Abstract
While it is not uncommon to form chiral crystals during crystallization, the formation of bulk porous homochiral materials from achiral building units is rare. Reported here is the homochiral crystallization of microporous materials through the chirality induction effect of natural alkaloids. The resulting material possesses permanent microporosity and has a uniform pore size of 9.3Å.
Homochirality is an essential feature of life and is essential to many biological processes such as self-replication. Despite its great importance, the generation of homochirality or the understanding of its mechanism remains one of the most challenging tasks. There is therefore a great need to study the generation of homochiral materials.
Recently, there has been an increasing interest in creating homochiral porous materials that may be utilized for enantioselective catalysis, separation etc.1,2 Most homochiral porous solids prepared so far consist of enantiopure organic ligands that impart homochirality to the resulting crystals.3–4 The synthesis of these materials can be considered as a "chirality conservation" process with the homochirality of the products coming from the incorporation of enantiopure starting materials.
In the absence of enantiopure building blocks, the chirality can also be generated from achiral precursors through crystallization.5 Numerous examples are known in which achiral components crystallize in chiral symmetry. In these crystals, chirality results from the spatial organization (e.g., helix) of achiral components. It is worth noting that individual crystals can be homochiral through a process called spontaneous resolution.5 However, the bulk sample tends to be a conglomerate, an equal mixture of crystals with opposite handedness.
The bulk homochiral crystallization from achiral precursors is an unusual and yet highly desirable process. Several well-known examples of homochiral crystallization (called symmetry breaking if no chiral source is present) from achiral precursors are known.6 In these examples, each experiment can lead to bulk homochiral crystals with a particular handedness, however, chirality control from run to run is generally not possible.
To develop a more predictable method for homochiral crystallization, it can be advantageous to employ an additional chiral source to induce a given handedness. The seeding during crystallization is a well-established procedure.5 However, it is generally not usable for exploratory synthesis involving homochiral crystals of unknown composition and structure. Recently, in an unusual discovery, Morris et al demonstrated the homochiral crystallization of an interesting compound in which the absolute configuration is controllable through the chirality of the chiral ionic liquid solvent containing L-asparate.7
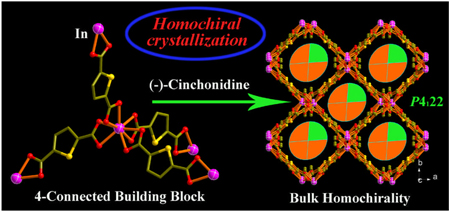
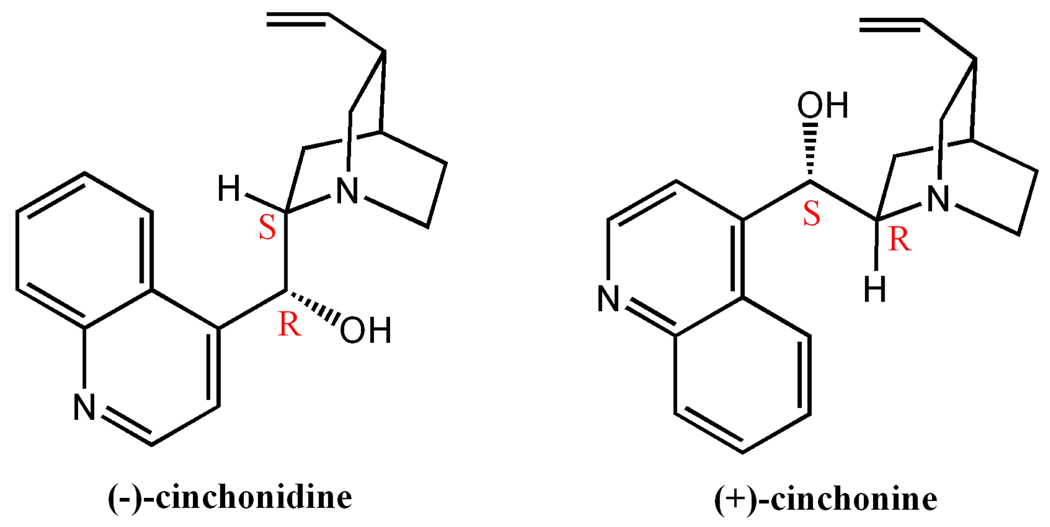
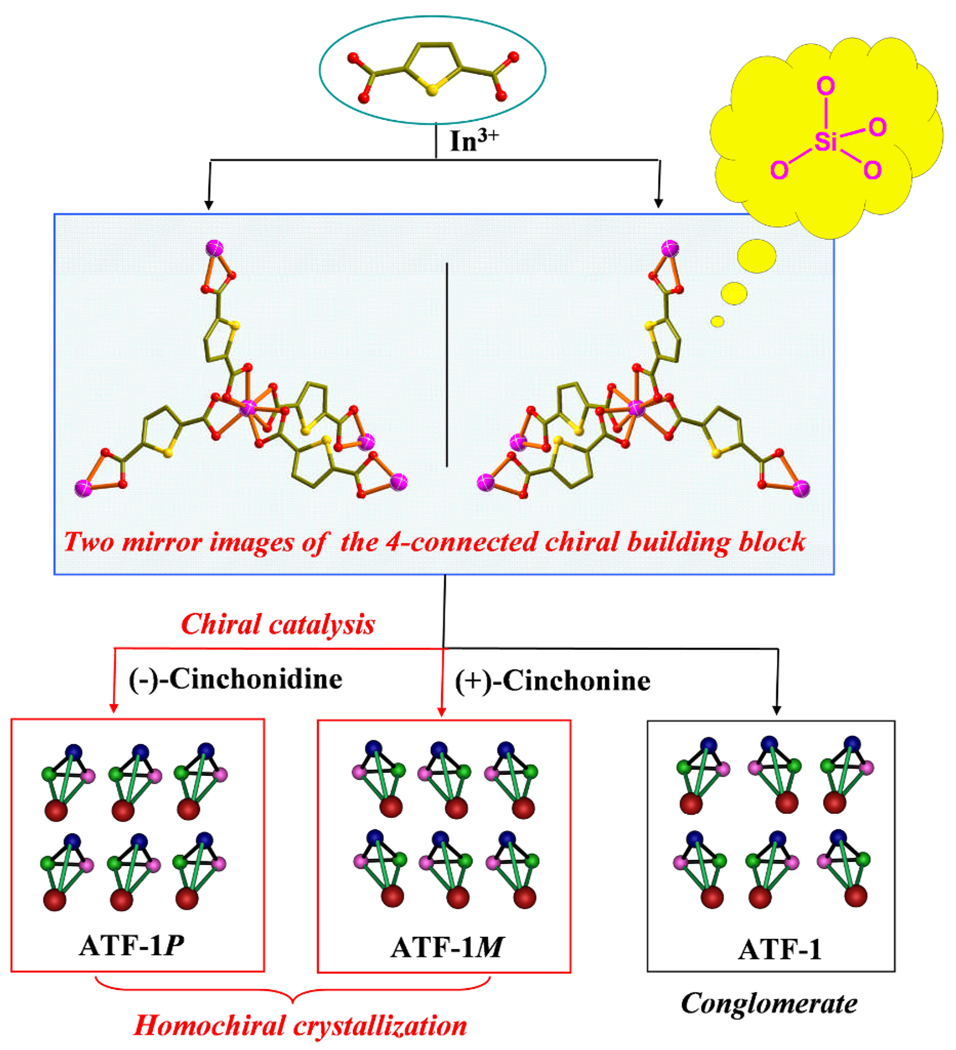
In this work, instead of using chiral solvents, we explore the chirality induction effect of spectator chiral solutes. We found that the use of alkaloids such as (−)-cinchonidine or (+)-cinchonine (Scheme 1) can induce homochiral crystallization from achiral precursors. The addition of a small amount of (−)-cinchonidine or (+)-cinchonine is enough to create the chiral catalytic effect that leads to the growth of homochiral crystals, (Me2NH2)[In(thb)2]˙x(DMF) (denoted ATF-1, ATF-1P or ATF-1M; ATF = Anionic Tetrahedral Iramework; P and M denote the handedness, H2thb = thiophene-2,5-dicarboxylic acid; DMF = dimethylformamide). These materials are rare very negatively charged chiral metal-organic frameworks that exhibit permanent microporosity.
Scheme 1.
Two naturally occurring alkaloids used as the catalysts.
Solvothermal reactions of In(NO3)3 ˙xH2O, H2thb with (or without) cinchonidine or cinchonine in two different solvents (1, DMF; 2, DEF = diethylformamide) leads to five different materials (Table 1). In all of them, the basic coordination chemistry is the same. Each In3+ site is 4-connected despite its 8-coordinate geometry, because each In3+ site is bonded to four thb ligands and each carboxylate group chelates to just one In3+ site (Figure 1).
Table 1.
A Summary of Crystal Data and Refinement Results.
| Formula | Space group | a, b (Å) | c (Å) | R(F) | wR2 | Flack parameter | |
|---|---|---|---|---|---|---|---|
| ATF-1P* | (Me2NH2)[In(thb)2]˙x(DMF) | P4122 | 13.565(5) | 15.503(13) | 0.0488 | 0.1145 | −0.004(61) |
| ATF-1M* | (Me2NH2)[In(thb)2]˙x(DMF) | P4322 | 13.5590(1) | 15.6782(2) | 0.0392 | 0.1457 | 0.014(64) |
| ATF-1 | (Me2NH2)[In(thb)2]˙x(DMF) | P4122 | 13.5770(4) | 15.5394(9) | 0.0378 | 0.0992 | 0.50(5) |
| ATF-2P* | (Et2NH2)[In(thb)2] ˙x(DEF) | P4122 | 13.5100(1) | 16.0684(3) | 0.0526 | 0.1788 | 0.035(86) |
| ATF-2 | (Et2NH2)[In(thb)2] ˙x(DEF) | P4322 | 13.5065(2) | 15.9076(4) | 0.0602 | 0.1916 | 0.55(10) |
H2thb = thiophene-2,5-dicarboxylic acid
superscript “*” indicates these materials were synthesized by using chiral catalysis.
Figure 1.
Schematic representation of the generation of conglomerate (ATF-1) or bulk homochirality (ATF-1P and ATF-1M) induced by (−)-cinchonidine or (+)-cinchonine from the basic 4-connected building block with achiral precursors.
Each distorted tetrahedral node In(COO−)4 in ATFs is a chiral building block (Figure 1) because of the spatial arrangement of ligands around In3+. The C-In-C angles of the In(COO−)4 unit are 99.4, 93.8, 135.3, 100.58, 135.3, 99.4 ° for ATF-1P. The absolute chirality of such a chiral building block may be affected by the presence of alkaloids, which may be the basis of the chirality induction effect reported here.
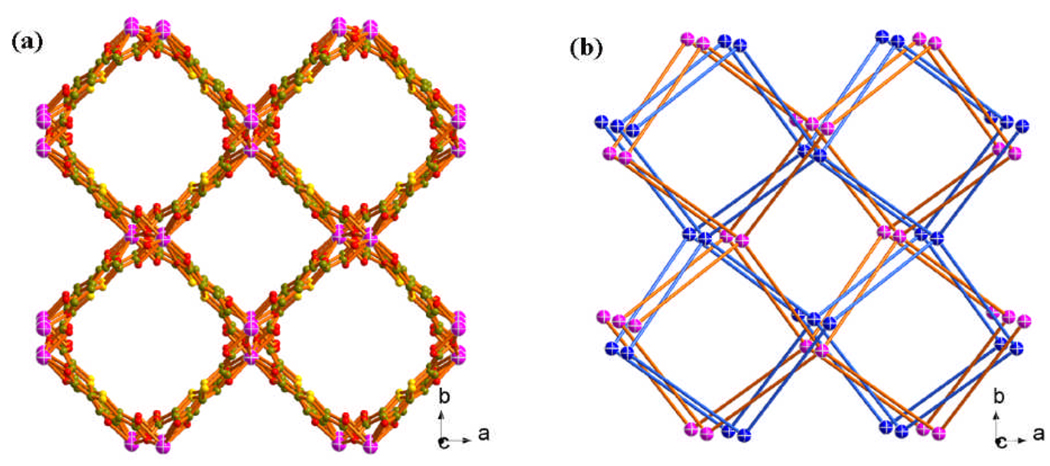
Each thb ligand links two In3+ sites with the In-thb-In angle of approximately 156 °, which is a little larger than the typical Si-O-Si angle in zeolites (about 145 °). The framework features of all ATFs are characteristic of the 4-connected net with the AX2 formula. All these materials have the 2-fold interpenetrating diamond-type anionic framework (Figure 2a and 2b). The negative framework charge is balanced by protonated amine cations such as Mt2NH2 + or Et2NH2 +.
Figure 2.
The 3D open framework (a) and the 2-fold interpenetrating diamond net (b) of ATF-1P.
ATF-1P was prepared by using (−)-cinchonidine as a co-solute and it crystallized in the P4122 space group. Crystal structures of nine randomly and consecutively selected crystals were refined using single-crystal X-ray diffraction data and all of them belong to the P4122 space group with the Flack parameters near zero, suggesting that the P4122 (rather than P4322) is the preferred configuration (Table S1) in the bulk sample. Without the chirality induction effect, such an observation is highly unlikely.
Similarly, chirality induction effect was observed with (+)-cinchonine, which results in the formation of ATF-1M with the opposite handedness in the P4322 space group (Table S2). In comparison, ATF-1 was prepared without the use of any alkaloid. The resulting crystals tend to be racemic twins (Table S3). This demonstrates the chiral catalytic effect of (−)-cinchonidine and (+)-cinchonine, which results in the predominance of one chiral form (P4144 or P4322) over the other one in the bulk sample. The enantioselectivity of chiral cosolutes such D-mannitol and D-sorbitol on crystallization of chiral crystals such as NaClO3 was reported earlier,8 but we are not aware of any similar studies on the enantioselectivity of chiral porous crystals.
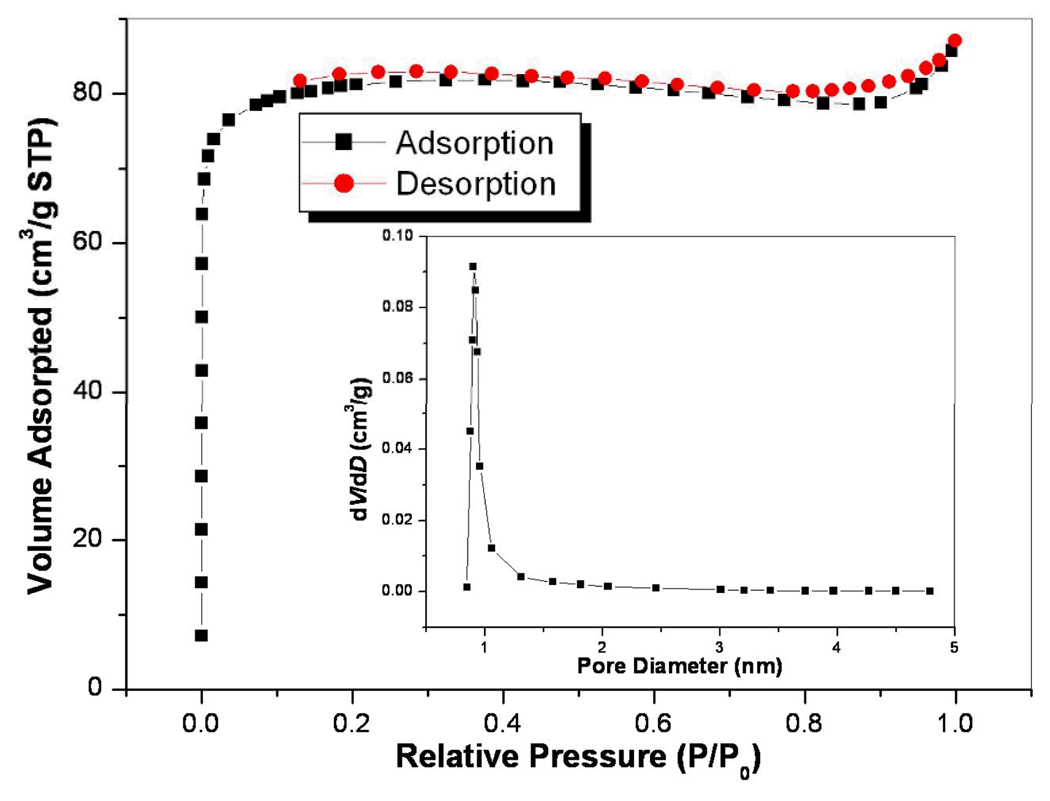
Although there are two interpenetrating nets in ATF-1, it still possesses two types of large square channels along the c axis. In the as-synthesized form, these large channels are filled with amine cations and disordered solvent molecules (Figure 2a). The solvent-accessible volume is 1442.2 Å3 per unit cell volume and the pore volume ratio is 50.2 % as calculated with the PLATON program.9 The permanent porosity of ATF-1 was confirmed by gas adsorption measurements performed on Micromeritics ASAP 2010 surface area and pore size analyzer (Figure 3). The sample was degassed at 150°C prior to the measurement. A type I isotherm was observed, indicating that ATF-1 is microporous. The Langmuir surface area of 360.3m2/g was calculated by using the data in the range of P/P0= 0.072–0.204. A single data point at relative pressure 0.204 gives a micropore volume of 0.126cm3/g by Horvath-Kawazoe equation. The median pore size of 9.3Å was also calculated.
Figure 3.
Nitrogen sorption isotherm at 77K. P/P0 is the ratio of gas pressure (P) to saturation pressure (P0), with P0=740 torr. Insert: the pore size distribution curve.
Supplementary Material
Acknowledgment
We thank the support of this work by NIH (X. B. 2 S06 GM063119-05) and NSF (P. F.).
Footnotes
Supporting Information Available: Experimental details, figures, TGA and XRD diagrams, and CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Guillou N, Livage C, Drillon M, Férey G. Angew. Chem. Int. Ed. 2003;42:5314. doi: 10.1002/anie.200352520. [DOI] [PubMed] [Google Scholar]; (b) Férey G. Chem. Soc. Rev. 2008;37:191. doi: 10.1039/b618320b. [DOI] [PubMed] [Google Scholar]; (c) Kepert CJ, Prior TJ, Rosseinsky MJ. J. Am. Chem. Soc. 2000;122:5158. [Google Scholar]; (d) Bradshaw DB, Prior TJ, Cussen EJ, Claridge JB, Rosseinsky MJ. J. Am. Chem. Soc. 2004;126:6106. doi: 10.1021/ja0316420. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cao G, Garcia ME, Alcalá M, Burgess LF, Mallouk TE. J. Am. Chem. Soc. 1992;114:7574. [Google Scholar]; (b) Garibay SJ, Stork JR, Wang Z, Cohen SM, Telfer SG. Chem. Commun. 2007:4881. doi: 10.1039/b712118k. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kesanli B, Lin W. Coord. Chem. Rev. 2003;246:305. [Google Scholar]; (b) Wu CD, Lin W. Angew. Chem. Int. Ed. 2005;44:1958. doi: 10.1002/anie.200462711. [DOI] [PubMed] [Google Scholar]; (c) Wu C-D, Hu A, Zhang L, Lin W. J. Am. Chem. Soc. 2005;127:8940. doi: 10.1021/ja052431t. [DOI] [PubMed] [Google Scholar]
- 4.(a) Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. doi: 10.1038/35010088. [DOI] [PubMed] [Google Scholar]; (b) Vaidhyanathan R, Bradshaw D, Rebilly J-N, Barrio JP, Gould JA, Berry NG, Rosseinsky MJ. Angew. Chem. Int. Ed. 2006;45:6495. doi: 10.1002/anie.200602242. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-García L, Amabilino DB. Chem. Soc. Rev. 2002;31:342. doi: 10.1039/b201099m. [DOI] [PubMed] [Google Scholar]
- 6.(a) Sun Q, Bai Y, He G, Duan C, Lin Z, Meng Q. Chem. Commun. 2006:2777. doi: 10.1039/b604066g. [DOI] [PubMed] [Google Scholar]; (b) Wu S-T, Wu Y-R, Kang Q-Q, Zhang H, Long L-S, Zheng Z, Huang R-B, Zheng L-S. Angew. Chem. Int. Ed. 2007;46:8475. doi: 10.1002/anie.200703443. [DOI] [PubMed] [Google Scholar]; (c) Ezuhara T, Endo K, Aoyama Y. J. Am. Chem. Soc. 1999;121:3279. [Google Scholar]; (d) Kondepudi DK, Kaufman RJ, Singh N. Science. 1990;250:975. doi: 10.1126/science.250.4983.975. [DOI] [PubMed] [Google Scholar]
- 7.Lin Z, Slawin AMZ, Morris RE. J. Am. Chem. Soc. 2007;129:4880. doi: 10.1021/ja070671y. [DOI] [PubMed] [Google Scholar]
- 8.Pagni RM, Compton RN. Crystal Growth & Design. 2002;2:249. [Google Scholar]
- 9.Spek AL. Acta Crystallogr. Sect. A. 1990;46:C34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.