Summary
Cohesins and their regulators are vital for normal chromosome cohesion and segregation. A number of cohesion proteins have also been localized to centrosomes and proposed to function there. We show that RNAi-mediated depletion of factors required for cohesion, including haspin, Sgo1 and Scc1, leads to the generation of multiple acentriolar centrosome-like foci and disruption of spindle structure in mitosis. Live-cell imaging reveals that, in haspin-depleted cells, these effects occur only as defects in chromosome cohesion become manifest, and they require ongoing microtubule dynamics and kinesin-5 (also known as Eg5) activity. Inhibition of topoisomerase II in mitosis, which prevents decatenation and separation of chromatids, circumvents the loss of cohesion and restores integrity of the spindle poles. Although these results do not rule out roles for cohesin proteins at centrosomes, they suggest that when cohesion is compromised, spindle-pole integrity can be disrupted as an indirect consequence of the failure to properly integrate chromosome- and centrosome-initiated pathways for spindle formation.
Keywords: Chromosome cohesion, Centrosome, Mitosis, Gsg2 Spindle assembly, Kinesin-5
Introduction
Proteins of the cohesin system provide cohesion between replicated chromosomes, regulate cohesion establishment during S phase and after DNA damage, and dissolve cohesion in a regulated manner during mitosis (Peters et al., 2008). The finding that Separase, a protease that cleaves cohesin Scc1 (also known as Rad21), is also required for centriole disengagement upon mitotic exit (Tsou and Stearns, 2006) suggests that other elements of the cohesin system might operate at centrosomes. In fact, a number of proteins that influence chromosome cohesion, including Separase, Scc1, SMC1, SA1 and haspin, have been reported to localize to centrosomes (Chestukhin et al., 2003; Dai et al., 2005; Gregson et al., 2001; Guan et al., 2008; Wang et al., 2008; Warren et al., 2000; Wong and Blobel, 2008) and it has been suggested that at least some of these proteins play a direct role in centrosome function. For example, human SMC1 has been reported to associate with NuMA at centrosomes, and immunodepletion of human SMC1 reduced the efficiency of mitotic aster formation in a HeLa cell extract system (Gregson et al., 2001), whereas its overexpression induced multipolar spindles in HeLa cells (Wong and Blobel, 2008). Furthermore, a splice variant of the cohesin protector Sgo1 is able to reduce the number of extra centrosome-like foci induced by Sgo1 RNAi in human cells (Wang et al., 2008). These observations have stimulated interest in the function of proteins of the cohesin system at centrosomes.
Haspin (also known as Gsg2) is a chromosomal kinase that phosphorylates histone H3 at threonine 3 (H3T3ph) and is required for normal chromosome cohesion during mitosis (Dai et al., 2006; Dai et al., 2005). Enhanced green fluorescent protein (EGFP)-haspin also localizes to spindle poles in mitosis (Dai et al., 2005). This prompted us to examine the effect of haspin RNAi on spindle formation. Although we observed spindle defects, our results argue that they are largely an indirect consequence of loss of chromosome cohesion, rather than being caused by a local failure of centrosome function.
Results
Haspin depletion increases the number of centrosome-like foci in mitosis
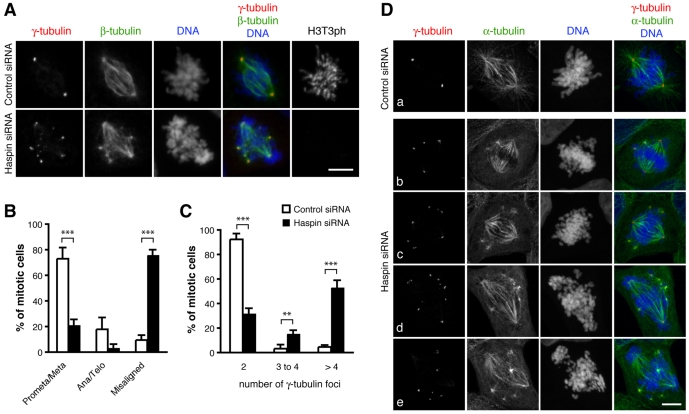
First, we confirmed by immunoblot analysis that haspin siRNA decreased haspin protein levels in U2OS cells (supplementary material Fig. S1A). After transfection with haspin siRNA, 76% of mitotic U2OS cells had low levels of H3T3ph and extensive chromosome misalignment (Fig. 1A,B), consistent with our previous findings (Dai et al., 2006; Dai et al., 2005). In cells with misaligned chromosomes, the majority of microtubules were incorporated into bipolar spindles but additional small clusters of short microtubules were noted adjacent to the fusiform spindles. These clusters were always associated with foci of γ-tubulin (Fig. 1A,D). Sixty-seven percent of mitotic haspin-depleted cells contained three or more foci of γ-tubulin, whereas 92% of control siRNA-transfected cells contained only two foci of γ-tubulin (Fig. 1C). Similar increases in numbers of γ-tubulin foci were noted in HeLa cells transfected with haspin siRNA (see later). Therefore, depletion of haspin caused centrosomal defects in mitosis that correlated with the presence of chromosome misalignment.
Fig. 1.
Haspin depletion disrupts chromosome alignment and formation of normal spindles. (A) U2OS cells transfected with control or haspin siRNAs were stained with antibodies to γ-tubulin, β-tubulin and H3T3ph, and with the DNA dye Hoechst 33342, and subjected to immunofluorescence microscopy. (B) Mitotic cells treated as in A were classified into mitotic stages (prometaphase/metaphase or anaphase/telophase), or as chromosomes misaligned (a dominant bipolar spindle and partial metaphase plate present, but other chromosomes found near the poles). (C-D) U2OS cells transfected with control siRNA (Da) or haspin siRNA (Db-e) were stained with antibodies to γ-tubulin and α-tubulin, and with the DNA dye DRAQ5 and subjected to immunofluorescence microscopy to determine the number of γ-tubulin foci present per mitotic cell. (C) Over 100 cells were counted for each sample. Means ± s.d. are shown, n=3; **P<0.01, ***P<0.001 by Student's t-test. Scale bars: 10 μm.
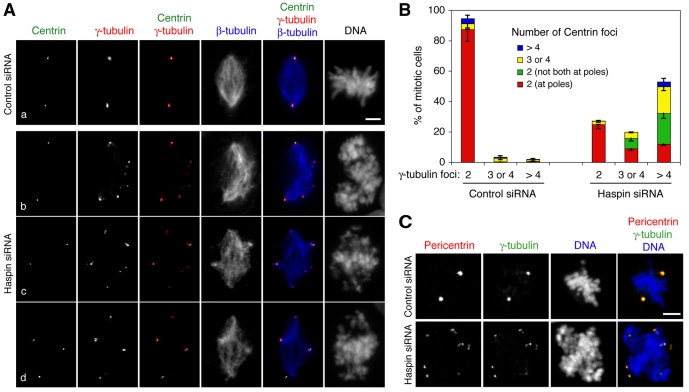
During mitosis, γ-tubulin is a major microtubule-nucleating component of the pericentriolar material (PCM) that surrounds the paired centrioles of each centrosome. γ-tubulin dynamically exchanges with a non-centrosomal pool (Khodjakov and Rieder, 1999) and it contributes to nucleation of microtubules initiated from chromosomes and other microtubules during spindle formation (Luders et al., 2006; Mahoney et al., 2006; Wilde and Zheng, 1999). One possible cause of the multiple γ-tubulin foci in haspin-depleted cells is the presence of additional centrosomes due to over-amplification in interphase or a failure of cytokinesis in a previous cell cycle. However, there was no change in the number of γ-tubulin foci observed at interphase or mitotic prophase (data not shown), and there was no significant increase in the number of mitotic cells with more than four centrioles following haspin depletion (Fig. 2A,B), suggesting that neither increased centrosome numbers nor centriole fragmentation were responsible. Furthermore, the average fluorescence intensity of individual γ-tubulin foci in haspin-depleted mitotic cells was only one third that of bona fide centrosomes in control cells (supplementary material Fig. S1B). Together, these findings suggested that, following haspin depletion, γ-tubulin is redistributed to multiple foci that arise during mitosis.
Fig. 2.
Haspin depletion causes formation of extra centrosome-like foci. (A) U2OS cells transfected with control (Aa) or haspin siRNAs (Ab-d) were stained with antibodies to γ-tubulin, β-tubulin and centrin, and with the DNA dye Hoechst 33342, and subjected to immunofluorescence microscopy. (B) Mitotic cells treated as in A were classified according to the number and location of γ-tubulin- and centrin-containing foci. Means ± s.d. are shown, n=3. Over 100 cells were counted for each sample. (C) U2OS cells transfected with control or haspin siRNAs were stained with antibodies to pericentrin and γ-tubulin, and with the DNA dye DRAQ5, and subjected to immunofluorescence microscopy. Scale bars: 5 μm.
To further characterize the extra foci, we examined the distribution of additional proteins known to be components of the PCM. In haspin-depleted cells, all γ-tubulin foci contained pericentrin (Fig. 2C) and Aurora A (see below), suggesting that they are PCM-like structures. We also examined the distribution of centrioles in haspin-depleted cells using the marker centrin (Paoletti et al., 1996). Depending on centrosome orientation, a normal mitotic cell has up to four visible distinct centrin dots (corresponding to centrioles) that are tightly paired at the two centrosomes. Haspin depletion caused a modest degree of centriole disengagement (Fig. 2Aa,2B), as evidenced by an increase in the percentage of mitotic cells possessing three or four separate centrin-containing foci (24% in haspin-depleted cells versus 7% in controls; P=0.01, Student's t-test). However, this centriole disengagement was insufficient to account for the presence of three or more γ-tubulin foci in nearly 70% of haspin-depleted mitotic cells (Fig. 2A,B; note the existence of γ-tubulin foci that do not contain centrin).
Ectopic centrosome-like foci can nucleate microtubule asters but do not prevent formation of a dominant bipolar spindle
A notable feature of haspin-depleted cells was the essentially universal presence of an identifiable bipolar spindle, even when the intensity of γ-tubulin foci at the bipolar spindle poles was weaker than at the other ectopic foci (Figs 1 and 2). In fact, in some cells, neither γ-tubulin nor centrin staining was evident at some bipolar spindle poles (Fig. 1De, Fig. 2Ac,d,2B). This raised the issue of what distinguished the dominant spindle poles in haspin-depleted cells containing multiple centrosome-like foci.
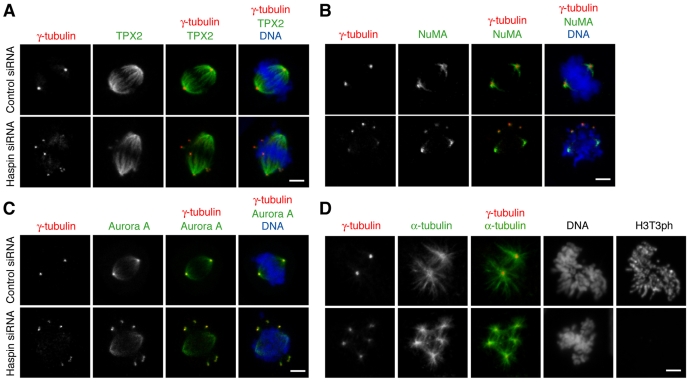
The formation of focused bipolar spindles relies on microtubule-associated motor and non-motor proteins (Manning and Compton, 2007). We asked whether three such proteins, TPX2, NuMA and Aurora A, were recruited equally to ectopic γ-tubulin foci and the major bipolar spindle poles after haspin depletion. TPX2 is a key mediator of the Ran-signaling pathway that localizes to spindle microtubules and is required for microtubule nucleation near kinetochores and for normal spindle formation (Bird and Hyman, 2008; Gruss et al., 2002; Tulu et al., 2006). In cells depleted of haspin, microtubules at ectopic γ-tubulin foci were able to recruit small amounts of TPX2, but most TPX2 was associated with the dominant bipolar spindle (Fig. 3A). Similarly, haspin depletion showed little effect on localization of the microtubule minus-end crosslinker NuMA to the dominant spindle pole microtubules, although relatively low levels of NuMA were also apparent at ectopic γ-tubulin foci (Fig. 3B). The kinase Aurora A has been implicated in both centrosome and chromosome-initiated microtubule nucleation (Sardon et al., 2008; Tsai and Zheng, 2005). In contrast to TPX2 and NuMA, Aurora A is localized in the PCM of centrosomes as well as at the minus ends of spindle microtubules. In haspin-depleted cells we found that, like TPX2 and NuMA, Aurora A was localized on microtubules at the two poles of the dominant bipolar spindle, even when no γ-tubulin foci were detectable at these poles. At the same time, the association of Aurora A with multiple γ-tubulin foci was more apparent than that of TPX2 and NuMA, confirming that the ectopic poles contain PCM components, including Aurora A (Fig. 3C). Therefore, the primary localization of TPX2 and NuMA and of microtubule-associated Aurora A to the dominant bipolar spindle was unaffected in cells depleted of haspin, despite the presence of ectopic centrosome-like foci.
Fig. 3.
Cells depleted of haspin possess a dominant bipolar spindle and extra centrosome-like foci that can nucleate microtubules. (A-C) U2OS cells transfected with control or haspin siRNAs were stained with the DNA dye DRAQ5 and antibodies to (A) γ-tubulin and TPX2, (B) γ-tubulin and NuMA, (C) γ-tubulin and Aurora A, and subjected to immunofluorescence microscopy. (D) Microtubule regrowth assays, in which spindle disassembly by cold treatment for 1 hour was followed by regrowth at 37°C for 2 minutes, were carried out on U2OS cells transfected with control or haspin siRNAs. The cells were then stained with antibodies to γ-tubulin, α-tubulin and H3T3ph, and with the DNA dye Hoechst 33342, and subjected to immunofluorescence microscopy. Scale bars: 5 μm.
The above findings raised the question of whether the ectopic γ-tubulin foci had intrinsically low capacity to focus large microtubule asters. To address this question, we performed microtubule regrowth assays in which spindle disassembly by cold treatment for 1 hour was followed by regrowth at 37°C for 2 minutes. In haspin-siRNA-transfected cells, each of the multiple γ-tubulin foci was able to nucleate microtubule asters with similar efficiency, providing no evidence that ectopic foci were functionally compromised (Fig. 3D). In addition, multiple γ-tubulin foci were able to support the formation of cold-stable kinetochore microtubules in haspin-depleted cells (supplementary material Fig. S2). These findings led us to ask why the presence of multiple functional centrosome-like foci did not prevent the formation of a dominant bipolar spindle. In particular, we wished to determine at what point during mitosis the multiple γ-tubulin foci became apparent.
Centrosome defects occur as chromosome cohesion is compromised
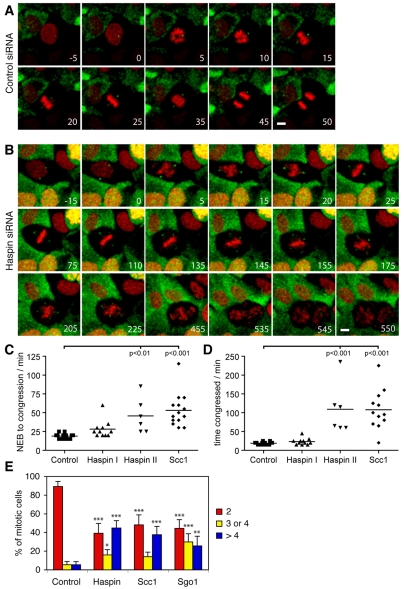
To monitor the dynamics of spindle poles and chromosome misalignment in mitosis, we generated a stable U2OS cell line expressing γ-tubulin-EGFP and histone H2B-mRFP (monomeric red fluorescent protein) and performed live-cell imaging. All observed control siRNA-treated cells underwent normal mitosis (Fig. 4A, supplementary material Movie 1), whereas cells that had been exposed to haspin siRNA fell into two groups: Group I cells completed a near-normal mitosis (supplementary material Movie 2), whereas group II cells that were presumably more fully depleted of haspin failed to undergo normal anaphase (Fig. 4B, supplementary material Movie 3 and Fig. S3). In both haspin-depleted groups, prophase cells contained two centrosomes that accumulated γ-tubulin and moved to opposite poles, as in controls. Chromosomes also condensed and congregated at a metaphase plate, although congression took somewhat longer than normal in haspin-depleted cells (28±12 and 46±23 minutes for group I and II cells, respectively, compared to 19±3 minutes for controls; Fig. 4C). Control and group I cells then underwent apparently normal anaphase after 19±3 minutes and 23±9 minutes in metaphase, respectively. By contrast, group II haspin-depleted cells remained at a metaphase-like stage for 1-4 hours (Fig. 4D). Following this, small numbers of chromosomes began to move away from the plate. At this time, extra γ-tubulin foci started to appear. Increasing numbers of chromosomes then became misaligned until no clear metaphase plate existed, and the γ-tubulin foci appeared to increase in number and mobility and to decrease in intensity. At times, there was no obvious γ-tubulin focus at at least one of the presumptive poles. Cells remained in this state for 6-12 hours. Finally, there was an anaphase-like movement of unequal clusters of chromosomes to opposite poles of the cell that was quickly followed by an abortive attempt at cytokinesis as the cells presumably exited mitosis and the chromosomes decondensed to form micronuclei (see Fig. 4B, 535-550 minutes; supplementary material Movie 3).
Fig. 4.
Live imaging of U2OS cells transfected with haspin, Scc1 and control siRNAs. (A-B) U2OS cells stably expressing H2B-mRFP (red) and γ-tubulin–EGFP (green) were transfected with control (A) or haspin (B) siRNAs, partially synchronized by thymidine treatment, and imaged by confocal fluorescence microscopy every 5 minutes. The frame immediately preceding nuclear envelope breakdown (NEB) is defined as t=0. Selected maximum intensity projections of Z-stacks are shown. For complete movies see supplementary material Movies 1 and 3. Scale bars: 10 μm. (C-D) The time from NEB to metaphase-like chromosome alignment (C) and the duration of metaphase-like alignment (D) are shown for siRNA-transfected mitotic cells visualized by live-cell imaging. Haspin I cells are those that completed mitosis (65%), and haspin II cells are those that failed to carry out normal anaphase (35%). The results of a Bonferroni multiple comparisons test are indicated. (E) The numbers of γ-tubulin foci in U2OS cells transfected with control, haspin, Scc1 or Sgo1 siRNAs were quantified as in Fig. 1C. Means ± s.d. are shown, n=5; *P<0.05, **P<0.01, ***P<0.001 by Bonferroni multiple comparisons test compared with the corresponding control.
As described for cells defective in other cohesion factors (see Discussion), it appears that haspin-depleted cells enter mitosis with cohered sister chromatids that can congress to form a metaphase-like plate, but that premature loss of cohesion leads to sister chromatid separation, overt loss of the metaphase-like alignment, and a prolonged mitotic arrest. Clearly, in haspin-depleted cells, chromosome misalignment and the increase in centrosome-like foci were preceded by a stage in which near-normal chromosome congression and formation of bipolar spindles could occur. The presence of a dominant bipolar spindle even in haspin-depleted cells with severe chromosome misalignment therefore appears to be the result of its formation earlier in mitosis, before frank cohesion defects become evident.
Depletion of other cohesion factors causes similar centrosome defects
The known role of haspin in maintaining centromeric cohesion between sister chromatids during mitosis (Dai et al., 2006), coupled with the coincidence in appearance of the chromosome alignment and spindle defects, suggested that spindle defects following haspin depletion might be an indirect consequence of chromosome cohesion loss rather than an intrinsic centrosomal defect. If loss of integrity of the spindle pole is a result of cohesion loss, depletion of other cohesion factors should cause similar increases in the number of γ-tubulin foci. Indeed, we found that the percentage of mitotic cells with more than four γ-tubulin foci was increased from 6% to 44%, 41% and 28% following depletion of haspin, Scc1 and Sgo1, respectively (Fig. 4E). Therefore, depletion of Scc1 and Sgo1 resulted in the appearance of spindle defects similar to those in haspin-depleted cells. Consistent with this, live-cell imaging studies confirmed that chromosome dynamics and the appearance of extra γ-tubulin foci in cells depleted of Scc1 were similar to those in cells depleted of haspin (supplementary material Fig. S4 and Movie 4). The results suggest that the formation of multiple γ-tubulin foci is associated with precocious loss of cohesion between sister chromatids, irrespective of the particular cohesion factor that is depleted.
Topoisomerase II inhibition partially rescues spindle pole integrity
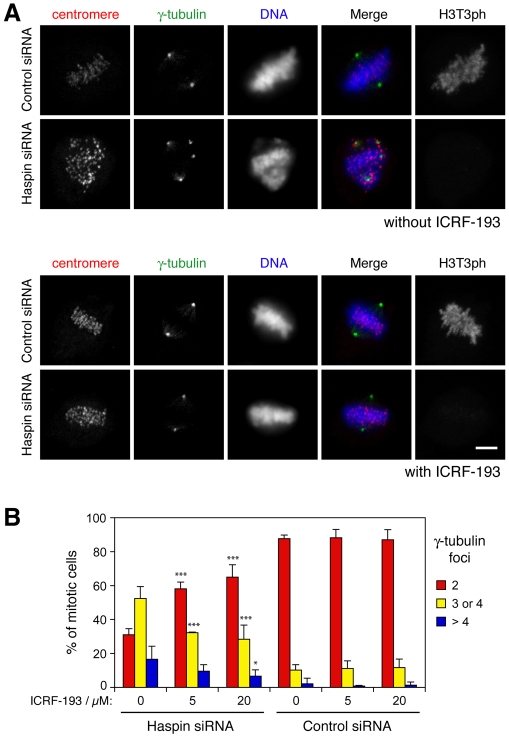
If the aberrant γ-tubulin localization and spindle structure observed following haspin depletion are indirect consequences of loss of cohesion, then artificially maintaining association between sisters in the absence of haspin should rescue the defect. To test this hypothesis, we treated cells with the catalytic topoisomerase II inhibitor ICRF-193 to prevent decatenation of chromatids during mitosis (Toyoda and Yanagida, 2006; Vagnarelli et al., 2004), and determined the numbers of cells with multiple γ-tubulin foci. Because such drugs can prevent entry into mitosis (Downes et al., 1994) and induce apoptosis (Vagnarelli et al., 2004), we treated partially synchronized HeLa cells with ICRF-193 for short periods as they entered mitosis. Synchronization reduced the efficiency of haspin depletion as previously reported (Dai et al., 2006), so only those cells that showed low or absent staining for H3T3ph were included in the analysis. ICRF-193 treatment decreased the percentage of haspin-depleted cells exhibiting three or more γ-tubulin foci in mitosis, but had little effect on the few control cells that had multiple foci (Fig. 5A,B), consistent with the idea that the formation of multiple centrosome-like foci in haspin-depleted cells is due at least in part to the loss of cohesion.
Fig. 5.
Cohesion is required for integrity of the spindle poles in haspin-depleted cells. (A) HeLa cells were transfected with haspin or control siRNAs, exposed to thymidine for 14 hours and released into drug-free medium for 11 or 13 hours. Then, 20 μM ICRF-193 was added for 1 hour prior to fixation. Cells were stained with antibodies to centromeres, γ-tubulin and H3T3ph and with the DNA dye Hoechst 33342, and subjected to immunofluorescence microscopy. (B) The numbers of γ-tubulin foci in mitotic HeLa cells with low or absent staining for H3T3ph treated as in A were quantified using immunofluorescence microscopy. Means ± s.d. are shown. Between 23 and 116 cells were classified on two coverslips from two experiments (i.e. four coverslips). *P<0.05, ***P<0.001 by Bonferroni multiple comparisons test compared with corresponding ICRF-193-untreated condition. Scale bars: 5 μm.
Role of microtubules in the formation of extra centrosome-like foci
Recently it has become clear that, in vertebrate cells, the formation of the mitotic spindle is the result of cooperation between centrosome, chromosome and spindle-initiated microtubule nucleation mechanisms (Karsenti and Vernos, 2001; Mahoney et al., 2006; O'Connell and Khodjakov, 2007; Toso et al., 2009). Early in mitosis, chromosome- and centrosome-initiated microtubule formation mechanisms probably act relatively independently, and might even be antagonistic in the absence of motor proteins that normally act to focus microtubule asters at the centrosomes (Manning and Compton, 2007). As mitosis progresses, microtubules initiated at chromosomes, spindle microtubules and centrosomes are integrated into a single bipolar spindle in a process that must balance the forces generated between microtubules formed by these different mechanisms (O'Connell and Khodjakov, 2007). It therefore seemed possible that loss of cohesion disrupted spindle structure in our studies because it prevented the integration of spindle assembly mechanisms that is required to maintain a fully bipolar state.
To determine whether the chromosome pathway of microtubule formation was operating in haspin-depleted cells, we conducted experiments in which chromatin-mediated microtubule aster formation was observed in mitotic cells released from arrest with the spindle poison nocodazole, as previously described (Tulu et al., 2006). In control and haspin-depleted cells, asters formed with similar efficiency in both centrosome and chromatin regions (supplementary material Fig. S5). Haspin depletion, therefore, did not obviously compromise chromosome-initiated microtubule nucleation.
To determine whether the generation of extra centrosome-like foci following loss of chromosome cohesion was dependent on microtubule activity, we released synchronized control and haspin-depleted cells into medium containing the microtubule-stabilizing compound taxol. Although taxol itself caused the formation of multiple microtubule asters in mitosis, both control and haspin-depleted cells normally each contained only two γ-tubulin foci in these conditions (supplementary material Fig. S6). Microtubule depolymerization with nocodazole also prevented the emergence of multiple spindle poles in haspin-depleted cells (supplementary material Fig. S5). Therefore, formation of multiple γ-tubulin foci was prevented if microtubule dynamics were suppressed.
Kinesin-5 (also known as Eg5) is a microtubule motor required for centrosome separation and bipolar spindle formation in mitosis (Blangy et al., 1995; Heck et al., 1993). We therefore determined the effect of kinesin-5 inhibition on spindle formation in haspin-depleted cells. U2OS cells were transfected with control or haspin siRNA and, after 48 hours, exposed to monastrol for 4 hours. Cells were then examined at 0, 20, and 60 minutes following removal of monastrol. As expected, monastrol treatment resulted in monopolar spindles with two adjacent centrosomes in over 90% of control mitotic cells (Fig. 6Aa,B). Most of the remaining mitotic cells had bipolar spindles with a centrosome at each pole. These probably represent cells that were in mitosis when the kinesin-5 inhibitor was added because monastrol is reported not to cause the collapse of pre-existing cellular bipolar spindles (Kapoor et al., 2000; Tanenbaum et al., 2008). After removal of monastrol, 86% of control cells adopted a normal bipolar configuration within 20 minutes as mitosis resumed (Fig. 6Ad,B).
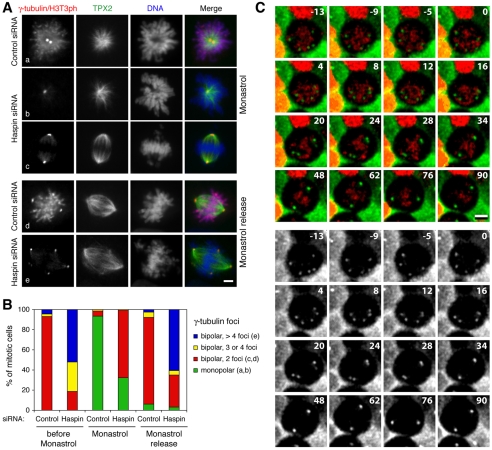
Fig. 6.
Kinesin-5 activity is required to form and maintain ectopic γ-tubulin foci in haspin-depleted cells. (A) U2OS cells transfected with control or haspin siRNAs were incubated in monastrol for 4 hours and then immediately fixed (Aa-c) or incubated in monastrol and then released into monastrol-free medium for 20 minutes prior to fixation (Ad,e). Cells were stained with antibodies to γ-tubulin and H3T3ph (together), TPX2, and with the DNA dye DRAQ5, and subjected to immunofluorescence microscopy. Scale bar: 5 μm. (B) Cells treated as in A were classified according to the number of γ-tubulin foci and spindle polarity as judged by TPX2 staining. Note that cells referred to as `bipolar' had clear dominant bipolar spindles, but in the noted cases also had extra γ-tubulin foci. (C) U2OS cells stably expressing H2B-mRFP (red) and γ-tubulin–EGFP (green or gray) were transfected with haspin siRNA. After 48 hours, cells were imaged by confocal fluorescence microscopy every 2 minutes for 10 minutes. Monastrol was added at t=0 minutes, and imaging was then continued every 2 minutes for another 92 minutes. Selected maximum intensity projections of Z-stacks are shown. For complete data see supplementary material Movie 5. Scale bar: 10 μm.
Among haspin-depleted mitotic cells, 81% had essentially bipolar spindles with three or more γ-tubulin foci prior to monastrol treatment. After monastrol treatment, there were a significant number of monopolar cells with two γ-tubulin foci among haspin-siRNA transfectants (32%), indicating that haspin-depleted cells that enter mitosis in monastrol fail to form bipolar spindles, as in controls, and do not generate extra γ-tubulin foci (Fig. 6Ab,B). The remaining 68% of haspin-depleted mitotic cells retained a bipolar configuration in monastrol, compared with 7% in controls. This probably reflects the extended duration of mitosis in haspin-depleted cells and the relatively high percentage of cells that were therefore already in mitosis when monastrol was added. Most striking, however, was the observation that essentially all of these bipolar cells had only two γ-tubulin foci, both at the spindle poles (Fig. 6Ac,B). Therefore, pre-existing ectopic γ-tubulin foci in haspin-depleted cells were largely eliminated by monastrol treatment. This interpretation was strikingly confirmed using live imaging to determine the effect of monastrol on haspin-depleted mitotic cells containing multiple pre-existing γ-tubulin foci. By 40 minutes after addition of monastrol to such cells, ectopic foci had approached and fused with the primary spindle poles to form fully bipolar spindles with only two γ-tubulin foci (Fig. 6C and supplementary material Movie 5). Within 20 minutes of monastrol removal, 66% of haspin-depleted cells returned to a bipolar configuration with three or more γ-tubulin foci, resembling the situation prior to monastrol treatment (Fig. 6Ae,B). These results suggest that both the formation and maintenance of ectopic γ-tubulin foci in haspin-depleted cells require the activity of the microtubule motor kinesin-5. Consistent with this, kinesin-5 localized to spindle microtubules, including those associated with ectopic γ-tubulin foci in haspin-depleted cells (supplementary material Fig. S7). Together, these findings suggest that the extra centrosome-like foci formed in haspin-depleted cells are dynamic structures whose formation and persistence require ongoing microtubule dynamics and microtubule motor activity.
Discussion
A number of studies have shown that manipulations leading to loss of chromosome cohesion, including RNAi directed at Scc1, Sgo1, EFO1/2, San, Pds5A/B, PHB2, CENP-F and now haspin, cause the appearance of extra spindle poles in mitosis (Holt et al., 2005; Hou et al., 2007; Hou and Zou, 2005; Losada et al., 2005; Salic et al., 2004; Takata et al., 2007; Wang et al., 2008). We suggest that in many cases this is due to secondary consequences of defects in chromosome cohesion that prevent the proper integration of chromosome-initiated pathways of microtubule nucleation with other pathways involved in formation of a fully bipolar spindle. Disruption of chromosome cohesion prevents stable attachment of chromosomes to the spindle during mitosis (Deehan Kenney and Heald, 2006; Sonoda et al., 2001). It is possible that ongoing nucleation of microtubules by such chromosomes and subsequent focusing of these microtubules into γ-tubulin-containing foci (Luders et al., 2006; Tulu et al., 2006) provides a persistent source of centrosome-like structures, which prevents convergence of spindle assembly on a truly bipolar structure.
Consistent with this proposal, the appearance of extra γ-tubulin foci in haspin-depleted cells requires ongoing microtubule activity. In particular, inhibition of the microtubule plus-end-directed motor kinesin-5 with monastrol prevents the emergence and maintenance of ectopic centrosome-like foci in haspin-depleted cells. This contrasts with the reported effects of monastrol on the structure of centrosomes when they are compromised directly by depletion of the centrosomal protein Kizuna, or by microinjection of anti-polyglutamylated tubulin antibodies. In these cases, monastrol treatment did not prevent the appearance of multiple γ-tubulin foci (Abal et al., 2005; Oshimori et al., 2006). The requirement for kinesin-5 activity to produce ectopic poles in haspin-depleted cells might be analogous to the role of the Drosophila kinesin-5, Klp61F, in the utilization of chromosome-initiated microtubules to form acentriolar poles within monastral bipolar spindles in S2 cells (Goshima and Vale, 2003). The ability of kinesin-5 to slide apart anti-parallel microtubules and to prevent the relative motion of parallel microtubules might serve to move chromatin-nucleated microtubules away from chromosomes and aid in their subsequent clustering to form spindle poles (Burbank et al., 2007; Kapitein et al., 2005; Walczak et al., 1998). Interestingly, we find that kinesin-5 activity is required to maintain separation of ectopic γ-tubulin foci from the dominant spindle poles, but not the separation of the two dominant poles from one another. In cell extracts, but not in intact cells, preformed bipolar spindles collapse to monopolar spindles in the presence of monastrol, suggesting that attachment of spindle poles to the cell cortex might account for the insensitivity of pre-existing bipolar spindles to kinesin-5 inhibition (Kapoor et al., 2000). Therefore, more robust cortical attachment of the original bipolar spindle formed early in mitosis compared to the attachment of later-appearing ectopic poles is one possible explanation for the relative monastrol-insensitivity of the dominant spindle poles in haspin-depleted cells.
Our results also provide new insight into the nature of mitotic defects caused by haspin depletion. Many haspin-siRNA-treated cells reach a metaphase-like chromosome alignment before asynchronous loss of chromosome cohesion apparently leads to decay of the metaphase plate. It is notable that depletions of other cohesion factors, including Scc1 (Diaz-Martinez et al., 2007a; Sonoda et al., 2001; Toyoda and Yanagida, 2006), Sgo1 (McGuinness et al., 2005; Salic et al., 2004; Wolf et al., 2006) and Sororin (Diaz-Martinez et al., 2007b), have all led to similar findings. The reason that cells with defects in cohesin-mediated chromatid cohesion are able to align chromosomes remains incompletely defined, but might result from cohesin-independent cohesion, perhaps via DNA catenations (Deehan Kenney and Heald, 2006; Toyoda and Yanagida, 2006; Vagnarelli et al., 2004), as well as from incomplete protein depletion. In addition, when regulators of mitotic cohesion such as Sgo1 are depleted, sister chromatids are likely to enter mitosis held together by cohesin-mediated links established in S phase, and cleavage-independent cohesin removal during early mitosis is probably required for sisters to fully separate (Peters et al., 2008). Tension across bi-oriented chromosomes might also contribute to complete chromatid disjoining when the cohesin pathway is defective. This is suggested by observed reductions in scattering (but not separation) of sister chromatids in the presence of the microtubule-depolymerizing agent nocodazole following Sgo1 depletion (Dai et al., 2006; McGuinness et al., 2005), and by the maintenance of chromosome cohesion on monopolar spindles lacking tension in cohesin-depleted Xenopus extracts treated with monastrol (Deehan Kenney and Heald, 2006).
The results of haspin depletion are consistent with a similar scenario in which cells enter mitosis with cohered sister chromatids that can congress to form a metaphase-like plate, but in which bi-orientation and attachment is unstable and incapable of satisfying the spindle checkpoint. Progressive loss of cohesion by cleavage-independent cohesin release, topoisomerase II resolution of DNA catenations and tension across the centromere then leads to sister chromatid separation, overt loss of the metaphase-like alignment and a prolonged mitotic arrest. Consistent with this, in haspin-depleted cells, equatorial but not polar chromosomes appear attached to cold-stable kinetochore microtubules (supplementary material Fig. S2), and nocodazole treatment reduces the scattering of separated chromatids (Dai et al., 2006).
We note that our results do not rule out direct functions for cohesion regulators at centrosomes. A splice variant of human Sgo1, for example, localizes to centrosomes but not centromeres and appears to be required for centriole cohesion (Wang et al., 2008). It remains possible that haspin has a local centrosomal function during mitosis because EGFP-haspin can be seen at centrosomes during mitosis (Dai et al., 2005). Nevertheless, our results make clear the importance of chromosome cohesion in allowing the convergence of various spindle assembly pathways on a bipolar structure, and invite caution when interpreting the cause of apparent centrosome defects arising when centromere integrity is disrupted. Finally, chromosome cohesion defects have been implicated in some human cancers (Barber et al., 2008). The results presented here indicate that ectopic spindle poles might arise and might contribute to instability of chromosome number when chromosome cohesion is compromised.
Materials and Methods
Cell culture and transfection
Human U2OS and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS at 37°C and 10% CO2. Human haspin, Sgo1 and control siRNAs were described previously (Dai et al., 2006; Dai et al., 2005). Human Scc1 (Losada et al., 2005) siRNA was synthesized by Integrated DNA Technologies (Coralville, IA). Transfections with 40 nM siRNA were carried out using Oligofectamine according to the manufacturer's recommendations (Invitrogen).
For microtubule regrowth assays, siRNA-transfected cells were incubated in cold medium on ice for 1 hour followed by 2 minutes at 37°C in warm medium. To examine kinetochore-associated microtubules, cells were incubated in ice-cold medium for 10 minutes prior to fixation. In other experiments, cells were treated with 100 μM monastrol (Biomol, Plymouth Meeting, PA) for 4 hours or 1.1 μM nocodazole (Sigma) for 3 hours, then fixed immediately or at various times after drug removal by three washes in medium, or cells were treated with 10 μM taxol (Sigma) for 3 hours before fixation. For topoisomerase II inhibition, siRNA-transfected HeLa cells were released from a 14-hour block in 2 mM thymidine and, after approximately 12 hours, ICRF-193 (Biomol) was added for 1 hour prior to fixation.
Antibodies and immunofluorescence
At 48 hours after siRNA transfection, cells were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) at room temperature for 10 minutes followed by ice-cold methanol for 5 minutes. Antibodies used were as follows: 1:2000 rabbit anti-H3T3ph B8634 (Dai et al., 2005); 1:4000 rabbit or mouse anti-γ-tubulin (Sigma); 1:10,000 mouse anti-α-tubulin (Sigma); 1:400 goat anti-β-tubulin (Abcam); 1:8000 anti-TPX2 (Abcam); 1:4000 mouse anti-kinesin-5 (Abcam); and 1:2000 human centromere autoantibodies (Immunovision, Springdale, AR). Cells were directly fixed with ice-cold methanol for co-staining of γ-tubulin with the following antibodies: 1:5000 mouse anti-centrin (Paoletti et al., 1996); 1:8000 rabbit anti-pericentrin (Abcam); 1:2000 mouse anti-aurora A (Abcam); and 1:3000 rabbit anti-NuMA (Gaglio et al., 1995). Secondary antibodies were donkey anti-mouse, anti-rabbit or anti-human IgG-Cy3 or IgG-Cy5 (Jackson ImmunoResearch), or anti-rabbit, anti-goat or anti-mouse IgG-Alexa488 (Invitrogen). To detect DNA, 50 μg/ml propidium iodide plus 100 U/ml RNAse A (Sigma), or 0.5 μg/ml Hoechst 33342 (Sigma), or 1:2000 DRAQ5 (Axxora LLC) were used. Fluorescence microscopy was carried out using a Nikon TE2000 inverted microscope equipped with a 60× Plan Apo 1.4 NA oil immersion objective, a C1 Plus Confocal Laser Scanner and a SPOT-RT CCD camera. When four-color imaging was required, antibody staining was visualized using confocal microscopy, and DNA staining with Hoechst 33342 was detected by wide-field microscopy. Stacks of confocal images with 0.3-0.8 μm Z-steps were acquired and rendered as maximum intensity projections using EZ-C1 software (Nikon), and processed with Adobe Photoshop.
Live-cell imaging
To generate the stable cell lines, U2OS cells were first transfected with pEGFP-N1–γ-tubulin vector (a gift of Alexey Khodjakov, Wadsworth Center, NY) using Fugene 6 (Roche) and selected in 1 mg/ml G418. Expanded colonies expressing γ-tubulin–EGFP were further transfected with 9:1 pmRFP-N1–H2B (Dodson et al., 2007) and pGK-puro plasmids. After selection in 1 μg/ml puromycin, EGFP-positive cells with moderate levels of mRFP expression were collected by Dako Moflo flow cytometric sorting (DFCI Flow Cytometry Core Facility, Boston, MA). For imaging, cells expressing γ-tubulin–EGFP and H2B-mRFP were cultured in 35-mm glass bottom dishes (World Precision Instruments) in DMEM containing 10% FBS. In some cases, 24 hours after siRNA transfection, cells were incubated in medium containing 2 mM thymidine for 16 hours before release into phenol-red-free DMEM (Hyclone, South Logan, UT) containing 10% FBS and 25 mM HEPES for 10 hours. Time-lapse confocal fluorescence imaging was performed using the microscope described above in a 37°C heated chamber with CO2 supply. Two color Z stacks with 0.8 μm steps were collected with a 100-nm pinhole every 5 minutes using a 40× Plan Fluor 1.3 NA oil immersion objective, or every 2 minutes using a 60× Plan Apo 1.4 NA oil immersion objective lens. ImageJ software (NIH, Bethesda, MD) was used to adjust brightness and contrast and to render maximum intensity projections. Time series were converted into movies with Adobe ImageReady.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/22/4168/DC1
We thank Duane Compton (Dartmouth Medical School, Hanover, NH), Alexey Khodjakov, Ciaran Morrison (National University of Ireland, Galway, Ireland) and Jeffrey Salisbury (Mayo Clinic Foundation, Rochester, MN) for gifts of antibodies and expression vectors. We also thank Nancy Kedersha, Tyler Hickman and Fangwei Wang for assistance with U2OS cell lines, live-cell imaging and immunoblotting, respectively. This work was funded by grants to J.M.G.H. from the American Cancer Society (RSG 05-13401-GMC) and the National Institutes of Health (R01 GM074210). Deposited in PMC for release after 12 months.
References
- Abal, M., Keryer, G. and Bornens, M. (2005). Centrioles resist forces applied on centrosomes during G2/M transition. Biol. Cell 97, 425-434. [DOI] [PubMed] [Google Scholar]
- Barber, T. D., McManus, K., Yuen, K. W., Reis, M., Parmigiani, G., Shen, D., Barrett, I., Nouhi, Y., Spencer, F., Markowitz, S. et al. (2008). Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc. Natl. Acad. Sci. USA 105, 3443-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, A. W. and Hyman, A. A. (2008). Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J. Cell Biol. 182, 289-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy, A., Lane, H. A., d'Herin, P., Harper, M., Kress, M. and Nigg, E. A. (1995). Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159-1169. [DOI] [PubMed] [Google Scholar]
- Burbank, K. S., Mitchison, T. J. and Fisher, D. S. (2007). Slide-and-cluster models for spindle assembly. Curr. Biol. 17, 1373-1383. [DOI] [PubMed] [Google Scholar]
- Chestukhin, A., Pfeffer, C., Milligan, S., DeCaprio, J. A. and Pellman, D. (2003). Processing, localization, and requirement of human separase for normal anaphase progression. Proc. Natl. Acad. Sci. USA 100, 4574-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, J., Sultan, S., Taylor, S. S. and Higgins, J. M. G. (2005). The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 19, 472-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, J., Sullivan, B. A. and Higgins, J. M. G. (2006). Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev. Cell 11, 741-750. [DOI] [PubMed] [Google Scholar]
- Deehan Kenney, R. and Heald, R. (2006). Essential roles for cohesin in kinetochore and spindle function in Xenopus egg extracts. J. Cell Sci. 119, 5057-5066. [DOI] [PubMed] [Google Scholar]
- Diaz-Martinez, L. A., Gimenez-Abian, J. F. and Clarke, D. J. (2007a). Cohesin is dispensable for centromere cohesion in human cells. PLoS ONE 2, e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Martinez, L. A., Gimenez-Abian, J. F. and Clarke, D. J. (2007b). Regulation of centromeric cohesion by sororin independently of the APC/C. Cell Cycle 6, 714-724. [DOI] [PubMed] [Google Scholar]
- Dodson, H., Wheatley, S. P. and Morrison, C. G. (2007). Involvement of centrosome amplification in radiation-induced mitotic catastrophe. Cell Cycle 6, 364-370. [DOI] [PubMed] [Google Scholar]
- Downes, C. S., Clarke, D. J., Mullinger, A. M., Gimenez-Abian, J. F., Creighton, A. M. and Johnson, R. T. (1994). A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature 372, 467-470. [DOI] [PubMed] [Google Scholar]
- Gaglio, T., Saredi, A. and Compton, D. A. (1995). NuMA is required for the organization of microtubules into aster-like mitotic arrays. J. Cell Biol. 131, 693-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G. and Vale, R. D. (2003). The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J. Cell Biol. 162, 1003-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson, H. C., Schmiesing, J. A., Kim, J. S., Kobayashi, T., Zhou, S. and Yokomori, K. (2001). A potential role for human cohesin in mitotic spindle aster assembly. J. Biol. Chem. 276, 47575-47582. [DOI] [PubMed] [Google Scholar]
- Gruss, O. J., Wittmann, M., Yokoyama, H., Pepperkok, R., Kufer, T., Sillje, H., Karsenti, E., Mattaj, I. W. and Vernos, I. (2002). Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871-879. [DOI] [PubMed] [Google Scholar]
- Guan, J., Ekwurtzel, E., Kvist, U. and Yuan, L. (2008). Cohesin protein SMC1 is a centrosomal protein. Biochem. Biophys. Res. Commun. 372, 761-764. [DOI] [PubMed] [Google Scholar]
- Heck, M. M., Pereira, A., Pesavento, P., Yannoni, Y., Spradling, A. C. and Goldstein, L. S. (1993). The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J. Cell Biol. 123, 665-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, S. V., Vergnolle, M. A., Hussein, D., Wozniak, M. J., Allan, V. J. and Taylor, S. S. (2005). Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J. Cell Sci. 118, 4889-4900. [DOI] [PubMed] [Google Scholar]
- Hou, F. and Zou, H. (2005). Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol. Biol. Cell 16, 3908-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, F., Chu, C. W., Kong, X., Yokomori, K. and Zou, H. (2007). The acetyltransferase activity of San stabilizes the mitotic cohesin at the centromeres in a shugoshin-independent manner. J. Cell Biol. 177, 587-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein, L. C., Peterman, E. J., Kwok, B. H., Kim, J. H., Kapoor, T. M. and Schmidt, C. F. (2005). The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435, 114-118. [DOI] [PubMed] [Google Scholar]
- Kapoor, T. M., Mayer, T. U., Coughlin, M. L. and Mitchison, T. J. (2000). Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, 975-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti, E. and Vernos, I. (2001). The mitotic spindle: a self-made machine. Science 294, 543-547. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A. and Rieder, C. L. (1999). The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada, A., Yokochi, T. and Hirano, T. (2005). Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J. Cell Sci. 118, 2133-2141. [DOI] [PubMed] [Google Scholar]
- Luders, J., Patel, U. K. and Stearns, T. (2006). GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137-147. [DOI] [PubMed] [Google Scholar]
- Mahoney, N. M., Goshima, G., Douglass, A. D. and Vale, R. D. (2006). Making microtubules and mitotic spindles in cells without functional centrosomes. Curr. Biol. 16, 564-569. [DOI] [PubMed] [Google Scholar]
- Manning, A. L. and Compton, D. A. (2007). Mechanisms of spindle-pole organization are influenced by kinetochore activity in mammalian cells. Curr. Biol. 17, 260-265. [DOI] [PubMed] [Google Scholar]
- McGuinness, B. E., Hirota, T., Kudo, N. R., Peters, J. M. and Nasmyth, K. (2005). Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, C. B. and Khodjakov, A. L. (2007). Cooperative mechanisms of mitotic spindle formation. J. Cell Sci. 120, 1717-1722. [DOI] [PubMed] [Google Scholar]
- Oshimori, N., Ohsugi, M. and Yamamoto, T. (2006). The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 8, 1095-1101. [DOI] [PubMed] [Google Scholar]
- Paoletti, A., Moudjou, M., Paintrand, M., Salisbury, J. L. and Bornens, M. (1996). Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 109, 3089-3102. [DOI] [PubMed] [Google Scholar]
- Peters, J. M., Tedeschi, A. and Schmitz, J. (2008). The cohesin complex and its roles in chromosome biology. Genes Dev. 22, 3089-3114. [DOI] [PubMed] [Google Scholar]
- Salic, A., Waters, J. C. and Mitchison, T. J. (2004). Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118, 567-578. [DOI] [PubMed] [Google Scholar]
- Sardon, T., Peset, I., Petrova, B. and Vernos, I. (2008). Dissecting the role of Aurora A during spindle assembly. EMBO J. 27, 2567-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda, E., Matsusaka, T., Morrison, C., Vagnarelli, P., Hoshi, O., Ushiki, T., Nojima, K., Fukagawa, T., Waizenegger, I. C., Peters, J. M. et al. (2001). Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev. Cell 1, 759-770. [DOI] [PubMed] [Google Scholar]
- Takata, H., Matsunaga, S., Morimoto, A., Ma, N., Kurihara, D., Ono-Maniwa, R., Nakagawa, M., Azuma, T., Uchiyama, S. and Fukui, K. (2007). PHB2 protects sister-chromatid cohesion in mitosis. Curr. Biol. 17, 1356-1361. [DOI] [PubMed] [Google Scholar]
- Tanenbaum, M. E., Macurek, L., Galjart, N. and Medema, R. H. (2008). Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J. 27, 3235-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso, A., Winter, J. R., Garrod, A. J., Amaro, A. C., Meraldi, P. and McAinsh, A. D. (2009). Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J. Cell Biol. 184, 365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda, Y. and Yanagida, M. (2006). Coordinated requirements of human topo II and cohesin for metaphase centromere alignment under Mad2-dependent spindle checkpoint surveillance. Mol. Biol. Cell 17, 2287-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M. Y. and Zheng, Y. (2005). Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr. Biol. 15, 2156-2163. [DOI] [PubMed] [Google Scholar]
- Tsou, M. F. and Stearns, T. (2006). Mechanism limiting centrosome duplication to once per cell cycle. Nature 442, 947-951. [DOI] [PubMed] [Google Scholar]
- Tulu, U. S., Fagerstrom, C., Ferenz, N. P. and Wadsworth, P. (2006). Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 16, 536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli, P., Morrison, C., Dodson, H., Sonoda, E., Takeda, S. and Earnshaw, W. C. (2004). Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores. EMBO Rep. 5, 167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C. E., Vernos, I., Mitchison, T. J., Karsenti, E. and Heald, R. (1998). A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903-913. [DOI] [PubMed] [Google Scholar]
- Wang, X., Yang, Y., Duan, Q., Jiang, N., Huang, Y., Darzynkiewicz, Z. and Dai, W. (2008). sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev. Cell 14, 331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, W. D., Steffensen, S., Lin, E., Coelho, P., Loupart, M., Cobbe, N., Lee, J. Y., McKay, M. J., Orr-Weaver, T., Heck, M. M. et al. (2000). The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr. Biol. 10, 1463-1466. [DOI] [PubMed] [Google Scholar]
- Wilde, A. and Zheng, Y. (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284, 1359-1362. [DOI] [PubMed] [Google Scholar]
- Wolf, F., Wandke, C., Isenberg, N. and Geley, S. (2006). Dose-dependent effects of stable cyclin B1 on progression through mitosis in human cells. EMBO J. 25, 2802-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, R. W. and Blobel, G. (2008). Cohesin subunit SMC1 associates with mitotic microtubules at the spindle pole. Proc. Natl. Acad. Sci. USA 105, 15441-15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.