Abstract
BACKGROUND:
Recent improvements in multidetector computed tomography (MDCT) with 64-slice scanners have allowed acquisition of a coronary study in 5 s to 6 s, with good temporal and spatial resolution. Previous studies have reported an underestimation of plaque burden by MDCT. Whether shorter scan times can allow correct assessment of plaque volume requires comparison with intravascular ultrasound (IVUS).
METHODS:
Patients (n=30) scheduled for coronary angiography also underwent MDCT and IVUS examinations within 96 h. MDCT examination was performed with a 64-slice scanner. Nitroglycerin was administered before all imaging procedures. MDCT, quantitative coronary angiography (QCA) and IVUS analyses were performed by observers blinded to other results. Plaque volumes were determined by MDCT and IVUS in one vessel, and maximum percentage diameter stenosis was identified in each coronary segment by MDCT and QCA.
RESULTS:
The mean (± SD) plaque volume was determined to be 179.1±78.9 mm3 by MDCT and 176.1±87.9 mm3 by IVUS. There was a strong positive correlation for plaque volume between MDCT and IVUS (r=0.84, P<0.0001). Percentage diameter stenosis assessed by MDCT and QCA also correlated well (r=0.88 per patient and r=0.87 per vessel, P<0.0001 for both). The maximum percentage diameter stenosis per vessel was 38.1±30.2% with MDCT and 34.1±27.6% with QCA. The sensitivity and specificity of MDCT in detecting stenoses above 50% per vessel were 100% and 91.0%, respectively.
CONCLUSIONS:
Plaque volumes measured by 64-slice MDCT and IVUS correlate well, without systematic underestimation. The sensitivity and specificity of MDCT to detect stenoses greater than 50% by QCA are excellent with the administration of nitroglycerin before imaging.
Keywords: Angiography, Atherosclerosis, Coronary disease, Tomography, Ultrasonics
Abstract
HISTORIQUE :
Les récentes améliorations apportées à la tomodensitométrie multicoupe (TDMC) 64 barrettes ont permis l’acquisition d’images coronariennes en cinq à six secondes avec une bonne résolution temporelle et spatiale. Des études antérieures ont fait état d’une sous-estimation des plaques d’athérome au moyen de cette technique. Pour déterminer si un examen plus court permet d’évaluer correctement le volume des plaques, il faut procéder à une comparaison avec l’échographie intravasculaire (ÉIV).
MÉTHODE :
Les patients (n = 30) devant subir une coronarographie coronarienne ont également subi une TDMC et une ÉIV à l’intérieur d’une période de 96 heures. L’examen par TDMC a été réalisé au moyen d’un appareil à 64 coupes. De la nitroglycérine a été administrée avant toutes les interventions d’imagerie. Des observateurs ont procédé aux analyses des résultats de TDMC, de coronarographie quantitative (CGQ) et d’ÉIV en aveugle pour ce qui est des autres résultats. Les volumes des plaques ont été calculés par TDMC et par ÉIV sur un vaisseau et le pourcentage maximum de sténose a été identifié dans chacun des segments coronariens par TDMC et CGQ.
RÉSULTATS :
Le volume moyen des plaques (± É.-T.) a été évalué à 179,1 ± 78,9 mm3 par TDMC et à 176,1 ± 87,9 mm3 par ÉIV. On a noté une forte corrélation positive entre la TDMC et l’ÉIV pour ce qui est du volume de la plaque (r = 0,84, P < 0,0001). Le pourcentage de sténose évalué par TDMC et CGQ a également été en corrélation (r = 0,88 par patient et r = 0,87 par vaisseau, P < 0,0001 pour les deux). Le pourcentage maximum de sténose par vaisseau a été de 38,1 ± 30,2 % avec la TDMC et de 34,1 ± 27,6 % avec la CGQ. La sensibilité et la spécificité de la TDMC pour ce qui est du dépistage des sténoses de plus de 50 % par vaisseau ont été respectivement de 100 % et de 91,0 %.
CONCLUSION :
Les volumes des plaques mesurées par TDMC 64 barrettes et par ÉIV sont en bonne corrélation, sans sous-estimation systématique. La sensibilité et la spécificité de la TDMC utilisée pour dépister les sténoses de plus de 50 % observées à la CGQ sont excellentes avec une administration préalable de nitroglycérine.
There is a clear need for noninvasive assessment of coronary artery disease. Initial studies with multidetector computed tomography (MDCT) using four-slice and 16-slice scanners pointed to the potential of this imaging modality, but the specificity was sometimes suboptimal compared with invasive angiography (1,2). However, from a clinical perspective, false-negative MDCT results are of greater concern because they may lead to inappropriate avoidance of further investigation and undue patient reassurance. Such underestimation of disease severity is often due to motion artefacts (3), despite relatively short scan times of 8 s to 11 s (4). Compared with intravascular ultrasound (IVUS), which is the current gold standard for atherosclerosis imaging (5), MDCT also underestimated atheroma burden (4,6,7).
Recent improvements in MDCT technology with 64-slice scanners have allowed for acquisition of a coronary study in 5 s to 6 s, with good temporal and spatial resolution. To determine whether shorter scan times can lead to less underestimation of true plaque burden, a comparison with IVUS examination of a long coronary arterial segment using standard central laboratory analysis is required (8). Also, nitroglycerin was not systematically administered before MDCT in many previous comparative studies, which sometimes involved its use before invasive angiography only (9). Administration of nitroglycerin has been advocated for better assessment of stenosis severity in such studies (10) because variable vasomotor tone may influence results (11,12), especially when they are based on dichotomous outcomes (typically, stenosis greater than 50%). Therefore, the main objective of the present study was to compare coronary arterial plaque volumes determined by 64-slice MDCT and IVUS. A secondary objective was to compare evaluations of stenosis severity by quantitative coronary angiography (QCA) and MDCT when a 64-slice scanner and systematic administration of nitroglycerin were used.
METHODS
Patient population
The study was previously approved by the Montreal Heart Institute (Montreal, Quebec) ethics committee and every patient gave written informed consent. Patients selected for the study needed to be at least 18 years of age, scheduled for clinically indicated, nonurgent coronary angiography and appropriate candidates to undergo diagnostic invasive angiography, MDCT and IVUS examinations. A total of 49 consecutive patients who were initially suitable and willing to participate were recruited for the study. Patients with a contraindication to contrast injection (known allergy to iodinated contrast, renal insufficiency defined as serum creatinine greater than 120 μmol/L before MDCT examination [n=1] or pregnancy), irregular heart rhythm such as atrial fibrillation, previous coronary bypass surgery or stenting, contraindication to beta blockade or severe concomitant illness (n=1) were excluded. Also, patients who, on a preliminary calcium scoring study, demonstrated a value larger than 800 U using an Agatston score were excluded (n=5). A total of eight patients was determined to be unsuitable for IVUS examination by the interventional cardiologist for technical reasons (eg, because of severe stenoses or angulated vessels), one patient refused the MDCT examination and three patients were excluded because of low MDCT examination quality (bad electrocardiogram [ECG] synchronization, incomplete volume acquisition or improper breath-hold). Therefore, 30 patients underwent MDCT, coronary angiography and IVUS studies within 96 h of the initial computed tomography (CT) examination.
64-slice MDCT procedure
MDCT examination was performed with a 64-slice scanner (GE LightSpeed VCT; GE Healthcare, USA). Patients with a resting heart rate of greater than 65 beats/min 1 h before MDCT received 50 mg of metoprolol orally. After the use of standard localizers as well as bismuth shield moulds (Dyna Medical Corporation, Canada) on patients’ eyes, thyroid and breast areas to reduce radiation exposure to these organs, a preliminary calcium scoring study with prospective gating was performed to exclude patients with calcium scores above 800 U, measured on coronary arteries using an Agatston score. Parameters for the calcium scoring study were a rotation time of 350 ms, 2.5 mm thickness at 20 mm intervals, a tube voltage of 120 kV and a tube current of 400 mA, from the carina to the diaphragm.
Sublingual nitroglycerin (0.4 mg) was administered just before the contrast-enhanced acquisition to reduce vasomotor tone (10–12). Then, a triphasic bolus of 60 mL of nonionic, iso-osmolar iodine contrast (iodixanol 320) was given, followed by 50 mL of mixed contrast (50%) and saline (50%). Next, 25 mL of saline was injected into an antecubital vein at a rate of 5 mL/s. Automatic detection of maximum enhancement (SmartPrep [GE Healthcare] acquisition) on the ascending aorta was used to determine timing of scan acquisition. The volume data set of 64 parallel slices using an algorithm of 180-degree multislice cardiac interpolation was obtained at 0.625 mm intervals. The rotation time was 350 ms, and the tube voltage was 120 kV at a current of 550 mA to 750 mA (depending on patient weight) from the carina to the diaphragm, without ECG dose modulation. All images were acquired on a single breath-hold of approximately 6 s using mono- or multisegmented algorithms. Images were reconstructed to a slice thickness of 0.625 mm with an interslice gap of 0.625 mm using retrospective ECG gating from 0% to 90% of the R-R interval at 10% increments. Automatic reconstruction of the 75% phase was also obtained.
Invasive coronary angiography and QCA
Coronary angiography was performed within 96 h of MDCT imaging according to a standard acquisition protocol and parameters were controlled to decrease variability in measurements (13). The images were recorded with a digital system (CD-R) at a speed of at least 15 frames/s using catheters with a size of at least 6 Fr. Intracoronary nitroglycerin (150 μg) was administered into each coronary artery before angiographic injection. The segments of interest were visualized in multiple transverse and sagittal views to clearly separate stenoses from branches, minimize foreshortening and obtain views as perpendicular as possible to the long axis of the segments to be analyzed. The quantitative analysis of coronary angiograms (QCA) was performed using the Clinical Measurement Solutions system (QCA-CMS; MEDIS Imaging Systems, The Netherlands). For each lesion, an end-diastolic frame was selected with an angulation that best showed the stenosis at its most severe degree, with minimal foreshortening and branch overlap. The automatic edge detection program determined the vessel contours by assessing brightness along scanlines perpendicular to the vessel centre. To provide absolute numbers for vessel sizes, a calibration factor expressed in mm/pixel was determined by applying the edge detection procedure for parallel boundaries on a nontapering section (of known size) of the contrast-filled catheter. The computer software automatically calculated the minimum lumen diameter, reference diameter, percentage diameter stenosis and stenosis length. All coronary segments from the revised National Institutes of Health Protocol for the Bypass Angioplasty Revascularization Investigation (BARI) (14) nomenclature with a reference diameter of 1.5 mm or larger, that were well opacified and showed diameter stenosis of at least greater than 15% were analyzed by two experienced technicians and a radiologist blinded to IVUS and MDCT results. Segments distal to a complete occlusion or a very severe obstruction with slow antegrade flow were not analyzed due to inadequate distal filling.
IVUS examination and analysis
The methods of the IVUS procedure were detailed previously (8). Briefly, single-vessel IVUS studies were performed using 40 MHz IVUS catheters (Boston Scientific, USA). IVUS examination was performed on one target coronary artery with a reference diameter of 2.5 mm or larger that was free of thrombus, did not have lumen reduction greater than 50% in diameter, was not previously submitted to angioplasty, was not a candidate for intervention at the time of catheterization and was not severely angulated. If more than one coronary artery met these criteria, the choice was left to the discretion of the interventional cardiologist based on the expected ease of guiding catheter engagement and IVUS catheter advancement, as well as the safety of the procedure. Intracoronary nitroglycerin (150 μg) was administered before the IVUS examination. The IVUS catheter was advanced distally, at least 40 mm beyond the coronary artery ostium, to a recognizable landmark (arterial branch). IVUS images were then recorded on super-VHS videotape while the transducer was pulled back automatically at a speed of 0.5 mm/s up to the guiding catheter with the use of a validated motorized device. A detailed running audio commentary was recorded during the pullback. A second pullback was then performed in the same coronary artery using the same guidelines to ensure high-quality imaging.
The matching of the coronary segment to be analyzed on IVUS and MDCT was performed by an independent observer during the coronary angiogram, with distal and proximal branches used as recognizable fiduciary sites. Experienced technicians supervised by a cardiologist blinded to MDCT and QCA results performed the quantitative IVUS analysis using a custom-developed system and software for geometric computations (Echo Plaque, Indec Systems Inc, USA). The lumen and external elastic membrane borders were traced manually on digitized cross-sections at every 1 mm in the 30 mm segment of interest. Measurements of lumen area, plaque area and external elastic membrane area were available for every traced cross-section of the analyzed segment. Plaque, lumen and total vessel volumes were computed for the entire length of the analyzed segment by multiplying the corresponding areas of each of the cross-sections by the distance between the neighbouring slices and then adding all the products.
MDCT data analysis
All reconstructed images (0% to 90% of the R-R interval) were transferred to a dedicated Advantage Windows workstation version 4.2, which was equipped with CardIQ vessel analysis software (GE Healthcare) for image analysis by two experienced observers blinded to IVUS and QCA results. The appropriate phase of the R-R interval acquisition was first determined by consensus to be the best phase free of motion artefacts for each artery to make subsequent volume rendering, multiplanar and curved reconstructions centred on the lumen using the CardIQ vessel analysis program. Nineteen studies were reconstructed at 75% of the R-R interval, seven at 80%, three at 70% and one at both 75% and 80% of the R-R interval. Image quality was determined for each coronary segment (using the revised National Institutes of Health segment nomenclature [14]) and ranked from 1 to 4, based on the presence of motion artefacts and adequate opacification to obtain good contrast-to-noise ratio, where grade 1 (excellent) was an artefact-free study with adequate contrast enhancement/vessel opacification and an unrestricted evaluation; grade 2 (good) was a study with minor artefacts, adequate contrast enhancement/vessel opacification and good diagnostic quality; grade 3 (adequate) was a study with moderate artefacts but adequate contrast enhancement and vessel opacification, and acceptable for routine clinical diagnosis of significant obstruction; and grade 4 (poor/not evaluable) was a study with severe artefacts and/or limited/poor contrast enhancement resulting in blurred or double vessel contours or no vessel visualization, impairing or making accurate evaluation impossible. Segments with poor/not evaluable image quality were recorded but not included in the correlation analysis.
For the comparison of MDCT and IVUS, orthogonal reconstructions to the long axis of the vessel lumen of the target artery were obtained from curved reformatted MDCT images and recorded at 1 mm intervals using fixed window-level settings. Optimal contrast-to-noise ratio was determined visually for each patient to optimize window-level settings to reduce beam-hardening artefacts from calcified plaque. Manual tracings of the lumen and vessel wall contours were performed on each cross-section, and plaque area was calculated by subtracting luminal area from vessel area, using the same method as that for IVUS. Plaque volume was then calculated for the entire length of the analyzed segment by multiplying the plaque area of each of the cross-sections by the distance between the neighbouring slices and then summing all of the products. When calcifications impaired adequate tracing of the lumen or vessel on one cross-section, the image was excluded from plaque volume determination. Plaque volumes were indexed to 30 mm for both MDCT and IVUS. A total of 12 patients had their MDCT examinations evaluated twice by the same reviewer and also by a second reviewer for evaluation of intraobserver and interobserver variability of measurements.
For the comparison of MDCT and QCA, the percentage of diameter stenosis was determined to the nearest 5% by consensus of two experienced readers in all segments with at least adequate MDCT image quality. In case of disagreement between the two observers, the lumen diameter was measured at the sites of stenosis and reference with the standard measurement tools available in the Advanced Vessel Analysis software package (GE Healthcare) to determine percentage diameter stenosis by MDCT.
Statistical analysis
Continuous variables are presented as mean ± SD, median and range. Categorical variables are presented as counts and percentages. The primary efficacy end point was the plaque volume measured by MDCT and IVUS. The primary end point was analyzed using the patient as the unit of analysis because there was only primary end point measurement per patient. The secondary end point was the percentage diameter stenosis as assessed by MDCT and QCA in multiple coronary segments. The analysis of this secondary end point was performed with the patient, the vessel and the segment as the units of analysis. For the per-patient analysis, the percentage diameter stenosis was calculated as the maximum percentage stenosis on all coronary segments available for a patient. A similar approach was used for the per-vessel analysis in which the percentage stenosis was computed as the maximum value in the coronary segments of a given vessel. This end point (percentage diameter stenosis) was considered to be a continuous variable and was also studied as a binary variable using thresholds of 50% and 75%. Pearson’s correlation coefficients were used to assess the relationship between continuous variables (MDCT versus IVUS and MDCT versus QCA). The ability of MDCT to identify significant stenosis assessed by QCA was also evaluated by calculating the sensitivity and specificity in detecting patients, vessels or segments with at least one stenosis greater than 50% on QCA and greater than 75% on QCA. All tests were two-sided and conducted at the 0.05 significance level. Analyses were performed with SAS release 8.2 (SAS Institute Inc, USA).
RESULTS
Of the 30 patients from the evaluable population (mean age 59 years, 23 men), 26 (87%) had a history of angina, five (17%) had a history of previous myocardial infarction, 28 (93%) had dyslipidemia, 14 (47%) had hypertension, 21 (70%) reported a history of smoking and no patient had known diabetes. The clinical indication for coronary angiography was stable angina in most (87%) patients.
MDCT was performed successfully in all 30 patients without any adverse events. The mean heart rate was 58 beats/min (range 45 beats/min to 73 beats/min) at the beginning of the examination. Only two patients had heart rates faster than 70 beats/min during MDCT. The mean calcium score was 202 U (range 0 U to 777 U) on the Agatston scale. Three patients (10%) had total calcium scores higher than 600. Of a total of 462 coronary segments, 43 were considered too small to allow measurements on MDCT. Twenty-two segments of the total of 462 (4.8%) were rejected (grade 4) for the MDCT-QCA comparison because of severe motion artefacts. The MDCT image quality was graded 3 in five arteries (17%) targeted for the IVUS analysis. Also, 21 segments were not analyzed by QCA and MDCT because of different reasons including slow antegrade flow or because the segments were considered absent. MDCT reconstructions were all obtained between 70% and 80% of the R-R interval for all arteries. Quantification of plaque volume and percentage diameter stenosis was possible with MDCT in all patients.
Primary efficacy analysis (MDCT versus IVUS)
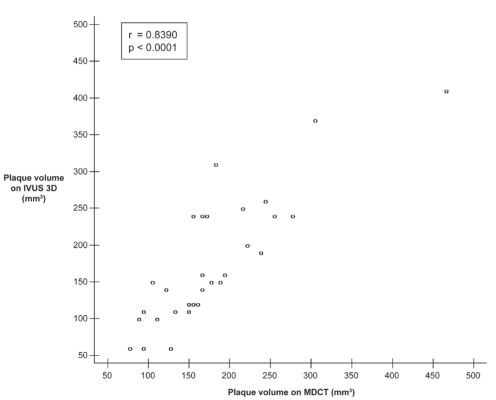
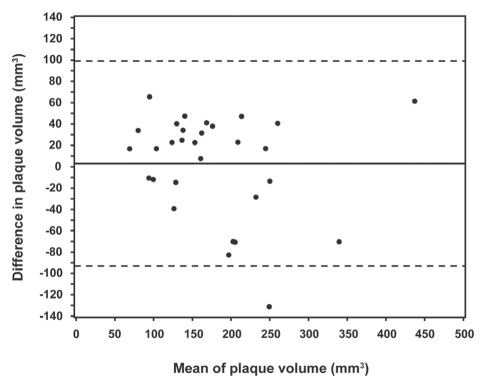
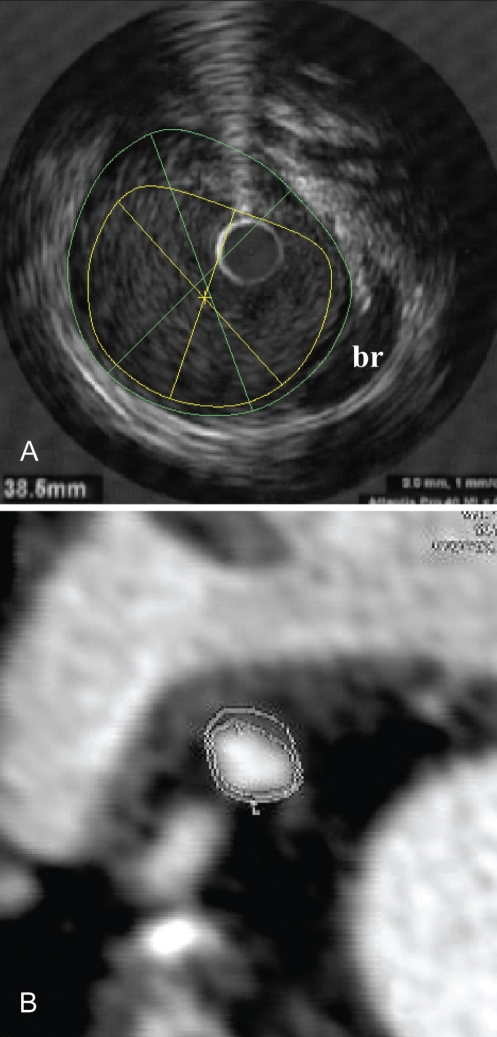
The target coronary artery for IVUS was the left anterior descending artery in 11 patients, the circumflex artery in six patients, an obtuse marginal branch in three patients and the right coronary artery in 10 patients. The mean plaque volume was 179.1±78.9 mm3 by MDCT (range 77.7 mm3 to 468.0 mm3) and 176.1±87.9 mm3 (range 60.9 mm3 to 406.5 mm3) by IVUS. There was a strong positive correlation between plaque volume on MDCT and IVUS, with an r value of 0.84 (P<0.0001, Figure 1). There was a slight overestimation (mean 3.0 mm3) of plaque volume with MDCT compared with IVUS (Figure 2). MDCT and IVUS images from one patient are shown in Figure 3.
Figure 1).
Correlation between multidetector computed tomography (MDCT) and three-dimensional intravascular ultrasound (IVUS 3D) for plaque volume
Figure 2).
Limits of agreement (Bland-Altman analysis) for the comparison of multidetector computed tomography (MDCT) and intravascular ultrasound (IVUS) for plaque volume. The vertical axis represents the value obtained by MDCT analysis minus the value obtained by IVUS analysis. The solid line indicates the mean difference between MDCT and IVUS, and the dashed lines represent mean ± 2 SD
Figure 3).
Multidetector computed tomography (MDCT) and intravascular ultrasound (IVUS) images of the same patient. Plaque, lumen and vessel areas were 7 mm2, 14 mm2 and 21 mm2, respectively, in this cross-section on both IVUS (A) and MDCT (B). br Arterial branch
The intraclass coefficients for intraobserver variability of lumen and vessel area measurements by MDCT were 0.98 and 0.95, respectively. The corresponding values for interobserver variability were 0.94 and 0.97, respectively.
Secondary efficacy analysis (MDCT versus QCA)
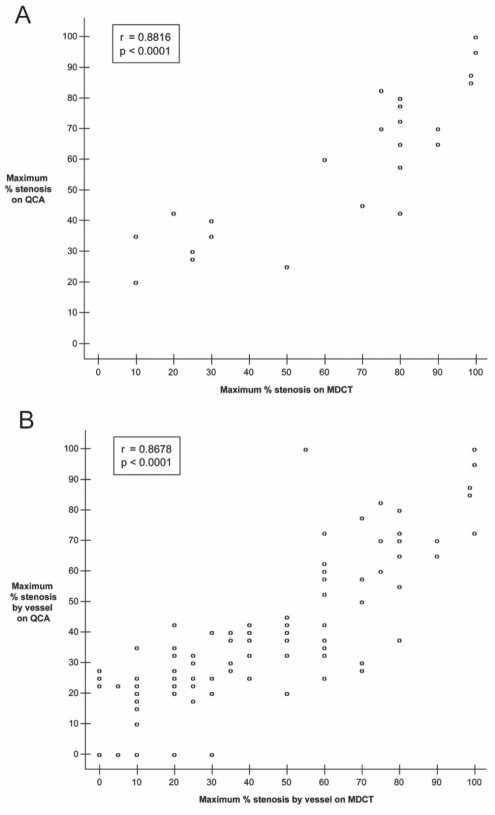
Using the patient as the unit of analysis, the mean percentage diameter stenosis was determined to be 69.6±30.0% by MDCT and 65.0±25.8% by QCA. There was an excellent correlation between the percentage diameter stenosis assessed by the two imaging techniques at the patient level (r=0.88, P<0.0001, Figure 4A). A total of 20 (66.7%) patients had at least one stenosis greater than 50% (Table 1) and 11 (36.7%) had at least one stenosis greater than 75% (Table 2) according to QCA. MDCT had a sensitivity and specificity of 100% and 80%, respectively, to correctly identify stenoses greater than 50%.
Figure 4).
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for percentage diameter stenosis in the per-patient analysis (A) and the per-vessel analysis (B). Six points and 40 points are superimposed in graphs A and B, respectively
TABLE 1.
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for stenosis greater than 50% in the per-patient analysis
|
At least one stenosis >50% by QCA |
|||
|---|---|---|---|
| Yes | No | ||
| At least one stenosis >50% by MDCT | Yes | 20 | 2 |
| No | 0 | 8 | |
Sensitivity 20/20=100%; specificity 8/10=80%
TABLE 2.
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for stenosis greater than 75% in the per-patient analysis
|
At least one stenosis >75% by QCA |
|||
|---|---|---|---|
| Yes | No | ||
| At least one stenosis >75% by MDCT | Yes | 10 | 6 |
| No | 1 | 13 | |
Sensitivity 10/11=90.9%; specificity 13/19=68.4%
With the vessel as the unit of analysis, 118 vessels were assessed by both MDCT and QCA in the 30 patients. When considered as a continuous variable and averaged over the 118 vessels, percentage diameter stenosis was determined to be 38.1±30.2% by MDCT and 34.1±27.6% by QCA. There was a strong positive association between the percentage diameter stenoses assessed by the two imaging techniques at the vessel level (r=0.87, P<0.0001, Figure 4B). According to QCA, 29 (24.6%) vessels showed a stenosis greater than 50% (Table 3) and 11 (9.3%) presented a stenosis greater than 75% (Table 4). The sensitivity and specificity of MDCT in detecting stenoses above 50% on QCA were 100% and 91.0% in the per-vessel analysis, respectively.
TABLE 3.
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for stenosis greater than 50% in the per-vessel analysis
|
Stenosis >50% by QCA |
|||
|---|---|---|---|
| Yes | No | ||
| Stenosis >50% by MDCT | Yes | 29 | 8 |
| No | 0 | 81 | |
Sensitivity 100%; specificity 91.0%
TABLE 4.
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for stenosis greater than 75% in the per-vessel analysis
|
Stenosis >75% by QCA |
|||
|---|---|---|---|
| Yes | No | ||
| Stenosis >75% by MDCT | Yes | 8 | 8 |
| No | 3 | 99 | |
Sensitivity 72.7%; specificity 92.5%
A total of 376 segments were assessed in the 30 patients by both MDCT and QCA. When considered as a continuous variable (n=376 segments), percentage diameter stenosis was 24.1±24.8% with MDCT and 19.5±22.4% with QCA, and the results were significantly correlated (r=0.74, P<0.0001). To avoid dilution of results by the large number of normal segments, analysis was also performed on the 208 segments with at least one stenosis greater than 0%; in this analysis, percentage stenosis was 34.9±25.9% with MDCT and 35.3±18.7% with QCA. When the coronary segment was taken as the unit of analysis (n=376 segments), 39 (10.4%) segments presented a stenosis greater than 50% (Table 5) and nine (2.4%) showed a stenosis greater than 75% (Table 6) on QCA. MDCT had a sensitivity and specificity to detect stenoses greater than 50% of 84.6% and 94.1% at the segment level, respectively. MDCT and QCA images from one patient are shown in Figure 5.
TABLE 5.
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for stenosis greater than 50% in the per-segment analysis*
|
Stenosis >50% by QCA |
|||
|---|---|---|---|
| Yes | No | ||
| Stenosis >50% by MDCT | Yes | 33 | 20 |
| No | 6 | 317 | |
Sensitivity 84.6%; specificity 94.1%.
After correcting for discrepancies due to different segment nomenclatures in QCA and MDCT analyses (see text), the sensitivity and specificity of MDCT were 90.0% and 94.9%, respectively
TABLE 6.
Correlation between multidetector computed tomography (MDCT) and quantitative coronary angiography (QCA) for stenosis greater than 75% in the per-segment analysis*
|
Stenosis >75% by QCA |
|||
|---|---|---|---|
| Yes | No | ||
| Stenosis >75% by MDCT | Yes | 6 | 10 |
| No | 3 | 357 | |
Sensitivity 66.7%; specificity 97.3%.
After correcting for discrepancies due to different segment nomenclatures in QCA and MDCT analyses (see text), the sensitivity and specificity of MDCT were 80.0% and 97.8%, respectively
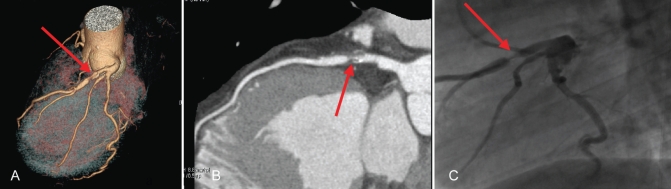
Figure 5).
Severe (70%) stenosis in the proximal left anterior descending artery (arrows) in volume rendering (A) and curved reformat (B) with multidetector computed tomography and conventional invasive angiography (C)
On review of the discrepancies between the QCA and MDCT results (after the blinded evaluations were completed), a total of seven segments (of 376) were identified, in which different nomenclatures or approaches were used for the same lesions identified by both laboratories (different segment numbers assigned; lesions distal to a subtotal occlusion measured by MDCT but not by QCA). When these were corrected, the correlation between MDCT and QCA at the segment level was 0.79 (P<0.0001), and the sensitivity and specificity of MDCT to detect stenosis greater than 50% on QCA were 90.0% and 94.9%, respectively.
DISCUSSION
The present study demonstrates good correlation between MDCT (with a 64-slice scanner allowing examinations in 5 s to 6 s) and IVUS for determining coronary artery plaque volume. There was also a strong correlation between MDCT and QCA for determination of percentage diameter stenosis, with excellent sensitivity and specificity of MDCT to detect stenoses greater than 50% on QCA in the per-patient and per-vessel analyses.
CT techniques have developed rapidly during the past decade. Evaluation of coronary calcifications with electron-beam CT paved the way, and thereafter, ECG-gating and multislice CT with four, eight, 16, 32 and now 64 detectors have refined the images with improved spatial and temporal resolution. Now that 64-slice CT is available on the market, spatial resolution is approximately 0.4 mm × 0.4 mm and temporal resolution is approximately 220 ms per slice. These parameters have improved the resolution on distal segments and reduced motion artefacts due to limited temporal resolution. Improved temporal resolution, with scanning times in the order of 6 s, has also improved image quality by reducing potential fluctuations of heart rate during a single acquisition. Only two studies have compared assessment of plaque burden by 64-slice MDCT and IVUS (4,7), while a few others compared 16-slice MDCT with IVUS (6,15). Underestimation of plaque burden by MDCT was reported in three of four studies (4,6,7), and in all studies that actually reported plaque volumes (6,7). It is probable that motion artefacts were partly responsible for this underestimation by MDCT, even if scan times were 8 s to 11 s (3,4). Plaque volumes evaluated by a 64-slice scanner were compared with those on IVUS in only one study involving 20 patients, in which an underestimation of plaque burden also occurred with MDCT, although a moderate correlation was still reported (7). In that study, 20 MHz IVUS catheters were used, resulting in lesser axial resolution as opposed to higher frequency catheters (40 MHz), and nitroglycerin was not used for IVUS and MDCT examinations (7). Our study adds to the current knowledge by reporting for the first time an excellent correlation of plaque volumes determined by 64-slice MDCT and IVUS (without systematic underestimation by MDCT), systematically using nitroglycerin (before MDCT and IVUS) and 40 MHz catheters. Our study is also the first to report the comparison of 64-slice MDCT and QCA, incorporating the careful use of nitroglycerin before both examinations as recommended (10), to eliminate differences in vasomotor tone (11,12). In contrast, some studies have described suboptimal specificity of MDCT compared with QCA (1,2). Reasons for these different results may include the use of older-generation (16-slice) scanners and the lack of nitroglycerin administration (1,2). In our study, the correlation between MDCT and QCA was slightly lower at the segment level than on a per-patient or per-vessel basis. One of the reasons for this observation is a difference in segment nomenclature between approaches in seven instances; for example, an obtuse marginal branch on one technique may have been defined as the distal circumflex artery when using the other imaging modality. Because of this, the per-vessel analysis (instead of the per-segment assessment) probably represents a better comparison of MDCT and QCA. When this analysis was considered, the sensitivity and specificity of MDCT to detect stenoses greater than 50% were 100% and 91.0%, respectively. It could be argued that the few false-positive results of MDCT may lead to unnecessary additional tests (possibly including invasive angiography). Nevertheless, from the clinician’s perspective, this occasional situation is of much less concern than if false-negative cases had been identified. Importantly, there were no patients in whom MDCT failed to identify a significant (greater than 50%) stenosis; hence, no patients would have been falsely reassured by the MDCT results.
There were no adverse events or renal dysfunctions associated with the imaging examinations in the present study. Because MDCT and invasive angiography were performed within a relatively short period of time (four days) and both required injections of contrast material, serum creatinine greater than 120 μmol/L at screening was a prespecified exclusion criterion. Nevertheless, MDCT is associated with exposure to radiation (16), which, in the case of the 64-slice scanner and protocol (without ECG dose modulation) used in the present study, is estimated to be 20 mSv. Interestingly, 16% of our patients were found to have significant extracardiac findings, emphasizing the value of carefully reviewing all the information provided by the MDCT examination. Important extracardiac abnormalities included pulmonary and upper abdominal pathology, including cancer and a large pulmonary embolus. Other abnormal findings observed in the present study (not reported in the Results section) included enlarged mediastinal lymph nodes, dilated ascending aorta, pericardial effusion and calcifications, pleural plaques, hepatic cysts and nodules. When taking into account all of these abnormalities, extracardiac findings were identified in more than one-half of patients, as recently described by Onuma et al (17).
Limitations
One limitation of the present study is the image quality with only an adequate grade in five target vessels for IVUS, as well as the 22 segments (4.8%) that could not be analyzed by MDCT (grade 4) for the MDCT-QCA comparison because of severe motion artefacts. The exclusion of these coronary segments did not involve the primary comparison of MDCT with IVUS. Another limitation of the present study is the selection bias caused by exclusion of patients with an Agatston score above 800 U, but these heavily calcified arteries are also more difficult to analyze with IVUS.
CONCLUSIONS
In patients with Agatston scores lower than 800 U and good-quality MDCT, coronary artery plaque volumes measured by 64-slice MDCT and 40 MHz IVUS correlate well, without systematic underestimation. The sensitivity and specificity of MDCT to detect angiographic stenoses above 50% are also excellent when nitroglycerin is used before each examination.
Acknowledgments
We gratefully acknowledge the expert work of the personnel of the IVUS core laboratory (Joanne Vincent, Ginette Grenier, Colombe Roy and Claudette Léger-Gauthier), the QCA core laboratory (Colette Desjardins and Marie-Josée Dussault) and the MDCT core laboratory (Brigitte Auclair, Janie Paquin, Sylvain Theberge and Brigitte Thibault).
Footnotes
FUNDING: This study was funded by GE Healthcare.
REFERENCES
- 1.Achenbach S, Giesler T, Ropers D, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography. Circulation. 2001;103:2535–8. doi: 10.1161/01.cir.103.21.2535. [DOI] [PubMed] [Google Scholar]
- 2.Garcia MJ, Lessick J, Hoffmann MHK, for the CATSCAN Study Investigators Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–11. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann U, Moselewski F, Cury RC, et al. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease. Circulation. 2004;110:2638–43. doi: 10.1161/01.CIR.0000145614.07427.9F. [DOI] [PubMed] [Google Scholar]
- 4.Leber AW, Knez A, Von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography. J Am Coll Cardiol. 2005;46:147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 5.Guedes A, Tardif JC. Intravascular ultrasound assessment of atherosclerosis. Curr Atheroscler Rev. 2004;6:219–24. doi: 10.1007/s11883-004-0035-4. [DOI] [PubMed] [Google Scholar]
- 6.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography. A segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 7.Leber AW, Becker A, Knez A, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system. A comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–7. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 8.Tardif JC, Gregoire J, L’Allier PL, et al. Progression of Lesions on UltraSound (A-PLUS) Investigators Effects of the acyl coenzyme A: Cholesterol acyltransferase inhibitor avasimibe on human coronary atherosclerotic lesions. Circulation. 2004;110:3372–8. doi: 10.1161/01.CIR.0000147777.12010.EF. [DOI] [PubMed] [Google Scholar]
- 9.Schoenhagen P, Tuzcu EM, Stillman AE, et al. Non-invasive assessment of plaque morphology and remodelling in mildly stenotic coronary segments: Comparison of 16-slice computed tomography and intravascular ultrasound. Coron Artery Dis. 2003;14:459–62. doi: 10.1097/00019501-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Schmermund A, Erbel R. Non-invasive computed tomographic coronary angiography: The end of the beginning. Eur Heart J. 2005;26:1451–3. doi: 10.1093/eurheartj/ehi322. [DOI] [PubMed] [Google Scholar]
- 11.Jost S, Sturm M, Hausmann D, Lippolt P, Lichtlen PR. Standardization of coronary vasomotor tone with intracoronary nitroglycerin. Am J Cardiol. 1996;78:120–3. doi: 10.1016/s0002-9149(96)00243-3. [DOI] [PubMed] [Google Scholar]
- 12.Lesperance J, Waters D. Measuring progression and regression of coronary atherosclerosis in clinical trials: Problems and progress. Int J Card Imaging. 1992;8:165–73. doi: 10.1007/BF01146835. [DOI] [PubMed] [Google Scholar]
- 13.Tardif JC, Gregoire J, Lesperance J, et al. Design features of the Avasimibe and Progression of coronary Lesions assessed by intravascular UltraSound (A-PLUS) clinical trial. Am Heart J. 2002;144:589–96. doi: 10.1067/mhj.2002.125329. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health Protocol for the Bypass Angioplasty Revascularization Investigation. Circulation. 1991;84(Suppl V):V1–27. [Google Scholar]
- 15.Moselewski F, Ropers D, Pohle K, et al. Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice multidetector computed tomography versus intravascular ultrasound. Am J Cardiol. 2004;94:1294–7. doi: 10.1016/j.amjcard.2004.07.117. [DOI] [PubMed] [Google Scholar]
- 16.Hausleiter J, Meyer T, Hadamitzky M, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice. Impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305–10. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 17.Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol. 2006;48:402–6. doi: 10.1016/j.jacc.2006.04.071. [DOI] [PubMed] [Google Scholar]