling in the ovary is also supported by a previous study of endothelin-2 (EDN2), a potent vasoactive hormone (Palanisamy et al., 2006). Gene-expression profiling using ovaries of mice subjected to gonadotrophin-induced superovulation in the presence or absence of CDB-2914, a synthetic PGR antagonist, revealed that EDN2 is produced downstream of PGR action in mural granulosa cells of pre-ovulatory follicles immediately preceding ovulation (Palanisamy et al., 2006). Blockade of EDN2 action using a selective antagonist for endothelin receptor B (ETR-B) resulted in a profound inhibition of ovulation in rats and mice (Ko et al., 2006; Palanisamy et al., 2006). Based on the observation that ETR-B is expressed in the mural and cumulus granulosa cells of pre-ovulatory follicles as well as the capillaries lining the inner border of the theca interna, it is postulated that EDN2 released by the mural granulosa cells acts, in a paracrine manner, on the cumulus cells of COCs and the endothelial cells of the capillaries of theca interna to control gene expression that in turn contribute to follicle-wall breakdown.
Richards and co-workers identified additional PGR-regulated genes in the ovary, such as cathepsin L (Ctsl), cyclic-GMP-dependent kinase II (Prkg2; cGKII) and pituitary adenylate-cyclase-activating polypeptide (Pacap) (Ko et al., 1999; Robker et al., 2000; Sriraman and Richards, 2004; Sriraman et al., 2005). In a recent study to identify the PGR-regulated genes that participate in the ovulatory process, we compared the global changes in ovarian gene-expression profiles of wild-type (WT) and Pgr-null mice during gonadotrophin-induced superovulation. This microarray analysis uncovered ∼300 genes whose expression was significantly altered in the Pgr-null ovaries compared with the WT ovaries at a time that shortly precedes follicular rupture (J.K., I.C.B. and M.K.B., unpublished results). These genes included several previously reported PGR-regulated genes, namely Adamts1, Edn2, Snap25, Pparg and Adam8, thereby strengthening the validity of our microarray analysis. When these microarray-derived genes were classified according to their known biological functions, they were found to encode diverse molecules such as proteases, transcription factors, growth factors, cell-adhesion molecules, modulators of vascular activities and regulators of inflammation. The PGR-regulated pathways are, therefore, linked to diverse biological processes, reflecting the overall complexity at the cellular and molecular levels that governs ovulation.
Novel insights into PGR regulation of inflammatory responses during ovulation
Ovulation has long been viewed as an inflammatory process. It has been proposed that the biological events driven by the LH surge in the ovary resemble an acute inflammatory response (Espey, 1980, 1994). Shortly after the pre-ovulatory LH surge, there is a significant rise in local circulation in the ovary, which persists until the time of follicle-wall degradation (Tanaka et al., 1989a, b). The LH-mediated pathways increase vasodilation and vascular permeability, creating a hyperemic condition within pre-ovulatory follicles. The increased vascular permeability drives the exudation of serum proteins and allows transmigration of leukocytes, primarily neutrophils and macrophages, from the blood vessels to the interior of the pre-ovulatory follicles (Brannstrom et al., 1993). In an inflamed tissue, migrating leukocytes secret proteases, damaging the tissue. Likewise, intrafollicular leukocytes may produce proteolytic enzymes that contribute to the disruption of the follicular wall at the time of ovulation.
Although it is unknown how this inflammatory condition results in the breakdown of the pre-ovulatory-follicle wall, especially at the molecular level, one can envision that the key molecules and signaling pathways implicated in inflammation may also play a critical role in the ovulatory process. Consistent with this hypothesis, recent ovarian gene-expression profiling experiments in our laboratory have indicated that several genes, such as Edn2, Pparg and Il6, are regulated by PGR during the ovulatory process (unpublished). Previous studies have shown that these genes mediate or control inflammatory response in a variety of tissues (Rose-John et al., 2006; Filipovich and Fleisher-Berkovich, 2008; Tontonoz and Spiegelman, 2008).
EDN2 regulates blood-vessel dynamics by controlling the constriction or dilation of the vessels and plays a role in inflammation (Meidan and Levy, 2007; Filipovich and Fleisher-Berkovich, 2008). Identification of Edn2 as a PGR-regulated gene essential for ovulation strengthened the notion that PGR-driven inflammatory reactions control ovulation (Palanisamy et al., 2006). It was shown recently that granulosa cells and COCs of pre-ovulatory follicles express innate immune-related genes, including several members of the Toll-like receptor surveillance system, the inflammatory cytokine IL6 and the scavenger receptor CD36 during the ovulatory process, pointing to an intimate link between ovulation and inflammation-like processes (Shimada et al., 2006a, b; Liu et al., 2008).
Our recent microarray study and those of others have revealed the induction of interleukin Il6 in granulosa cells of ovulating follicles (Hernandez-Gonzalez et al., 2006; Liu et al., 2009; J.K., I.C.B. and M.K.B., unpublished results). Our recent unpublished studies using mouse primary granulosa cell cultures showed that addition of IL6 strongly enhances mRNA expression of Ptgs2, a vital inflammatory molecule and a known regulator of ovulation. We further observed that treatment with IL6 also up-regulated mRNA expression of vascular endothelial growth factor A (Vegfa), which promotes vascular permeability and, thus, likely contributes to the inflammation process during ovulation (unpublished). Recent studies indicated that IL6 produced during the ovulatory process regulates ovarian cumulus cell function (Liu et al., 2009). These results are consistent with the hypothesis that IL6 functions downstream of PGR to induce or modulate inflammatory pathways that contribute to ovulation. It is, however, unclear whether the regulation of IL6 by PGR is direct or indirect.
Recently, we identified peroxisome proliferator-activated receptor γ (PPARγ), a ligand-inducible transcription factor and regulator of inflammation, as a novel PGR-regulated gene with an important role in ovulation (Kim et al., 2008). An extensive literature describes PPARγ as a key regulator of adipocyte differentiation, lipid and glucose homeostasis, insulin sensitization and inflammation (Lee and Evans, 2002; Koutnikova et al., 2003; Lazar, 2005; Tontonoz and Spiegelman, 2008). There is also accumulating evidence in favor of a role for PPARγ in reproduction (Cui et al., 2002; Komar, 2005; Minge et al., 2008).
During superovulation, PPARγ is expressed primarily in the granulosa cells of pre-ovulatory follicles in a PGR-dependent manner. To address the functional role of this gene during ovulation, we created a conditional knockout mouse model by crossing mice harboring ‘floxed’ Pparg gene with progesterone receptor Cre knock-in (Pgr-Cre) mice. This resulted in the generation of females (Ppargflox/flox PgrCre/+), in which the Pparg gene undergoes Cre-mediated excision in the mural granulosa cells of pre-ovulatory follicles. When Pparg conditional-null mice were subjected to gonadotrophin-induced superovulation, there was a drastic reduction in the number of released oocytes in mutant mice compared with Ppargflox/flox (control) mice. Upon histological examination of the ovaries of control and Pparg conditional-null mice at 18–19 h post-hCG, numerous corpora lutea were seen in the control ovary, whereas only a few corpora lutea and a large number of unruptured pre-ovulatory follicles were found in the mutant ovary. Loss of PPARγ signaling in the mural granulosa cells of mutant ovaries thus leads to impairment in follicular rupture. Gene-expression analysis identified a subset of PGR-regulated genes including Edn2, Prkg2 and Il6 as targets of regulation by PPARγ in the ovary. These results supported the concept that PPARγ functions as a mediator of certain of the biological actions of PGR in the ovulatory pathway.
It has been reported that PPARγ can be activated by an array of endogenous metabolites derived from fatty acids such as arachidonic acid and linoleic acid (Forman et al., 1995; Kliewer et al., 1995; Nagy et al., 1998; Schopfer et al., 2005). These fatty acid metabolites include prostaglandins, hydroxyeicosatetraenoic acids and hydroxyoctadecadienoic acids, which are not only potent signaling molecules but also inflammatory agents produced during ovulation (Tanaka et al., 1989a, b). In the ovary, PTGS2, an LH/hCG-induced enzyme that converts arachidonic acid into prostaglandins, plays a critical role in ovulation (Lim et al., 1997; Davis et al., 1999). Administration of indomethacin, a non-steroidal anti-inflammatory agent and an inhibitor of PTGS2, to rats undergoing gonadotrophin-induced ovulation, effectively inhibits ovulation (Tanaka et al., 1991). Interestingly, suppression of the synthesis of PTGS2-mediated inflammatory molecules by indomethacin or the selective inhibitor NS-398 decreased the expression of PPARγ-regulated genes, such as Edn2, Prkg2 and Il6. Since these genes also operate downstream of PGR (Sriraman et al., 2005; Kim et al., 2008), this study uncovered an important link between PGR- and PTGS2-driven pathways. It is conceivable that metabolism of long-chain unsaturated fatty acids by PTGS2 in mural granulosa cells produces inflammatory molecules that serve as endogenous activating ligand(s) of PPARγ in the ovarian follicles and regulates the expression of its downstream target genes to control ovulation. This finding further supports the inflammation hypothesis of ovulation and offers an insight as to how seemingly distinct pathways regulated by PGR-PPARγ and -PTGS2, respectively, converge functionally to control the events leading to ovulation.
It is important to note that synthetic PPARγ ligands have been clinically used to treat polycystic ovary syndrome (PCOS), which is the most common cause of anovulatory infertility. Recent clinical studies indicated that treatment of subjects with PCOS with rosiglitazone, a potent PPARγ agonist, restores normal ovulation in 55–85% of these patients (Dereli et al., 2005; Cataldo et al., 2006). Despite the remarkable effectiveness of this treatment, the precise mechanisms underlying the therapeutic effects of the PPARγ agonist have remained unclear. In light of the importance of PPARγ in the ovulatory process, it is tempting to speculate that the treatment of PCOS by rosiglitazone may work via modulation of ovarian functions of PPARγ (Froment et al., 2005). This hypothesis, however, needs to be tested by further studies.
PGR regulates hypoxia signaling during ovulation
We recently identified genes encoding the hypoxia-inducible factors (HIFs), HIF-1α, HIF-2α and HIF-1β, as novel PGR-regulated genes in the granulosa cells of the pre-ovulatory follicles (Kim et al., 2009). These transcription factors serve as critical regulators of the tissue's response to changes in oxygen levels (Semenza, 2003). They form heterodimeric complexes consisting of one α subunit and one β subunit, each represented by three isoforms that are encoded by distinct genes (Semenza, 2003; Pouyssegur et al., 2006; Keith and Simon, 2007). Under conditions of abundant oxygen (>8–10%), HIF-α proteins are expressed but rapidly degraded by an oxygen-dependent proteasomal activity. When the oxygen level in the tissue falls below the normal (<5%), the degradation of HIF-α proteins is prevented, leading to the accumulation of these proteins in the nucleus. The active HIF-α protein forms a heterodimeric complex with its binding partner HIF-1β, which is constitutively expressed, independent of oxygen levels. The HIF-αβ heterodimer then binds to the regulatory regions of target genes and controls their expression.
In mice, inhibition of the HIF transcriptional activity by echinomycin, a small-molecule inhibitor that suppresses the binding of HIF-αβ heterodimers to target genes, blocked gonadotrophin-induced superovulation by preventing the follicle-wall breakdown of pre-ovulatory follicles. Since a similar defect in follicular rupture is also observed in the Pgr-null mice, our results are consistent with the concept that HIFs are critical mediators of the ovulatory effects of PGR. Consistent with this scenario, the HIF-target genes in the ovary included Adamts1, Edn2, Vegfa and Cxcr4, which are also identified as PGR-regulated genes in our microarray analysis. Most importantly, Adamts1, Edn2 and Vegfa are known to play critical roles during ovulation (Hazzard et al., 2002; Mittaz et al., 2004; Fraser et al., 2005; Shozu et al., 2005; Ko et al., 2006; Palanisamy et al., 2006).
Interestingly, the exposure of primary granulosa cells to the hypoxia mimetic cobalt chloride enhanced the levels of HIF-1α and HIF-2α proteins, which in turn stimulated the expression of HIF-target genes such as Edn2, Vegfa and Cxcr4 (Kim et al., 2009). This result raised the possibility that a hypoxic condition is generated within the ovulatory follicles downstream of gonadotrophin action during ovulation. Presently there is no direct evidence indicating that such a condition is actually created in vivo within the intrafollicular environment during ovulation. It is nevertheless conceivable that the rapid proliferation of granulosa cells in the growing follicles in response to gonadotrophin stimulation in conjunction with the lack of vasculature in the interior mural granulosa layers may give rise to a local hypoxic environment within the follicles (Neeman et al., 1997). This condition would prevent degradation of HIF-α proteins, leading to accumulation of the HIF-αβ heterodimeric complexes in the nucleus and subsequent induction of HIF-target genes to regulate the ovulatory process.
Is there a role of PGR in COC expansion?
A well-known biological consequence of the pre-ovulatory LH surge is the expansion of the COC (Eppig, 2001; Richards et al., 2002). This process involves accumulation of hyaluronan-rich extracellular matrix (ECM), leading to the generation of an expanded COC. The COC expansion is closely linked to ovulation because the lack of key structural components of the expanded COC matrix or factors regulating this process impairs oocyte release from the pre-ovulatory follicle (Richards et al., 2002; Richards, 2005). The previous report that versican, which is a hyaluronan-binding proteoglycan in the COC ECM and a potential cross-linker of the matrix components, is cleaved by the PGR-regulated protease ADAMTS-1 raised the possibility that PGR has a role in cumulus expansion that occurs prior to ovulation (Russell et al., 2003).
The role of PGR in cumulus expansion, however, remains unclear. Initial morphological examinations indicated that the COCs undergo expansion in Pgr-null mice (Lydon et al., 1995). However, our recent microarray-based gene-expression analyses at the time of ovulation revealed that the expression of many genes, such as Tsg6, Ptx3 and Has2, which are known to be critical for expansion of COC matrix, is reduced in ovaries of Pgr-null mice compared with WT mice (unpublished observation). It has also been shown that expression of Areg (amphiregulin) and Ereg (epiregulin), EGF-like factors critical for COC expansion, is reduced in COCs and ovaries of Pgr-null mice (Shimada et al., 2006a, b). Moreover, IL6, a PGR-regulated cytokine, induces expansion of COCs and the expression of genes known to be involved in this process (Liu et al., 2009). It is thus conceivable that in the Pgr-null mice, subtle alterations, which are not discernable by morphological examination, occur in the composition of the COC matrix. These changes may contribute to functional abnormalities in the matrix that is generated, which in turn may hinder the rupture of pre-ovulatory follicles. If confirmed by further analysis, impairment in the formation of a functional COC matrix would represent an additional mechanism by which PGR regulates ovulation.
Conclusions
The failure of Pgr-null mice to ovulate in response to a gonadotrophin surge has uncovered an essential role of progesterone-dependent pathways in the regulation of ovulation. The present challenge is to determine how these PGR-controlled pathways work together to bring about follicle-wall breakdown. Gene-expression profiling experiments, aided by functional analysis using various knockout mouse models, have provided an initial blueprint of the signaling network that mediates the effects of PGR during ovulation (Fig. 1). It is increasingly becoming clear that ovulation, which resembles an acute inflammatory response, is associated with the production of several PGR-regulated factors that are potential regulators of inflammation. These include PPARγ, EDN2, ADAMTS-1, IL6, PTGS2, VEGF-A and possibly additional molecules. Continued exploration of these pathways will clarify the precise mechanisms by which the PGR-regulated factors act within the ovarian tissue in concert with each other to direct the program leading to ovulation. Moreover, this research has the potential to help develop novel contraceptives that will target key factors mediating the effects of PGR during ovulation. These contraceptives are likely to be non-steroidal in nature and, therefore, may have lesser side effects than their steroidal counterparts. The knowledge gained from this research may also help advance our understanding of the basis of various anovulatory disorders, such as PCOS, ultimately providing therapies to improve fertility in these patients.
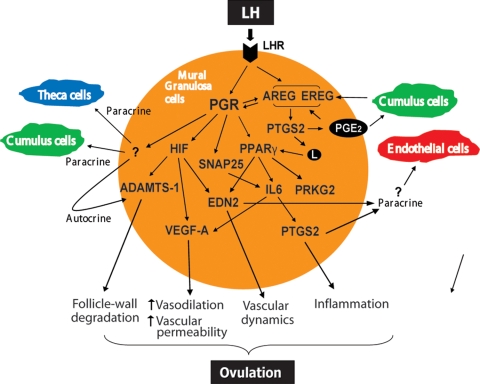
Figure 1:
An emerging blueprint of PGR-regulated gene networks that control ovulation in the mouse. The schematic describes the PGR-regulated factors that are discussed in this review. The PGR, expressed in mural granulosa cells of pre-ovulatory follicles, regulates downstream genes such as peroxisome proliferator-activated receptors (PPARγ) and HIFs, which are important mediators of its function during ovulation. These genes in turn regulate the production of critical factors that are secreted and act on various ovarian cell types, such as cumulus granulosa cells, theca cells and vascular endothelial cells. Both PPARγ and HIFs control the production of endothelin (EDN2), which likely contributes to the follicle-wall degradation by influencing the dynamic changes in the vasculature during ovulation. PPARγ also controls the synthesis of IL6, which acts by regulating VEGF-A, which enhances vascular permeability, and PTGS2, which generates inflammation-inducing fatty-acid metabolites. The fatty-acid metabolites produced by PTGS2 promote ovulation by acting as agonists of PPARγ, thereby forming a classical regulatory loop. SNAP25, another PGR-regulated gene, may also participate in the ovulatory process by regulating the release of cytokines, including IL6, from granulosa cells. PGR also mediates the induction of the EGF-like growth factors, such as AREG and EREG, in granulosa cells. These factors in turn induce PTGS2, which generates prostaglandin E2 (PGE2). AREG and PGE2 can then act on cumulus cells, via paracrine mechanisms, to induce AREG and PTGS2. Since ovulation also involves physical degradation of the follicular wall, the actions of proteases, such as ADAMTS-1, are essential during this process. The coordinated actions of the PGR-regulated pathways eventually bring about the breakdown of the pre-ovulatory follicle, releasing the oocyte. PRKG2, cyclic-GMP-dependent protein kinase; L, fatty-acid metabolites.
Funding
The authors are supported by U54 HD299901 (I.C.B.) and the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD55787 (M.K.B. and I.C.B.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
References
- Andreini C, Banci L, Bertini I, Elmi S, Rosato A. Comparative analysis of the ADAM and ADAMTS families. J Proteome Res. 2005;4:881–888. doi: 10.1021/pr0500096. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Mayrhofer G, Robertson SA. Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biol Reprod. 1993;48:277–286. doi: 10.1095/biolreprod48.2.277. [DOI] [PubMed] [Google Scholar]
- Cataldo NA, Abbasi F, McLaughlin TL, Basina M, Fechner PY, Giudice LC, Reaven GM. Metabolic and ovarian effects of rosiglitazone treatment for 12 weeks in insulin-resistant women with polycystic ovary syndrome. Hum Reprod. 2006;21:109–120. doi: 10.1093/humrep/dei289. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Lydon JP. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids. 2000;65:571–577. doi: 10.1016/s0039-128x(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–3262. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- Cui Y, Miyoshi K, Claudio E, Siebenlist UK, Gonzalez FJ, Flaws J, Wagner KU, Hennighausen L. Loss of the peroxisome proliferation-activated receptor gamma (PPARgamma) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J Biol Chem. 2002;277:17830–17835. doi: 10.1074/jbc.M200186200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology. 1999;140:2685–2695. doi: 10.1210/endo.140.6.6715. [DOI] [PubMed] [Google Scholar]
- Dereli D, Dereli T, Bayraktar F, Ozgen AG, Yilmaz C. Endocrine and metabolic effects of rosiglitazone in non-obese women with polycystic ovary disease. Endocr J. 2005;52:299–308. doi: 10.1507/endocrj.52.299. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod. 1980;22:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- Espey LL. Ovulation. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. CA, USA: Academic Press; 1998. pp. 605–614. [Google Scholar]
- Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- Filipovich T, Fleisher-Berkovich S. Regulation of glial inflammatory mediators synthesis: possible role of endothelins. Peptides. 2008;29:2250–2256. doi: 10.1016/j.peptides.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 1214-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Wilson H, Rudge JS, Wiegand SJ. Single injections of vascular endothelial growth factor trap block ovulation in the macaque and produce a prolonged, dose-related suppression of ovarian function. J Clin Endocrinol Metab. 2005;90:1114–1122. doi: 10.1210/jc.2004-1572. [DOI] [PubMed] [Google Scholar]
- Froment P, Gizard F, Staels B, Dupont J, Monget P. A role of PPARgamma in reproduction? Med Sci (Paris) 2005;21:507–511. doi: 10.1051/medsci/2005215507. [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67:1305–1312. doi: 10.1095/biolreprod67.4.1305. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol. 2006;20:1300–1321. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–1782. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150:3392–3400. doi: 10.1210/en.2008-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Ko C, In YH, Park-Sarge OK. Role of progesterone receptor activation in pituitary adenylate cyclase activating polypeptide gene expression in rat ovary. Endocrinology. 1999;140:5185–5194. doi: 10.1210/endo.140.11.7149. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function—implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol. 2005;3:41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA. 2003;100:14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–562. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- Lazar MA. PPAR gamma, 10 years later. Biochimie. 2005;87:9–13. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Lee CH, Evans RM. Peroxisome proliferator-activated receptor-gamma in macrophage lipid homeostasis. Trends Endocrinol Metab. 2002;13:331–335. doi: 10.1016/s1043-2760(02)00668-9. [DOI] [PubMed] [Google Scholar]
- Li X, O'Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- Li X, Lonard DM, O'Malley BW. A contemporary understanding of progesterone receptor function. Mech Ageing Dev. 2004;125:669–678. doi: 10.1016/j.mad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shimada M, Richards JS. The involvement of the Toll-like receptor family in ovulation. J Assist Reprod Genet. 2008;25:223–228. doi: 10.1007/s10815-008-9219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, de Matos DG, Fan HY, Shimada M, Palmer S, Richards JS. IL6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2009;150:3360–3368. doi: 10.1210/en.2008-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutradis D, Bletsa R, Aravantinos L, Kallianidis K, Michalas S, Psychoyos A. Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum Reprod. 1991;6:1238–1240. doi: 10.1093/oxfordjournals.humrep.a137519. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Meidan R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab. 2007;18:379–385. doi: 10.1016/j.tem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Minge CE, Robker RL, Norman RJ. PPAR gamma: coordinating metabolic and immune contributions to female fertility. PPAR Res. 2008;2008:243791. doi: 10.1155/2008/243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70:1096–1105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C. Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle. Int J Exp Pathol. 1997;78:57–70. doi: 10.1046/j.1365-2613.1997.d01-247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Park OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991;5:967–978. doi: 10.1210/mend-5-7-967. [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Shao R, Markstrom E, Friberg PA, Johansson M, Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: stage-dependent PR-mediated regulation of apoptosis and cell proliferation. Biol Reprod. 2003;68:914–921. doi: 10.1095/biolreprod.102.009035. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol. 2006;a 20:3228–3239. doi: 10.1210/me.2006-0194. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;b 20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, Richards JS. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–2502. doi: 10.1210/me.2007-0042. [DOI] [PubMed] [Google Scholar]
- Shozu M, Minami N, Yokoyama H, Inoue M, Kurihara H, Matsushima K, Kuno K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J Mol Endocrinol. 2005;35:343–355. doi: 10.1677/jme.1.01735. [DOI] [PubMed] [Google Scholar]
- Sorensen JB. SNARE complexes prepare for membrane fusion. Trends Neurosci. 2005;28:453–455. doi: 10.1016/j.tins.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Richards JS. Cathepsin L gene expression and promoter activation in rodent granulosa cells. Endocrinology. 2004;145:582–591. doi: 10.1210/en.2003-0963. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS. Cyclic GMP dependent protein kinase II is induced by LH and PR dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol. 2006;20:348–361. doi: 10.1210/me.2005-0317. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Eichenlaub-Ritter U, Bartsch JW, Rittger A, Mulders SM, Richards JS. Regulated expression of ADAM8 (a disintegrin and metalloprotease domain 8) in the mouse ovary: evidence for a regulatory role of luteinizing hormone, progesterone receptor, and epidermal growth factor-like growth factors. Biol Reprod. 2008;78:1038–1048. doi: 10.1095/biolreprod.107.066340. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPbeta in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Espey LL, Okamura H. Increase in ovarian blood volume during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 1989;a 40:762–768. doi: 10.1095/biolreprod40.4.762. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Espey LL, Okamura H. Increase in ovarian 15-hydroxyeicosatetraenoic acid during ovulation in the gonadotropin-primed immature rat. Endocrinology. 1989;b 125:1373–1377. doi: 10.1210/endo-125-3-1373. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Espey LL, Kawano T, Okamura H. Comparison of inhibitory actions of indomethacin and epostane on ovulation in rats. Am J Physiol. 1991;260:E170–E174. doi: 10.1152/ajpendo.1991.260.2.E170. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Higuchi Y, Yoshiyama K, Shimizu E, Kataoka M, Hijiya N, Matsuura K. ADAM family proteins in the immune system. Immunol Today. 1999;20:278–284. doi: 10.1016/s0167-5699(99)01464-4. [DOI] [PubMed] [Google Scholar]