Abstract
Bone is the preferred site of prostate cancer metastasis, contributing to the morbidity and mortality of this disease. A key step in the successful establishment of prostate cancer bone metastases is activation of osteoclasts with subsequent bone resorption causing the release of several growth factors from the bone matrix. CD11b+ cells in bone marrow are enriched for osteoclast precursors. Conditioned media from prostate cancer PC-3 cells induces CD11b+ cells from human peripheral blood to differentiate into functional osteoclasts with subsequent bone resorption. Analysis of PC-3 conditioned media revealed high amounts of IL-6 and IL-8. CD11b+ cells were cultured with M-CSF and RANKL, IL-6, IL-8 and CCL2, alone or in combination. All of these conditions induced osteoclast fusion, but cells cultured with M-CSF, IL-6, IL-8 and CCL2 were capable of limited bone resorption. Co-incubation with IL-6 and IL-8 and the RANK inhibitor, RANK-Fc, failed to inhibit osteoclast fusion and bone resorption, suggesting a potential RANKL-independent mechanism of functional osteoclast formation. This study demonstrates that functional osteoclasts can be derived from CD11b+ cells derived from human PBMCs. Prostate cancer cells secrete factors, including IL-6 and IL-8, that play an important role in osteoclast fusion by a RANKL-independent mechanism.
Keywords: Osteoclast, CD11b, Prostate Cancer, RANKL, IL-6, IL-8, CCL2
Prostate cancer is the most commonly diagnosed malignancy in US men [Walczak and Carducci, 2007]. Advanced prostate cancer is first treated through chemical or surgical castration because a large percentage of the cancer cells are androgen dependent. The large majority of patients, however, relapse within a few years of treatment because of the emergence of a castration resistant clone of cancer cells. Castration independent prostate cancer is an incurable disease with little effective therapy and there is a great need for novel therapeutic strategies that target the molecular basis of castration-independent, chemo-resistant prostate cancer [Isaacs, 2005]. Previous studies have demonstrated that 83%–90% of men had evidence of bone metastasis when they died of prostate cancer [Keller et al., 2007; Kingsley et al., 2007; Loberg et al., 2005]. Bone is the preferred metastasis site of advanced prostate cancer, and in many patients, the only clinically evident site of metastasis at the time of death [Taichman et al., 2007]. There is a great need of novel therapeutic strategies that target the molecular basis of advanced prostate cancer – bone interactions. A key step in the successful establishment of prostate cancer bone metastases is osteoclast formation and bone resorption followed by the release of several growth factors from the bone matrix that promotes tumor growth [Taichman et al., 2007]. The mechanisms by which prostate cancer cells promote osteoclast formation and bone resorption remain unclear.
Previous reports suggested that RANKL, Interleukin-6 (IL-6), Interleukin-8 (IL-8) and C-C chemokine ligand 2 (MCP-1, CCL2) mediate osteoclast formation from human bone marrow mononuclear cells (HBMCs) and human peripheral blood mononuclear cells [Kudo et al., 2003; Lu et al., 2007]. Furthermore, prostate cancer cells have been demonstrated to secrete these factors that promote osteoclastogenesis of HBMCs [Lu et al., 2007]. The purpose of this study was to further delineate the cell – types and mechanisms involved in prostate cancer induced osteoclastogenesis.
Monocyte-macrophages are known to be the precursors of osteoclasts [Fujikawa et al., 1996; Quinn et al., 1998]. The cell surface antigen CD11/CD18 is known to be a heterodimeric structure, consisting of a common β subunit (CD18) and a distinct β subunit (CD11a, CD11b, CD11c). CD11b/CD18 is also known as Mac-1/C R3, and αMβ2-integrin and is expressed in human monocytes, macrophages, neutrophils and eosinophils [Murdoch et al., 2008]. Hayashi et al. demonstrated that CD11/CD18 is required for nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation [Hayashi et al., 2008].
RANKL induces osteoclast formation and activation in the presence of macrophage colony stimulating factor (M-CSF) through NFATc-1 and MMP9 activation [Boyce and Xing, 2008; Del Fattore et al., 2008; Takayanagi, 2005]. Furthermore, IL-6 and IL-8 are known to be highly expressed in prostate cancer metastatic sites [Lu et al., 2007]. CCL2 is a member of the CC beta chemokine family and is classically known for activating chemotaxis for monocyte/macrophages and other inflammatory cells via its receptor CCR2. The precise mechanism underlying the role of CCL2 on osteoclast formation remains to be explored.
In this study, human recombinant chemokines were used to identify which factors were important in osteoclast formation and bone resorption from human peripheral mononuclear CD11b+ cells.
Materials and Methods
Reagents
Recombinant human M-CSF, IL-6, IL-8 and neutralizing antibodies for IL-6 and IL-8 and RANKFc were purchased from R&D systems (Minneapolis, MN). RANKL and rhMCP-1 were purchased from PEPROTECH Inc. (Rocky Hill, NJ) and APOLLO (Alexandria, Sydney, Australia) respectively.
Cell Culture
Human prostate cancer PC-3 cells were cultured in RPMI 1640 (GIBCO) supplemented with 10% fetal bovine serum (GIBCO) under a humidified atmosphere of 5% CO2 at 37°C. For preparing conditioned media to induce osteoclasts, PC-3 cells were cultured in 150 mm2 tissue culture flasks to 90% confluency. Cells were then washed with PBS and cultured with 1% serum media for 48 hrs. The conditioned media was normalized for cell number (1.0 × 106 cells/ ml). The conditioned media was centrifuged and supernatant was collected and stored in −20°C until use.
Cytokine Array
Cytokine arrays were purchased from R&D systems and used per manufacturer’s instructions (Minneapolis, MN). Briefly, after blocking, membranes were incubated overnight with 1 ml of the conditioned medium collected from PC-3 cells. Membranes were then washed and immunoreactive spots were detected with chemiluminescence.
ELISA Assays
ELISA analysis for IL-6, IL-8 and CCL2 (R&D systems) were performed following the manufacture’s instructions. ELISA analysis kit for RANKL was purchased from Biomedica Medizinprodukte GmbH & Co KG (Austria).
Quantitative RT-PCR
RNA was extracted according to manufacturer’s instructions by using RNAeasy Micro Kit (Qiagen Inc., USA). Briefly, cells were lysed directly in the wells and homogenized by passing through a needle several times. RNA was precipitated with 70% ethanol and applied to RNAeasy MiniElute columns. Columns were washed with buffers and 80% ethanol. Total RNA was eluted from the columns with RNAase-free distilled water. One microgram of total RNA of each sample was reverse transcribed by using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Branchburg NJ). Primers (NFATc-1, MMP9) were purchased from Applied Biosystems. The housekeeping transcript, GAPDH, was used as a control for standardization. PCR was carried out with TaqMan Universal PCR Master Mix (Applied Biosystems).
Osteoclast Formation Assays
Human peripheral mononuclear cells (PBMCs) were isolated by Ficoll-Paque (Amersham Biosciences) density gradient centrifugation from healthy male volunteers. Cells of the monocyte fraction (CD11b+) were isolated from the PBMCs by MACS CD11b Micro Beads (Miltenyi Biotec) as recommended by the manufacturer. 5×105 CD11b cells were seeded on coverslips (Nalge Nunc International, Rochester NY) in MEM supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 mg/ml) and M-CSF (25 ng/ml) in 24 wells. 24 h after seeding, media was changed to fresh media and incubated in the presence of M-CSF (25 ng/ml), RANKL (30 ng/ml), IL-6 (5 ng/ml), IL-8 (30 ng/ml), RANKFc (1 µg/ml) or combination of PC-3 conditioned medium and MEM ((1:1) and anti-IL-6 (0.6 µg/ml) and anti-IL-8 antibody (1.8 µg/ml). Cells were cultured for 14 days and then fixed and stained with a TRAP staining kit (Sigma). Coverslips were also fixed in methanol and stained immunocytochemically using an antibody against vitronectin receptor (abcam Cambridge MA). TRAP+ cells that resembled RANKL-induced multinuclear cells were considered to be osteoclasts and counted under the microscope. Stained cell images were obtained using a BX51 microscope (Olympus, Japan).
Flow Cytometry
CD11b+ cells were stained with anti-human CD11b-FITC (Pharmingen) antibodies and their matching isotype controls according to the manufacturer’s protocols. The cells were incubated with antibodies for 30 min at 4°C and washed with PBS. The samples were analyzed by using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson).
Bone Resorption Assays
5×105 CD11b+ cells were seeded on the resorbable artificial bone film-coated disks (Becton and Dickinson, Bedford, MA) and incubated as described above. After 3 weeks of culture, cells were removed by sodium 5% hypochlorite solution and bone resorption area images were obtained by a BX51 microscope. The mean area of resorption from 5 randomly selected fields was analyzed with Adobe Photoshop software (Adobe).
Results
Identification of cytokines and RANKL secretion by prostate cancer, PC-3 cells
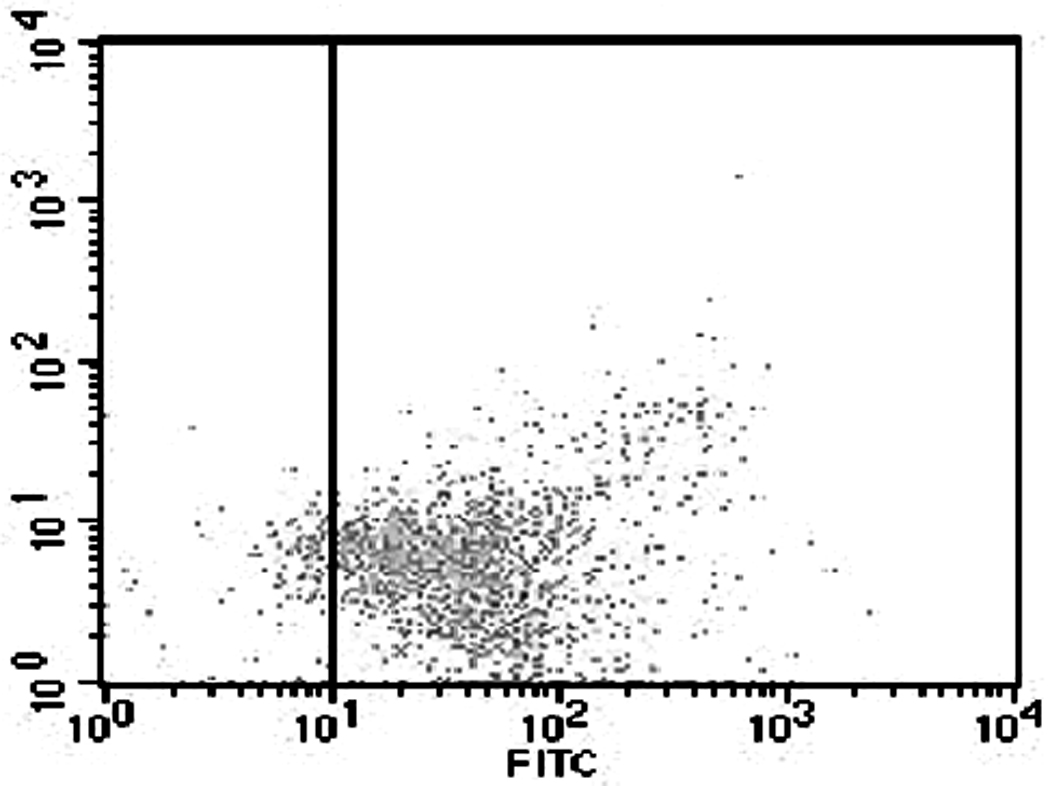
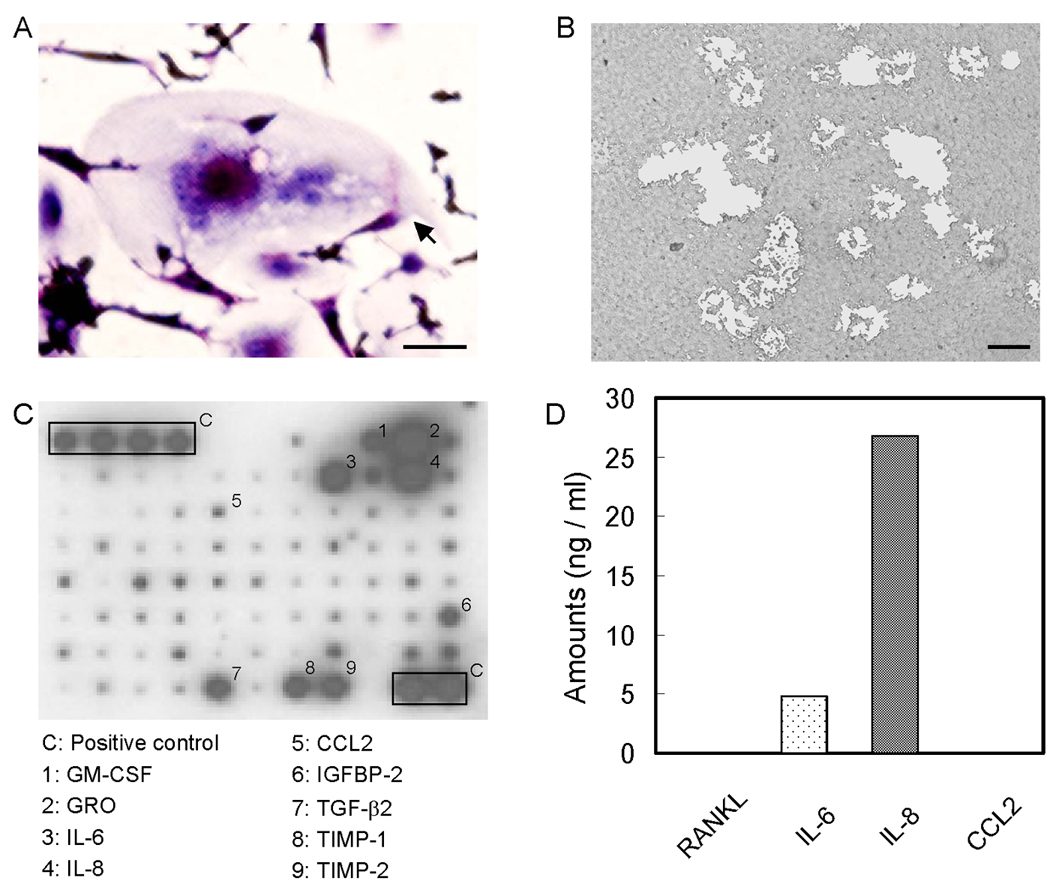
The fact that conditioned medium of several prostate cancer cell lines induces osteoclast from HBMCs or the general PBMC population is well established. Prior to the osteoclast formation experiment, we investigated the population of cells isolated by CD11b beads. More than 90% of isolated cells were CD11b positive (Fig. 1). Osteoclasts were induced from the CD11b+ fraction of PBMCs by PC-3 conditioned media (Fig. 2 A). These osteoclasts were considered to be functional as evidenced by bone resorption in vitro (Fig. 2 B). Identification of factors from prostate cancer cells is essential to explain the mechanism of prostate cancer induced bone resorption. A limited cytokine antibody array demonstrated that PC-3 cells secrete high amounts of GM-CSF, GRO, IL-6, IL-8, IGF-BP2, TGF-β2, TIMP-1 and TIMP-2 but very little CCL2 (Fig. 2 C). Of these factors, IL-6, IL-8 and CCL2 are reported to be important for osteoclast formation. To quantify these factors in PC-3 conditioned medium, the amount of IL-6, IL-8, CCL2 and RANKL by ELISA was measured. IL-6 and IL-8 were highly secreted in PC-3 conditioned medium; however, the level of RANKL (less than 5pg/ml) and CCL2 (undetectable) were low (Fig. 2 D).
Figure 1.
Prostate cancer promotes CD11b+ cells to differentiate into osteoclasts. PC-3 conditioned media-induced osteoclast formation (A; TRAP staining, B; bone resorption) Bars; 200 µm. 0.5×106 CD11b+ cells were cultured with M-CSF (25 ng/ml) and 50% conditioned media from PC-3 cells in 24 well plates for 14 days. C. Anti-body based cytokine analysis of PC-3 conditioned media. D. ELISA measurement of RANKL, CCL2, IL-6 and IL-8 in PC-3 conditioned media.
Figure 2.
Cell population analysis of isolated cells from PBMCs. 80 – 95% of isolated cells using CD11b beads were CD11b positive. Isolated cells were incubated anti-human CD11b antibody that was conjugated with FITC and analyzed by flow cytometry.
IL-6 and IL-8 promote CD11b positive cells to osteoclast like cell but not bone resorption
At day 14 after incubation in the presence of each factors plus M-CSF, osteoclast fusion was detected by TRAP staining and vitronectin. As shown in Figure 3 A, 3B and 3D; IL-6, IL-8 alone and in combination induced TRAP-positive multinuclear cells from CD11b+ cells. The number of IL-6 and IL-8 induced osteoclast-like cells were similar and approximately half of the number of those seen in M-CSF + RANKL stimulated conditions or in the IL-6 + IL-8 + M-CSF stimulated conditions. Co-incubation with M-CSF, IL-6, IL-8 and RANKFc, a biologic inhibitor of RANKL, failed in inhibiting osteoclast fusion and bone resorption. This result implies that IL-6 and IL-8 have a potential RANKL independent mechanism of osteoclast fusion. M-CSF and CCL2 induced CD11b+ derived osteoclast fusion; however, the number of nuclei in CCL2 induced multinuclear cells was fewer compared to RANKL, IL-6 and IL-8 induced multinuclear cells (Figure 3A and 3D).
Figure 3.
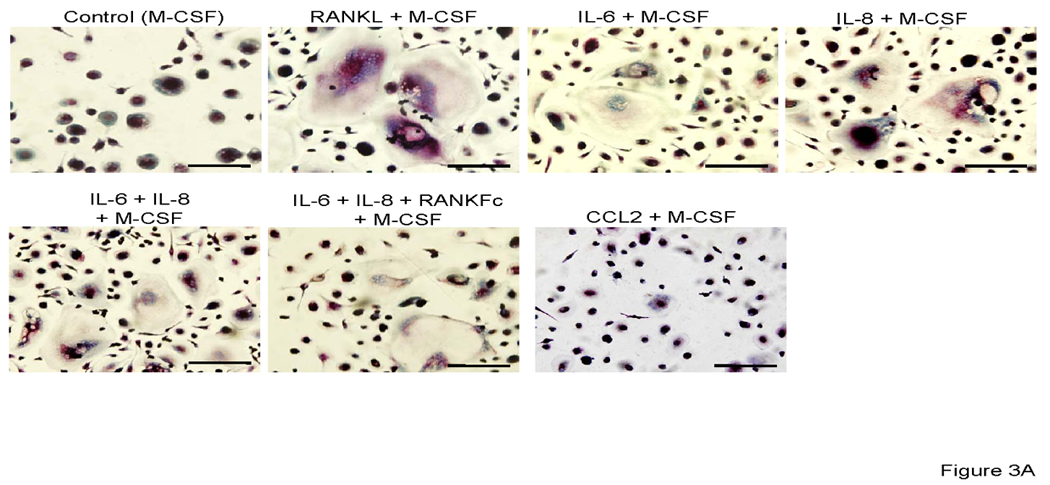
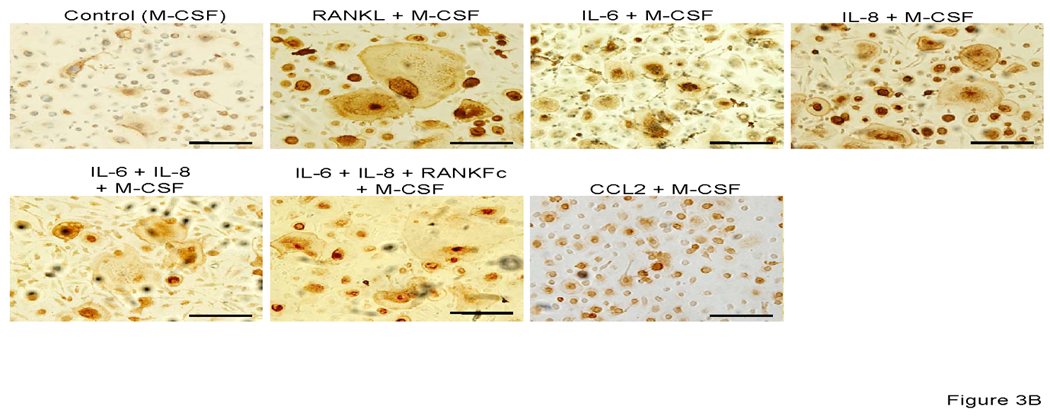
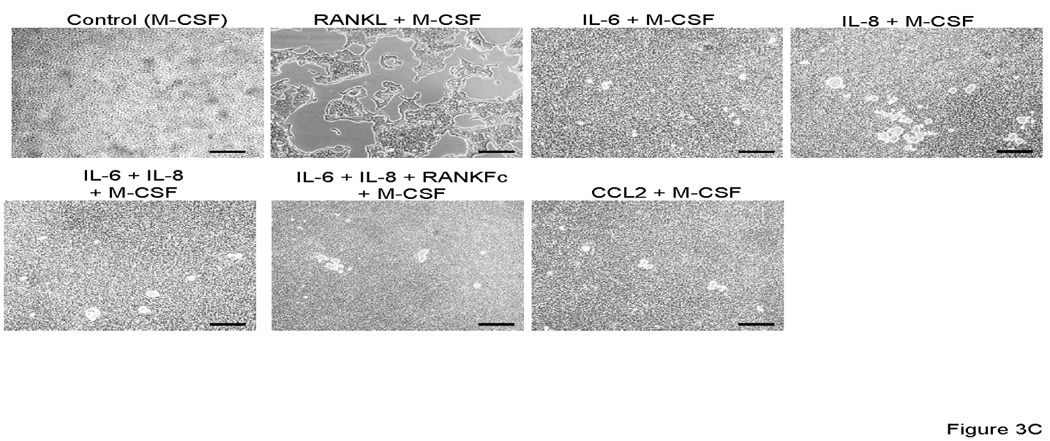
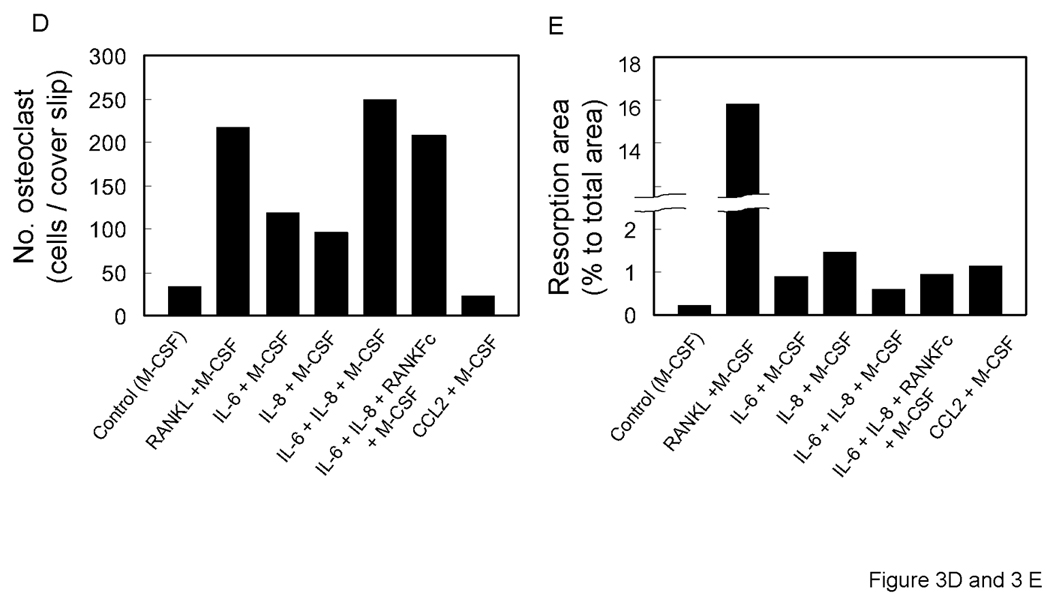
Representative micrographs of TRAP positive (A) and vitronectin (B) multinuclear cells that are induced by each stimulator. 0.5×106 CD11b+ cells were all cultured with M-CSF (25 ng/ml) and IL-6 (5 ng/ml), IL-8 (30 ng/ml), RANKFc (1 µg/ml) and CCL2 (100 ng/ml) or 50% conditioned media from PC-3 cells in 24 well plates for 14 days. C. Representative micrographs of artificial bone resorption by osteoclasts. 21 days after incubation with each stimulator, cells were removed and bone resorption area was visualized. Bars; 100 µm. Graphical representation of number of osteoclast-like multinuclear TRAP positive cells (C) and bone resorption area (D). After TRAP staining multi nucleated and expanded cells per slide were counted. The mean area of bone resorption from 5 randomly selected fields was quantified.
To confirm the ability of CD11b+ derived osteoclast-like cells were functional and capable of bone resorption, CD11b+ cells were cultured on artificial bone discs. As expected, the soluble RANKL induced strongest bone resorption (Figures 3C, 3D, 3E). CD11b+ cells cultured with other factors (IL-6, IL-8, CCL2 and their combination), demonstrated limited or no resorption as compared to RANKL stimulation (Figures 3C, 3D, 3E). Interestingly, osteoclast-like cells that were induced by co-incubation with IL-6 and IL-8 made significantly less pit formation, regardless of the same amount of TRAP positive multinuclear cells compared with RANKL stimulation, perhaps suggesting that CD11b+ require key factors like soluble RANKL for of bone resorption activity after formation of TRAP positive multinuclear, osteoclast-like cells (Fig. 3 C and D).
PC-3 conditioned media promotes CD11b positive cells to differentiate to osteoclasts
PC-3 conditioned medium has been shown to promote HBMCs to osteoclasts [Lu et al., 2007]. CD11b + cells from mice are known precursors of osteoclasts [Li et al., 2004]. To investigate the effect of PC-3 conditioned media on osteoclast formation, human CD11b+ cells were cultured with conditioned media. PC-3 conditioned media promoted CD11b+ cells to fuse to form TRAP positive multinuclear cells and the cells had the capability of bone resorption (Figure 4). The results from the cytokine array and osteoclast formation assay indicated that RANKL, IL-6 and IL-8 may play important roles in CD11b+ osteoclast fusion and bone resorption. To determine the role of RANKL, IL-6 and IL-8 in PC-3 conditioned media induced CD11b+ osteoclast formation, CD11b+ cells were cultured with conditioned medium in the presence of RANK-Fc and neutralizing antibodies for IL-6 and IL-8. RANKFc and the combination of anti-IL-6 and anti-IL-8 failed to inhibit conditioned media induced osteoclast formation (Figure 4). To verify that prostate cancer cells induced osteoclast differentiated from CD11b+ cells, bone resorption area was observed (Figure 4A). The resorption was qualitatively different than the resorption demonstrated by stimulation with M-CSF + RANKL (Figure 3A). RANKFc had no effect on conditioned media induced osteoclast resorption while combination treatment of anti-IL-6 and anti-IL-8 antibodies slightly reduced PC-3 conditioned media induced bone resorption. These results imply that IL-6 and IL-8 may have a role in CD11b + osteoclastogenesis.
Figure 4.
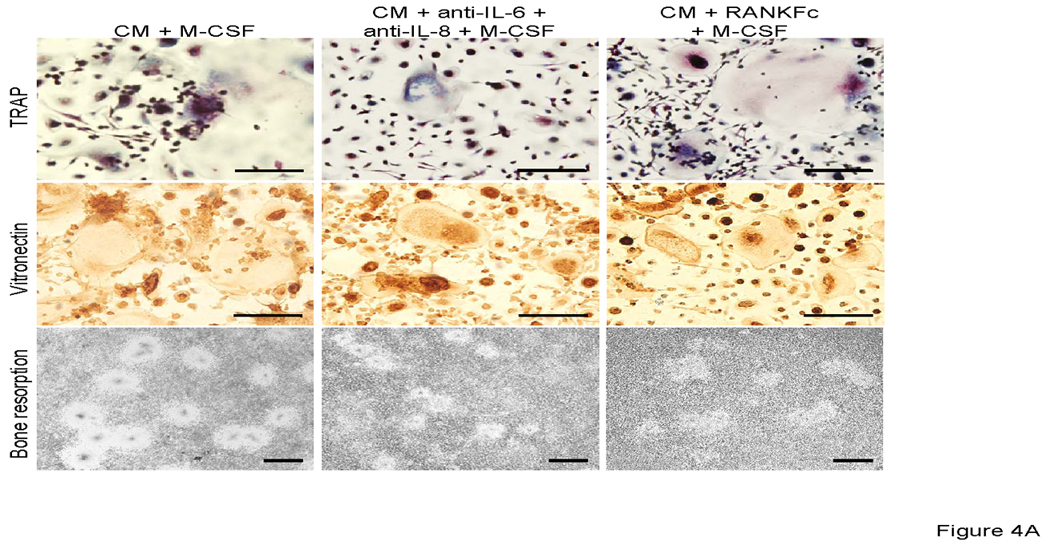
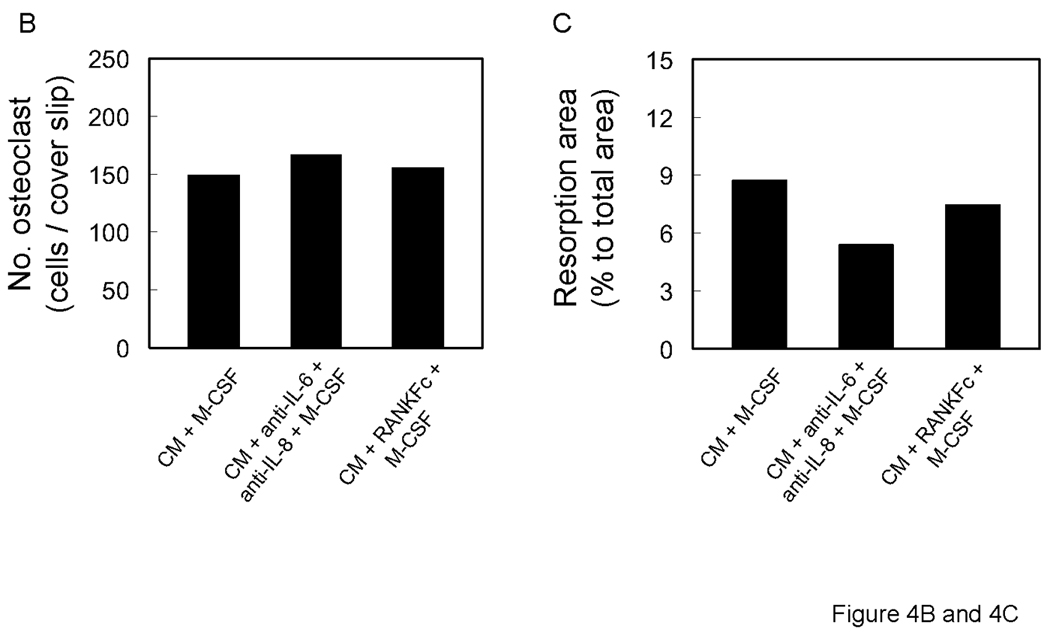
A. Representative micrographs of TRAP positive (upper panels), vitronectin positive (middle panels) multinuclear cells and bone resorption (lower panel). 0.5×106 CD11b+ cells were all cultured with M-CSF (25 ng/ml) and neutralizing antibodies for IL-6 and IL-8 and RANKFc with 50% conditioned media from PC-3 cells. Graphical representation of number of osteoclast-like multinuclear TRAP positive cells (B) and bone resorption area (C).
Expression of NFATc-1 and MMP-9 by each simulation
NFATc-1 and MMP-9 are known to be activated during osteoclast fusion and function [Boyce and Xing, 2008; Del Fattore et al., 2008; Takayanagi, 2005; Kim et al., 2006]. Osteoclast-related gene expression after 21 days incubation of CD11b+ cells with each factor was examined by quantitative RT-PCR. Both NFAT-c-1 and MMP-9 expression were increased by RANKL compared to MCSF alone (Fig. 5). Expression levels induced by other factors did not show significant difference. Interestingly, expression levels of NFaTc-1 decreased to 63% as compared to control (MCSF) conditioned media.
Figure 5.
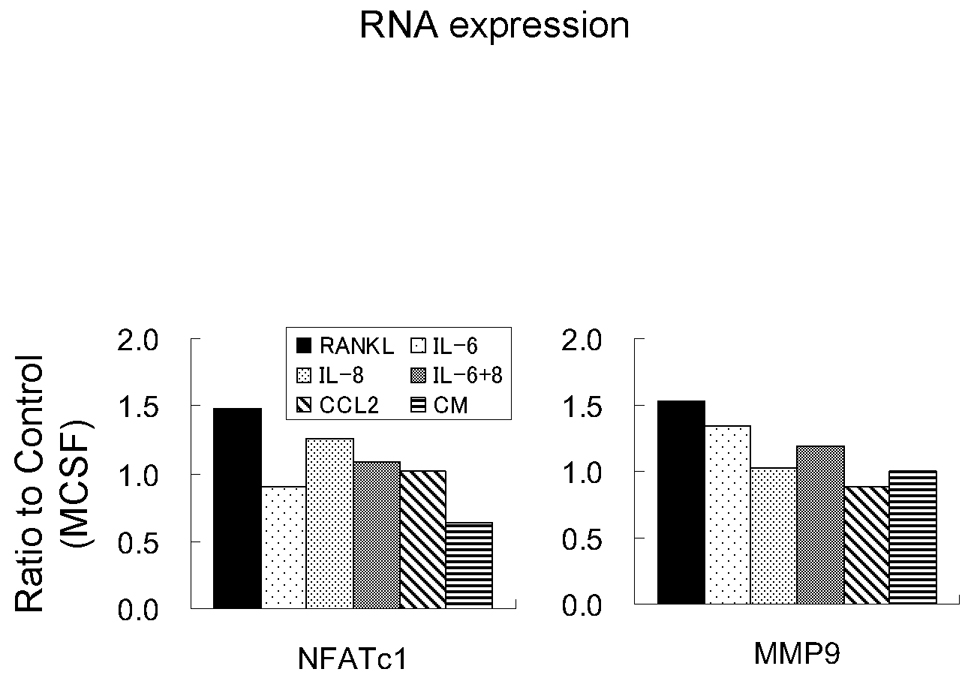
Graphical representation of molecular characterization of osteoclastogenesis. 21 days after incubation, mRNA was extracted and amplified. cDNA was used for quantitative real time PCR using primers of NFATc-1 and MMP9.
Discussion
The results of the present study demonstrated that functional osteoclasts can be induced to be differentiated from human peripheral blood derived CD11b+ cells. Recently, it is reported that reduction of activity of CD11b by siRNA and neutralizing antibody inhibited CD11b+ mononuclear cells osteoclastogenesis by M-CSF and RANKL [Hayashi et al., 2008]. Hence, CD11b+ is thought to be a marker of osteoclast precursors. Detailed studies of prostate cancer induced CD11b positive cell-osteoclastogenensis have not been reported. Results demonstrated that RANKL, IL-6 and IL-8 promote osteoclast fusion from CD11b+ cells. RANKL has been previously demonstrated to promote osteoclastogenesis through the up-regulation of NFATc-1 in HMBCs and PBMCs [Lu et al., 2007; Boyce and Xing, 2008; Del Fattore et al., 2008; Takayanagi, 2005]. This study extends these results and demonstrated that RANKL plays an important role in both osteoclast fusion and bone resorption of human CD11b+ cells with up-regulation of NFATc-1.
IL-6 and IL-8 are known as key factors in HBMC and PBMC osteoclastogenesis [Lu et al., 2007]. Kudo and co-workers had reported that IL-6 induced osteoclast formation by a RANKL independent mechanism [Kudo et al., 2003]. In this study, IL-6, IL-8 and CCL2 did not induce RANKL expression from CD11b+ cells (data not shown). RANKL expression is not induced by IL-6 in human bone marrow and osteoblasts [Han et al., 2001; Hofbauer et al., 1999]. The findings demonstrate that IL-6 effects osteoclast fusion but not bone resorption, suggesting other factor(s) are necessary for bone resorption. IL-8 is also multifunctional cytokine correlating with tumor growth, chemotactic activities and osteoclastogenesis. Bendre et al. had reported IL-8 secreted by tumor cells induce osteolysis independent of the RANKL pathway [Bendre et al., 2005]. Osteoclasts without bone resorption activity has been reported [Kim et al., 2006]. TRAP-positive multinuclear cells induced by IL-6 and IL-8 with negative activity for bone resorption may represent a precursor stage of functional osteoclasts. The results of no induced RANKL expression by IL-6 and IL-8 as well as co-culture with IL-6 and IL-8 and RANKFc may indicate that osteoclast fusion occurs by a RANKL-independent mechanism in CD11b+ cells. Direct osteoclast differentiation by IL-6 through NFATc-1 activation has been previously reported [Del Fattore et al., 2008]. Up-regulation of NFATc-1 and bone resorption by IL-6 were not observed in this study. This may be due to the different concentrations of IL-6 used in the different studies.
CCL2 is a member of the CC beta chemokine family and is classically known for activating chemotaxis for monocyte/macrophages and other inflammatory cells. CCL2 has been demonstrated to promote osteoclast fusion and function; however, the exact cell type that is induced to differentiate into functional osteoclasts from PBMCs remains unclear [Lu et al., 2007; Kim et al., 2006; Kim et al., 2005; Li et al., 2007]. These results suggest CCL2 had limited effect on both osteoclast fusion and bone resorption of CD11b+ cells in vitro.
The studies conducted here confirm that conditioned medium from prostate cancer induces osteoclast formation [Lu et al., 2007]. The results demonstrate that osteoclast formation occurs via a RANKL independent mechanism. Prostate cancer induced osteoclastogenesis, however, also appears to be independent of IL-6 and IL-8. Further experiments are needed to address the role of other factors produced by prostate cancer cells that effect osteoclast formation. For example, it has been demonstrated that PTHrP produced by prostate cancer cells can facilitate osteoclastogenesis and osteoblastogenesis [Li et al., 2007; Liao et al., 2008]. This study demonstrates that functional osteoclasts can be derived from CD11b+ cells derived from human PBMCs. Prostate cancer cells secrete factors, including IL-6 and IL-8, that play an important role in osteoclast fusion by a RANKL-independent mechanism.
Acknowledgments
Grant Information: K.J. Pienta is supported by NIH grant PO1 CA093900, an American Cancer Society Clinical Research Professorship, NIH SPORE in prostate cancer grant P50 CA69568, Cancer Center support grant P30 CA46592, South West Oncology Group CA32102, and the Prostate Cancer Foundation.
References
- Walczak JR, Carducci MA. Prostate cancer: a practical approach to current management of recurrent disease. Mayo Clin Proc. 2007;82:243–249. doi: 10.4065/82.2.243. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. New strategies for the medical treatment of prostate cancer. BJU Int. 2005;96(Suppl 2):35–40. doi: 10.1111/j.1464-410X.2005.05945.x. [DOI] [PubMed] [Google Scholar]
- Keller ET, Dai J, Escara-Wilke J, Hall CL, Ignatoski K, Taichman RS, Keller J. New trends in the treatment of bone metastasis. J Cell Biochem. 2007;102:1095–1102. doi: 10.1002/jcb.21540. [DOI] [PubMed] [Google Scholar]
- Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol. 2005;23:8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058–4060. doi: 10.1210/endo.137.9.8756585. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Neale S, Fujikawa Y, McGee JO, Athanasou NA. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62:527–531. doi: 10.1007/s002239900473. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Nakahama K, Sato T, Tuchiya T, Asakawa Y, Maemura T, Tanaka M, Morita M, Morita I. The role of Mac-1 (CD11b/CD18) in osteoclast differentiation induced by receptor activator of nuclear factor-kappa B ligand. FEBS Lett. 2008;582:3243–3248. doi: 10.1016/j.febslet.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Teti A, Rucci N. Osteoclast receptors and signaling. Arch Biochem Biophys. 2008;473:147–160. doi: 10.1016/j.abb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med. 2005;83:170–179. doi: 10.1007/s00109-004-0612-6. [DOI] [PubMed] [Google Scholar]
- Li P, Schwarz EM, O'Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50:265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- Kim MS, Day CJ, Selinger CI, Magno CL, Stephens SR, Morrison NA. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J Biol Chem. 2006;281:1274–1285. doi: 10.1074/jbc.M510156200. [DOI] [PubMed] [Google Scholar]
- Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, Swain F, Ramani V, Mohammad KS, Wessner LL, Martinez A, Guise TA, Chirgwin JM, Gaddy D, Suva LJ. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-[kappa]B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J Biol Chem. 2007;282:33098–33106. doi: 10.1074/jbc.M611781200. [DOI] [PubMed] [Google Scholar]
- Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ, Pienta KJ, McCauley LK. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer. 2008;123:2267–2278. doi: 10.1002/ijc.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]


