Summary
ATR is an essential kinase activated in response to DNA-replication stress, with a known target being the RPA2 subunit of human replication protein A (RPA). We find that S33-RPA2 phosphorylation by ATR occurs primarily in the late-S and G2 phases, probably at sites of residual stalled DNA-replication forks, with S33-P-RPA2 contained within nuclear repair centers. Although cells in which endogenous RPA2 was `replaced' with an RPA2 protein with mutations T21A and S33A (T21A/S33A-RPA) had normal levels of DNA replication under non-stress conditions, the mutant cells were severely deficient in the amount of DNA synthesis occurring during replication stress. These cells also had abnormally high levels of chromatin-bound RPA, indicative of increased amounts of single-stranded DNA (ssDNA) and showed defective recovery from stress. Cells replaced with the mutant RPA2 also generated G1 cells with a broader DNA distribution and high levels of apoptosis following stress, compared with cells expressing wild-type RPA2. Surprisingly, cells expressing the wild-type RPA2 subunit had increased levels of stress-dependent DNA breaks. Our data demonstrate that RPA phosphorylation at the T21 and S33 sites facilitates adaptation of a DNA-replication fork to replication stress.
Keywords: ATR, DNA damage, DNA replication, RPA, Replication stress
Introduction
In response to genotoxic stress, eukaryotic cells induce signaling pathways that result in the regulation of cell-cycle progression and mobilization of repair proteins. An important, although poorly understood, pathway involves the response to replication stress (Ben-Yehoyada et al., 2007). During S phase, conditions that cause uncoupling of the replicative DNA helicase from the DNA-polymerase machinery (e.g. by DNA-polymerase stalling) lead to the generation of significant lengths of single-stranded DNA (ssDNA) (Byun et al., 2005). The ssDNA becomes bound by replication protein A (RPA) and the resulting RPA-ssDNA entity serves to recruit the ATR checkpoint kinase and the associated ATR-interacting protein (ATRIP) (Namiki and Zou, 2006; Zou and Elledge, 2003). Binding leads to kinase activation and phosphorylation of downstream effector molecules such as the tumor suppressors BRCA1 (Yarden et al., 2002) and p53 (Tibbetts et al., 1999), the histone variant H2AX (yielding γH2AX) (Ward and Chen, 2001), and the Chk1 checkpoint kinase (Guo et al., 2000; Liu et al., 2000; Zhao and Piwnica-Worms, 2001). Study of the function of ATR and its homologs (e.g. Mec1 in Saccharomyces cerevisiae) indicate that it stabilizes stalled or damaged DNA-replication forks (Lopes et al., 2001; Tercero and Diffley, 2001; Sogo et al., 2002) and arrests the progression of non-damaged forks (Seiler et al., 2007; Unsal-Kacmaz et al., 2007).
RPA is the primary eukaryotic ssDNA-binding protein and is essential for chromosomal DNA replication and certain DNA repair reactions (Binz et al., 2004; Iftode et al., 1999; San Filippo et al., 2008). In addition to being crucial for recruitment and activation of ATR, RPA is also an ATR target (Block et al., 2004; Olson et al., 2006). Human RPA is a heterotrimer composed of the RPA1, RPA2 and RPA3 subunits (also termed RPA70, RPA32 and RPA14, respectively, for their molecular masses). The protein is primarily phosphorylated within the N-terminal 33 residues of RPA2, in which approximately nine sites have been identified (Binz et al., 2004). Two of the sites (T21 and S33) are consensus for phosphatidylinositol 3-kinase-like kinase (PIKK)-family members (ATM, ATR and DNA-PK), whereas others are targets of the cyclin-Cdk complex (S23 and S29) or DNA-PK (S4, S8, S11, S12 and S13; Fig. 1A) (Anantha et al., 2007; Block et al., 2004; Dutta and Stillman, 1992; Fang and Newport, 1993; Liu et al., 2006; Manthey et al., 2007; Niu et al., 1997; Oakley et al., 2003; Olson et al., 2006; Pan et al., 1994; Zernik-Kobak et al., 1997). Treatment of cells with the DNA-damaging agent camptothecin (CPT) causes initial RPA phosphorylation on S33 by ATR; this phosphorylation subsequently stimulates phosphorylation by cyclin-Cdk and DNA-PK to yield hyperphosphorylated RPA (Anantha et al., 2007). A variety of other damage conditions, including UV irradiation or ionizing irradiation (IR), treatment with DNA-damaging chemicals and use of DNA-polymerase inhibitors [hydroxyurea (HU), aphidicolin], stimulate RPA phosphorylation by ATM, ATR, DNA-PK and cyclin-Cdk (Anantha et al., 2007; Gibbs et al., 1996; Liu et al., 2000; Pan et al., 1994; Wang et al., 2001; Zernik-Kobak et al., 1997) [also see review by Binz et al. (Binz et al., 2004) and references therein].
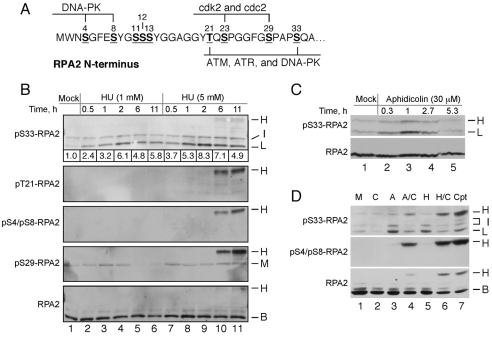
Fig. 1.
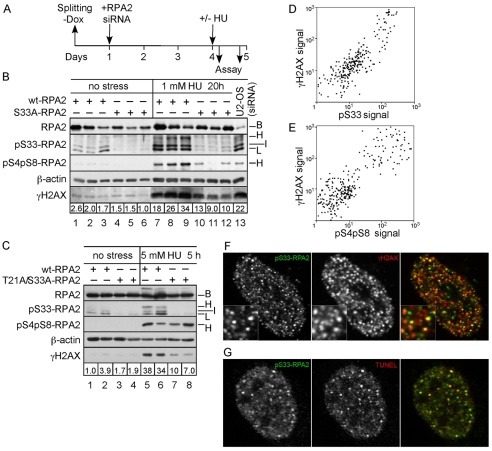
RPA2 S33 is phosphorylated in response to replication stress. (A) Human RPA2 phosphorylation sites. Phosphorylation sites are indicated by underlines, with known or putative kinases shown. (B) Time course of RPA modification in response to HU treatment. U2-OS cells were treated with 1 or 5 mM HU for various times, as indicated. Lysates were then prepared and subjected to western analysis for the presence of S33-P-, T21-P-, S4-P-S8-P- and S29-P-RPA2, and total RPA2. The relative intensities of the S33-P-positive `L' bands in each lane, normalized to total RPA2 levels, are indicated beneath the S33-P panel. (C) RPA modification of S33 in response to aphidicolin treatment. U2-OS cells were mock-treated or treated with 30 μM aphidicolin for various times, as indicated. Lysates were then probed by western blotting for S33-P-RPA2 and total RPA2. (D) U2-OS cells were subjected to various treatments as follows: mock (`M'), 5 mM caffeine for 5 hours (`C'), 5 μM aphidicolin for 5 hours (`A'), 5 mM caffeine and 5 μM aphidicolin for 5 hours (`A/C'), 2.5 mM HU for 5 hours (`H'), 2.5 mM HU and 5 mM caffeine for 5 hours (`H/C'), and 2 μM camptothecin for 1 hour (`Cpt'). Lysates were subjected to western analysis using antibodies specific for S33-P-RPA2, S4-P-S8-P-RPA2 and total RPA2. (B-D) The migration of non-phosphorylated RPA2 (`B' for basal), RPA2 phosphorylated to yield species with low (`L') or intermediate (`I') mobilities, and hyperphosphorylated RPA2 (`H') are indicated.
Although significant understanding exists regarding the kinases that are responsible for RPA phosphorylation, the role of phosphorylation in regulating RPA activity remains unclear. RPA phosphorylation does not have profound effects on ssDNA binding or support of SV40 DNA replication (Brush et al., 1994; Henricksen et al., 1996; Oakley et al., 2003; Pan et al., 1995; Vassin et al., 2004). Phosphorylation of RPA also has not been found to directly influence checkpoint activation in response to interphase DNA damage (Morgan and Kastan, 1997; Olson et al., 2006). By contrast, tests of RPA2 mutants that mimic hyperphosphorylation indicate that phosphorylation inhibits RPA from participating in chromosomal DNA replication (Vassin et al., 2004; Olson et al., 2006). Because these mutant factors are competent to bind to sites of DNA damage, it has been argued that hyperphosphorylation inhibits RPA loading onto ssDNA by the replication machinery (Vassin et al., 2004). Mitotic RPA2 hyperphosphorylation in response to mitotic DNA damage facilitates release of cells into G1 and increases cell viability, apparently by a signaling mechanism (Anantha and Borowiec, 2009; Anantha et al., 2008). In response to genotoxic stress, it was shown that cells expressing an RPA2 variant that was mutated at both Cdk2 sites (S23 and S29) had aberrant RPA foci and increased persistence of the DNA-damage marker γH2AX following genotoxic stress (Anantha et al., 2007). Such data indicate that RPA phosphorylation stimulates chromosomal DNA repair, although the molecular basis for these effects are not understood.
To elucidate the functional role of RPA phosphorylation, we examined the effect of mutation of the two PIKK sites (T21 and S33). Testing cells in which the endogenous RPA2 was `replaced' with an ectopic version, we found that cells expressing the mutant RPA2 were severely defective in DNA synthesis under conditions of DNA-replication stress, causing high levels of apoptosis. This decrease in synthesis occurred concomitantly with an increase in the amount of RPA bound to DNA, an event also seen in cells deficient for ATR. Our data demonstrate that RPA phosphorylation minimizes ssDNA generation under replication-stress conditions in part by stimulating repair DNA synthesis.
Results
RPA2 S33 is phosphorylated in response to replication stress
RPA is phosphorylated in response to a variety of genotoxic stresses. In this study, we focused on mild DNA-replication stress to understand the cellular response to impeded replication-fork progression through particular chromosomal regions (e.g. fragile loci) in non-stressed cells or during the early response to DNA lesions.
Human U2-OS cells were first treated with HU, and the effect on RPA2 phosphorylation was examined at various time points using antibodies specific to RPA2 phosphorylated at S33 (S33-P), T21 (T21-P), S29 (S29-P), and doubly modified S4 and S8 (S4-P-S8-P). Previous characterization of these antibodies found no significant recognition of non-phosphorylated RPA2 or of bands that co-migrate with RPA2 (e.g. Anantha et al., 2007). In the absence of treatment, only two weak S33-P-positive bands were seen, and termed the low (L) and intermediate (I) forms (Fig. 1B, lane 1). The I form also cross-reacted with the S29-P antibody. Test of a `low' concentration of HU that has partial inhibitory properties on nucleotide incorporation (1 mM; see below) demonstrated that the L form of S33-P-RPA2 was induced at early times post-treatment (30 minutes), peaked at 2 hours and decreased thereafter (Fig. 1B, lanes 2-6). Other than a slight increase in S29-P formation (Fig. 1B, compare lanes 1 and 3) that diminished at late times of treatment, no other modification was detected. A similar biphasic profile was noted using aphidicolin to cause DNA-replication stress (Fig. 1C). Previous studies testing various RPA2 phosphorylation mutants indicated that the L species corresponds to RPA2 phosphorylated solely on S33, whereas the S33-P-positive species migrating with intermediate mobility is also modified at the S23 and/or S29 cyclin-Cdk sites (Anantha et al., 2007). Thus, conditions of DNA-replication stress cause an increase in RPA2 phosphorylation at the S33, S29 and S23 sites. In addition, these data indicate that activation of the replication-stress checkpoint under these conditions is transient in that the cell can respond in a manner that eventually causes a reduction in the checkpoint signal.
In response to replication stress caused by treatment with 5 mM HU, the S33-P signal initially gained intensity in the first 2 hours of treatment and then partially diminished (Fig. 1B, lanes 7-11). At the two later time points (i.e. 6 and 11 hours; Fig. 1B, lanes 10 and 11, respectively), an additional band migrating even more slowly was detected (termed `H'). This RPA2 species also reacted with the T21-P, S29-P and S4-P-S8-P antibodies, and therefore is hyperphosphorylated RPA2. The H species has been previously found to be formed in response to agents that cause double-strand DNA breaks (DSBs), including CPT and bleomycin, and that also lead to the formation of γH2AX (Vassin et al., 2004; Anantha et al., 2007; Anantha et al., 2008). Notably, evidence from cell-free and cell-culture studies indicate that phosphorylation of S4 and S8 is primarily catalyzed by the DSB-responsive DNA-PK (Zernik-Kobak et al., 1997; Anantha et al., 2007). We interpret these data to indicate that extended incubation in high HU concentrations cause a fraction of DNA-replication forks to collapse, generating DSBs and RPA2 hyperphosphorylation (see also below).
We treated cells with aphidicolin or HU in the presence of caffeine. Our previous work has demonstrated that the use of caffeine under DNA-damage conditions blocks the checkpoint response, inducing replication-fork collapse and hence more-extensive damage (Vassin et al., 2004). Note that the effect of caffeine treatment is functionally similar to that caused by deficiencies in Mec1p and Rad53p (the budding yeast homologs of ATR and Chk1, respectively), following exposure to DNA-replication stress (Lopes et al., 2001; Tercero and Diffley, 2001). Treatment with either aphidicolin (Fig. 1D, lane 4) or HU (Fig. 1D, lane 6) in combination with caffeine gave rise to a hyperphosphorylated RPA2 species that was reactive to both the S33-P and S4-P-S8-P antibodies. Incubation of cells with CPT to cause DSBs similarly caused the formation of a S33-P and S4-P-S8-P-positive hyperphosphorylated RPA2 (Fig. 1D, lane 7). These data indicate that replication stress (i.e. treatment with aphidicolin or HU) induces limited RPA2 modification including formation of S33-P. By contrast, more-extensive damage causes RPA2 hyperphosphorylation, which includes S4 and S8 modification (as well as formation of S29-P and T21-P; see Fig. 3B) (Anantha et al., 2007).
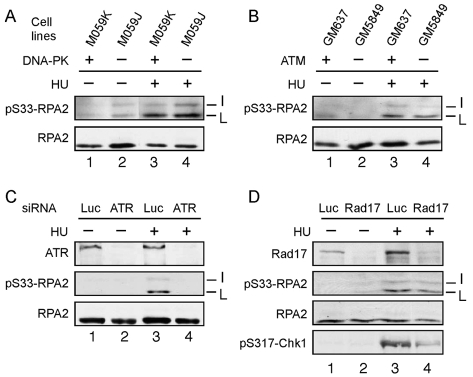
Fig. 3.
S33-RPA2 phosphorylated by ATR in response to replication stress. (A,B) The M059J (DNA-PK–/–) or M059K (DNA-PK+/+) paired cell lines (A), and GM637 (ATM+/+) or GM5849 (ATM–/–) lines (B) were either mock-treated or treated with 2.5 mM HU for 3 hours to cause replication stress. (C) U2-OS cells were transfected with an siRNA directed against ATR or a control siRNA against luciferase (Luc). After 48 hours, cells were either mock-treated or treated with 2.5 mM HU for 3 hours. (D) U2-OS cells were transfected with an siRNA directed against Rad17 or luciferase. For all panels, cells were lysed following treatment and the lysates subjected to SDS-PAGE and western blotting using antibodies specific to total RPA2, S33-P-RPA2, ATR, Rad17 or S317-P-Chk1, as indicated. The total RPA2 signal was used as a loading control. The positions of the various RPA2 species are indicated as in the Fig. 1 legend.
S33-P-RPA2 is formed in late-S and G2 phases
We examined the cell-cycle dependence of S33 phosphorylation in the absence of induced genotoxic stress. Cells were arrested in mitosis and then released into the cell cycle for various times (Fig. 2A). At each time point, both the cell-cycle distribution and the phosphorylation status of S33 were quantitated (Fig. 2B and 2C, respectively). Cells in G1 showed low levels of S33-P (4 and 8 hours), which increased as cells entered S phase (16 hours). The amount of S33-P-RPA2 continued to increase and reached a peak at 23 hours when cells were predominantly in late S and in G2. Throughout the cell cycle, the predominant S33-P (L) band migrated near the basal RPA2 form. This S33-P-RPA2 species was also detected using the general anti-RPA2 antibodies as a weak band migrating just above basal RPA2 (Fig. 2C, marked with `*') and with similar kinetics of appearance. Weaker S33-P-positive RPA2 species migrating in the I and H forms were also detected. These data indicate that a fraction of the RPA pool becomes phosphorylated on S33-RPA2 in a normal cell cycle, as cells proceed through S phase into G2. Use of epifluorescence microscopy similarly demonstrated the presence of a small number of S33-P-RPA2-positive foci (usually in the range of two to five foci), which were present selectively in the nuclei of late-S and G2 cells (supplementary material Fig. S1). From the results described above (see Fig. 1), we argue that this phosphorylation reflects the presence of residual stalled DNA-replication forks in a normal S phase.
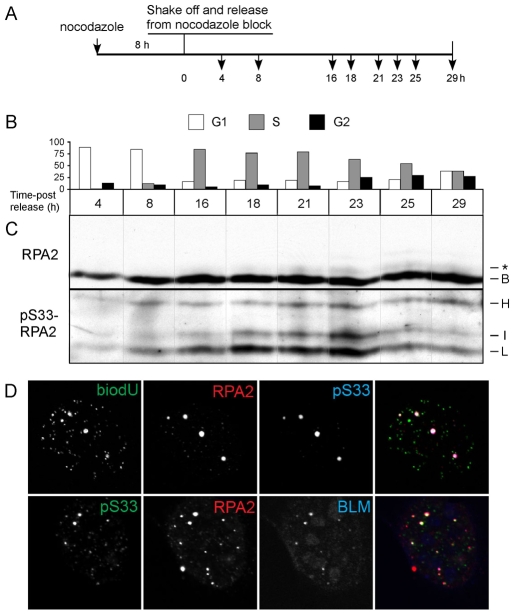
Fig. 2.
S33-P-RPA2 foci are formed in the late-S and G2 phases, and colocalize with BLM foci. (A) Schema of treatment. (B,C) U2-OS cells were treated with nocodazole for 8 hours and the mitotic cells then shaken off and resuspended in media lacking nocodazole. Cells were allowed to grow for various times, and aliquots taken and either subjected to FACS analysis for cell-cycle position (B) or lysed and analyzed by western blotting for total RPA2 and S33-P-RPA2 (C). (C) The positions of the various RPA2 species are indicated as described in the Fig. 1 legend. The `*' band indicates the putative RPA2 species phosphorylated only on S33, and migrating slightly above basal RPA2. (D) Nocodazole-arrested U2-OS cells were released for 23 hours, yielding a population of cells mostly in either the late-S or G2 phases. Just prior to fixation, an aliquot of cells were incubated with biotin-11-dUTP (biodU) to label sites of DNA synthesis. Cells were extracted to remove most soluble RPA, such that only DNA-bound RPA (at sites of replication or repair) remained. Images show representative cells stained for: (top) incorporated biodU, total RPA2 and S33-P-RPA2; and (bottom) S33-P-RPA2, total RPA2 and BLM. The signals are shown in grey tones for the left three images. The right image for each row shows the combined staining for all three markers. The colors of the three markers in the merged images are indicated by the color of letters for each stain.
The significance of S33 phosphorylation was further characterized by immunofluorescence microscopy. To determine whether S33-P-RPA2 is found at sites of DNA synthesis, cells were permeabilized and incubated with biotin-11-dUTP (biodU) to label sites of incorporation. Following fixation, cells were also stained for general RPA2 and S33-P-RPA2. Note that biodU is used here rather than BrdU because the procedure for detecting incorporated BrdU generally involves harsh conditions (e.g. 2 M HCl) that can interfere with subsequent detection of proteins. As shown in an image of a typical late-S–G2 cell (Fig. 2D, top panel), a small number of S33-P-positive foci were detected that colocalized with total RPA2 and sites of biodU incorporation. Additional images of non-stressed cells demonstrate similar patterns of colocalizing S33-P-RPA2 and biodU foci (supplementary material Fig. S2), with such patterns observed in a majority of cells. We conclude that S33-P-RPA2 formation is associated with sites of DNA synthesis, probably stalled (or slowly advancing) DNA-replication forks.
BLM is a recQ-like DNA helicase whose gene is mutated in Bloom syndrome, a disease resulting in cancer predisposition and developmental defects (Ouyang et al., 2008). Under genotoxic-stress conditions, BLM moves to DNA-damage foci (Davalos and Campisi, 2003). Testing the colocalization of RPA and BLM, we observed that nearly all BLM-positive foci also showed S33-P staining (Fig. 2D, bottom panel). S33-P-RPA2 was also found to colocalize with γH2AX (see below). These data indicate that S33-P forms at sites of DNA damage.
ATR phosphorylates S33 in response to replication stress
We determined the kinase(s) responsible for S33 phosphorylation under replication-stress conditions. Because S33 is a PIKK site, we restricted our analysis to DNA-PK, ATM and ATR. The role of DNA-PK in modifying S33 was tested using the M059J (DNA-PK–/–) or M059K (DNA-PK+/+) paired cell lines (Allalunis-Turner et al., 1993). Aliquots of each cell type were mock-treated or treated with HU to cause replication stress, and the lysates then subjected to western blot analysis for S33-P-RPA2. The absence of DNA-PK did not have any notable effects on the L and I forms of phosphorylated RPA2 (Fig. 3A). A similar test of ATM using cells defective for ATM kinase activity (ATM–/–) and a paired wild-type ATM control cell line (ATM+/+) showed no significant difference between the control and mutant cell lines on S33-P formation. Finally, the influence of ATR on S33 phosphorylation was examined using RNA interference to knock down ATR levels (Fig. 3C). Transfection of a small interfering RNA (siRNA) specific for ATR was observed to reduce ATR protein levels by >90%, compared with control cells transfected with a luciferase siRNA (Luc). Treatment with HU demonstrated that the appearance of both the S33-P-positive L and I species of RPA2 was selectively inhibited in the cells lacking ATR (Fig. 3C, lane 4). These data indicate that ATR, but not ATM or DNA-PK, is the primary S33 kinase in response to replication stress. A similar conclusion was recently made by Olson et al. (Olson et al., 2006).
Chk1 is a key effector kinase activated by ATR phosphorylation (Paulsen and Cimprich, 2007). Chk1 modification by ATR is also indirectly dependent upon the Rad17-RFC complex. Rad17-RFC loads the Rad9-Hus1-Rad1 (9-1-1) complex onto DNA at sites of damage (Kondo et al., 2001; Melo et al., 2001; Zou et al., 2002), with the bound 9-1-1 supporting recruitment and activation of Chk1 (Zou et al., 2002). Because RPA is a direct ATR target, we examined whether S33 phosphorylation is also dependent on the Rad17-RFC complex. The human Rad17 protein was down-modulated by use of a specific siRNA (Fig. 3D, lane 2), in comparison with cells transfected with a luciferase (control) siRNA (Fig. 3D, lane 1). HU treatment of control cells showed robust phosphorylation of S317-Chk1 and S33-RPA2 (Fig. 3D, lane 3). Rad17-deficient cells were found to have defective S317-Chk1, but not S33-RPA2, phosphorylation (Fig. 3D, lane 4). These data demonstrate that S33-RPA2 phosphorylation is independent of Rad17-RFC- and presumably 9-1-1-complex loading at sites of DNA-replication stress.
Response of DNA replication to HU treatment
We examined in more detail the effect of HU treatment on chromosomal DNA synthesis and chromatin association of RPA in U2-OS cells. At various times post-treatment, cells were incubated with BrdU and then fixed. Cells were then stained with anti-BrdU and -RPA2 antibodies and imaged. Under our fixation conditions, soluble RPA is extracted, so that the retained RPA fraction is primarily chromatin-bound (Dimitrova and Gilbert, 2000). We quantitated both the fraction of BrdU- and RPA-positive cells, and the average BrdU and RPA signals in these cells. A representative experiment demonstrating the appreciable BrdU staining signal in cells exposed to 1.5 mM HU is included (supplementary material Fig. S3). Following treatment with a low HU concentration (1 mM), the fraction of BrdU-positive cells initially decreased from >55% to ∼15%, before recovering to near control levels (Fig. 4A). The level of BrdU incorporation in these cells showed an even more dramatic drop at initial times of treatment, followed by a weak recovery in which cells were incorporating BrdU at ∼15% of control levels (Fig. 4B). The formation of S4-P S8-P was low under these conditions (see Fig. 1B, lanes 2 to 6), indicating that replication-fork collapse does not appreciably occur under these conditions. By contrast, when a high HU concentration (5 mM) was used, a nearly complete abolition of DNA synthesis occurred, and only a marginal recovery phase was seen. Nearly all cells stained positive for RPA over the course of the experiment, regardless of HU concentration, although those treated with high HU also showed a corresponding increase in formation of S4-P-S8-P-RPA2, indicative of DSB formation following replication-fork collapse (Fig. 4C).
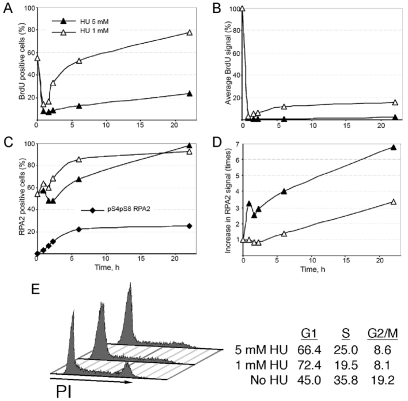
Fig. 4.
Effects of HU on chromosomal DNA replication and RPA chromatin loading. U2-OS cells on coverslips were incubated with 1 or 5 mM HU for various times. (A,B) For measurement of DNA synthesis, cells were pulsed with BrdU and then processed as indicated in Materials and Methods to quantitate both the percentage of BrdU-positive cells (A) and the average BrdU signal in BrdU-positive cells (B). For the latter determination, the signal in cells not treated with HU (i.e. 0-hour time point) was normalized to 100%. See supplementary material Fig. S3 for representative images of cells stained for BrdU. (C,D) Following HU treatment, cells were fixed and then stained with anti-RPA2 antibodies and imaged by epifluorescence microscopy. Both the fraction of RPA2-positive cells (in percent; C) and the relative RPA2 signal in RPA2-positive cells (D) were determined. (D) The average RPA2 signal in mock-treated cells was normalized to a value of 1.0. (E) Effect of HU on cell-cycle distribution. U2-OS cells were either mock-treated or subjected to treatment with 1 or 5 mM HU for 48 hours. Cells were then analyzed by FACS for DNA content. The relative percentages of cells in G1, S and G2-M phases were quantitated and are shown on the right.
Of note, the average RPA2 signal per cell increased following HU treatment, with the use of high HU levels found to cause an ∼sevenfold increase in chromatin-bound RPA at late times post-treatment (Fig. 4D). Because the extraction procedure retains only chromatin-bound RPA, the level of non-extracted RPA provides a general indicator of the amount of ssDNA present in cells. Chromatin-associated RPA has been validated as an indicator of ssDNA by others (e.g. Raderschall et al., 1999). This increase in ssDNA probably occurs as a consequence of DNA-polymerase stalling even while the replicative DNA helicase continues to unwind the duplex DNA at the fork.
The cell-cycle profiles of U2-OS cells were determined after a 48-hour exposure to HU. Following treatment with 1 mM HU, we saw a decline in the fractions of both S (from 35.8 to 19.5%)- and G2-M (from 19.2 to 8.1%)-phase cells, whereas G1-phase cells increased (from 45.0 to 72.4%) (Fig. 4E). Although cells treated with 5 mM HU had a similar decrease in G2-M cells (to 8.6%), the loss in S-phase cells was somewhat reduced (to 25.0%). These data indicate that a significant fraction of replicating cells exposed to 1 mM HU are eventually able to exit S phase and reach the subsequent G1 phase. Cells incubated with the higher (5 mM) HU concentration evinced a smaller fraction of cells able to exit S phase.
The S33A-RPA2 mutation reduces DNA synthesis catalyzed during DNA-replication stress
To examine the functional role of RPA phosphorylation, we generated stable cell lines that allow inducible expression of an untagged mutant or wild-type RPA2 subunit. Clones were chosen that express RPA2 at a level similar to that of the endogenous factor (e.g. see supplementary material Fig. S4). Expression is coupled with knock down of endogenous RPA2 using an siRNA duplex that targets a 3′ UTR sequence specific to the endogenous RPA2 mRNA. To minimize stress caused by replacement, the ectopic RPA2 is first induced, followed by knock down of the endogenous protein (Fig. 5A). The `replaced' cells are then assayed 72 hours post-siRNA transfection. The efficiency of the approach and key controls were previously presented (Anantha et al., 2007; Anantha et al., 2008) (see also below).
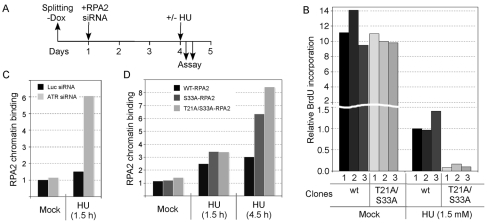
Fig. 5.
The T21A-S33A-RPA2 mutation inhibits DNA repair synthesis and increases ssDNA accumulation during replication stress. (A) Schematic indicating the steps involved in cellular RPA2 replacement. `–Dox' indicates the removal of doxycycline to cause the induction of ectopic RPA2 expression. Three U2-OS clones each that express either wt-RPA2 or a mutant RPA2 variant were invariably tested. Ectopic RPA2 was induced and, 24 hours later, the endogenous RPA2 was silenced. 3 days later, cells were either mock-treated (no stress) or treated with HU for various times and processed. (B) U2-OS clones were replaced with either wt-RPA2 or T21A-S33A-RPA2, and then either mock- or HU-treated (1.5 mM) for 4.5 hours. Cells were then incubated with BrdU and processed as described in Materials and Methods for analysis of BrdU incorporation. Approximately 500 cells were analyzed for each clone, quantitating the average BrdU signal from all cells. (C,D) U2-OS cells were transfected with siRNA molecules against luciferase (Luc; control) or ATR (C), or cloned cells replaced with either wt-, S33- or T21A-S33A-RPA2 (D). Cells were then either mock- or HU-treated (5 mM) for 1.5 or 4.5 hours (as indicated). Following extraction and fixation, cells were stained for RPA2 and imaged by epifluorescence microscopy. The average signals of retained, and hence chromatin-bound, RPA2 were determined.
Using this replacement strategy, we examined the biological effects of RPA2 subunits mutated at both PIKK-phosphorylation sites (i.e. S33 and T21) to alanines. Replaced cells were treated with HU and then incubated with BrdU to allow quantitation of chromosomal DNA synthesis and fixed. Cells were stained with anti-BrdU antibodies and imaged, quantitating the average BrdU signal from all cells. In the absence of exogenous stress, the mutant clones (expressing RPA2 with mutations T21A and S33A; T21A/S33A-RPA) were seen to have a similar overall BrdU signal as the two wild-type RPA2 (wt-RPA2) clones (Fig. 5B). These data demonstrate that the T21A/S33A mutation does not have significant effects on the overall level of DNA synthesis during chromosomal DNA replication.
Next, the same clones were treated with a low concentration of HU to cause replication stress, and similarly analyzed. The level of BrdU incorporation for the HU-treated cells replaced with wt-RPA2 was ∼10% of that found in the same cells not exposed to HU. Significantly, under HU-treatment conditions, the S33A mutation caused a further >tenfold reduction in the level of DNA synthesis compared with wt-RPA2 cells. The T21A/S33A-RPA2 clones had both a reduced fraction of BrdU-positive cells and a lower BrdU signal in these cells. Thus, expression of the T21A/S33A-RPA2 mutant results in the inhibition of DNA synthesis selectively following HU treatment. Because of the reproducibility of the approach, hereafter we generally show the results from only a single representative clone, even though three clones were invariably analyzed for each RPA2 variant.
Lack of T21/S33-RPA2 phosphorylation increases ssDNA formation during replication stress
We examined the consequences of the T21A/S33A-RPA2 mutation on the generation of ssDNA during DNA-replication stress. We first tested the effect of ATR down-modulation on RPA-chromatin association. U2-OS cells were either treated with an siRNA specific for ATR, or a luciferase control. Following mock or HU treatment, cells were extracted and fixed, and the level of retained RPA quantitated after immunofluorescence microscopy (Fig. 5C). In the absence of genotoxic stress, there was only a minor effect of ATR down-modulation on the level of chromatin-associated RPA. Following exposure to HU, whereas control knockdown cells had a 1.5-fold increase in RPA staining, ATR-knockdown cells had a >sixfold increase in RPA staining. These data indicate that ATR activity serves to reduce the amount of ssDNA generated in cells undergoing replication stress. A similar phenomenon has been observed in yeast defective in the Mec1 pathway (Lopes et al., 2001; Sogo et al., 2002).
We next tested the effects of mutation of the two consensus PIKK sites on RPA-chromatin association (Fig. 5D). Under mock treatment conditions, no significant differences were observed in the level of RPA-chromatin association, indicative of similar levels of ssDNA being present in each cell line on average. Following a 1.5-hour treatment with HU, cells replaced with S33A-RPA2 or T21A/S33A-RPA2 each had a ∼3.3-fold increase in RPA-chromatin association, which is modestly higher than the 2.5-fold increase seen in cells replaced with wt-RPA2. After a longer treatment with HU (4.5 hours), whereas the wt-RPA2 cells showed a threefold increase in chromatin-associated RPA, the S33A-RPA2 and T21A/S33A-RPA2 cells had 6.3-fold and 8.4-fold increases in chromatin-bound RPA, respectively. These higher levels of retained RPA indicate that cells replaced with S33A-RPA2 and T21A/S33A-RPA2 have more ssDNA present under replication-stress conditions compared with cells replaced with wt-RPA2.
Mutation of the T21 and S33 sites inhibits recovery from genotoxic stress
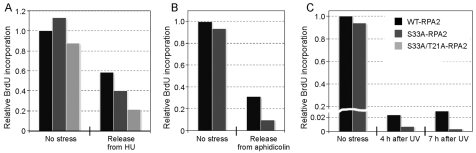
We examined the role of the T21 and S33 sites in recovery from genotoxic stress, first testing HU. Replaced cells were first synchronized in G1 with mimosine and then released. After allowing cells to proceed into S phase, cells were treated with 5 mM HU for 4 hours, released from the HU block for 40 minutes and the relative BrdU incorporation then determined (Fig. 6A). Whereas the level of DNA synthesis in wt-RPA2 cells recovered to ∼60% of the level found in non-stressed cells, only a ∼22% recovery was detected in cells replaced with the T21A/S33A-RPA2 mutant. The recovery of the S33A-RPA2 mutant was intermediate between the wt-RPA2 and T21A/S33A-RPA2 cells. Similarly, 30 minutes after release from a 7-hour treatment with aphidicolin, cells replaced with S33A-RPA2 showed a significantly reduced level of DNA synthesis compared with wt-RPA2 cells (Fig. 6B). The S33A-RPA2 cells were also defective in recovery from UV irradiation (Fig. 6C). These data demonstrate that cells expressing RPA2 phosphorylation mutants are defective in recovery from replication stress.
Fig. 6.
Effect of mutation of RPA2 PIKK sites on recovery from DNA-replication stress. U2-OS clones were replaced with either wt-, S33- or T21A/S33A-RPA2. (A) Cells were synchronized in G1 with 0.5 mM mimosine for 24 hours and then released from the block for 7 hours. Cells were then either mock- or HU-treated (5 mM) for 4 hours and released into fresh media for 40 minutes. (B) Replaced cells were mock or aphidicolin-treated (30 μM) for 7 hours and then released into fresh media for 40 minutes. (C) Replaced cells were subjected to UV irradiation (70 J/m2 of UVB) and further incubated for 4 or 7 hours. In all cases, cells were then incubated with 20 μM BrdU for 30 minutes, fixed and processed as described in Materials and Methods for analysis of BrdU incorporation.
Correlation between S33-P and γH2AX signals
We examined the levels of the DNA-damage marker γH2AX in cell lines replaced with wt- or S33A-RPA2 (Fig. 7A). Cells were treated with a low concentration of HU (1 mM for 20 hours), which allows residual DNA replication yet causes replication stress (e.g. see Fig. 4B). Lysates were prepared and subjected to western analysis against various targets (Fig. 7B). Although mock-treated wt-RPA2 cells had only background levels of S33-P, this signal increased after HU treatment. Whereas the signal was mostly found at the L and I positions, indicative of replication-fork stalling, a clear S33-P signal migrating at the hyperphosphorylated (H) position was also detected (Fig. 7B, lanes 7-9). Importantly, cells expressing wt-RPA2 also showed significant signals of both S4-P-S8-P-RPA2 and γH2AX.
Fig. 7.
Cells replaced with the T21A/S33A-RPA2 mutant have a reduced γH2AX signal during replication stress. (A) Schematic indicating the experimental protocol. (B) Three U2-OS clones each were replaced with wt- or S33A-RPA2. At 3 days post-silencing, cells were either mock-treated or incubated with 1 mM HU for 20 hours. Lysates were then prepared and subjected to western analysis against γH2AX, RPA2 (total), S33-P-RPA2, S4-P-S8-P-RPA2 and β-actin (loading control). Also included as a control is a lysate from U2-OS cells that were silenced but not replaced (lane 13). Numbers below γH2AX panel indicate the intensity of γH2AX staining, normalized to β-actin levels. (C) Identical to panel B except that two clones each of cells replaced with wt- or T21A/S33A-RPA2 were treated with 5 mM HU for 5 hours. (D) U2-OS cells were treated with 1 mM HU for 10 hours and then fixed. Cells were stained with antibodies against S33-P-RPA2 and γH2AX. Approximately 300 cells were analyzed by immunofluorescence microscopy for the intensity of two stains, and these signals plotted against each other. (E) Similar to panel D, except that cells were treated with 5 mM HU for 5 hours and assayed for S4-P-S8-P-RPA2 and γH2AX. (F,G) Aliquots of cells described in D were stained for S33-P-RPA2 and either γH2AX (F) or a modified TUNEL assay to directly detect DNA ends (G). Cells were then imaged by immunofluorescence microscopy. The right image for each panel shows the combined staining for the two markers, with the colors of each marker indicated by the color of letters for each stain.
As expected, cells replaced with S33A-RPA2 showed a great loss of the S33-P signal, indicating the efficiency of the replacement strategy. These cells also showed a reduced S4-P-S8-P signal, also not unexpected because we found previously that S4 and S8 phosphorylation is stimulated by S33-P formation (Anantha et al., 2007). Surprisingly, clones replaced with S33A-RPA2 showed a ∼2.5-fold decrease in the γH2AX signal, compared with the γH2AX levels in cells replaced with wt-RPA2 (Fig. 7B, lanes 10-12). Similarly, cells expressing T21A/S33A-RPA2 (Fig. 7C, lanes 7 and 8) had an ∼fourfold lower γH2AX signal compared with wt-RPA2 cells (Fig. 7C, lanes 5 and 6), selectively following HU (5 mM) treatment.
As mentioned above, past studies have failed to detect any notable effects of RPA phosphorylation on checkpoint kinase activation following interphase DNA damage (Morgan and Kastan, 1997; Olson et al., 2006). The greater levels of γH2AX are therefore indicative of more DNA breaks, rather than any defects in checkpoint signaling. We interpret these results to indicate that RPA phosphorylation can stimulate DNA synthesis during replication stress (see Fig. 5B), but this incorporation heightens the possibility of DNA breakage. Nevertheless, we demonstrate below that RPA phosphorylation enhances overall cell viability during stress.
To examine the link between the S33-P and γH2AX signals in greater detail, U2-OS cells were subjected to HU treatment (1 mM for 10 hours), dually stained with antibodies against S33-P and γH2AX, and then analyzed by immunofluorescence microscopy. Nearly 300 cells were analyzed for the levels of both the S33-P and γH2AX signals, and these were then plotted against each other (Fig. 7D). As expected, the two signals demonstrated significant correlation. Note that the low concentration of HU in this experiment favor RPA2 phosphorylation up to the L and I levels, rather than formation of hyperphosphorylated RPA (see Fig. 1A). The S4-P-S8-P signal for hyperphosphorylated RPA was also examined following treatment with 5 mM HU (Fig. 7E). A similar relationship was found between the cellular levels of S4-P-S8-P-RPA2 and γH2AX, even though the actual levels of staining showed a weaker correspondence as compared with the S33-P and γH2AX signals.
The correlation between S33-P and γH2AX signals also occurred at the level of nuclear foci, because immunofluorescence imaging of HU-treated cells showed that a significant fraction of S33-P-positive foci colocalized with γH2AX (Fig. 7F). Because γH2AX is only an indirect indicator of DNA damage, we verified this association by an independent assay, using immunofluorescence microscopy in combination with a modified TUNEL assay to directly detect DNA nicks and/or ends. Using this assay, S33-P-positive foci were found also to contain DNA ends, and hence were sites of DSBs or nicks (Fig. 7G). In summary, we conclude that cells expressing wt-RPA2 support increased DNA synthesis under DNA-replication-stress conditions, yet this synthesis correlates with an increased level of DNA breaks.
RPA phosphorylation-site mutations alter cell-cycle profile and reduce viability
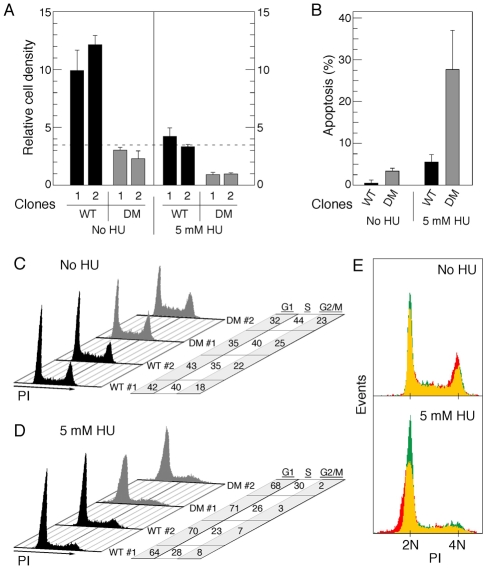
We examined whether the loss of RPA2 phosphorylation at the two consensus PIKK sites had additional functional consequences. Strong differences were noted when cell proliferation was tested in U2-OS cells replaced with wt-RPA2 or T21A/S33A-RPA2. Whereas clones expressing wt-RPA2 demonstrated a ∼threefold expansion when assayed 48 hours after plating, the T21A/S33A-RPA2 clones showed little change in growth (Fig. 8A). Under replication-stress conditions (5 mM HU for 48 hours), the wt-RPA2 clones had minimal changes in cell density. By contrast, only 30% of cells expressing T21A/S33A-RPA2 survived, with visible accumulation of cell debris (data not shown). These data indicated that the T21A/S33A mutation had deleterious effects on cell viability. To test this directly, U2-OS cells replaced with the wt-RPA2 or T21A/S33A-RPA2 variants were either mock-treated or exposed to 5 mM HU for 24 hours, and then assayed for apoptosis (Fig. 8B). The level of apoptosis was low in both cell lines under control conditions, even though an ∼sevenfold higher fraction of T21A/S33A-RPA2 cells were TUNEL-positive (apoptotic), compared with cells replaced with wt-RPA2. Following HU treatment, a massive fraction of the T21A/S33A-RPA2 cells underwent apoptosis (>27%), compared with ∼6% of the wt-RPA2 cells. Thus, the lack of proper RPA phosphorylation significantly reduces cell viability, both under control and replication-stress conditions.
Fig. 8.
Mutation of RPA2 PIKK sites impairs cell growth and alters the cell-cycle profile. (A) Two U2-OS clones each were replaced with either wt- or T21A/S33A-RPA2 (DM, double mutant). At 72 hours post-siRNA transfection, cells were plated in triplicate to an equivalent relative density (0.35; indicated by dashed line) and then either mock-treated or incubated with 5 mM HU. 48 hours later, cells were released from the plate by trypsin-EDTA treatment and cell count in each plate determined. (B) U2-OS clones replaced with either wt- or T21A/S33A-RPA2 (DM) were, at 72 hours post-siRNA transfection, mock-treated or incubated with 5 mM HU for 24 hours. Cells were then processed to quantify the percentage of cells undergoing apoptosis, using a TUNEL assay. (C,D) Similar aliquots of cells described in A were either mock- or HU-treated (5 mM for 48 hours) and then subjected to FACS analysis for DNA content. Cell-cycle profiles and relative distributions (in percent of total) in the G1, S and G2-M phases are shown. (E) Overlay of representative cell distributions from clones replaced with wt-RPA2 (green) or T21A/S33A-RPA2 (red), prepared as described in B and C. Regions of overlap are shown by yellow coloring.
The cell-cycle distribution of the different lines was analyzed by FACS, using similar conditions and gating the populations to eliminate cellular debris. In the absence of HU, cells expressing wt-RPA2 or T21A/S33A-RPA2 had similar distributions, although we did reproducibly observe a slightly higher fraction of cells replaced with the doubly-mutated RPA2 in the S and G2-M phases, as compared with wt-RPA2 cells (Fig. 8C). A similar finding was previously made in our studies of cells expressing RPA2 mutated at the two cyclin-Cdk sites (Anantha et al., 2007). Following a 48-hour treatment with HU, similar fractions of wt-RPA2 and T21A/S33A-RPA2 cells exited S phase and became synchronized at the G1-S boundary of the subsequent cell cycle (Fig. 8D), similar to the effect of HU on the parental U2-OS cells (Fig. 4E). Two significant differences were noted between HU-treated wt-RPA2 and T21A/S33A-RPA2 cells. First, the residual G2-M-phase cells replaced with T21A/S33A-RPA2 were nearly completely depleted in comparison with the wt-RPA2 cells (i.e. 2 vs 7%, respectively). Second, we found that the distribution of G1-phase cells expressing T21A/S33A-RPA2 was significantly wider than the wt-RPA2 cells, with a clear shift of mutant cells towards the <2N side of the G1 peak. This effect is more clearly visible when the two distributions were overlaid (Fig. 8E). From these data, we suggest that the mutation of RPA2 phosphorylation sites causes a defect in the proper transmission of chromosomal DNA to the subsequent cell cycle, following severe replication stress.
Discussion
Conditions of DNA-replication stress cause the stalling of one or more replicative DNA polymerases, resulting in uncoupling of the advancing DNA helicase from the polymerase machinery. These events increase the steady-state amount of ssDNA, as seen both in this study and previously by others (Byun et al., 2005; Lopes et al., 2001; Sogo et al., 2002), and lead to activation of the ATR kinase (Zou and Elledge, 2003; Namiki and Zou, 2006). The resulting phosphorylation of RPA by ATR facilitates additional phosphorylation events on RPA by other kinases, dependent on the degree of stress and cell-cycle position (Anantha et al., 2007; Anantha et al., 2008; Anantha and Borowiec, 2009) (and this work). We demonstrate that the phosphorylated RPA formed under replication-stress conditions stimulates DNA synthesis and reduces the amount of ssDNA generated as assayed by chromatin-bound RPA. In the absence of stress-dependent RPA phosphorylation, cells undergo high levels of apoptosis and a reduction in the rate of cell proliferation under both normal and stress conditions.
Increased ssDNA accumulation following replication stress was observed both in cells expressing RPA2 phosphorylation mutants and in cells that were down-modulated for ATR. Because the mutant cells also show reduced DNA synthesis during stress, a simple interpretation is that these two events are directly coupled. That is, phosphorylated RPA stimulates stress-dependent DNA synthesis by an as-yet-unidentified DNA polymerase on the persistent ssDNA, thereby minimizing ssDNA accumulation (Fig. 9). This model is consistent with our finding that, in response to a low concentration of HU, cells can partially recover DNA synthesis from levels observed soon after stress onset (see Fig. 4B). Thus, an important conclusion from this study is that RPA not only signals the presence of stress and stimulates the activation of ATR, but the accompanying phosphorylation of RPA facilitates events at the site of the stalled fork that alleviate the consequences of stress. In other words, the initial modification of RPA in response to stress stimulates changes to the replication and/or repair machineries that eventually lead to a reduction in stress signaling and loss of RPA phosphorylation.
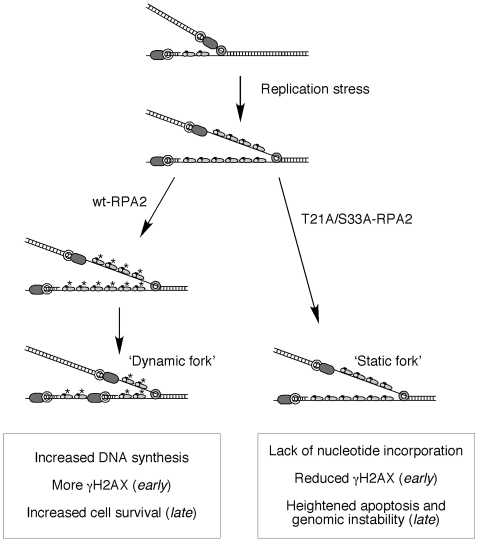
Fig. 9.
Model indicating the roles of RPA phosphorylation in response to DNA-replication stress. See text for details.
We found that the down-modulation of ATR caused more RPA-chromatin binding, and hence ssDNA accumulation, compared with either of the two RPA2 mutants tested (see Fig. 5). These data indicate that, although RPA2 is an important ATR target for counteracting the effects of replication stress, it is clearly not the only target. Recent analysis of ATM and ATR targets that are modified during genotoxic stress (i.e. IR) found numerous substrates involved in DNA replication and repair, including subunits of the MCM (the putative replicative helicase) and RFC (PCNA clamp loader) complexes, and the DNA polymerase-ε catalytic subunit (Matsuoka et al., 2007). It is apparent that alteration of the activities of such factors could have complementary effects to RPA phosphorylation in the response to replication stress and the minimization of ssDNA accumulation.
One of the more intriguing findings of our study is that cells replaced with wt-RPA2 show higher levels of both γH2AX and DNA ends during replication stress compared with cells replaced with the mutant RPA2 variants. Note that RPA phosphorylation does not have significant effects on checkpoint activation in interphase cells (Morgan and Kastan, 1997; Olson et al., 2006), indicating that this result is not a result of defective cell-cycle checkpoints. In our experiments, replication stress was induced by treatment with the ribonucleotide-reductase inhibitor HU, which reduces cellular dNTP pools (Yarbro, 1992). It is possible that the replication complex catalyzing DNA synthesis under such conditions is prone to undergo collapse and further damage, including DSBs. Alternatively, the observed increase in DNA breaks might not be reflective of true damage, but rather a normal event in the processing of stalled DNA-replication forks. Regardless of the cause, it is clear that the events stimulated by RPA phosphorylation significantly increase cell viability both under stress and non-stress conditions.
Although we interrogated the role of RPA phosphorylation in the presence of HU, formation of S33-P-RPA2 was also detected in non-stressed cells, particularly at the S-G2-phase transition of the cell cycle. These data presumably reflect the gradual accumulation of stalled DNA-replication forks as cells progress through S phase. S33-P-RPA2 colocalized with the BLM protein, as well as with sites of DNA synthesis, indicating that the molecular events regulated by phosphorylated RPA probably occur within nuclear repair foci. That said, mutation of the RPA2 phosphorylation sites was not seen to have any dramatic effects on BLM-foci formation (data not shown), indicating that BML localization to sites of DNA-replication stress is independent of modification of these RPA2 residues.
Lastly, our data suggest that abnormal RPA phosphorylation increases genomic instability. We reproducibly observed that chronically stressed cells replaced with the T21A/S33A-RPA2 mutant yielded a distribution of G1 cells with a broader DNA distribution than that seen with wt-RPA2 cells. The mutant distribution was skewed to the <2N side of the G1 peak, potentially indicative of a significant loss of chromosomal DNA. Although identification of the molecular basis for the effect is ongoing, two possibilities come to mind. First, replication forks stalled by HU treatment are not properly resuscitated in cells expressing the T21A/S33A-RPA2 mutant. These stalled replication structures are subsequently repaired by error-prone pathways, leading to loss and gain of DNA from chromosomal DNA regions and thereby generating the distribution of G1 cells we observe by FACS. Alternatively, recent work by our laboratory found a role for RPA phosphorylation in stimulating cellular transit through a damaged mitosis (Anantha and Borowiec, 2009; Anantha et al., 2008). We found that mitotic DNA damage in cells replaced with an RPA2 phosphorylation mutant (i.e. in which the two cyclin-Cdk sites at S23 and S29 were changed to alanines) had an increased failure of cytokinesis compared with cells replaced with wt-RPA2. These data raise the possibility that defective RPA2 phosphorylation reduces the fidelity of mitotic chromosomal segregation under conditions of severe DNA-replication stress, thereby increasing cellular aneuploidy. Further investigation will be required to analyze the spectrum of mutagenic events that our data suggest is induced by defective RPA phosphorylation under conditions of DNA-replication stress.
Materials and Methods
Cell culture
U2-OS cell lines were cultured in McCoy's medium supplemented with 10% FBS. Cell lines positive (MO59K) or null (MO59J) for the DNA-PK catalytic subunit were cultured in DME/F12 (1:1; v:v) containing 10% FBS. Human fibroblast cell lines positive (GM637) or null (GM5849) for ATM (Coriel Institute, Camden, NJ) were cultured in McCoy's medium with 15% FBS. For synchronization experiments, cells were arrested in prometaphase by treatment with 100 ng/ml nocodazole (Sigma-Aldrich) for 8 hours and the mitotic cells isolated by shake-off.
All experiments involved the use of untagged RPA2. To generate the S33A-RPA2 and T21A/S33A-RPA2 variants, the parental pRetro-Off retroviral vector (Clontech) containing the wt-RPA2 coding sequence (Anantha et al., 2007) was subjected to site-directed mutagenesis using the Stratagene QuikChange Kit. U2-OS clones containing the integrated pRetro-Off RPA2 expression vector were prepared and isolated as described by Anantha et al. (Anantha et al., 2007). For RPA2 replacement, the appropriate U2-OS clone was first grown for 24-48 hours in medium lacking doxycycline to induce expression of the ectopic RPA2. Cells were then transfected (Hiperfect; Qiagen) with an siRNA molecule that selectively targets the endogenous RPA2 mRNA (Anantha et al., 2007), and the cells then tested 72 hours post-transfection. A representative western analysis of RPA2 protein levels following endogenous RPA2 silencing and ectopic RPA2 induction is shown (supplementary material Fig. S4).
Immunoblotting and antibodies
For western analysis, cells were directly lysed in SDS-PAGE sample buffer and the lysate proteins were separated by SDS-PAGE. Proteins were immobilized onto Protran nitrocellulose membranes (0.2-μm pore size). The antibodies used in this study were against general RPA2 (NeoMarkers), γH2AX (Upstate), S4-P-S8-P-RPA2 and S33-P-RPA2 (Bethyl Laboratories), T21-P-RPA2 (Abcam), ATR (Novus Biologicals), PML (Abnova), BLM (Santa Cruz), Rad17 (Santa Cruz), and S317-P-Chk1 (Bethyl Laboratories). The S29-P-RPA2 antibody was custom-synthesized as described (Anantha et al., 2007).
Immunofluorescence microscopy
For detection of chromatin-bound RPA, S33-P-RPA2 or BLM, cells were first extracted with CSK buffer (10 mM HEPES-KOH, pH 7.4, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2) containing 0.5% (v/v) Triton X-100 for 5 minutes on ice. Cells were then washed with CSK buffer lacking Triton X-100 and fixed with 4% (w/v) paraformaldehyde for 30 minutes. To quantitate chromatin-bound RPA, cells were stained with a mouse anti-RPA2 primary antibody and an anti-mouse Cy3-conjugated secondary antibody. A total of seven fields containing 300-400 cells were analyzed for each experiment, using IPLab software (BD Biosciences) to quantitate the Cy3 (i.e. RPA2) signal within the DAPI-stained nuclear region. In all cases, the average background signal in cells considered to be RPA2-negative was subtracted to determine the `true' RPA2 signal. When `RPA2-positive' cells were assayed, only those cells with RPA2 signals above background were analyzed.
BrdU incorporation
Cells were incubated in media containing 20 μM BrdU for 20 minutes and 1 hour for untreated and HU-treated cells, respectively, to yield a BrdU signal in the dynamic range. Cells were briefly extracted with CSK buffer containing 0.5% (v/v) Triton X-100 and fixed as above. Cells were then treated with 2 M HCl for 50 minutes and subjected to immunofluorescence microscopy using rat anti-BrdU primary antibodies (Harlan Sera-Lab) and Alexa-Fluor-499-conjugated secondary antibodies (Invitrogen). Photographs were taken on Zeiss AxioCam camera with a 40× objective and analyzed as described above for RPA2-signal quantitation. See supplementary material Fig. S3 for a representative experiment.
BiodU incorporation
U2-OS cells were arrested with nocodazole and the mitotic cells then released into nocodazole-free medium for 18 hours. Cells (on 18-mm coverslips) were washed at 37°C with ABC buffer (50 mM KCl, 20 mM creatine phosphate, pH 7.4, 3 mM MgCl2, 1 mM ATP and 11 mM K2HPO4) and then incubated at 37°C for 5-10 minutes with ABC buffer containing 250 μM each of dNTPs (dATP, dCTP and dGTP), 100 μM each of rNTPS (UTP, CTP and GTP), 50 μM biotin-11-dUTP, 0.5 U/μl streptolysin-O (Sigma-Aldrich) and 20 μg/ml creatine phosphokinase. Cells were washed with cold CSK buffer, incubated on ice for 2 minutes with CSK buffer containing 0.5% (v/v) Triton X-100 and then washed again with cold CSK buffer. Cells were fixed with 4% (w/v) paraformaldehyde for 30 minutes at room temperature, washed three times with PBS and then stained with a goat anti-biotin primary antibody (Pierce).
To measure apoptosis, two U2-OS clones were each treated to replace endogenous RPA2 with wt-RPA2 or T21A/S33A-RPA2. At 72 hours post-siRNA transfection, cells were mock-treated or incubated with 5 mM HU for 24 hours. Apoptosis was then assayed using the In Situ Cell Death Detection Kit (Roche), as per the manufacturer's instructions.
Flow cytometry
FACS analysis for DNA content was performed as described by Anantha et al. (Anantha et al., 2007). FACS was performed on a Becton Dickinson flow cytometer using the Cell Quest software. Cell-cycle analysis was performed using the Mod-Fit software.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/22/4070/DC1
We thank Priscilla Maldonado for critical reading of the manuscript. This work was supported by an Exceptional Project Award Grant from the Breast Cancer Alliance, NIH grant R01 GM083185, the New York University Cancer Institute, the Rita J. and Stanley Kaplan Comprehensive Cancer Center, and National Cancer Institute Grant P30CA16087. Deposited in PMC for release after 12 months.
References
- Allalunis-Turner, M. J., Barron, G. M., Day, R. S., 3rd, Dobler, K. D. and Mirzayans, R. (1993). Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat. Res. 134, 349-354. [PubMed] [Google Scholar]
- Anantha, R. W. and Borowiec, J. A. (2009). Mitotic crisis: the unmasking of a novel role for RPA. Cell Cycle 8, 357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantha, R. W., Vassin, V. M. and Borowiec, J. A. (2007). Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair. J. Biol. Chem. 282, 35910-35923. [DOI] [PubMed] [Google Scholar]
- Anantha, R. W., Sokolova, E. and Borowiec, J. A. (2008). RPA phosphorylation facilitates mitotic exit in response to mitotic DNA damage. Proc. Natl. Acad. Sci. USA 105, 12903-12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehoyada, M., Gautier, J. and Dupre, A. (2007). The DNA damage response during an unperturbed S-phase. DNA Repair (Amst) 6, 914-922. [DOI] [PubMed] [Google Scholar]
- Binz, S. K., Sheehan, A. M. and Wold, M. S. (2004). Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amst) 3, 1015-1024. [DOI] [PubMed] [Google Scholar]
- Block, W. D., Yu, Y. and Lees-Miller, S. P. (2004). Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucl. Acids Res. 32, 997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush, G. S., Anderson, C. W. and Kelly, T. J. (1994). The DNA-activated protein kinase is required for the phosphorylation of replication protein A during simian virus 40 DNA replication. Proc. Natl. Acad. Sci. USA 91, 12520-12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun, T. S., Pacek, M., Yee, M. C., Walter, J. C. and Cimprich, K. A. (2005). Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19, 1040-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos, A. R. and Campisi, J. (2003). Bloom syndrome cells undergo p53-dependent apoptosis and delayed assembly of BRCA1 and NBS1 repair complexes at stalled replication forks. J. Cell Biol. 162, 1197-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova, D. S. and Gilbert, D. M. (2000). Stability and nuclear distribution of mammalian replication protein A heterotrimeric complex. Exp. Cell Res. 254, 321-327. [DOI] [PubMed] [Google Scholar]
- Dutta, A. and Stillman, B. (1992). cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 11, 2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F. and Newport, J. W. (1993). Distinct roles of cdk2 and cdc2 in RP-A phosphorylation during the cell cycle. J. Cell Sci. 106, 983-994. [DOI] [PubMed] [Google Scholar]
- Gibbs, E., Pan, Z. Q., Niu, H. and Hurwitz, J. (1996). Studies on the in vitro phosphorylation of HSSB-p34 and -p107 by cyclin-dependent kinases. Cyclin-substrate interactions dictate the efficiency of phosphorylation. J. Biol. Chem. 271, 22847-22854. [DOI] [PubMed] [Google Scholar]
- Guo, Z., Kumagai, A., Wang, S. X. and Dunphy, W. G. (2000). Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14, 2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricksen, L. A., Carter, T., Dutta, A. and Wold, M. S. (1996). Phosphorylation of human replication protein A by the DNA-dependent protein kinase is involved in the modulation of DNA replication. Nucl. Acids Res. 24, 3107-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftode, C., Daniely, Y. and Borowiec, J. A. (1999). Replication protein A (RPA): the eukaryotic SSB. Crit. Rev. Biochem. Mol. Biol. 34, 141-180. [DOI] [PubMed] [Google Scholar]
- Kondo, T., Wakayama, T., Naiki, T., Matsumoto, K. and Sugimoto, K. (2001). Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science 294, 867-870. [DOI] [PubMed] [Google Scholar]
- Liu, J. S., Kuo, S. R., McHugh, M. M., Beerman, T. A. and Melendy, T. (2000). Adozelesin triggers DNA damage response pathways and arrests SV40 DNA replication through replication protein A inactivation. J. Biol. Chem. 275, 1391-1397. [DOI] [PubMed] [Google Scholar]
- Liu, J. S., Kuo, S. R. and Melendy, T. (2006). DNA damage-induced RPA focalization is independent of gamma-H2AX and RPA hyper-phosphorylation. J. Cell Biochem. 99, 1452-1462. [DOI] [PubMed] [Google Scholar]
- Lopes, M., Cotta-Ramusino, C., Pellicioli, A., Liberi, G., Plevani, P., Muzi-Falconi, M., Newlon, C. S. and Foiani, M. (2001). The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412, 557-561. [DOI] [PubMed] [Google Scholar]
- Manthey, K. C., Opiyo, S., Glanzer, J. G., Dimitrova, D., Elliott, J. and Oakley, G. G. (2007). NBS1 mediates ATR-dependent RPA hyperphosphorylation following replication-fork stall and collapse. J. Cell Sci. 120, 4221-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, S., Ballif, B. A., Smogorzewska, A., McDonald, E. R., 3rd, Hurov, K. E., Luo, J., Bakalarski, C. E., Zhao, Z., Solimini, N., Lerenthal, Y. et al. (2007). ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160-1166. [DOI] [PubMed] [Google Scholar]
- Melo, J. A., Cohen, J. and Toczyski, D. P. (2001). Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev. 15, 2809-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, S. E. and Kastan, M. B. (1997). Dissociation of radiation-induced phosphorylation of replication protein A from the S-phase checkpoint. Cancer Res. 57, 3386-3389. [PubMed] [Google Scholar]
- Namiki, Y. and Zou, L. (2006). ATRIP associates with replication protein A-coated ssDNA through multiple interactions. Proc. Natl. Acad. Sci. USA 103, 580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, H., Erdjument-Bromage, H., Pan, Z. Q., Lee, S. H., Tempst, P. and Hurwitz, J. (1997). Mapping of amino acid residues in the p34 subunit of human single-stranded DNA-binding protein phosphorylated by DNA-dependent protein kinase and Cdc2 kinase in vitro. J. Biol. Chem. 272, 12634-12641. [DOI] [PubMed] [Google Scholar]
- Oakley, G. G., Patrick, S. M., Yao, J., Carty, M. P., Turchi, J. J. and Dixon, K. (2003). RPA phosphorylation in mitosis alters DNA binding and protein-protein interactions. Biochemistry 42, 3255-3264. [DOI] [PubMed] [Google Scholar]
- Olson, E., Nievera, C. J., Klimovich, V., Fanning, E. and Wu, X. (2006). RPA2 Is a Direct Downstream Target for ATR to Regulate the S-phase Checkpoint. J. Biol. Chem. 281, 39517-39533. [DOI] [PubMed] [Google Scholar]
- Ouyang, K. J., Woo, L. L. and Ellis, N. A. (2008). Homologous recombination and maintenance of genome integrity: cancer and aging through the prism of human RecQ helicases. Mech. Ageing Dev. 129, 425-440. [DOI] [PubMed] [Google Scholar]
- Pan, Z. Q., Amin, A. A., Gibbs, E., Niu, H. and Hurwitz, J. (1994). Phosphorylation of the p34 subunit of human single-stranded-DNA-binding protein in cyclin A-activated G1 extracts is catalyzed by cdk-cyclin A complex and DNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 91, 8343-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z.-Q., Park, C. H., Amin, A. A., Hurwitz, J. and Sancar, A. (1995). Phosphorylated and unphosphorylated forms of human single-stranded DNA-binding protein are equally active in simian virus 40 DNA replication and in nucleotide excision repair. Proc. Natl. Acad. Sci. USA 92, 4636-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen, R. D. and Cimprich, K. A. (2007). The ATR pathway: fine-tuning the fork. DNA Repair (Amst) 6, 953-966. [DOI] [PubMed] [Google Scholar]
- Raderschall, E., Golub, E. I. and Haaf, T. (1999). Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc. Natl. Acad. Sci. USA 96, 1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Filippo, J., Sung, P. and Klein, H. (2008). Mechanism of Eukaryotic Homologous Recombination. Annu. Rev. Biochem. 77, 229-257. [DOI] [PubMed] [Google Scholar]
- Seiler, J. A., Conti, C., Syed, A., Aladjem, M. I. and Pommier, Y. (2007). The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses. Mol. Cell. Biol. 27, 5806-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo, J. M., Lopes, M. and Foiani, M. (2002). Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297, 599-602. [DOI] [PubMed] [Google Scholar]
- Tercero, J. A. and Diffley, J. F. (2001). Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412, 553-557. [DOI] [PubMed] [Google Scholar]
- Tibbetts, R. S., Brumbaugh, K. M., Williams, J. M., Sarkaria, J. N., Cliby, W. A., Shieh, S. Y., Taya, Y., Prives, C. and Abraham, R. T. (1999). A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13, 152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsal-Kacmaz, K., Chastain, P. D., Qu, P. P., Minoo, P., Cordeiro-Stone, M., Sancar, A. and Kaufmann, W. K. (2007). The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol. Cell. Biol. 27, 3131-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassin, V. M., Wold, M. S. and Borowiec, J. A. (2004). Replication protein A (RPA) phosphorylation prevents RPA association with replication centers. Mol. Cell. Biol. 24, 1930-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Guan, J., Perrault, A. R., Wang, Y. and Iliakis, G. (2001). Replication protein A2 phosphorylation after DNA damage by the coordinated action of ataxia telangiectasia-mutated and DNA-dependent protein kinase. Cancer Res. 61, 8554-8563. [PubMed] [Google Scholar]
- Ward, I. M. and Chen, J. (2001). Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276, 47759-47762. [DOI] [PubMed] [Google Scholar]
- Yarbro, J. W. (1992). Mechanism of action of hydroxyurea. Semin. Oncol. 19, 1-10. [PubMed] [Google Scholar]
- Yarden, R. I., Pardo-Reoyo, S., Sgagias, M., Cowan, K. H. and Brody, L. C. (2002). BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat. Genet. 30, 285-289. [DOI] [PubMed] [Google Scholar]
- Zernik-Kobak, M., Vasunia, K., Connelly, M., Anderson, C. W. and Dixon, K. (1997). Sites of UV-induced phosphorylation of the p34 subunit of replication protein A from HeLa cells. J. Biol. Chem. 272, 23896-23904. [DOI] [PubMed] [Google Scholar]
- Zhao, H. and Piwnica-Worms, H. (2001). ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21, 4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L. and Elledge, S. J. (2003). Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542-1548. [DOI] [PubMed] [Google Scholar]
- Zou, L., Cortez, D. and Elledge, S. J. (2002). Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16, 198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.