Abstract
BACKGROUND:
Current guidelines support an early invasive strategy in the management of high-risk non-ST elevation acute coronary syndromes (NSTE-ACS). Although studies in the 1990s suggested that high-risk patients received less aggressive treatment, there are limited data on the contemporary management patterns of NSTE-ACS in Canada.
OBJECTIVE:
To examine the in-hospital use of coronary angiography and revascularization in relation to risk among less selected patients with NSTE-ACS.
METHODS:
Data from the prospective, multicentre Global Registry of Acute Coronary Events (main GRACE and expanded GRACE2) were used. Between June 1999 and September 2007, 7131 patients from across Canada with a final diagnosis of NSTE-ACS were included the study. The study population was stratified into low-, intermediate- and high-risk groups, based on their calculated GRACE risk score (a validated predictor of in-hospital mortality) and according to time of enrollment.
RESULTS:
While rates of in-hospital death and reinfarction were significantly (P<0.001) greater in higher-risk patients, the in-hospital use of cardiac catheterization in low- (64.7%), intermediate- (60.3%) and high-risk (42.3%) patients showed an inverse relationship (P<0.001). This trend persisted despite the increase in the overall rates of cardiac catheterization over time (47.9% in 1999 to 2003 versus 51.6% in 2004 to 2005 versus 63.8% in 2006 to 2007; P<0.001). After adjusting for confounders, intermediate-risk (adjusted OR 0.80 [95% CI 0.70 to 0.92], P=0.002) and high-risk (adjusted OR 0.38 [95% CI 0.29 to 0.48], P<0.001) patients remained less likely to undergo in-hospital cardiac catheterization.
CONCLUSION:
Despite the temporal increase in the use of invasive cardiac procedures, they remain paradoxically targeted toward low-risk patients with NSTE-ACS in contemporary practice. This treatment-risk paradox needs to be further addressed to maximize the benefits of invasive therapies in Canada.
Keywords: Acute coronary syndromes, Cardiac catheterization, Guidelines, Risk stratification
Abstract
HISTORIQUE :
Les lignes directrices actuelles appuient une stratégie effractive précoce dans la prise en charge des syndromes coronariens aigus sans élévation du segment ST (SCA-SÉST) à haut risque. Même si, dans les années 1990, des études ont laissé supposer que les patients à haut risque recevaient un traitement moins énergique, les données sur la prise en charge courante des profils de SCA-SÉST demeurent limitées au Canada.
OBJECTIF :
Examiner l’utilisation de la coronarographie et de la revascularisation en milieu hospitalier par rapport au risque chez des patients moins sélectionnés ayant une SCA-SÉST.
MÉTHODOLOGIE :
Les auteurs ont utilisé les données de l’étude prospective multicentrique sur le registre mondial des événements coronariens aigus (étude GRACE principale et GRACE élargie2). Entre juin 1999 et septembre 2007, 7 131 patients de partout au Canada ayant un diagnostic définitif de SCA-SÉST ont participé à l’étude. Cette population était stratifiée en groupes à faible risque, à risque moyen et à haut risque, d’après l’indice de risque calculé pour l’étude GRACE (un prédicteur validé de la mortalité en milieu hospitalier) et le moment de leur inscription à l’étude.
RÉSULTATS :
Les taux de décès et de nouvel infarctus en milieu hospitalier étaient considérablement plus élevés (P<0,001) chez les patients à plus haut risque, mais l’utilisation du cathétérisme cardiaque en milieu hospitalier était inversement proportionnelle (P<0,001) chez les patients à faible risque (64,7 %), à risque moyen (60,3 %) et à haut risque (42,3 %). Cette tendance a persisté malgré l’augmentation des taux globaux de cathétérisme cardiaque au fil du temps (47,9 % de 1999 à 2003, par rapport à 51,6 % en 2004 et 2005 et à 63,8 % en 2006 et 2007; P<0,001). Après rajustement compte tenu des variables confusionnelles, les patients à risque moyen (RRR 0,80 [95% IC 0,70 à 0,92], P=0,002) et à haut risque (RRR 0,38 [95% IC 0,29 à 0,48], P<0,001) demeuraient moins susceptibles de subir un cathétérisme cardiaque en milieu hospitalier.
CONCLUSION :
Malgré l’augmentation temporelle de l’utilisation des interventions cardiaques effractives, dans la pratique actuelle, ces interventions demeurent paradoxalement ciblées vers les patients à faible risque ayant un SCA-SÉST. Il faut étudier davantage ce paradoxe entre le traitement et le risque pour maximiser les bienfaits des thérapies effractives au Canada.
In 2002, and again in 2007, the American College of Cardiology/American Heart Association (1,2) published evidence-based practice guidelines supporting the use of an early invasive strategy in the management of high-risk non-ST elevation acute coronary syndromes (NSTE-ACS). However, observational studies have suggested that high-risk NSTE-ACS patients receive less aggressive treatment than their low-risk counterparts (3,4). This discrepancy, termed the ‘treatment-risk paradox’, was evident in the invasive management of NSTE-ACS in Canadian acute coronary syndrome (ACS) registries I and II (5,6), which spanned 1999 to 2001, and 2002 to 2003, respectively. Although the gap between evidence and routine practice has been recognized (7–9), there are limited recent data on the temporal characteristics and ‘treatment-risk paradox’ of early invasive therapies for NSTE-ACS in the ‘real-world’ Canadian population. Furthermore, the impact of the publication of randomized trial evidence (10–12), including the Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) trial (13), which challenged the benefits of a routine invasive approach, and treatment guidelines (1,2) on contemporary practice patterns in the ‘real world’, has not been well characterized.
Therefore, the objectives of the present study were to examine the use of coronary angiography and revascularization strategies in relation to risk in a Canadian NSTE-ACS population, and determine temporal changes of the in-hospital management of NSTE-ACS in relation to patient risk using data collected from Canadian patients enrolled in the Global Registry of Acute Coronary Events (GRACE). A better understanding of these issues may help to maximize the benefits afforded by invasive therapies for NSTE-ACS in Canada.
METHODS
Study design and population
Full details of the GRACE design and methods have been published (14,15). In brief, GRACE was a multinational, prospective registry designed to study an unbiased representation of patients with ACS, irrespective of geographical regions. Eligible patients were at least 18 years of age and were admitted to the hospital for a presumptive diagnosis of ACS, with at least one of the following: abnormal cardiac biomarkers, electrocardiogram changes consistent with ACS and/or documented history of coronary artery disease. Patients with ACS precipitated or accompanied by surgery, trauma or serious comorbidity were excluded. At each site, trained personnel collected data on patient demographics, clinical presentation, treatment, use of cardiac procedures and outcome on standardized case report forms. To ensure the enrollment of an unselected population, each site was instructed to recruit the first 10 to 20 consecutive patients per month (depending on patient throughput at each site).
Subsequent to the initiation of the main GRACE in 1999, an expanded version of the project (GRACE2) provided an opportunity for many additional hospitals and countries to enroll their ACS patients, starting in 2003. In total, 53 hospitals across Canada participated in GRACE and GRACE2; 18 (34.6 %) had on-site cardiac catheterization facilities and 11 (21.2 %) performed coronary artery bypass grafting (CABG). Between June 1999 and September 2007, 13,352 patients with suspected ACS (including ST elevation myocardial infarction) were recruited; of these, 7927 received a final diagnosis of NSTE-ACS based on standardized criteria. Due to missing data (which constituted less than 3% of cases for most variables), we could not determine the GRACE risk score for 796 patients who were excluded in the present study. Compared with the remaining cohort, these 796 patients had lower systolic blood pressure (P=0.01), higher Killip class (P<0.001), lower rate of positive biomarkers (P=0.015), more frequent cardiac arrest on presentation (P<0.001) and higher in-hospital mortality (P=0.048).
Where required, study investigators received approval from their local hospital ethics or institutional review board. Informed consent was obtained from patients in hospitals requiring such permission.
Patient stratification
The cohort for the present study (n=7131) was stratified, on the basis of predefined cut-off points of the GRACE risk score, into low-, intermediate- and high-risk groups (16). The GRACE risk score was derived using data from 11,389 patients in the international GRACE patient population, and was designed to predict in-hospital mortality (c-statistic 0.83) (17–19). The GRACE risk model for in-hospital mortality is composed of the following predictor variables on presentation: age, heart rate, systolic blood pressure, cardiac arrest, Killip class, creatinine, ST segment deviation and biomarker status (20). All predictor variables were collected on admission to facilitate early risk stratification and guide management decisions. Patients in the present Canadian analysis were also categorized into three enrollment time periods – 1999 to 2003 (n=1296), 2004 to 2005 (n=2846) and 2006 to 2007 (n=2989) – to explore temporal trends in patient management in relation to the publication of randomized trials (10–13) and guidelines (1,2).
Statistical analysis
Continuous variables are presented as medians with interquartile ranges, and categorical variables as frequencies or percentages. Group comparisons of continuous and categorical variables were made by the Kruskal-Wallis test and χ2 test, respectively. Kendall’s tau-b test was used for nonparametric correlations.
Multivariable logistic regression analysis was performed to determine the ORs (95% CIs) of independent predictors of in-hospital cardiac catheterization, adjusting for other hospital and patient factors previously shown to be related to the in-hospital use of invasive cardiac procedures (3,21–23). Generalized estimating equations were used to account for the clustering of patients within hospitals. Model discrimination and calibration were assessed by the c-statistic and the Hosmer-Lemeshow goodness-of-fit test, respectively. Because patients who died shortly after admission might not have had a chance to undergo cardiac catheterization, a sensitivity analysis was performed, excluding patients who died within 48 h of admission. Because of regional variations in the use of invasive cardiac procedures (and recruitment in GRACE2 only began in hospitals outside of Ontario in 2003), a separate analysis, restricted to patients enrolled in Ontario hospitals (n=3611), was conducted.
Statistical analysis was performed using SPSS version 15.0 (SPSS Inc, USA). A two-sided P<0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
Table 1 shows the baseline characteristics, medical history and presenting clinical features of the study population, stratified according to the three risk groups by GRACE risk score: 36.3% were classified as low risk, 31.4% as intermediate risk and 32.3% as high risk. The median (interquartile range) GRACE risk score of the overall cohort was 121 (97 to 151). Compared with the low- and intermediate-risk groups, patients in the high-risk category were older, more often women, more likely to have a history of hypertension, diabetes mellitus, previous congestive heart failure, previous transient ischemic attack or stroke, and peripheral vascular disease (Table 1).
TABLE 1.
Baseline demographics and presenting characteristics of patients stratified into Global Registry of Acute Coronary Events (GRACE) risk groups
| Characteristic | Overall* (n=7131) |
GRACE risk group (score) |
P (for trend) | ||
|---|---|---|---|---|---|
| Low (≤108) (n=2589) | Intermediate (109–140) (n=2236) | High (≥141) (n=2306) | |||
| Age, years | 68 (57 to 77) | 56 (50 to 63) | 70 (62 to 77) | 78 (71 to 84) | <0.001 |
| Women, % | 34.9 | 28.6 | 36.1 | 40.7 | <0.001 |
| Medical history, % | |||||
| Smoker | 24.0 | 33.2 | 22.2 | 15.2 | <0.001 |
| Dyslipidemia | 56.2 | 57.7 | 59.0 | 51.7 | <0.001 |
| Hypertension | 63.6 | 56.2 | 66.2 | 69.3 | <0.001 |
| Diabetes mellitus | 28.8 | 23.5 | 29.2 | 34.5 | <0.001 |
| Angina | 51.8 | 47.5 | 53.6 | 55.0 | <0.001 |
| Previous myocardial infarction | 36.6 | 30.2 | 36.7 | 43.7 | <0.001 |
| Previous percutaneous coronary intervention | 19.0 | 21.2 | 20.7 | 14.9 | <0.001 |
| Previous coronary artery bypass graft | 14.5 | 11.0 | 16.1 | 17.1 | <0.001 |
| Previous congestive heart failure | 11.6 | 3.0 | 8.6 | 24.0 | <0.001 |
| Previous transient ischemic attack/stroke | 9.8 | 5.2 | 8.9 | 15.8 | <0.001 |
| Previous peripheral vascular disease | 9.8 | 5.3 | 9.6 | 15.2 | <0.001 |
| Presenting characteristics | |||||
| Heart rate, beats/min | 78 (66 to 92) | 74 (64 to 86) | 76 (65 to 89) | 88 (73 to 109) | <0.001 |
| Systolic blood pressure, mmHg | 145 (127 to 164) | 152 (137 to 172) | 145 (128 to 162) | 134 (116 to 154) | <0.001 |
| Killip class, % | |||||
| I | 84.6 | 98.6 | 92.2 | 61.7 | <0.001 |
| II | 10.2 | 1.3 | 6.9 | 23.3 | |
| III/IV | 5.2 | 0.1 | 0.9 | 15.1 | |
| Serum creatinine, μmol/L | 92 (78 to 114) | 85 (74 to 97) | 92 (78 to 110) | 108 (87 to 141) | <0.001 |
| Cardiac arrest, % | 0.5 | 0.0 | 0.2 | 1.4 | <0.001 |
| ST deviation, % | 31.8 | 8.5 | 29.0 | 60.6 | <0.001 |
| Abnormal cardiac biomarker, % | 42.1 | 27.8 | 41.7 | 58.4 | <0.001 |
Data presented as median (interquartile range) unless otherwise indicated.
GRACE risk score not calculated for 10% (n=796) of patients due to incomplete data
In-hospital outcomes
Overall, the rates of in-hospital mortality and death/myocardial re-infarction (MI) were 2.8% and 5.7%, respectively. When analyzed as a continuous variable, GRACE risk score demonstrated excellent discrimination for in-hospital death (c-statistic 0.85, P<0.001) and the composite end point of death/re-MI (c-statistic 0.71, P<0.001). The trend of increasing in-hospital mortality rates in low- (0.3%), intermediate- (1.0%) and high-risk (7.2%) patients was significant (P<0.001) (Table 2) and comparable with the published rates for the GRACE risk score. In addition, there was an increasing gradient of risk of death/re-MI across the higher GRACE risk groups. This significant (P<0.001) trend toward increasing in-hospital death and re-MI among higher-risk patients was sustained within each time period examined.
TABLE 2.
In-hospital procedures and outcomes by Global Registry of Acute Coronary Events (GRACE) risk score
| Characteristic | Overall (n=7131) |
GRACE risk group (score) |
|||
|---|---|---|---|---|---|
| Low (≤108) (n=2589) | Intermediate (109–140) (n=2236) | High (≥141) (n=2306) | P (for trend) | ||
| Cardiac catheterization, % | 56.1 | 64.7 | 60.3 | 42.3 | <0.001 |
| Time to cardiac catheterization, days* | 3 (2 to 5) | 3 (2 to 5) | 3 (2 to 5) | 4 (2 to 6) | <0.001 |
| Percutaneous coronary intervention, % | 27.4 | 31.7 | 30.0 | 20.0 | <0.001 |
| Coronary artery bypass grafting, % | 3.1 | 2.6 | 3.6 | 3.1 | 0.36 |
| Percutaneous coronary intervention/coronary artery bypass grafting, % | 29.0 | 32.8 | 32.3 | 21.7 | <0.001 |
| Death, % | 2.8 | 0.3 | 1.0 | 7.2 | <0.001 |
| Death/myocardial re-infarction, % | 5.7 | 2.8 | 3.4 | 11.3 | <0.001 |
Data presented as median (interquartile range)
Medication use within the first 24 h of admission
Table 3 illustrates the relationship between GRACE risk score and acute medication use in NSTE-ACS. High-risk patients were significantly less likely than intermediate- and low-risk patients to receive acute treatment (less than 24 h) such as acetylsalicylic acid, thienopyridine, low molecular weight heparin, glycoprotein IIb/IIIa inhibitor, beta-blocker and statin. Conversely, high-risk patients were more frequently treated with calcium channel blockers.
TABLE 3.
Medication use by Global Registry of Acute Coronary Events (GRACE) risk groups
| Treatment within first 24 h | Overall (n=7131), % |
GRACE risk group (score) |
P (for trend) | ||
|---|---|---|---|---|---|
| Low (≤108) (n=2589), % | Intermediate (109–140) (n=2236), % | High (≥141) (n=2306), % | |||
| Acetylsalicylic acid | 91.2 | 94.5 | 91.9 | 86.8 | <0.001 |
| Thienopyridine | 58.7 | 63.4 | 61.5 | 50.7 | <0.001 |
| Unfractionated heparin | 28.4 | 25.2 | 29.4 | 31.1 | <0.001 |
| Low molecular weight heparin | 62.5 | 69.1 | 62.3 | 55.2 | <0.001 |
| Glycoprotein IIb/IIIa inhibitor | 7.0 | 7.5 | 8.1 | 5.2 | 0.003 |
| Angiotensin-converting enzyme inhibitor | 55.3 | 54.6 | 56.0 | 55.5 | 0.52 |
| Beta-blocker | 77.7 | 82.0 | 78.4 | 72.2 | <0.001 |
| Angiotensin receptor blocker | 10.8 | 8.7 | 12.4 | 11.7 | 0.001 |
| Calcium channel blocker | 25.6 | 20.4 | 25.9 | 31.3 | <0.001 |
| Statin | 65.5 | 68.2 | 67.4 | 60.5 | <0.001 |
In-hospital procedures
The overall rates of cardiac catheterization, percutaneous coronary intervention (PCI) and CABG were 56.1%, 27.4% and 3.1%, respectively, during the initial hospitalization. Patients in low-, intermediate- and high-risk groups received cardiac catheterization at a median (interquartile range) of three (two to five), three (two to five) and four (two to six) days after admission, respectively (Table 2). The time from admission to cardiac catheterization was positively correlated with GRACE risk score (P<0.001), with a significantly longer time among high-risk patients. There was an inverse relationship between time from hospitalization to cardiac catheterization, and the time period of enrollment (P<0.001).
Similarly, there was an inverse relationship between the use of stress test and GRACE risk score: 26.8%, 21.7% and 16.3% of the low-, intermediate- and high-risk patients, respectively, underwent a stress test during the index hospitalization (P for trend <0.001).
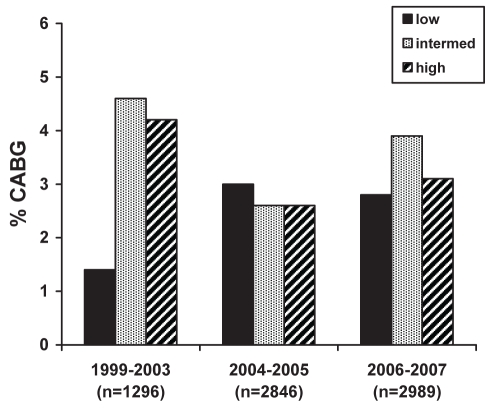
The rates of in-hospital cardiac procedures significantly increased over time. Cardiac catheterization rates were 47.9% (1999 to 2003), 51.6% (2004 to 2005) and 63.8% (2006 to 2007) (P<0.001), while PCI rates increased from 14.1% to 25.4% to 35.2% (P<0.001) during the same respective time periods (Table 4). However, the rates of CABG remained relatively unchanged at 3.2%, 2.8% and 3.2%, respectively (P=0.76). Overall, the rate of coronary revascularization (PCI or CABG) also showed a significant temporal increase.
TABLE 4.
In-hospital procedures by time period of enrollment
| Procedure | Overall (n=7131) | 1999–2003 (n=1296) | 2004–2005 (n=2846) | 2006–2007 (n=2989) | P (for trend) |
|---|---|---|---|---|---|
| Cardiac catheterization, % | 56.1 | 47.9 | 51.6 | 63.8 | <0.001 |
| Time to cardiac catheterization, days* | 3 (2 to 5) | 4 (2 to 6) | 3 (2 to 5) | 3 (2 to 5) | <0.001 |
| PCI, % | 27.4 | 14.1 | 25.4 | 35.2 | <0.001 |
| CABG, % | 3.1 | 3.2 | 2.8 | 3.2 | 0.76 |
| PCI/CABG, % | 29.9 | 17.3 | 27.4 | 37.9 | <0.001 |
Data presented as median (interquartile range). CABG Coronary artery bypass grafting; PCI Percutaneous coronary intervention
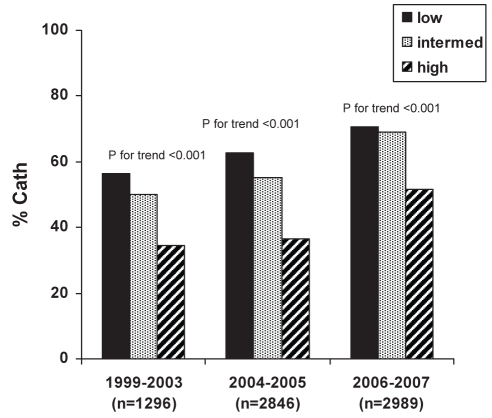
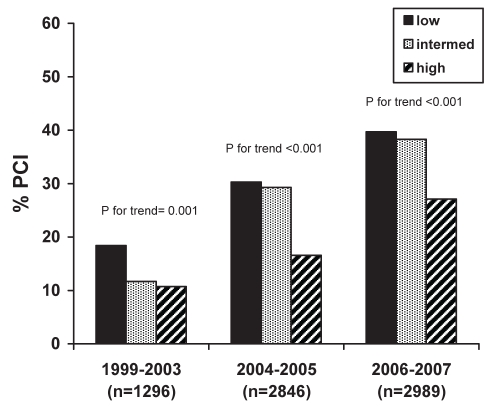
Despite the temporal trend toward an increasing use of invasive cardiac procedures, overall, there was an inverse relationship between GRACE risk group and either cardiac catheterization, PCI or any revascularization (PCI/CABG) (Table 2). This relationship persisted across the study time periods for rates of cardiac catheterization (Figure 1) and PCI (Figure 2), but not for CABG (Figure 3). Patients with a higher calculated GRACE risk score consistently had lower rates of cardiac catheterization and PCI. These patterns were consistently observed in hospitals with or without on-site cardiac catheterization facilities.
Figure 1).
Rates of in-hospital cardiac catheterization (Cath) across enrollment time periods in low-, intermediate- and high-risk patients as per the Global Registry of Acute Coronary Events (GRACE) risk score
Figure 2).
Rates of in-hospital percutaneous coronary intervention (PCI) across enrollment time periods in low-, intermediate- and high-risk patients as per the Global Registry of Acute Coronary Events (GRACE) risk score
Figure 3).
Rates of in-hospital coronary artery bypass grafting (CABG) across enrollment time periods in low-, intermediate- and high-risk patients as per the Global Registry of Acute Coronary Events (GRACE) risk score
In a multivariable analysis adjusting for other confounders, intermediate- and high-risk patients were significantly less likely to undergo cardiac catheterization during the index hospitalization than low-risk patients (Table 5). The model c-statistic was 0.68 and the P-value for the Hosmer-Lemeshow goodness-of-fit test was 0.60, demonstrating adequate discrimination and calibration, respectively. After further adjustment for previous heart failure and coronary revascularization, GRACE risk score maintained a strong negative association with the use of cardiac catheterization (adjusted OR 0.87 [95% CI 0.76 to 0.99], P=0.039 for the intermediate-risk group; and adjusted OR 0.45 [95% CI 0.35 to 0.58], P<0.001 for the high-risk group). Finally, similar results were obtained in sensitivity analyses that excluded early deaths within 48 h of admission (adjusted OR 0.80 [95% CI 0.70 to 0.92], P=0.002 for the intermediate-risk group; and adjusted OR 0.38 [95% CI 0.30 to 0.49], P<0.001 for the high-risk group) or the elderly (age of 75 years or older), and in a separate analysis restricted to patients admitted to hospitals in Ontario.
TABLE 5.
Independent predictors of in-hospital cardiac catheterization
| Independent predictor | Adjusted OR (95% CI) | P |
|---|---|---|
| Female sex | 0.75 (0.66–0.86) | <0.001 |
| Presence of on-site cardiac catheterization facility | 3.17 (2.01–5.01) | <0.001 |
| Time of enrollment | ||
| 1999–2003 | Reference | |
| 2004–2005 | 1.41 (0.97–2.04) | 0.071 |
| 2006–2007 | 2.44 (1.67–3.58) | <0.001 |
| GRACE risk score | ||
| Low | Reference | |
| Intermediate | 0.80 (0.70–0.92) | 0.002 |
| High | 0.38 (0.29–0.48) | <0.001 |
GRACE, Global Registry of Acute Coronary Events
DISCUSSION
The results of the present study illustrate that in a relatively unselected population of NSTE-ACS patients, patients at higher baseline risk according to GRACE risk score (which is a powerful predictor of adverse outcomes) had, paradoxically, a lower adjusted rate of cardiac catheterization. Furthermore, this inverse relationship persisted across several time periods. These findings imply that, despite guideline recommendations, physicians fail to selectively target an invasive approach toward high-risk NSTE-ACS patients.
Although previous studies have examined the use of cardiac catheterization in the 1990s, it is important to re-evaluate more recent temporal trends in management patterns since the publication of clinical trials (10–13) and practice guidelines (1,2) for NSTE-ACS. Several landmark trials demonstrated the benefits of an early invasive strategy, especially in high-risk patients. The FRagmin and Fast Revascularization during InStability in Coronary artery disease (FRISC II) (10), Treat Angina With Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction 18 (TACTICS-TIMI 18) (11) and Randomized Intervention Trial of unstable Angina (RITA)-3 (12) studies all showed benefit with an early invasive strategy with respect to a reduction in the incidence of MI. More importantly, an early invasive strategy conferred a significant mortality benefit over the long term in both FRISC II (one year) and RITA-3 (five years). In contrast, the most recent ICTUS trial (13), which evaluated an early invasive strategy in a high-risk NSTE-ACS population with elevated levels of cardiac troponin, failed to show a reduction in the composite end point of death, MI or re-hospitalization for ACS at both one year and three years. These new data challenge the benefits of a routine early invasive strategy and are reflected in the current NSTE-ACS guidelines (2), which suggest that an initial conservative strategy is acceptable for patients in the absence of high-risk features.
Concurrent with the evolving evidence base from clinical trials, the past decade also had a proliferation in the use of invasive cardiac procedures (24,25). The most recent large-scale study of management patterns in relation to risk stratification in a Canadian population of NSTE-ACS reflects data from 1999 to 2003 (6). Thus, there are only limited contemporary data on the ‘real-world’ management of NSTE-ACS in Canada, since the publication of the ICTUS trial and the increased availability of invasive cardiac procedures (24,25).
In the present study, the overall rates of cardiac catheterization increased significantly over time, while median time to cardiac catheterization showed a significant decline in the more recent patient cohort. This likely reflects an increased access to these procedures in our health care system. However, when cardiac catheterization rates were examined in relation to GRACE risk score, the high-risk patients had a lower adjusted rate of cardiac catheterization and the longest time to this procedure; this relationship persisted over time. These high-risk patients experienced a significantly higher rate of in-hospital death and re-MI, suggesting that they would likely benefit from a more invasive approach (2). These findings are consistent with earlier data from the Canadian ACS I and ACS II registries (6), but remain discordant with the current evidence-based practice guidelines (2). In addition to higher GRACE risk scores, there were several other independent negative predictors of in-hospital cardiac catheterization, including a history of heart failure, previous CABG and female sex. This longer time to cardiac catheterization among high-risk patients implies a treatment gap that could potentially be narrowed with a more effective triage system, in which clinicians routinely include a risk score assessment to identify the NSTE-ACS patients requiring the most urgent invasive investigations. Importantly, we observed a similar inverse relationship between the administration of evidence-based antithrombotic therapies and patient risk. The GRACE risk score, externally validated in ACS patients (16,19,20), was endorsed in the latest management guidelines (2) as a useful risk-stratification tool for the management of NSTE-ACS. This would likely improve the effectiveness of an early invasive strategy and potent antithrombotic therapies by selectively targeting them toward higher-risk patients, who may derive the greatest therapeutic benefit.
In the present study, 3119 (43.9%) patients were not referred for in-hospital cardiac catheterization during the index hospitalization. Although we did not specifically address the question of why patients were not referred for invasive investigations, this has been the focus of previous investigations. Data from the Canadian ACS registry (26) show that the reason most frequently cited by physicians (68.4%) was that the patient was not high risk and/or current clinical evidence did not support an early invasive strategy. Of note, 59.1% of these patients were determined to be intermediate-to-high risk according to a validated risk score. Moreover, in-hospital revascularization is associated with lower one-year mortality among these high-risk patients (19,27). Our study further supports the notion that physicians do not tailor invasive cardiac procedures appropriately according to the patients’ true risk of adverse outcome (6,20).
Limitations
Several limitations of the present study should be addressed. While GRACE is a prospective, large multinational registry with standard criteria for ACS and an emphasis on quality assurance, the present study was a retrospective analysis. However, this allowed us to more critically examine practice management in the ‘real world’ without influencing physicians’ clinical decisions. Second, the number of patients enrolled in the early time period (1999 to 2003) was appreciably smaller. Thus, the early practice pattern observed in the present study may be less representative of the general population. Third, the registry aimed to recruit consecutive patients, but this cannot be verified, so the patient population may not be truly unselected. Fourth, although the GRACE risk score offers a fairly accurate risk assessment based on various historical and clinical features, there are immeasurable factors, such as other medical comorbidities and patient preference, that might influence clinical decisions. Therefore, the present study may have overestimated the number of high-risk patients who would be eligible for cardiac catheterization. However, underestimation of risk, rather than comorbidities, appears to be the main reason for withholding invasive cardiac procedures in the management of high-risk ACS (26). Fifth, due to missing data, we could not determine GRACE risk score for 796 patients who were excluded from the study, which could have introduced bias. Finally, we did not evaluate the relationship between in-hospital invasive treatment and long-term outcome, although this was the focus of previous studies (19,27).
CONCLUSIONS
Fewer invasive cardiac procedures were performed among high-risk NSTE-ACS patients stratified by GRACE risk score. This inverse relationship was strong and consistently observed across all time periods examined. This treatment-risk paradox prevents the most effective use of invasive cardiac procedures in those high-risk patients who may derive the greatest therapeutic benefits. Strategies to eradicate this treatment-risk paradox, such as improved and objective risk stratification to inform management decisions, should be considered carefully.
Acknowledgments
The authors thank all the study investigators, coordinators and patients who participated in GRACE.
APPENDIX
GRACE Investigators: Shaun Goodman/Brigita Zile, St Michael’s Hospital, Toronto; James Cha/Jill Bilich, Lakeridge Regional Hospital, Oshawa; Maria De Villa/Carolyn Vardy, St Joseph’s Hospital, Toronto; Roland Leader/Carrie Harrison, Ajax Hospital, Ajax; Rajen Chetty/Debbie Rickeard, Hotel-Dieu Grace Hospital, Windsor; Fred Spencer/Pam Stevens/Imelda Esporlas-Jewer, McMaster Health Sciences Centre, Hamilton; Allan Hess, York County Hospital, Newmarket; Robert Bauer, Humber River Regional Hospital, Toronto; Teosar Bhesania, Humber River Regional Hospital-Finch Site, Toronto; Eric Gangbar, York Central Hospital, Richmond Hill; and Hans Strauss, The Credit Valley Hospital, Mississauga, Ontario.
GRACE2 Investigators: Richard Gallo/Nathalie Gendron/Colette Anctil, Institut de Cardiologie de Montreal, Montreal, Quebec; Jean-Pierre Picard, Hopital Hotel-Dieu de Sorel, Sorel-Tracy, Quebec; Real Brossoit/Celine Peck, Centre Hospitalier de Granby, Granby, Quebec; Jean Diodati/Celine Groulx, Hopital de Sacre-Coeur de Montreal, Montreal, Quebec ; Jean-Pierre Dery/Marie-Mai Larivere/Suzanne Keilani, Hopital Laval, Sainte-Foy, Quebec; Francois Grondin/Noella Bilodeau and Francine Dumont, Hotel Dieu de Levis, Levis, Quebec; Gaeten Houde/Stephanie Rousseau, CHA-Hopital de l’Enfant-Jesus, Quebec, Quebec; Richard Haichin/Violeta Toyota, Hopital Royal Victoria-McGill, Montreal, Quebec; Thao Huynh/Analia Robinowicz, Montreal General Hospital-McGill, Montreal, Quebec; YK Chan/Dianna Zaniol/Donna Lee Mallette, NSH/Greater Niagara General Site, Niagara Falls, Ontario; Ashok Mukherjee/Bev Bozek, Rouge Valley Health System/Scarborough Cardiology Research, Scarborough, Ontario; Sven Pallie/Sheila Krekorian, NHS/St Catharines General Hospital, St Catharines, Ontario; J Paul DeYoung/Marian Watt, Cornwall Community Hospital, Cornwall, Ontario; Denis Chauret/Micheline Marquis/Ava Eargibay, Montfort Hospital, Ottawa, Ontario; Christopher Lai/Grace Fox, Thunder Bay Regional Health Sciences, Thunder Bay, Ontario; James Cherry/Barbara Ross, Scarborough General Hospital, Scarborough, Ontario; Petr Polasek/Violette Stedham/Michelle Mantle, Kelowna General Hospital/Kelowna Cardiology Research, Kelowna, British Columbia; Jan Kornder/Helen Elliott, Surrey Memorial Hospital, Surrey, British Columbia; Donald Hilton/Marion Johnson/Bev Lowe, Cowichan District Hospital, Duncan, British Columbia; Kevin Lai/Gail Hannah, Nanaimo Regional Hospital, Nanaimo, British Columbia; Francis Ervin/Zhanna Yarkayeva, Ridge Meadows Hospital, Maple Ridge, British Columbia; Carolyn Baer/Rhonda Steeves, Moncton Hospital, Moncton, New Brunswick; Christian Paillard/Cecile Paillard, Clinique de Medecine Interne, Edmundston, New Brunswick; Wadea Tarhuni/Diane Ireland/Denise Bunnell, Moose Jaw Cardiac Centre, Moose Jaw, Saskatchewan; Kon Son/Sherry Deyoung, Chilliwack General Hospital, Chilliwack, British Columbia; Graham Wong/Joanne Gamache, Gordon and Leslie Diamond Health Care Centre, Vancouver, British Columbia; Gabor Gyenes/Linda Harris and Joann Flathers, University of Alberta Hospital, Edmonton, Alberta; Gerry Simkus/Carol Marchand, Royal Columbian Hospital, New Westminster, British Columbia; Peter Giannoccaro/Peggy Beresford, Peter Lougheed Centre, Calgary, Alberta; Thomas Ashton/Susan Valley, New Brunswick Heart Centre Research, Penticton, New Brunswick; Vernon Paddock/Fabia Fitzgerald/Elizabeth Collings, New Brunswick Heart Centre, Saint John, New Brunswick; John Imrie/Jennifer J McGregor, Lions Gate Hospital, North Vancouver, British Columbia; Teddi Orenstein/Pat Berdan, Richmond Hospital, Richmond, British Columbia; Rodney Zimmerman/Gisele Patterson and Cheryl Altwasser, Regina General Hospital, Regina, Saskatchewan; Carlton Harnarine/Jennifer Pasma, Chatham Kent Health Alliance, Chatham, Ontario; Samer Mansour/Carole Lemany, Hopital Hotel-Dieu, Montreal, Quebec; Yves Pesant/Veronique Sardin, Centre de Sante et de Service Sociaux de St-Jerome, St-Jerome, Quebec; Christian Constance/Marie-France Gauthier, Hopital Maisonneuve-Rosemont, Montreal, Quebec; Wojciech Sobkowski/Nancy Nero, Welland Hospital, Welland, Ontario; Richard Audet/Vincent Wellemans, Centre Hospitalier Baie des Chaleurs, Maria, Quebec; and Marc Lamothe, Hotel-Dieu d’Arthabaska, Victoriaville, Quebec.
Footnotes
CONTRIBUTORS: Sean Jedrzkiewicz drafted the manuscript. All of the authors contributed to the study design, data interpretation and critical revision of the manuscript. All authors approved the final version submitted for publication.
SOURCE OF FUNDING: This research was supported by an unrestricted grant from sanofi-aventis (Paris, France and Laval, Quebec) and by Bristol-Myers Squibb Canada (Montreal, Quebec). The industry sponsors had no involvement in the study conception or design; collection, analysis and interpretation of data; the writing, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction – summary article: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol 200750e1–57.17692738 [Google Scholar]
- 3.Bhatt DL, Roe MT, Peterson ED, et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: Results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292:2096–104. doi: 10.1001/jama.292.17.2096. [DOI] [PubMed] [Google Scholar]
- 4.Fox KA, Anderson FA, Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: Do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE) Heart. 2007;93:177–82. doi: 10.1136/hrt.2005.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zia MI, Goodman SG, Peterson ED, et al. Paradoxical use of invasive cardiac procedures for patients with non-ST segment elevation myocardial infarction: An international perspective from the CRUSADE Initiative and the Canadian ACS Registries I and II. Can J Cardiol. 2007;23:1073–9. doi: 10.1016/s0828-282x(07)70876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan AT, Yan RT, Tan M, et al. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med. 2007;167:1009–16. doi: 10.1001/archinte.167.10.1009. [DOI] [PubMed] [Google Scholar]
- 7.McAlister FA, Oreopoulos A, Norris CM, et al. Exploring the treatment-risk paradox in coronary disease. Arch Intern Med. 2007;167:1019–25. doi: 10.1001/archinte.167.10.1019. [DOI] [PubMed] [Google Scholar]
- 8.Roe MT, Peterson ED, Newby LK, et al. The influence of risk status on guideline adherance for patients with non-ST segment elevation acute coronary syndromes. Am Heart J. 2006;151:1205–13. doi: 10.1016/j.ahj.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Fox KAA, Goodman SG, Anderson FA, et al. From guidelines to clinical practice: The impact of hospital and geographical characteristics on temporal trends in the management of acute coronary syndromes. Eur Heart J. 2003;24:1414–24. doi: 10.1016/s0195-668x(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 10.FRagmin and Fast Revascularization during InStability in Coronary artery disease (FRISC II) Investigators Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999;354:708–15. [PubMed] [Google Scholar]
- 11.Cannon CP, Weintraub WS, Demopoulos LA, et al. TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)-Thrombolysis in Myocardial Infarction 18 Investigators Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–87. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 12.Fox KA, Poole-Wilson PA, Henderson RA, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: The British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–51. doi: 10.1016/s0140-6736(02)09894-x. [DOI] [PubMed] [Google Scholar]
- 13.De Winter RJ, Windhausen F, Cornel JH, et al. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med. 2005;353:1095–104. doi: 10.1056/NEJMoa044259. [DOI] [PubMed] [Google Scholar]
- 14.The Grace Investigators Rationale and design of the GRACE (Global Registry of Acute Coronary Events) project: A multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141:190–9. doi: 10.1067/mhj.2001.112404. [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, Goldberg RJ, Gore JM, et al. for the GRACE Investigators Baseline characteristics, management practices, and in hospital outcome of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–63. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 16.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163:2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 17.University of Massachusetts Medical School Center for Outcomes Research. < http://www.outcomes-umassmed.org/grace/grace_risk_table.cfm> (Version current at January 7, 2008).
- 18.Yan AT, Jong P, Yan RT, et al. Canadian Acute Coronary Syndromes registry investigators. Clinical trial-derived risk model may not generalize to real-world patients with acute coronary syndrome. Am Heart J. 2004;148:1020–7. doi: 10.1016/j.ahj.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 19.de Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: Sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26:865–72. doi: 10.1093/eurheartj/ehi187. [DOI] [PubMed] [Google Scholar]
- 20.Yan AT, Yan RT, Tan M, et al. Risk scores for risk stratification in acute coronary syndromes: Useful but simpler is not necessarily better. Eur Heart J. 2007;28:1072–8. doi: 10.1093/eurheartj/ehm004. [DOI] [PubMed] [Google Scholar]
- 21.Alter DA, Naylor CD, Austin PC, Tu JV. Long-term MI outcomes at hospitals with or without on-site revascularization. JAMA. 2001;285:2101–8. doi: 10.1001/jama.285.16.2101. [DOI] [PubMed] [Google Scholar]
- 22.Every NR, Parsons LS, Fihn SD, et al. MITI Investigators, Myocardial Infarction Triage and Intervention Long-term outcome in acute myocardial infarction patients admitted to hospitals with and without on-site cardiac catheterization facilities. Circulation. 1997;96:1770–5. doi: 10.1161/01.cir.96.6.1770. [DOI] [PubMed] [Google Scholar]
- 23.Stukel TA, Lucas FL, Wennberg DE. Long-term outcomes of regional variations in intensity of invasive vs medical management of Medicare patients with acute myocardial infarction. JAMA. 2005;293:1329–37. doi: 10.1001/jama.293.11.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas FL, DeLorenzo MA, Siewers AE, Wennberg DE. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113:374–9. doi: 10.1161/CIRCULATIONAHA.105.560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alter DA, Stukel TA, Newman A. Proliferation of cardiac technology in Canada: A challenge to the sustainability of Medicare. Circulation. 2006;113:380–7. doi: 10.1161/CIRCULATIONAHA.105.560466. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Tan M, Yan AT, et al. Use of cardiac catheterization for non-ST-segment elevation acute coronary syndromes according to initial risk: Reasons why physicians choose not to refer their patients. Arch Intern Med. 2008;168:291–6. doi: 10.1001/archinternmed.2007.78. [DOI] [PubMed] [Google Scholar]
- 27.Yan AT, Yan RT, Tan M, et al. Canadian Acute Coronary Syndromes Registry Investigators In-hospital revascularization and one-year outcome of acute coronary syndrome patients stratified by the GRACE risk score. Am J Cardiol. 2005;96:913–6. doi: 10.1016/j.amjcard.2005.05.046. [DOI] [PubMed] [Google Scholar]