Abstract
BACKGROUND:
The Incremental Decrease in End-Points Through Aggressive Lipid-Lowering (IDEAL) trial demonstrated incremental cardiovascular benefit of treatment with high-dose atorvastatin (80 mg/day) versus standard-dose simvastatin (20 mg/day to 40 mg/day) in 8888 patients with a previous myocardial infarction (MI) over a median follow-up period of 4.8 years.
OBJECTIVES:
To assess the cost-effectiveness of high-dose atorvastatin versus standard-dose simvastatin treatment in patients with a history of MI from a Canadian societal perspective.
METHODS:
In a within-trial analysis, end point-related events, resources used and productivity losses occurring during the IDEAL trial were aggregated by treatment arm on an intention-to-treat basis to calculate the incremental cost per event avoided. Additionally, quality-adjusted survival was projected using a lifetime Markov model. Transition probabilities, workdays lost, use of study medication and cardiovascular hospitalization rates were based on IDEAL trial data. Hospitalization, study medication and productivity costs were included. Probabilistic and deterministic sensitivity analyses were performed.
RESULTS:
Compared with standard-dose simvastatin, atorvastatin 80 mg led to 0.099 fewer events per patient and cost savings over 4.8 years of treatment. Over a lifetime horizon, atorvastatin 80 mg led to 0.023 quality-adjusted life years (QALYs) gained per patient at an incremental cost of $26,795/QALY gained. The incremental cost-effectiveness ratio remained below $50,000/QALY in 78% of 1000 simulations. Exclusion of indirect costs resulted in an incremental cost-effectiveness ratio of $38,834/QALY. Results were relatively sensitive to baseline age, but robust with respect to sex, baseline low-density lipoprotein cholesterol levels, diabetes status and hospitalization costs.
CONCLUSION:
From a Canadian societal perspective, high-dose atorvastatin is cost-effective compared with standard-dose simvastatin in patients with a previous MI.
Keywords: Atorvastatin, Clinical outcomes, Cost-effectiveness, Cost utility, Markov model, Simvastatin
Abstract
HISTORIQUE :
L’essai IDEAL sur la diminution incrémentielle des points de virage par l’abaissement énergique des lipides a démontré les bienfaits cardiovasculaires incrémentiels du traitement d’atorvastatine à forte dose (80 mg/jour) par rapport au traitement de simvastatine à dose standard (de 20 mg/jour à 40 mg/jour) chez 8 888 patients ayant déjà subi un infarctus du myocarde (IM) pendant une période de suivi médiane de 4,8 ans.
OBJECTIFS :
Évaluer le rapport coût-efficacité du traitement d’atorvastatine à forte dose par rapport à celui de simvastatine à dose standard chez des patients ayant des antécédents d’IM selon une perspective sociétale canadienne.
MÉTHODOLOGIE : Dans l’analyse de l’essai, les auteurs ont regroupé les événements liés aux points de virage, les ressources utilisées et les pertes de productivité observées pendant l’essai IDEAL selon le volet de traitement et d’après l’intention de traiter afin de calculer le coût incrémentiel par événement évité. De plus, ils ont projeté la survie rajustée à la qualité au moyen du modèle de Markov au cours d’une vie. Ils ont fondé les probabilités de transition, les jours de travail perdus, l’utilisation des médicaments à l’étude et les taux d’hospitalisation en soins cardiovasculaires sur les données de l’essai IDEAL. Enfin, ils ont inclus l’hospitalisation, les médicaments à l’étude et les coûts de productivité et ont effectué des analyses de sensibilité probabilistes et déterministes.
RÉSULTATS : Par rapport à la simvastatine à dose standard, 80 mg/jour d’atorvastatine ont favorisé 0,099 moins d’événements par patient et des économies pendant les 4,8 années du traitement. Dans l’horizon d’une vie, 80 mg d’atorvastatine suscitaient un gain de 0,023 année de vie pondérée par la qualité (AVPQ), à un coût incrémentiel de 26 795 $/AVPQ. Le rapport coût-efficacité incrémentiel est demeuré sous les 50 000 $/AVPQ dans 78 % des 1 000 simulations. L’exclusion des coûts indirects a donné un rapport coût-efficacité incrémentiel de 38 834 $/AVPQ. Les résultats étaient relativement sensibles à l’âge de départ, mais robustes pour ce qui est du sexe, des taux de cholestérol à lipoprotéines de basse densité de départ, du statut de diabète et des coûts d’hospitalisation.
CONCLUSION :
D’une perspective sociétale canadienne, l’atorvastatine à forte dose est rentable par rapport à la simvastatine à dose standard chez des patients ayant déjà subi un IM.
Coronary artery disease (CAD) is a major public health problem in Canada. In the 2000/2001 Canadian Community Health Survey, 2.1% of people aged 12 years or older reported having had a myocardial infarction (MI), 1.9% reported suffering from angina and 1.0% had congestive heart failure (CHF) (1). The overall prevalence of self-reported heart disease was 5.0% (1). In 1999, CAD was responsible for 20% of deaths in Canada – one-half of which were due to acute MI (2). Heart disease also affects patients at an individual level. Among those with angina, CHF or MI, approximately 17.4% report problems walking, 35.9% live with pain, 59.2% need help with activities of daily living, and 5.4% are not working because of illness or disability (3).
CAD is a major driver of cost in the Canadian health care system. (2,4). Between 1994/1995 and 1999/2000, age- and sex-standardized hospitalization rates for chest pain, angina and acute MI increased by 11%, 8% and 6%, respectively (5). The use of diagnostic and therapeutic cardiac procedures rose even more rapidly. Between 1992 and 2001, the age- and sex-adjusted number of percutaneous coronary interventions (PCIs) per 1000 adult Ontarians increased by 180%, the number of catheterizations by 138% and the number of coronary artery bypass grafts (CABGs) by 57% (4). As a consequence, direct expenditures for these types of procedures (including echocardiography, stress testing, perfusion imaging, catheterization, PCI and CABG) almost doubled in Ontario from $207 million in 1992 to $391 million in 2001 (presented in 2000/2001 CAD dollars) (4).
Lowering low-density lipoprotein cholesterol (LDL-C) levels is a recommended treatment strategy for the prevention of cardiovascular (CV) events (6). Guidelines released by the Canadian Cardiovascular Society in 2003 (7) recommended intensive lipid lowering to an LDL-C target of below 2.0 mmol/L for patients at high risk for CAD, such as those with established CAD. A recent meta-analysis (8) of 14 randomized trials based on data from 90,056 patients receiving statins found that the five-year incidence of major coronary events, coronary revascularization procedures and stroke was reduced by approximately 20% per mmol/L (corresponding to 38.7 mg/dL) drop in LDL-C, irrespective of baseline lipid levels.
The Incremental Decrease in End-Points Through Aggressive Lipid-Lowering (IDEAL) study (9) – a prospective, randomized, open-label, blinded end point trial – confirmed the incremental clinical benefit of intensive versus moderate LDL-C lowering. The trial compared high-dose atorvastatin (80 mg/day) treatment with standard-dose simvastatin (20 mg/day to 40 mg/day) treatment in 8888 patients from Northern Europe (Denmark, Finland, Iceland, the Netherlands, Norway and Sweden) who had a history of acute MI. Over the median follow-up of 4.8 years, mean LDL-C levels decreased from 3.15 mmol/L (121.5 mg/dL) at baseline to 2.69 mmol/L (104 mg/dL) for patients allocated simvastatin, and to 2.10 mmol/L (81 mg/dL) for patients allocated atorvastatin. Patients randomly assigned to high-dose atorvastatin experienced fewer major coronary events, defined as coronary death, nonfatal acute MI or cardiac arrest with resuscitation (hazard ratio [HR] 0.89 [95% CI 0.78 to 1.01], P=0.07), and significantly fewer nonfatal acute MIs (HR 0.83 [95% CI 0.71 to 0.98], P=0.02), CAD events (HR 0.84 [95% CI 0.76 to 0.91], P<0.001), CV events (HR 0.84 [95% CI 0.78 to 0.91], P<0.001) and major CV events (HR 0.87 [95% CI 0.78 to 0.98], P=0.02).
An economic evaluation based on data collected during the IDEAL trial indicated that intensive lipid lowering with atorvastatin 80 mg would be cost-effective compared with treatment with generic simvastatin in Denmark, Norway and Sweden (10). In Finland, this strategy was found to be cost-effective in high-risk patients (10). Given the availability of generic simvastatin in Canada at an acquisition cost lower than that of atorvastatin, we questioned whether treatment with high-dose atorvastatin would represent a cost-effective use of health care resources in Canada. Therefore, the objective of the present study was to assess, from a Canadian societal perspective, the cost-effectiveness of intensive lipid lowering with high-dose atorvastatin (80 mg/day) compared with moderate lipid lowering with standard-dose generic simvastatin (20 mg/day to 40 mg/day) in patients with a history of MI, based on data collected during the IDEAL trial.
METHODS
Study description
Two approaches (a within-trial analysis and a trial-based Markov model) were used to assess the cost-effectiveness of intensive lipid lowering with high-dose atorvastatin (80 mg/day) versus moderate lipid lowering with standard-dose simvastatin (20 mg/day to 40 mg/day).
For the within-trial analysis, all resources used (included end point-related hospitalization and study drugs) and productivity losses (defined as time not working due to sick leave) during the IDEAL trial period (median follow-up 4.8 years) were aggregated by treatment arm on an intention-to-treat basis and multiplied by their respective Canadian costs. Likewise, all end point-related events, including stroke, transient ischemic attack, nonfatal myocardial infarction, heart failure, angina pectoris, cardiac arrest, PCI, CABG, cardiac transplant, other cardiothoracic procedures and other vascular procedures, were aggregated to arrive at a mean number of events per patient over the trial period in each treatment arm. Cost-effectiveness was calculated as the incremental cost per event avoided. No discounting was applied in the within-trial analysis due to the short time span.
The Markov cost-effectiveness model followed patients with a history of MI treated with either simvastatin (20 mg/day to 40 mg/day) or atorvastatin (80 mg/day) through four health states over a lifetime horizon (Figure 1). A detailed description of the model has been published (10). All patients start in the ‘at risk’ state. (For a detailed description of patients in the ‘at risk’ state, see below.) During each one-year cycle, patients could experience a nonfatal MI and transition into the ‘post-MI’ state, undergo a revascularization procedure (PCI or CABG) and transition into the ‘postrevascularization’ state, or die from any cause. Patients in the ‘post-MI’ and ‘postrevascularization’ states could remain in their respective health states or die.
Figure 1).
Markov cost-effectiveness model. MI Myocardial infarction
To avoid extrapolating the benefit of atorvastatin beyond the duration of the clinical trial, patients were assumed to receive atorvastatin for five years and then be switched to simvastatin. Health outcomes were quantified as life-years, based on predicted survival in the two treatment arms, and quality-adjusted life years (QALYs), based on survival adjusted for quality of life using published utility weights collected from Swedish patients. Cost-effectiveness was calculated as the incremental cost per life-year gained and the incremental cost per QALY gained. All costs and benefits were discounted at 5% per year as recommended by the guidelines of the Canadian Agency for Drugs and Technologies in Health (11).
The trial-based Markov model was adapted using Canadian patient characteristics and costs. Based on published studies of Canadian patients with a history of MI (12–14), the average initial age of the model cohort (ie, patients entering the model in the ‘at-risk’ state) was assumed to be 62 years, the proportion of women was 30% and the proportion of patients with diabetes was 25%. The baseline LDL-C level was assumed to be 2.75 mmol/L (106 mg/dL) based on a study (15) that reported baseline lipid levels for patients treated with atorvastatin and simvastatin in clinical practice in Southwestern Ontario.
Markov model transition probabilities
Transition probabilities between health states were based on patient-level data collected during the IDEAL trial and adapted using Canadian patient characteristics. The risks of experiencing an MI or undergoing a revascularization procedure – depending on sex, baseline age, diabetes status and LDL-C – were estimated by applying Weibull regression models to data from patients randomly assigned to simvastatin. The coefficients for parameters used in Weibull regression models to populate Markov model transition probabilities have been published (10). The corresponding risks in the atorvastatin arm were estimated by applying a RR ratio of 0.83 for MI and 0.77 for revascularization to the simvastatin-based risk estimates (9). The RR reduction was assumed to remain constant over time. However, after five years, patients in both arms were assumed to have the same risk as the simvastatin-treated patients; thus, the benefit of high-dose atorvastatin treatment was assumed to cease after the trial treatment period. The risk of death was assessed separately for patients in the ‘at-risk’ and ‘post-MI’ states by applying Weibull regression models to trial data from patients who had experienced no events (events were counted as censoring) or had experienced a nonfatal MI. Because there were too few deaths after revascularization during the trial, the model assumed the same mortality for patients in the ‘postrevascularization’ as in the ‘at-risk’ state. Mortality risk was assumed to be the same for both treatments arms.
Health care resource use
Resource use was based on patient-level data collected during the IDEAL trial and included use of study drug, concomitant medication and end point-related hospitalization. During the IDEAL trial, patients randomly assigned to atorvastatin received the 80 mg dose on 90.7% of the study days on which a study medication was given and received the 40 mg dose on 9.3% of the days. Patients randomly assigned to simvastatin received the 20 mg dose on 79.3% of study medication days and the 40 mg dose on 20.7%. Because there was no significant difference in the use of concomitant medication between the treatment arms, these data were not included in the model. End point-related hospitalizations are hospital admissions with a primary diagnosis of stroke, transient ischemic attack, nonfatal MI, heart failure, cardiac arrest or angina, or hospitalizations for heart transplantation, CABG, PCI, or other cardiothoracic or vascular procedure. During the IDEAL trial, all end points were assigned a diagnosis-related group (DRG) based on the NordDRG classification logic (16). In cases in which NordDRG classification was ambiguous, a weighted average based on the frequency of the NordDRG in the general Swedish population was used. The mean number of end point-related admissions per patient by NordDRG code is shown in Table 1.
TABLE 1.
Mean number of hospitalizations per patient during the Incremental Decrease in End-Points Through Aggressive Lipid-Lowering (IDEAL) trial
| Diagnosis or procedure (NordDRG code) |
Number of hospitalizations per patient, mean ± SD |
|
|---|---|---|
| Atorvastatin (80 mg/day) | Simvastatin (20–40 mg/day) | |
| Stroke (014) | 0.035±0.183 | 0.045±0.185 |
| Transient ischemic attack (015) | 0.013±0.114 | 0.013±0.111 |
| Nonfatal MI* (121, 122) | 0.054±0.226 | 0.052±0.221 |
| Heart failure (127) | 0.032±0.174 | 0.038±0.191 |
| Angina pectoris (140) | 0.038±0.191 | 0.044±0.205 |
| Cardiac arrest (129) | 0.002±0.037 | 0.002±0.037 |
| PCI (112) | 0.097±0.296 | 0.127±0.333 |
| CABG (106, 107) | 0.052±0.223 | 0.069±0.253 |
| Cardiac transplant (103) | 0 | 0.001±0.021 |
| Other cardiothoracic procedure (108) | 0 | 0.001±0.021 |
| Other vascular procedure (478, 479) | 0.028±0.164 | 0.040±0.194 |
Myocardial infarction (MI) without a procedure performed during the same hospitalization. Including all hospitalizations with MI, the total number was 0.08 per patient in the atorvastatin arm and 0.09 in the simvastatin arm. Note that patients can have more than one event during a hospitalization. CABG Coronary artery bypass graft; DRG Diagnosis-related group; PCI Percutaneous coronary intervention
The work status of each patient (ie, working, on sick leave or in early retirement) was recorded at each visit during the IDEAL trial for assessment of indirect (productivity) costs.
Costing
The cost per 40 mg or 80 mg atorvastatin pill used in the model was $2.236 and the cost per generic simvastatin 20 mg or 40 mg pill was $1.100, as listed in the current Ontario Drug Benefit Formulary (17). Dispensing fees were not included.
Each type of end point-related hospitalization was assigned a total cost of hospitalization (including overhead) based on the Ontario Case Costing Initiative (OCCI) 2003/2004 acute inpatient cost data (18) (Table 2). For diagnoses, this was achieved by identifying the underlying International Classification of Diseases – 10th Revision (ICD-10) diagnoses for each NordDRG code using the NordDRG Users’ Manual (16) and matching the ICD-10 diagnosis to the corresponding OCCI cost. Because each NordDRG code covers several ICD-10 diagnoses, the OCCI cost for each ICD-10 diagnosis was weighted by the corresponding number of patients in the OCCI database to obtain the weighted average OCCI cost for each NordDRG code. For procedures, it was assumed that the terms ‘PCI’ (NordDRG 112) and ‘CABG’ (NordDRG 106, 107) covered similar procedures in the OCCI as in the NordDRG classification system. Weighted average costs for PCI and CABG were obtained based on the number of patients undergoing these types of procedures (each identified by a Canadian Classification of Health Interventions code) listed in the OCCI database under the descriptors ‘PCI’ and ‘CABG’. Because there was no corresponding ICD-10 code, the Canadian cost for ‘other cardiothoracic procedures’ (NordDRG 108) was assumed to be approximately 1.39 times the cost of CABG based on the relative cost of these two types of procedures in Sweden. The cost of ‘other vascular procedures’ (NordDRG 478, 479) was assumed to be the same as the cost of PCI. The OCCI costs were adjusted to 2006 Canadian dollars using the health and personal care component of the Canadian Consumer Price Index (19).
TABLE 2.
End point-related hospitalization costs
| Diagnosis or procedure (NordDRG code) | Main ICD-10 or CCI codes | Cost*, $ |
|---|---|---|
| Stroke (014) | I600–03, I605–16, I618–21, I629–30, I632–35, I638–39, I64, I694 | 16,724 |
| Transient ischemic attack (015) | G450–52, G458–59, I650–53, I658–64, I660–69 | 6,027 |
| Nonfatal myocardial infarction (121, 122) | I210–14, I219–I221, I228–36, I238 | 11,444 |
| Heart failure (127) | I110, I130, I500 | 9,835 |
| Angina pectoris (140) | I201, I2080, I2088, I209 | 4,499 |
| Cardiac arrest (129) | I460, I469 | 17,934 |
| PCI (112) | 1IJ50GQBD, 1IJ50GQOA | 8,856 |
| CABG (106, 107) | 1IJ76LAXXA, 1IJ76LAXXG, 1IJ76LAXXQ | 21,603 |
| Cardiac transplant (103) | 1HY85LAXXK, 1HZ85LAXXK | 141,893 |
| Other cardiothoracic procedure (108) | NA | 30,018† |
| Other vascular procedure (478, 479) | NA | 8,856‡ |
Based on the Ontario Case Costing Initiative total hospitalization cost 2003/2004 (including overhead), adjusted to 2006 Canadian dollars using the Consumer Price Index – Health and Personal Care component;
Cost was assumed to be 1.39 times the cost of coronary artery bypass graft surgery (CABG);
Cost was assumed to be equal to the cost of percutaneous coronary intervention (PCI). CCI Canadian Classification of health Interventions; DRG Diagnosis-related group; ICD-10 International Classification of Diseases – 10th Revision; NA Not applicable
The cost of productivity loss was based on the average weekly earnings in 2004 ($706.02) and 2005 ($728.17), available from Statistics Canada (20). These values were extrapolated to 2006 earnings ($751.00), assuming a constant earning growth rate. As suggested by the Canadian Agency for Drugs and Technologies in Health guidelines (11), a 20% employer contribution was added to account for benefits such as employment insurance and pensions, resulting in a total cost of productivity loss of $901.21 per week.
End point-related hospitalization and productivity costs applied to each state in the Markov cost-effectiveness model are shown in Table 3. For patients in the ‘at-risk’ state, annual resource use (ie, end point-related hospitalization) was based on data collected during the time before an MI or revascularization occurred; thus, these costs included the impact of all end point-related events (as defined earlier), except for the two that are explicitly modelled (ie, nonfatal MI and revascularization). Based on trial data, resource use and productivity losses increased sharply once an MI or revascularization occurred and remained elevated (although they did decline) for three to four years after the event, reflecting the higher risk of other end point-related events in patients who had recently experienced an MI or revascularization. Productivity loss after an MI or a revascularization procedure was recorded as a change in productivity from baseline. Consequently, productivity loss for patients in the ‘at-risk’ state was set at $0. Annual costs were modelled to return to those for the ‘at-risk’ state in the fourth or fifth year after the event for the ‘post-MI’ or ‘postrevascularization’ states, respectively. The cost of the event itself was included in the first-year postevent cost.
TABLE 3.
Annual end point-related hospitalization and productivity costs applied to Markov model health states
| Health state |
Cost, $, mean ± SD |
|
|---|---|---|
| End point-related hospitalization | Productivity loss* | |
| At risk | 414±2,525 | 0 |
| Postmyocardial infarction | ||
| 1st year | 17,357±10,472 | 9,156±1155 |
| 2nd year | 1,500±5,334 | 5,966±1223 |
| 3rd year | 660±3,965 | 6,254±1352 |
| 4th year | 414±2,525 | 6,281±1910 |
| 5th year and following | 414±2,525 | 0 |
| Postrevascularization | ||
| 1st year | 15,799±7,911 | 8,832±679 |
| 2nd year | 762±3,346 | 5,002±728 |
| 3rd year | 415±2,509 | 4,055±822 |
| 4th year | 399±2,384 | 3,749±968 |
| 5th year and following | 414±2,525 | 0 |
Productivity loss after a myocardial infarction or a revascularization procedure was recorded as the change in productivity from baseline. Consequently, productivity loss for the ‘at-risk’ state was set at $0
Utilities
Utility values, stratified by age and sex, were based on the general Swedish population (using the European Quality of Life-5 Dimensions [EQ-5D] instrument and the United Kingdom EQ-5D index tariff) and corrected by subtracting a utility decrement of 0.035 to account for the effect of pre-existing CAD in the model population (Table 4). The utility decrement was obtained from a cross-sectional survey (also using the EQ-5D instrument) of patients hospitalized at coronary care units in Sweden (21). This survey was also used as a source for utility decrements applied to the first year after an MI or a revascularization procedure. Utilities were assumed to return to the pre-event estimates for the second and following years after an event. It was assumed that utilities would be similar between Canadian and Swedish patients.
TABLE 4.
Health utilities applied to the Markov model
Sensitivity analyses
For the within-trial analysis, a threshold analysis was performed by varying the acquisition cost of simvastatin to obtain the value that would lead to a total incremental cost in the atorvastatin arm versus the simvastatin arm of $0. The degree of uncertainty of the cost-effectiveness ratios was estimated by a procedure that involved drawing 1000 samples from the original dataset (ie, bootstrapping) and, based on the distributions of these samples, calculating CIs using angular transformation (ie, using the arcsine of the original data).
For the Markov model, uncertainty was assessed using deterministic and probabilistic sensitivity analyses. For the probabilistic sensitivity analysis, distributions of each model parameter (ie, all transition probabilities, resource use data and utility data) were constructed using non-parametric bootstrapping (ie, no specific type of distribution was assumed). For transition probability and resource use data, 1000 bootstrap replicates were drawn from the trial data. For the utility data, bootstrapping was performed using published data (21). Using these distributions, 1000 second-order Monte Carlo simulations were performed. For each simulation, values for all model parameters were independently drawn from their underlying distributions and the respective cost-effectiveness ratio was calculated. Results are presented on a cost-effectiveness plane and as a cost-effectiveness acceptability curve, which showed the probability of atorvastatin 80 mg being cost-effective as a function of the societal willingness to pay for a QALY gained.
For the deterministic sensitivity analysis, patient characteristics (baseline age, sex, diabetes status and baseline LDL-C), hospitalization costs, utility decrements and discount rate were varied around their base-case values. Baseline age varied between 50 and 70 years in yearly increments. The proportions of women and diabetic patients in the model cohort varied between 0% and 100%. For baseline LDL-C, the 75th percentile value of the IDEAL patient population (3.64 mmol/L [142 mg/dL]) was used as the upper range limit (10). The lower range limit was based on the 2003 Canadian Lipid Study – Observational (CALIPSO) (22) involving 321 Canadian physicians, which reported a mean LDL-C level of 2.38 mmol/L (91.9 mg/dL) among 2526 patients at high risk for CAD who were treated with statins. The costs of end point-related hospitalization and productivity losses varied between 85% and 115% (plus or minus 15%) of the base-case values. Utility decrements for MI and revascularization varied between 200% and 50% (plus 100% or minus 50%) of the base-case value. The discount rate varied between 0% and 3%, as recommended by current Canadian guidelines.
RESULTS
Results of the within-trial analysis are shown in Table 5. Over 4.8 years of treatment, patients in the atorvastatin arm experienced an average of 0.099 fewer events than patients in the simvastatin arm, while incurring $1,549 higher study drug costs. However, this cost increase was more than offset by fewer end point-related hospital stays and lower productivity losses, resulting in a net cost savings of $81 per patient in the atorvastatin arm. Because patients in the atorvastatin arm incurred fewer costs and had fewer events, atorvastatin treatment was dominant. Threshold analysis indicated that the total incremental cost per event avoided would be $0 if the simvastatin cost was reduced to $1.0489 per pill (ie, a 4.6% decrease from the current list price). The incremental cost per event avoided was $4,844 (95% CI $359 to $14,743) when considering direct costs only (ie, after exclusion of productivity losses). The incremental cost per event avoided was $16,843 after exclusion of simvastatin costs (ie, $0/pill). Productivity loss represented the biggest cost driver in the within-trial analysis, highlighting the large impact of CV diseases on daily life.
TABLE 5.
Costs and outcomes per patient over 4.8 years of treatment: Within-trial analysis
| Atorvastatin (80 mg/day) | Simvastatin (20–40 mg/day) | Difference | |
|---|---|---|---|
| Cost, $ | |||
| Study drug | 3,298 | 1,748 | 1,549 |
| End point-related hospitalization | 4,039 | 5,109 | −1,070 |
| Productivity loss | 20,154 | 20,714 | −561 |
| Total | 27,491 | 27,572 | −81 |
| Average number of events per patient | 0.462 | 0.561 | 0.099 |
| Incremental cost per event avoided, $ (95% CI) | |||
| Societal perspective | Dominant (−22,369 to 24,562) | ||
| Direct costs only | 4,844 (359 to 14,743) | ||
All costs are expressed in 2006 Canadian dollars
Table 6 shows the results of the Markov model over a lifetime horizon, with costs and benefits discounted at 5%. Patients in the atorvastatin arm were projected to live an average of 0.0356 years longer and have 0.0234 more QALYs than patients in the simvastatin arm. This improvement came at an increase of $1,375 in study drug costs; 33.9% of this incremental cost was offset by reduced expenditures for end point-related hospitalization and an additional 20.5% by lower productivity losses, leading to a total incremental cost of $627 per patient in the atorvastatin arm. The resulting incremental cost-effectiveness ratio (ICER) was $17,573 per life-year gained or $26,795/QALY gained from the societal perspective. Exclusion of indirect costs (productivity losses) resulted in an ICER of $25,469 per life-year gained or $38,834/QALY gained.
TABLE 6.
Markov model output, base-case
| Atorvastatin (80 mg/day) | Simvastatin (20–40 mg/day) | Difference | |
|---|---|---|---|
| Projected health outcomes per patient | |||
| Life-years | 11.5525 | 11.5169 | 0.0356 |
| Quality-adjusted life years | 8.7369 | 8.7135 | 0.0234 |
| Projected costs per patient, $ | |||
| Study drug | 4,837 | 3,462 | 1,375 |
| End point-related hospitalization | 4,137 | 4,603 | −466 |
| Productivity loss | 1,071 | 1,353 | −282 |
| Total | 10,045 | 9,418 | 627 |
| Cost per life-year gained, $ | |||
| Societal perspective | 17,573 | ||
| Direct costs only | 25,469 | ||
| Cost per quality-adjusted life year gained, $ | |||
| Societal perspective | 26,795 | ||
| Direct costs only | 38,834 | ||
Costs and benefits were discounted at 5% per year
Figure 2 displays results of the Markov model deterministic sensitivity analysis. The model was most sensitive to baseline age. The ICER reached a maximum ($35,686/QALY) for an age of 64 years (corresponding to an age when 100% of IDEAL patients had just retired) and a minimum ($8,931/QALY) for an age of 50 years. (The ICER was $28,076/QALY for a baseline age of 70 years.) The ICER increased with decreasing baseline LDL-C, reaching $30,337/QALY when the baseline LDL-C equalled the value reported by the CALIPSO investigators for statin-treated high-risk patients (2.38 mmol/L). Atorvastatin treatment was more cost-effective for patients with diabetes than for patients without diabetes ($18,733 versus $30,428/QALY) and slightly more cost-effective in men than in women ($26,059 versus $28,613/QALY). Reducing the discount rates to 3% and 0% resulted in a drop of the ICER to $22,393/QALY and $16,914/QALY, respectively. Variations in end point-related hospitalization costs, the cost of productivity loss and utility decrements had a minor impact on the ICER.
Figure 2).
Markov model deterministic sensitivity analysis. *Corresponds to the 75th percentile low-density lipoprotein cholesterol (LDL-C) baseline value of the Incremental Decrease in End-Points Through Aggressive Lipid-Lowering (IDEAL) trial population (10); †Corresponds to the mean LDL-C value of Canadian patients at high risk for coronary artery disease treated with statins as reported in the Canadian Lipid Study – Observational (CALIPSO) (22). MI Myocardial infarction; QALY Quality-adjusted life years
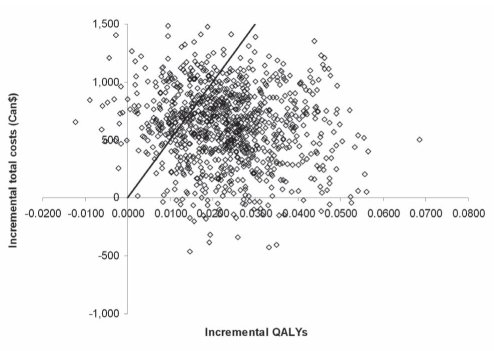
The cost-effectiveness plane showed incremental costs of atorvastatin 80 mg treatment plotted against incremental QALYs as output from 1000 Monte Carlo simulations (Figure 3). Atorvastatin 80 mg treatment was more effective than simvastatin 20 mg to 40 mg treatment in 98.8% of simulations (ie, 1.2% of points lie left of the y-axis). The cost-effectiveness acceptability curve (Figure 4) indicated that the probability of atorvastatin 80 mg treatment being cost-effective was 78% for a willingness to pay of $50,000/QALY (ie, in 78% of 1000 probabilistic sensitivity analysis simulations, the ICER was below $50,000/QALY). If society was willing to pay $70,000/QALY gained, the probability of atorvastatin 80 mg being cost-effective reached 87%. The cost-effectiveness acceptability curve reached 95.0% for a willingness to pay of $120,000/QALY gained and then converged asymptotically toward 96.8%.
Figure 3).
Cost-effectiveness plane. The line indicates the $50,000 per quality-adjusted life year (QALY) threshold
Figure 4).
The graph shows the fraction of simulations that produce a result that would be considered cost-effective within a range of societal willingness to pay to gain a quality-adjusted life year (QALY)
DISCUSSION
The results of the present study indicate that treatment of patients who have a history of MI with high-dose atorvastatin (80 mg/day) is cost-effective compared with standard-dose simvastatin (20 mg/day to 40 mg/day) treatment from a Canadian societal perspective. The within-trial analysis suggests that treatment with atorvastatin would be cost-saving compared with simvastatin treatment over a median of 4.8 years. When considering direct costs only, the incremental cost per event avoided was estimated at $4,844. After exclusion of simvastatin costs from the analysis, the incremental cost per event avoided for atorvastatin treatment was $16,843.
The lifetime Markov model projected that 54% of the incremental drug cost in the atorvastatin treatment arm would be offset by savings stemming from reduced hospitalization for CV events and less productivity loss, resulting in an ICER of $26,795/QALY gained. The ICER remained below $50,000/QALY in 78% of 1000 probabilistic sensitivity analysis simulations. The model is very conservative against atorvastatin because the effects and associated costs of simvastatin are only assumed after a five-year switch from atorvastatin. If the results were extrapolated over those five years, the clinical and economic benefits of atorvastatin would have been much greater.
The results of the present study support the economic value of intensive versus moderate lipid lowering in the secondary prevention of CV events. Previous Canadian studies have indicated that, compared with no treatment or placebo, lipid lowering through statin therapy is a cost-effective treatment for the secondary prevention of CV events (23,24) and may be cost-effective for primary prevention in selected patients (23). For use of statins for primary prvention, a study reported ICERs (versus no treatment) below $45,000 per life-year saved in men and ICERs ranging from $37,000 to $141,000 in women, depending on type of statin, age and number of risk factors (23). For secondary prevention, an analysis (24) based on results from the Scandinavian Simvastatin Survival Study (4S) reported incremental costs for simvastatin versus placebo treatment ranging from US$4,487 to US$8,532 per life-year saved for high-risk men and from US$5,138 to US$8,389 for high-risk women from a public payer perspective (1996 values). These values translate to approximately $7,600 to $14,400 after adjustment to 2006 Canadian dollars, a range that is comparable with the $20,365 per life-year saved for intensive versus moderate lipid lowering for secondary prevention of CV events reported in the present study.
Findings of the within-trial analysis may be compared with results of corresponding analyses based on the IDEAL trial for Sweden and the United States (25). In Sweden, where generic simvastatin is available at a cost of up to 21- to 24-fold lower than patented atorvastatin, the incremental cost-effectiveness of atorvastatin (80 mg/day) versus simvastatin (20 mg/day to 40 mg/day) was estimated to be US$22,225 per event avoided; in the United States, use of atorvastatin (80 mg/day) was found to be associated with cost savings, given the current cost of branded simvastatin (25). Our results lie somewhat between those for Sweden and the United States, reflecting that generic simvastatin is available in Canada, but its cost difference from patented atorvastatin is not as large as that seen in Sweden. Findings of the Markov model are comparable with results of the corresponding models for Denmark, Finland, Norway and Sweden, where ICERs ranged from €35,210 to €62,639 per QALY gained, depending on the country (10).
The economic analyses presented were based on actual resource consumption and clinical outcomes observed during a head-to-head active comparator trial, which allowed for a direct comparative assessment of the cost-effectiveness of the two statin therapies. Although trial-based cost-effectiveness analyses of statins have been published previously (26–35), they were based on placebo-controlled trials and, as such, did not compare different statin therapies. Previous attempts to comparatively assess the cost-effectiveness of different statin therapies had to rely on indirect comparisons, drawing data from disparate clinical trials and using, for example, LDL-C reduction as a surrogate end point (23,36,37). With the availability of results from head-to-head statin clinical studies, such as the IDEAL trial, it has now become possible to overcome previous limitations and base cost-effectiveness analyses on actual clinical outcomes and resource use data collected from patients randomly assigned to different statin therapies. Another strength of the present study was the inclusion of indirect costs, which allowed for a comprehensive assessment of the economic impact of intensive therapy in a patient population that had already experienced an MI and was close to retirement age (38).
The results of the Markov model were sensitive to baseline age, a consequence of two counteracting factors: the proportion of retired patients and CV risk. The proportion of retired patients increased with age, which reduced productivity differences due to differences in event rates between treatment groups; however, CV risk increased with age, which improved the cost-effectiveness of treatments that were more effective. Because 100% of patients were retired at 64 years of age, the ICER based on this and older baseline ages included direct costs only and declined with advancing age.
As can be expected for a treatment that is more effective and more costly than the comparator, the cost-effectiveness of atorvastatin improved with increasing baseline risk of CV events and mortality (ie, with increasing proportions of diabetic patients, older patients and patients with a higher baseline LDL-C level). During the IDEAL trial, there was no significant difference between men and women with respect to the risk of revascularization, MI and MI-linked mortality; however, women had a reduced non-MI-linked mortality, which was reflected in the slightly higher ICER for women.
Limitations
The present study needs to be considered in light of its limitations. Resource use patterns may differ between patients participating in clinical trials and those treated in clinical practice, where patients may not be followed as closely as they are in clinical studies. In addition, while the model was adjusted to account for differences in characteristics between Canadian and Swedish patients with a history of MI – including age, sex distribution, diabetes status and baseline LDL-C – it was not modified to reflect potential differences in utilities, productivity losses and life expectancy. Comparison of World Health Organization life tables (39) indicates that life expectancy is quite similar in these two countries; small variations are not expected to have a major impact on results presented here. Utility values and productivity costs varied extensively in the deterministic sensitivity analysis to indicate the extent to which these parameters affect the ICER. Retirement age distributions may differ between Canada and Sweden, and impact the ICER through their effect on productivity costs (ie, patients who have retired cannot incur productivity losses). Older retirement ages are expected to favour atorvastatin treatment because productivity losses due to sickness would disproportionally increase among patients receiving the less effective treatment. Treatment practices may also differ between Northern Europe and Canada. This may apply not so much to hospital admissions for acute events such as MI or stroke, but more to procedures such as CABG and PCI. In addition, although all care was taken to correctly match NordDRG codes to the respective OCCI costs, some costing discrepancies, particularly with respect to procedures, may have occurred. Also, the OCCI data include costs of physician services only for physicians paid by the hospital, but not for those paid by the Ministry of Health (40). However, the model showed little sensitivity to hospitalization costs, which precludes cost mismatches and exclusion of some physician costs being major sources of error. Furthermore, only the first MI or revascularization was modelled explicitly, which could have led to an underestimation of the total cost. Adverse experiences were not included in the analysis because there were no significant differences in the overall frequency of adverse events and serious adverse events between patients randomly assigned to atorvastatin or simvastatin in the IDEAL trial (9). However, patients assigned to atorvastatin were more likely to discontinue study medication due to an adverse event, primarily because of myalgia, diarrhea, abdominal pain and nausea, which occurred significantly more often in the atorvastatin than in the simvastatin arm (9). These types of adverse experiences usually do not require resource-intensive therapies and it is likely that their inclusion would not have had a major impact on model output. Other important cost parameters not included in the model were expenditures for rehabilitation and long-term care, which may be significant in this elderly patient population with a history of MI, but were not collected during the IDEAL trial. Inclusion of these costs is likely to improve the cost-effectiveness of intensive lipid lowering with atorvastatin. Another model feature that disfavours atorvastatin is the assumption that the incremental benefit of atorvastatin disappears after five years of treatment, when all patients are switched to standard-dose simvastatin. It may be speculated that the effect of intensive lipid lowering may persist for some time after switching to less intensive therapy.
CONCLUSION
Cost-effectiveness analysis, based on clinical outcomes and resource use data collected during a head-to-head active comparator trial, indicated that treatment with high-dose atorvastatin (80 mg/day) is cost-effective compared with standard-dose simvastatin (20 mg/day to 40 mg/day) from a Canadian societal perspective. Furthermore, a within-trial analysis suggested that atorvastatin would result in cost savings compared with simvastatin over a median of 4.8 years.
Acknowledgments
The authors thank Donna Rindress for her thoughtful comments.
Footnotes
CONFLICT OF INTEREST STATEMENT: This study was funded by Pfizer Inc. Monika Wagner and Mireille Goetghebeur are employees of BioMedCom Consultants, who were paid consultants to Pfizer Inc in connection with the Canadian adaptation of the model and development of the manuscript. Elizabeth Merikle is an employee of Pfizer Canada. Peter Lindgren and Bengt Jönsson are employees of European Health Economics who were paid consultants to Pfizer in connection with the development of the manuscript.
REFERENCES
- 1.Chow CM, Donovan L, Manuel D, Johansen H, Tu JV. Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol. 2005;21:1265–71. [PubMed] [Google Scholar]
- 2.Heart and Stroke Foundation of Canada . The Growing Burden of Heart Disease and Stroke in Canada 2003. Ottawa: Heart and Stroke Foundation of Canada; 2003. [Google Scholar]
- 3.Manuel DG, Leung M, Nguyen K, Tanuseputro P, Johansen H. Burden of cardiovascular disease in Canada. Can J Cardiol. 2003;19:997–1004. [PubMed] [Google Scholar]
- 4.Alter DA, Stukel TA, Newman A. Proliferation of cardiac technology in Canada: A challenge to the sustainability of Medicare. Circulation. 2006;113:380–7. doi: 10.1161/CIRCULATIONAHA.105.560466. [DOI] [PubMed] [Google Scholar]
- 5.Hall RE, Tu JV. Hospitalization rates and length of stay for cardiovascular conditions in Canada, 1994 to 1999. Can J Cardiol. 2003;19:1123–31. [PubMed] [Google Scholar]
- 6.Genest J, Frohlich J, Fodor G, McPherson R. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: Summary of the 2003 update. CMAJ. 2003;169:921–4. [PMC free article] [PubMed] [Google Scholar]
- 7.McPherson R, Frohlich J, Fodor G, Genest J, Canadian Cardiovascular Society Canadian Cardiovascular Society position statement – recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–27. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: A randomized controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren P, Graff J, Olsson AG, Pedersen TJ, Jonsson B. Cost-effectiveness of high-dose atorvastatin compared with regular dose simvastatin. Eur Heart J. 2007;28:1448–53. doi: 10.1093/eurheartj/ehm020. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Agency for Drugs and Technologies in Health Guidelines for the economic evaluation of health technologies: Canada. < http://www.cadth.ca> (Version current at October 2, 2009).
- 12.Bata IR, Gregor RD, Wolf HK, Brownell B. Trends in five-year survival of patients discharged after acute myocardial infarction. Can J Cardiol. 2006;22:399–404. doi: 10.1016/s0828-282x(06)70925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan RT, Yan AT, Tan M, et al. Age-related differences in the management and outcome of patients with acute coronary syndromes. Am Heart J. 2006;151:352–9. doi: 10.1016/j.ahj.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Alter DA, Chong A, Austin PC, et al. Socioeconomic status and mortality after acute myocardial infarction. Ann Intern Med. 2006;144:82–93. doi: 10.7326/0003-4819-144-2-200601170-00005. [DOI] [PubMed] [Google Scholar]
- 15.Petrella RJ, Merikle E, Jones J. Prevalence and treatment of dyslipidemia in Canadian primary care: A retrospective cohort analysis. Clin Ther. 2007;29:742–50. doi: 10.1016/j.clinthera.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Nordic Classification Centre Nordic Centre for Classifications in Health Care NordDRG Users’ Manual. 2006. < http://www.nordclass.uu.se/verksam/norddrgmanual/NordDRG_2006_SWE> (Version current at October 2, 2009).
- 17.Ontario Ministry of Health and Long-Term Care Ontario Drug Benefit Formulary/Comparative Drug Index. < http://www.health.gov.on.ca/english/providers/program/drugs/odbf_eformulary.html> (Version current at October 2, 2009).
- 18.Ontario Ministry of Health and Long-Term Care Ontario Case Costing Initiative. < http://www.occp.com/> (Version current at October 2, 2009).
- 19.Statistics Canada Table 5: the Consumer Price Index for Canada, major components and special aggregates (not seasonally adjusted), 1987–2006. < http://www.statcan.gc.ca/pub/62-001-x/00506/t/4125989-eng.htm> (Version current at October 2, 2009).
- 20.Statistics Canada Earnings, average weekly, by province and territory. < http://www40.statcan.ca/l01/cst01/labr79.htmx> (Version current at October 2, 2009).
- 21.Lindgren P, Stenestrand U, Hambraeus K, Wallentin L, Jonsson B.PCI reduces utility loss after MI in Sweden Eur Heart J 200627Suppl 177316399777 [Google Scholar]
- 22.Bourgault C, Davignon J, Fodor G, et al. Statin therapy in Canadian patients with hypercholesterolemia: The Canadian Lipid Study – Observational (CALIPSO) Can J Cardiol. 2005;21:1187–93. [PubMed] [Google Scholar]
- 23.Russell MW, Huse DM, Miller JD, Kraemer DF, Hartz SC. Cost effectiveness of HMG-CoA reductase inhibition in Canada. Can J Clin Pharmacol. 2001;8:9–16. [PubMed] [Google Scholar]
- 24.Grover SA, Coupal L, Paquet S, Zowall H. Cost-effectiveness of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the secondary prevention of cardiovascular disease: Forecasting the incremental benefits of preventing coronary and cerebrovascular events. Arch Intern Med. 1999;159:593–600. doi: 10.1001/archinte.159.6.593. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren P, Jonsson B. Trial based economic evaluation of intensive atorvastatin compared to standard simvastatin in the IDEAL trial. 65th Annual Conference of the AHA; Tampa. May 11 to 14, 2006. [Google Scholar]
- 26.Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: Economic analysis of a randomised trial in 20,536 individuals. Lancet. 2005;365:1779–85. doi: 10.1016/S0140-6736(05)63014-0. [DOI] [PubMed] [Google Scholar]
- 27.Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20,536 people. BMJ. 2006;333:1145. doi: 10.1136/bmj.38993.731725.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szucs TD, Klose G, Dusing R. [Cost-effectiveness of atorvastatin for the prevention of coronary disease. An analysis of the ASCOT study.] Dtsch Med Wochenschr. 2004;129:1420–4. doi: 10.1055/s-2004-826883. [DOI] [PubMed] [Google Scholar]
- 29.Glasziou PP, Eckermann SD, Mulray SE, et al. Cholesterol-lowering therapy with pravastatin in patients with average cholesterol levels and established ischaemic heart disease: Is it cost-effective? Med J Aust. 2002;177:428–34. doi: 10.5694/j.1326-5377.2002.tb04883.x. [DOI] [PubMed] [Google Scholar]
- 30.Buller N, Gillen D, Casciano R, Doyle J, Wilson K. A pharmacoeconomic evaluation of the Myocardial Ischaemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study in the United Kingdom. Pharmacoeconomics. 2003;21(Suppl 1):25–32. doi: 10.2165/00019053-200321001-00003. [DOI] [PubMed] [Google Scholar]
- 31.Casciano R, Tarride JE, Breton MC, Stern L, Langer A. A pharmacoeconomic evaluation of the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study in Canada. Can J Clin Pharmacol. 2004;11:e179–90. [PubMed] [Google Scholar]
- 32.Gomez-Gerique JA, Casciano R, Stern L, Rejas J. A pharmacoeconomic evaluation of the effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes in Spain. Eur J Health Econ. 2004;5:278–84. doi: 10.1007/s10198-003-0222-1. [DOI] [PubMed] [Google Scholar]
- 33.Lange AP, Szucs TD. [Cost-effectiveness of atorvastatin in the early treatment of acute coronary syndromes in Germany based on the MIRACL study.] Med Klin (Munich) 2004;99:500–5. doi: 10.1007/s00063-004-1076-8. [DOI] [PubMed] [Google Scholar]
- 34.Olsson A, Casciano R, Stern L, Svangren P. A pharmacoeconomic evaluation of aggressive cholesterol lowering in Sweden. Int J Cardiol. 2004;96:51–7. doi: 10.1016/j.ijcard.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GG, Ganz P, Waters D, Arikian S. Pharmacoeconomic evaluation of the effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. Am J Cardiol. 2003;92:1109–12. doi: 10.1016/j.amjcard.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Huse DM, Russell MW, Miller JD, et al. Cost-effectiveness of statins. Am J Cardiol. 1998;82:1357–63. doi: 10.1016/s0002-9149(98)00641-9. [DOI] [PubMed] [Google Scholar]
- 37.Elliott WJ, Weir DR. Comparative cost-effectiveness of HMG-CoA reductase inhibitors in secondary prevention of acute myocardial infarction. Am J Health Syst Pharm. 1999;56:1726–32. doi: 10.1093/ajhp/56.17.1726. [DOI] [PubMed] [Google Scholar]
- 38.Grover SA, Ho V, Lavoie F, Coupal L, Zowall H, Pilote L. The importance of indirect costs in primary cardiovascular disease prevention: Can we save lives and money with statins? Arch Intern Med. 2003;163:333–9. doi: 10.1001/archinte.163.3.333. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization Life tables for WHO Member states. < http://www.who.int/whosis/database/life_tables/life_tables.cfm> (Version current at October 2, 2009).
- 40.Ontario Ministry of Health and Long-Term Care Ontario Guide to Case Costing. < http://www.occp.com/> (Version current at October 2, 2009).
- 41.Burstrom K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res. 2001;10:621–35. doi: 10.1023/a:1013171831202. [DOI] [PubMed] [Google Scholar]