Abstract
The importance of reactive oxygen species (ROS) in innate immunity was first recognized in professional phagocytes undergoing a “respiratory burst” upon activation. This robust oxygen consumption is related to a superoxide-generating enzyme, the phagocytic NADPH oxidase (Nox2 or phox). The oxidase is essential for microbial killing, since patients lacking a functional oxidase suffer from enhanced susceptibility to microbial infections. ROS derived from superoxide attack bacteria in the isolated niche of the neutrophil phagosome. The oxidase is electrogenic, alters ion currents across membranes, induces apoptosis, regulates cytokine production, influences gene expression, and promotes formation of extracellular traps. Recently, new homologues of Nox2 were discovered establishing the Nox family of NADPH oxidases that encompasses seven members. Nox1 is highly expressed in the colon epithelium, and can be induced by LPS or IFN-γ. Nox4 was implicated in innate immunity since LPS induces Nox4-dependent ROS generation. Duox1 and Duox2 localize to the apical plasma membrane of epithelial cells in major airways, salivary glands, and the gastrointestinal tract, and provide extracellular hydrogen peroxide to lactoperoxidase to produce antimicrobial hypothiocyanite ions. Th1 and Th2 cytokines regulate expression of Dual oxidases in human airways and may thereby act in host defense or in proinflammatory responses.
Introduction
Reactive oxygen species (ROS) are a group of chemically reactive ions, radicals and molecules derived from oxygen. The spectrum of functions they participate in ranges from hormone biosynthesis to cell signaling and aging to microbial killing. ROS can originate from mitochondria as by-products of the mitochondrial respiratory chain, however this process is highly unspecific. The Nox/Duox protein family represents well-defined, controllable, and dedicated sources of two reactive oxygen species: superoxide and hydrogen peroxide [1–3]. These basal forms can be transformed into other ROS. Oxidative defense mechanisms involving ROS produced by these enzymes have important roles in innate immunity, as well [4]. Members of this family have existed since the divergence of the animal and plant kingdoms, and have served ancient functions in innate immunity that arose probably since the development of multi-cellular life forms. Much information has accumulated in the past 80 years about the first-recognized role of ROS in innate immunity (killing of microbes by phagocytes) [5], although only recently has the expression of these ROS generators in a broad range of cell types in the human body been appreciated. Despite these many years of intense investigation, the principles behind ROS-dependent killing processes are still subjects of passionate discussions, and a wide range of new possibilities regarding novel roles of Nox/Duox proteins in innate immune defenses have emerged recently. In this chapter we summarize the current knowledge about the innate immune functions of reactive oxygen species produced by the Nox/Duox family proteins.
Structural and functional properties of the members of the Nox/Duox protein family
The phenomenon of robust oxygen consumption upon activation of neutrophils by a range of stimuli is commonly referred to as the “respiratory burst”, which is required for efficient microbial killing. This burst has nothing to do with mitochondrial respiration, as it involves the direct reduction of molecular oxygen by one electron and production of superoxide anions as a consequence. The reaction is catalyzed by a protein complex called NADPH oxidase or the phagocyte oxidase. The enzyme converts cytoplasmic NADPH into NADP+ by liberating two electrons and one proton. The proton remains in the cytoplasm whereas the two electrons are transported through the plasma/phagosomal membrane and bind to two oxygen molecules resulting in the formation of two superoxide anions in the extracellular or intraphagosomal space. The central component of the complex is the flavocytochrome b558, a membrane-embedded heterodimer consisting of a larger glycoprotein (gp91phox, product of the CYBB gene, located on chromosome X) and a smaller, 22 kD protein (p22 phox, product of CYBA gene) (Fig. 2.). Four cytosolic components belong to the complex: p47phox, p67phox, p40phox and Rac1 or Rac2. Gp91phox (or Nox2, according to the new nomenclature of this oxidase family) possesses the entire electron transport machinery [1–3]. The cytoplasmic C-terminal portion has binding sites for FAD and NADPH (Fig. 1.). The hydrophobic N-terminal part of gp91phox contains six transmembrane helices. The third and fifth helices each have two histidine residues that anchor two heme prosthetic groups between these helices within the lipid bilayer. These histidine residues are well conserved in all Nox family members and are essential for trans-membrane electron transport. The electrons obtained from NADPH are passed first to FAD, then to the two heme groups sequentially, and finally on to molecular oxygen on the extracellular or intraphagosomal side of the membrane. The extracellular loops II and III contain N-linked glycosylation sites.
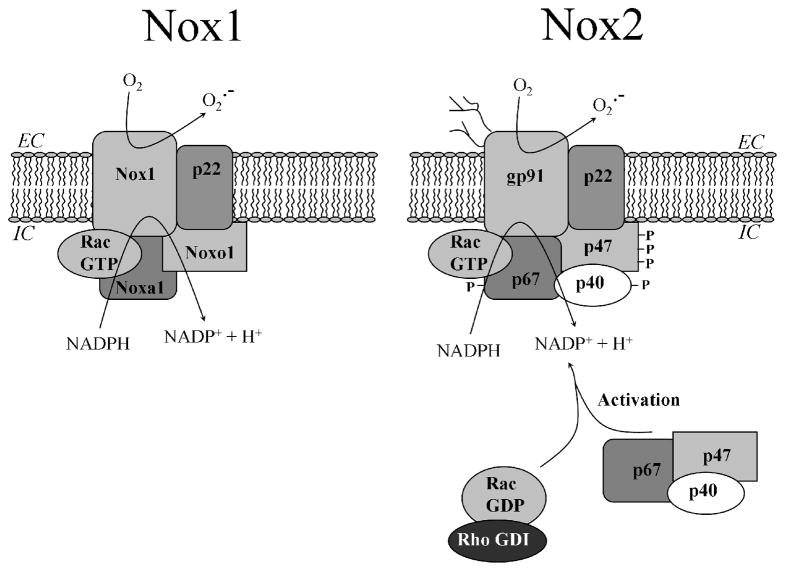
Figure 2. Activated complexes of multi-component Nox1- and Nox2-based NADPH oxidases.
The Nox1 and Nox2(gp91phox) flavocytochromes form heterodimeric complexes with a common p22phox chain. Both oxidases are regulated by homologous organizer (Noxo1 or p47phox) and activator (Noxa1 or p67phox) proteins, and require GTP-bound Rac. The cytosolic subunits of Nox2 (p47phox, p67phox and p40phox) are preassembled in the cytosol and translocate to the flavocytochrome upon activation. In resting cells, Rac is found in a GDP-bound state stabilized by RhoGDI. When activated, both oxidases produce superoxide anions. Nox1 is localized to the plasma membrane of colon epithelial cells and produces superoxide into the extracellular space, whereas Nox2 is assembled and activated on phagosomes of phagocytic cells.
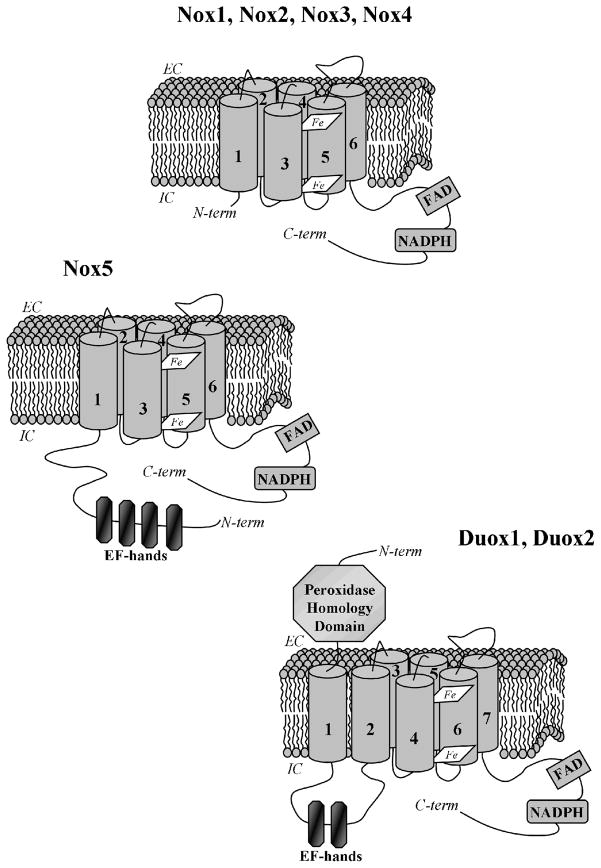
Figure 1. Structures of Nox/Duox family NADPH oxidases.
All NADPH oxidase catalytic components have similar structural elements required for transmembrane electron transfer from the cytosol to molecular oxygen: the C-terminal intracellular (IC) tails containing NADPH and FAD-binding sites and six transmembrane segments anchoring two heme groups. In addition, Nox5 has an N-terminal extension containing four EF-hands responsible for calcium binding. The dual oxidases have an additional transmembrane helix, an extracellular (EC) N-terminal domain with peroxidase homology, and two EF-hands within their first intracellular loop.
Research on the genetic causes of the immune defect called chronic granulomatous disease (CGD) has advanced our understanding of the function of the Nox2 system. In CGD the NADPH oxidase is defective because of mutations in genes encoding in any one of four essential subunits of the complex [5]. Because of the diminished superoxide production, these patients are subject to recurrent bacterial and fungal infections. In about two thirds of cases, the gene for gp91phox (Nox2), located on the X chromosome, is affected, leading to disease development almost exclusively in males. Defects or deficiencies in three other subunits account for the remaining 30% of CGD cases (p47phox: 20%, p67phox: 5% and p22phox: 5%, respectively), although there are no reports to date of mutations in p40phox associated with CGD. A dominant negative point mutation in Rac2 causing oxidase defects has also been recognized in one patient, confirming its essential role in NADPH oxidase function.
The smaller subunit of the flavocytochrome, p22phox consists of 194 amino acids. It functions on the one hand in providing a docking site for the cytosolic subunits by binding p47phox (Fig. 2.), while on the other hand in subcellular targeting and stabilizing the flavocytochrome heterodimer [5, 6]. The cytosolic components contain several modular domains, including Src homology (SH3) domains, tetratricopeptide repeat (TPR) motifs, Phox and Bem 1 (PB1) domains, phox homology (PX) domains and proline-rich motifs, capable of intra- and intermolecular protein-protein and protein-lipid interactions. These domains are crucial in binding of the cytosolic subunits to each other and to the membrane-bound components, thereby promoting assembly of the functional oxidase. In resting neutrophils the three cytosolic phox subunits form a cytosolic complex (Fig. 2.). p47phox is made up by 390 amino acids and is heavily phosphorylated during activation, which is required to disrupt autoinhibitory interactions, thereby enabling binding to p22phox and membrane lipids. It binds to p22phox, gp91phox and p67phox, promoting interactions of p67phox with the flavocytochrome. p67phox contains 526 amino acids, is phosphorylated and binds GTP-Rac upon activation, thereby promoting electron flow through the flavocytochrome. Both, p40phox and p47phox harbor PX domains that bind to activated phosphatidylinositol-phosphates in activated membranes of neutrophils. p40phox binds to p67phox (which does not contain PX domains) and is involved in stabilizing associations of p47phox and p67phox with the phagosomal membrane. Rac translocates to the membrane during activation, independently from the other three components (Fig. 2.). In resting cells, Rac is maintained in an inactive cytoplasmic complex with guanine nucleotide dissociation inhibitor (GDI). Upon activation, they dissociate and Rac is transported to the membrane, following GDP to GTP exchange promoted by a guanine nucleotide exchange factors (GEFs) activated by PI(3,4,5)P3 and G-proteins.
With the rapid expansion of human genome sequence databases in the late 1990’s, investigators soon appreciated that Nox 2 was representative of an entire NADPH oxidase (Nox/Duox) family that includes six other homologues of Nox2 produced in a variety of human tissues: Nox1, Nox3, Nox4, Nox5, Duox1 and Duox2. Their structures and functions have been reviewed recently [1–3]. All these oxidases share common structural and functional features that were well-characterized in studies on the prototype, Nox2: all have conserved NADPH and FAD binding sites in their C-terminal domain, and two membrane-embedded heme moieties (Fig. 1.). Each Nox/Duox family member is capable of consuming NADPH and transporting electrons through the membrane. The five Nox enzymes produce superoxide anion, whereas the two Duox enzymes (derived from dual oxidase) produce hydrogen peroxide. The structure of Nox1, Nox3 and Nox 4 are very similar to Nox2, having six transmembrane helices and a C-terminal flavodomain, while Nox5 has an additional, cytosolic N-terminal portion containing four calcium-binding EF-hand motifs. Duox1 and Duox2 have an additional transmembrane segment and an extracellular, N-terminal domain showing significant peroxidase homology (Fig. 1.).
Nox1 is the first new member of the family and the closest homologue of Nox2, sharing 56% sequence identity (564 amino acids). In addition to their structural similarities, Nox1 and Nox2 show striking functional similarities. As in the case of Nox2, Nox1 requires p22phox and Rac1 for complete activity (Fig. 2.). In cellular models of X-linked CGD, Nox1 can cross-function in replacing Nox2 for ROS production, although co-transfection of Nox2 cytosolic subunits, p47phox and p67phox, with Nox1 results in lower superoxide production. Novel homologues of the cytosolic subunits capable of supporting higher Nox1 activity were discovered: Noxo1 (Nox organizer 1, the homologue of p47phox) and Noxa1 (Nox activator 1, the homologue of p67phox) (Fig. 2.) [7]. The most significant structural difference between Noxo1 and p47phox is that Noxo1 lacks sequence homologous to the autoinhibitory region of p47phox that becomes hyperphosphorylated and allows binding to p22phox and membrane phospholipids. Thus, the Nox1/Noxo1/Noxa1 system differs from the Nox2/p47phox/p67phox system in that it is less subject to tight controls and exhibits significant constitutive activity, which is further enhanced by phorpbol-12-myristate-13-acetate (PMA). The activity of Nox1 in reconstituted systems is influenced by Rac1 or Rac-binding Noxa1 mutants, or by Rac1-targeted RNA interference. Rac1 binds to Noxa1, GTP is required for this binding, and Rac1 may thereby control Nox1 activity in a way similar to that seen with Nox2 (Fig. 2.).
Nox3 is also a multi-component oxidase having similar structure to Nox2 (Fig. 1.). Nox3 has a high, basal activity when expressed alone in heterologous systems, which can be further enhanced by expressing either one or both of the Nox1 or Nox2 supportive cytosolic partners. So far, no role of Nox3 in innate immunity has been suggested, although Nox3 is considered critical in gravity and balance perception, based on the effects on Nox3 mutations in mice. Noxo1 mutations were also identified that cause a similar imbalance phenotype, therefore Noxo1 is considered the likely physiological partner of Nox3.
Nox4 shares 39% homology with Nox2. Nox4 requires p22phox for stabilization and ROS production, but its activity is not altered by any of the other Nox1 or Nox2 cytosolic partners, Noxo1, Noxa1, p47phox, p67phox, or Rac. Nox4 exhibits high constitutive activity. In HEK293 cells, Nox4 is retained within intracellular compartments, thus any superoxide produced intracellularly dismutates rapidly before being detected as extracellular hydrogen peroxide. Originally, Nox4 was described in the kidney [8], where it is most abundant, but it was found also in many other tissues and cell types of the human body: osteoclasts, fibroblasts, fetal tissues, hepatocytes and vascular cells. The wide tissue distribution of Nox4 suggests very diverse functions of this enzyme ranging from oxygen sensing to fibrotic processes. There are data accumulating that suggest roles for Nox4 in innate immunity as well.
Nox5 is a unique member of the family since it has an extra cytoplasmic domain containing four EF-hand motifs (Fig. 1.). Elevations in intracellular calcium concentrations are enough to activate Nox5 through binding of calcium ions to the EF-hands. Nox5 is mostly expressed in pachytene spermatocytes of testis, spleen and lymph nodes. In the latter two, it is expressed in lymphocyte regions of the organs and might have signaling roles in lymphocytes. Surprisingly, Nox5 was lost from the murine genome. No roles for Nox5 in innate immune functions have been proposed to date.
Duox 1 and Duox 2 are the largest members of the family with a molecular weight of around 180 kD; they share 83% sequence similarity. Their names derive from “dual oxidase” because they possess an extracellular peroxidase-like domain in addition to their C-terminal Nox-like portions (Fig. 1.). Although their ectodomains show extended sequence similarities with other peroxidases, and peroxidase function has been suggested, critical heme-binding residues are absent and no heme is detected in the expressed proteins (unpublished), which is an essential requisite for peroxidase activity. The first cytosolic loop of Duox upstream of the Nox-like portion contains two EF-hands, consistent with the activation of H2O2 release by calcium-mobilizing agonist. The dual oxidases were first identified in the thyroid gland as the primary sources of H2O2 in the thyroid follicle [9]. The hydrogen peroxide produced by Duox2 is essential for organification of iodide and thyroid hormone biosynthesis, since biallelic lesions in Duox2 lead to severe hypothyroidism [10]. The role of Duox1 in the thyroid is still unclear despite its high expression in this tissue.
Attempts to express reconstituted active dual oxidases in heterologous systems were unsuccessful for some time because of incomplete processing and failure to transport these proteins to their final destination, the plasma membrane. Recently, this mystery was solved with the discovery of essential maturation factors, Duox activator 1 (Duoxa1) and Duox activator 2 (Duoxa2), required for full processing of Duox to the plasma membrane. All four genes (Duox1/2 and Duoxa1/2) are localized within a compressed genomic region on chromosome 15, which suggests that expression of the oxidases and their corresponding maturation factors may be coordinated by the same bidirectional promoter sequences. In addition to their high thyroid expression, Dual oxidases are expressed on epithelial surfaces of the airways, salivary gland ducts, and along the digestive tract of the human body [11]. This extrathyroid expression pattern has prompted the interest of several groups exploring the proposed innate immune function of Duox on mucosal surfaces as a source of extracellular hydrogen peroxide that can support the antimicrobial activity of lactoperoxidase [11, 12].
Oxygen metabolites
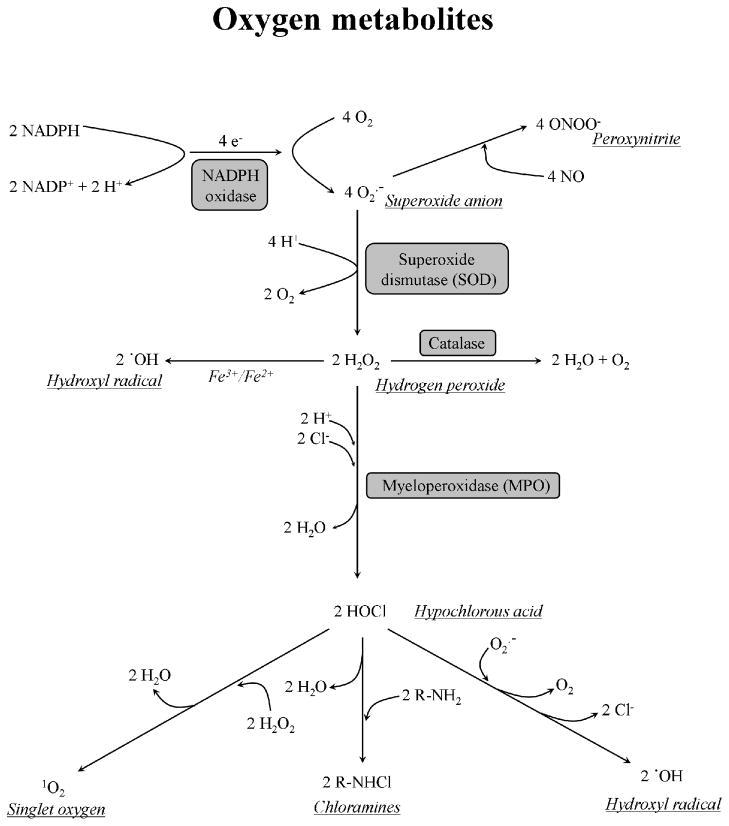
A common feature of the Nox/Duox family oxidases is the consumption of NAD(P)H and oxygen, transport of electrons via FAD and two heme moieties. Nox1-5 produce superoxide anions whereas the dual oxidases appear to produce hydrogen peroxide as a primary product (superoxide is barely detectable). Superoxide or hydrogen peroxide are transformed further into other derivatives, either spontaneously or catalyzed by pro- and antioxidant enzymes (see Fig. 3). All of these metabolites have in common that they originate from oxygen, hence the name reactive oxygen species, intermediates, or metabolites. Oxygen is a reactive molecule that can react with a wide range of elements and organic compounds. Despite this thermodynamic reactivity, oxygen is rather an inert gas from a kinetic point of view [12]. The stability of oxygen is due to the fact that it has to overcome a kinetic barrier to become chemically reactive. This barrier function ensures that oxygen does not react immediately with organic compounds and the cells are not abruptly depleted of oxygen; this is why oxygen is stable in the cells and can reach certain concentrations (partial tensions). Oxygen can be reduced to water by obtaining up to four electrons and four protons: O2 > O2.- > H2O2 > .OH > H2O. The intermediate forms of this reduction chain are more reactive than oxygen. Oxygen can also be excited by absorbing of energy. In this case one unpaired electron of the molecule changes its spin and O2 becomes more reactive. The respiratory burst of neutrophils reduces two oxygen molecules into two superoxide anions by transporting two electrons from one NADPH (Fig. 3.). At neutral pH (as in the phagosome) the unprotonated form of superoxide is dominant. Superoxide anions usually donate an electron and reduce other chemicals, for example cytochrome C. Two superoxide anions can react with each other and result in oxygen and hydrogen peroxide by consuming 2 protons, as the pH increases. This dismutation of superoxide occurs spontaneously and is faster at lower pH values, however the reaction can be catalyzed enzymatically by superoxide dismutases (SOD) (Fig. 3.). Superoxide is believed to be only weakly toxic, as it reacts rather slowly with different biocompounds. Superoxide does not reach very far away from the site of production and it is membrane-impermeable. Hydrogen peroxide is produced during the respiratory burst in tremendous amounts by spontaneous or SOD-mediated dismutation of superoxide. The toxicity of H2O2 is well known, as it reacts quickly with a wide range of biologically important compounds but the derivatives of H2O2 are usually far more reactive. Hydrogen peroxide is membrane-permeable and relatively stable, so it can diffuse away from the site of production. Hydrogen peroxide is more toxic to microorganisms when released in very high concentrations than when produced in a constant but very slow manner. Both, the microbes and neutrophils are exposed to hydrogen peroxide in the phagosome, against which they needed to develop defense mechanisms. Catalases split H2O2 into water and oxygen (Fig. 3.). Another ROS scavenger system is the glutathione cycle. Reduced glutathione (GSH) is oxidized by H2O2 into oxidized glutathione (GSSG). This latter is reduced back to GSH by the glutathione reductase oxidizing NADPH into NADP. The hexose-monophosphate shunt breaks down glucose to CO2 to reduce NADP back to NADPH.
Figure 3. Oxygen metabolites of phagocytes.
A variety of reactive oxygen species are formed inside the phagosome. The phagocyitic NADPH oxidase (Nox2-based) produces intraphagosomal superoxide by consuming cytosolic NADPH and transporting electrons across the membrane. The superoxide anions can be further dismutated into hydrogen peroxide by superoxide dismutase or converted into peroxynitrite by nitric oxide. Catalase dismantles H2O2 into water and oxygen. Myeloperoxidase catalyzes formation of hypochlorous acid (HOCl) from chloride and H2O2. See text for further details.
The toxicity of hydrogen peroxide can be enhanced by different mechanisms [13]. First, by reacting with peroxidases and halides or pseudohalides to form hypohalous acids or pseudohypohalous acids; second, by converting into hydroxyl radical in the presence of ferrous iron (Fenton reaction) (Fig. 3.). A number of peroxidases exist in the body, which differ in terms of structure, synthesis, and localization, but they have one thing in common: they dramatically increase the rate of hydrogen peroxide-dependent reactions. By themselves they are not toxic, but by converting nontoxic compounds using H2O2 into highly reactive, toxic metabolites they become antimicrobial. Preferred peroxidase substrates include halides or pseudohalides that have very little antimicrobial activity, although the hypohalous acids formed are highly reactive, unstable and very toxic. As much as a few percent of the dry weight of neutrophils is made up of myeloperoxidase (MPO). MPO is not expressed in other cell types, except in monocytes. It gives pus its characteristic green color due to the huge amounts of MPO released from neutrophils. MPO appears early during neutrophil development in the bone marrow and in mature neutrophils resides in the primary granules. Upon phagocytosis these granules fuse with the phagosome containing bacteria, so that MPO is released into the phagosomal lumen. MPO is able to catalyze the conversion of Cl-, I-, Br-, and SCN-, but chloride is the most abundant phagosomal substrate, HOCl is the primary MPO product in neutrophil phagosomes (Fig. 4.). The membrane permeable hypochlorous acid reacts rapidly with a wide range of cellular components, as it can 1) chlorinate phenols, amines (chloramines), unsaturated bounds, 2) oxidize iron centers, sulfhydryl groups, heme-proteins, sulfur-ether groups, lipids and 3) cross-link and covalently chlorinate or iodinate proteins. The chloramines are weaker oxidizing agents but last longer. Hydroxyl radical, one of the most reactive oxygen metabolites, is formed by the Fenton-reaction (H2O2+Fe2+>Fe3+ +OH-+.OH), the Haber-Weiss reaction (H2O2+O2.- >(FE)> O2+OH-+.OH) or by the autooxidation of Fe2+ (Fig. 3.) Through different mechanisms singlet oxygen can be produced and is highly reactive and oxidizes a broad range of target molecules. There are other reactive species (CO3.-, HO2., Cl2, Br2, NO., NO2., N2O3, ONOO-, HNO2, O3, ONOOCO2-) formed in the neutrophil phagosome that contribute to microbial killing or, in the case of uncontrolled neutrophil function, damage of our own tissues.
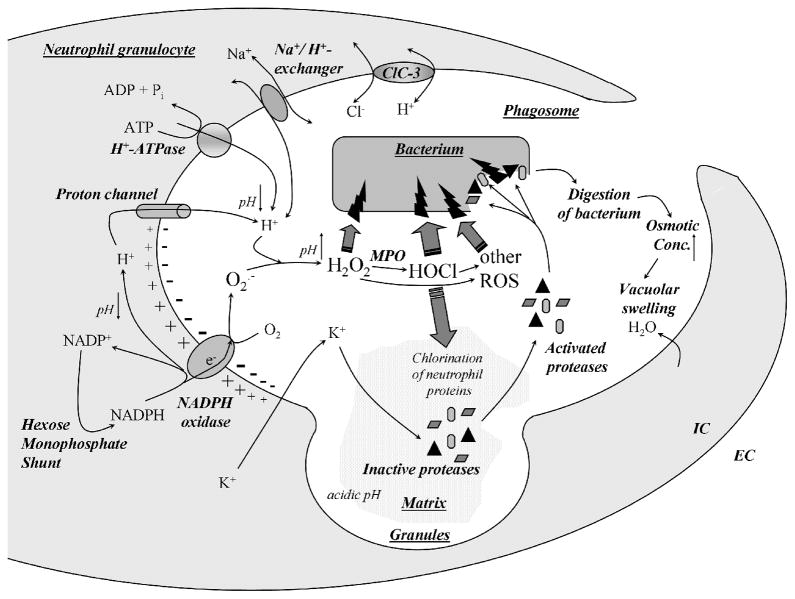
Figure 4. The neutrophil phagosome.
Upon engulfment of bacteria into the neutrophil phagosome, granules fuse with the phagosome and the phagocytic NADPH oxidase is assembled and activated on the phagosomal membrane. By transporting electrons from cytosolic NADPH, the phagosomal membrane depolarizes as superoxide is produced in the phagosome lumen. Superoxide gives rise to the whole spectrum of reactive oxygen species that are highly reactive and attack bacteria. The Nox2-generated depolarization drives protons and potassium ions into the phagosome. The protons maintain a neutral pH in the phagosome required for optimal protease activity and sustained oxidase function. Potassium ions liberate and activate latent proteases from the granule matrix, allowing them to attack and destroy bacteria.
Many of the highly toxic ROS described above are normally contained within intracellular compartments, such as the phagosome, where they can serve as effective microbicidal agents while inflicting minimum damage to host tissues. However, several of the novel Nox family members are expressed at high levels in epithelial cells and are aimed towards extracellular compartments (i.e., mucosal surfaces) [4]. In such cases, the spectrum of ROS generated is limited, generally less toxic, and less able to cause damage to tissues. In the thyroid follicle, Duox2 delivers hydrogen peroxide, not superoxide, from the apical surface of thyrocytes, where it forms a complex with thyroperoxidase. This system is designed to iodinate tyrosine residues on thyroglobulin within the confines of the thyroid follicle. On mucosal surfaces, Duox1 and Duox2 were proposed to function in partnership with lactoperoxidase (LPO), which is a well known antimicrobial enzyme in many exocrine secretions (milk, saliva, airway surface liquid, tears) [11]. The predominant LPO substrate oxidized by hydrogen peroxide in these secretions is the pseudohalide thiocyanate (SCN-; 100–500 micromol/liter), which is oxidized into a sulfhydryl-reactive hypothiocyanite (OSCN-) ion. LPO does not use Cl-, nor generate OCl-, which is less stable and more toxic than OSCN-. OSCN- is abundant enough in saliva and other mucosal secretions to act as an effective microbicidal or microbistatic agent, while being less toxic to cells than HOCl or hydrogen peroxide. Indeed, LPO concentrations in these secretions are also high enough to maintain hydrogen peroxide concentrations at low levels, thus LPO also serves as a protective antioxidant enzyme in these secretions.
Nox 2
Neutrophils are highly specialized professional phagocytic cells. Their essential function is to detect and migrate towards microbial intruders, and then phagocytose and kill them. Mature neutrophils are equipped with a broad range of antimicrobial agents, peptides and enzymes (among them is the NADPH oxidase) that are stored in their granules and are released into the phagosome. Only a certain subset of these antimicrobial weapons is effective against certain microbes, not all of them. Activation of the phagocyte oxidase is required for efficient killing of certain pathogens (Staphylococcus aureus, Burkholderia cepacia, Aspergillus fumigatus), whereas it is not needed to eradicate most of microbes. This is proven by CGD neutrophils that are able to fight successfully against most of their intruders; killing of just a few species mentioned above is impaired, which shows that the Nox2-mediated oxidative defenses are efficient against only certain bacteria and fungi [5].
In resting neutrophils most of the Nox2 proteins is localized primarily to secondary granules and only a small fraction to the plasma membrane [6]. Upon activation, secondary granules fuse with plasma and phagosomal membranes. Nox2 is expressed in other phagocytes besides neutrophils (eosinophils, monocytes/macrophages) and non-phagocytic cells (fibroblasts, cardiomyocytes, hematopoietic stem cells, and endothelial cells). Many different stimuli are capable of activating the neutrophil NADPH oxidase. Chemoattractants (fMLF, C5a, LTB4, PAF) and chemokines (IL-8) induce a weak and always transient signal, where superoxide production is aimed outward into the extracellular space. Among them fMLF, a bacterial tripeptide is the most powerful. In the presence of costimulatory molecules (TNF-alfa, IgG), integrins are also able to stimulate superoxide production in attached neutrophils. The stimuli of highest importance are living bacteria. Bacterial cells are phagocytosed by neutrophils, trapped in the phagosome where the NADPH oxidase assembles at the phagosomal membrane and superoxide production occurs inside of the phagosome, not in the extracellular lumen (Fig. 4.). This isolation of superoxide release into the phagosome is important since reactive oxygen species can damage our own tissues too. In the case of phagocytosis, NADPH oxidase activation occurs due to Fc or complement receptors. “Non-physiological” stimuli (PMA, calcium ionophores) can also induce superoxide production, which usually takes much longer and does not have to be initiated through membrane receptors (PMA activates PKC, calcium ionophores increase the intracellular calcium concentration). The initiation of superoxide production involves phosphorylation of p47phox and p67phox and their translocation to the flavocytochrome in the membrane. When assembled, Nox2 is able to start producing superoxide anions.
Priming is a phenomenon caused by certain agonists that do not directly activate the NADPH oxidase by themselves, but can augment superoxide production triggered by other stimuli. Priming agents can be released by bacteria (LPS), endothelial cells, or other cells (TNF-alfa, IFN-γ, GM-CSF), which pre-activate neutrophils to mount a more robust respiratory burst to kill microbes in infected tissues. There are two types of priming agents: those acting rapidly within a few minutes (LTB4, PAF, C5a) and long-acting ones (LPS, TNF-alfa, GM-CSF, IL-18) that need 20–60 min to exert their effects. The proposed mechanism for priming include enhanced phosphorylation of cytosolic components, decreased inhibition of the autoinhibitory region of p47phox, and assembly of p40phox, p47phox and p67phox into complexes in the cytosol before translocation to the membrane [14].
Although much is known about mechanisms of NADPH oxidase activation, less is known about its termination [14]. Desensitization or internalization of activating receptors can be one limiting factor. When the oxidase is switched on, oxygen consumption of neutrophils is augmented by as much as 100-fold. Under hypoxic conditions, limited oxygen accessibility prevents full oxidase activation. With rapid oxygen utilization, NADPH consumption speeds up as well. The cell lacks adequate NADPH stores to cover the needs of the respiratory burst, and new NADPH molecules must be generated by the hexose-monophosphate shunt (HMS). Depleted NADPH pools can also shut down activation of Nox2, as it is the case of glucose-6-phosphate dehydrogenase (G6PD) deficiency, where cells are unable to fully support the needs of the NADPH oxidase. Under 1% of normal G6PD activity, bactericidal capabilities of neutrophils are impaired, leading to CGD-like symptoms in these patients. Extreme membrane depolarization caused by the oxidase can also limit its activity. Other ways of controlling oxidase activity include changes in phosphorylated/dephosphorylated state of the oxidase, reactivation of oxidase components, involvement of the cytoskeleton, disassembly/reassembly of the complex.
In producing superoxide the phagocyte NADPH oxidase transports electrons from the cytoplasm into the extracellular or intraphagosomal space across the membrane. By doing this, the membrane depolarizes, since negative charges leave the cell interior. Full activation of the oxidase leads to such high membrane potential values that the oxidase can be inhibited. To keep the oxidase working continuously, compensatory ion movements are required to diminish this huge oxidase-induced depolarization. The onset of compensatory ion movements is delayed compared to oxidase activation, and this difference results in the depolarization of the membrane. Protons have been suggested as compensating charges and were detected by many groups that measured proton currents after NADPH oxidase activation in phagocytes [15]. However, other ion movements including outward potassium currents and inward chloride currents have been proposed to participate in compensating for the oxidase-triggered depolarization. Most probably protons are the main compensatory ions in neutrophils. In the reaction catalyzed by the oxidase, one proton is liberated from NADPH that remains in the cytosol, thereby increasing the cytosolic [H+] and lowering the pH temporarily. The superoxide produced in phagosomes increases the phagosomal pH immediately after onset of Nox2 activation as it consumes protons in its dismutation to hydrogen peroxide (Fig. 4.). Later on, due to the compensatory proton movements, protons are translocated from the cytosol into the phagosome so that the cytosol becomes more alkaline and the phagosome turns more acidic. The molecular identity of the proton channel and possible potassium transporter(s) are not known.
The neutrophil phagosome
Most of the information regarding NADPH oxidase function has been obtained from work on the oxidase in the plasma membrane, where it is more easily studied than in phagosomes. However, the latter is physiologically more relevant to microbial killing, but difficult to investigate as it is a very small compartment (a few femtoliters) with an environment that is always changing. The prevailing model for the role of the phagocyte NADPH oxidase in bacterial killing is that ROS attack and kill bacteria through their chemical reactivity (Fig. 4.). This conclusion has been drawn by the fact that CGD patients suffer from bacterial and fungal infections, and CGD neutrophils or healthy neutrophils under hypoxic or anoxic conditions kill certain microbes less efficiently in vitro than their healthy counterparts or in normoxia, respectively. Many in vitro studies have proven that reactive oxygen species (H2O2, HOCl…) are toxic to a broad range of microbes, but the conditions used in these experiments did not always mimic the physiological situation in the neutrophil phagosome, which are hard to determine. O2.- concentrations in the phagosome were suggested to be 4 M (!) at maximal NADPH oxidase activity in neutrophils phagocytosing bacteria. Introduction of glucose-oxidase in liposomes into CGD neutrophils greatly improved their killing capacity, which shows this extra portion of intracellular hydrogen peroxide increases the toxicity of deficient neutrophils towards bacteria.
What happens during phagocytosis? Bacteria taken up by different mechanisms end up in the phagosome niche surrounded by membrane mostly derived from the plasma membrane. Granules fuse with the nascent phagosome in a sequential manner: 1. gelatinase (primary), 2. specific (secondary) and 3. azurophil (tertiary) granules. The gelatinase and the specific granules contain the flavocytochrome in their membranes, so it translocates to the phagosomal membrane. The granules pour their acidic contents into the phagosome (Fig. 4.). Unlike in macrophages, where the phagosomal pH drops to 4–5 (which is required for optimal function of the macrophage proteases), in neutrophils the phagosomal pH stays close to neutral (6.5–7.0). This is the optimum range for neutrophil proteases (i.e., elastase and cathepsin G: around 7.5). The maintenance of a neutral phagosomal pH in PMNs is due to alkalinization caused by the NADPH oxidase. In CGD neutrophils or in healthy neutrophils treated with DPI (flavoenzyme inhibitor that inhibits all the Nox/Duox family members) phagosomal pH drops quickly to 5.0–5.5. Maintaining a neutral pH in the phagosome of CGD neutrophils improves their killing capacity. These observations show that the NADPH oxidase influences the outcome of killing not only by production of ROS, but also by maintaining the phagosomal pH near neutral. The pH and ionic composition of the phagosome is influenced by other ion channels and transporters: proton-ATPase, sodium/proton-exchanger and the anion transporter ClC-3 (Fig. 4.).
The classical model of reactive oxygen species as toxic metabolites responsible for killing has been challenged recently [16]. This model suggests that ROS are only by-products of the activation of the phagocyte oxidase that must be scavenged in order to prevent neutrophils themselves from damage. The main function of the oxidase would be to create a large depolarization that drives potassium ions into the phagosomal membrane, causing an increase in ionic strength (Fig. 4.). Potassium ions would release and thereby activate inactive proteases bound up within their granule matrix. These activated proteases would be ultimately responsible for killing of bacteria, not ROS. Due to digestion of bacteria, the osmotic concentration increases in the phagosome, water is driven from the cytoplasm and the volume of the phagosome subsequently increases (Fig. 4.). This model has been proposed based on the facts that 1. most of the targets of MPO-catalyzed chlorination/iodination by Nox2-derived hydrogen peroxide are neutrophil and not bacterial proteins; 2. MPO-deficient individuals have little or no immune problems (mostly Candida infections); 3. CGD phagosomes do not increase in volume and 4. the granule matrix is not dissolved in CGD neutrophils. The role of MPO in killing is controversial; when missing, its function might be compensated by other systems, since in vitro MPO-deficient mouse and human neutrophils are less efficient in killing of both Staphylococcus aureus and Candida albicans. The classical dogma about ROS is still being questioned, and currently the exact mechanism on how neutrophils actually kill microbes is not completely understood. Most probably, both theories are right, and both Nox2-derived ROS attack directly the microbe and Nox2-dependent membrane potential changes lead to activation of certain proteases.
Beyond intracellular killing
Evidence suggests that activation of the phagocyte oxidase also has consequences in intracellular signaling related to its function as an electrogenic (electron transporting) enzyme. In cases of stimuli that trigger both superoxide generation and calcium influx (fMLF, LTB4), calcium signals are higher in CGD neutrophils or in neutrophil-like model cells lacking gp91phox than in their Nox2-containing counterparts. Because the depolarization is missing in deficient cells, there is no restraint on the contemporary inward calcium currents. This would have consequences on cell behavior, since calcium is involved in many processes.
Studies on global gene expression changes between phagocytosing healthy and XCGD neutrophils revealed more than 200 genes whose expression is ROS-dependent [17]. In general, CGD neutrophils have augmented expression of pro-inflammatory and decreased expression of anti-inflammatory genes compared with those from healthy individuals. XCGD neutrophils present prolonged inflammation (through earlier activation and recruitment) and delayed resolution of the inflammatory process (delayed apoptosis), which might be the reason for the granuloma formation typical for these patients. It has been shown indeed that proinflammatory cytokine production is higher in CGD-neutrophils than in healthy cells. ROS are known to induce apoptosis in neutrophils. CGD neutrophils exhibit delayed apoptosis. Both the lack of ROS and the delayed higher calcium signals may be responsible for this in NADPH oxidase deficient neutrophils.
It has been shown recently, that neutrophils not only kill bacteria intracellularly but also extracellularly. The neutrophil extracellular traps (NETs) are extracellular fibers containing granule proteins and chromatin that can be released by soluble (IL-8, PMA) and microbial stimuli under low serum conditions [18]. The structure of NETs consists of DNA not proteins. NETs contain histones and many granule proteins (including myeloperoxidase). These proteins together kill bacteria, fungal hyphae and degrade microbial toxins. The NADPH oxidase is required for this killing since CGD neutrophils do not produce NETs [26]. ROS most probably induce cell death in neutrophils, which leads to NET formation, in a process that is neither necrosis, nor apoptosis. The activation of NETs is caspase-independent, in which no DNA fragmentation nor phosphatydilserine exposure occurs. First, the cell nucleus loses its shape, heterochromatin and euchromatin become homogenous. Secondly, the nuclear and granular membranes desintegrate, their contents mix and the cell membrane becomes ruptured.
Nox2: eosinophil granulocytes
Eosinophils are thought to function primarily in the innate immune system as effector cells against parasitic invaders. However, this view is changing with recognition of their role in antigen presentation, initiation, and modification of adaptive and innate immune processes. Eosinophils are able to engulf and kill microorganisms, although their phagocytic capacity is decreased compared with neutrophils. They express larger amounts of the phagocytic NADPH oxidase and have a more intensive respiratory burst with most stimuli. In most cases, they attack targets of much larger size, so they release the granule contents and superoxide anions into the surrounding extracellular milieu. Eosinophil granules contain basic proteins, among them large amounts of eosinophil peroxidase (EPO). Following phagocytosis, EPO is detected in the phagosome around the engulfed microorganism. EPO uses H2O2 produced by the NADPH oxidase to convert halide anions (Cl-, Br-, I-) into microbicidal hypohalous acids. Its affinity for Cl- is lower than that for iodide or bromide, but chloride is more abundant. The cell-free EPO/H2O2/halide system is toxic to bacteria (S. aureus, E. coli. L. pneumophila, M. leprae) and to parasites. CGD eosinophils do not produce superoxide and CGD is associated with eosinophilic inflammatory conditions and mild eosinophilia.
Nox2: monocytes/macrophages
Blood monocytes express lower amounts of the phagocyte NADPH oxidase and produce less superoxide than neutrophils. The oxidase is translocated to phagosomal membranes upon phagocytosis and releases superoxide radicals into the phagosome where MPO (lower amounts than in PMNs) converts halides into toxic hypohalous ions. MPO-deficient monocytes kill Aspergillus fumigatus and Candida albicans less efficiently than their healthy counterparts. Both, MPO- and Nox2-deficient monocytes have deficient killing of Toxoplasma gondii; this deficiency can be corrected in the absence of MPO, but not of Nox2, by addition of EPO into phagosomes. Unlike neutrophils, reactive nitrogen species have a more important role in killing mechanisms of monocytes and macrophages than oxygen-dependent killing. In mice, reactive nitrogen species alone are enough to control Leishmania donovani infection in visceral macrophages. When monocytes mature into macrophages, their antimicrobial capacity decreases along with diminished output of the oxidative mechanisms. Mouse CGD macrophages phagocytosing apoptotic neutrophils have diminished production of anti-inflammatory mediators (PGD(2) and TGF-beta). Nox2-derived ROS repress 5-lipoxygenase expression and activity in mouse alveolar macrophages resulting in diminished leukothriene synthesis.
Nox2: dendritic cells
Dendritic cells are professional antigen-presenting cells. They either present endogenous antigens bound to MHC I class molecules to CD8+ T lymphocytes, and activate a cytotoxic immune response, or display exogenous antigens together with MHC II class molecules to CD4+ T lymphocytes, resulting in a humoral immune response. The question of how viruses, which do not infect dendritic cells, activate CD8+ lymphocytes can be explained by “antigen cross-presentation”. Antigens of phagocytosed/endocytosed origin are digested, coupled to MHCI molecules in the ER, transported to the cell surface and presented to CD8+ T cells, leading to a Th1 response. How this happens is not understood in detail, although an important point in the process is breakdown of antigens to the required size (8–9 amino acids), but not further. One way in which dendritic cells accomplish this is by maintaining the phagosomal pH around neutral (like neutrophils), thereby restricting their protease activity. Dendritic cells contain proteases with acidic pH optimum, like macrophages. Dendritic cells express all the Nox2 components, but much smaller amounts (5%) than neutrophils that are insufficient for microbial killing, but enough to influence the phagosomal pH. Gp91phox-deficient dendritic cells have a more acidic phagosome, and are much less efficient in peptide cross-presentation than their healthy counterparts [19]. These findings reveal a novel role of Nox2 unrelated to microbial killing that bridges functions between the innate and adaptive immune systems and raises new questions about the pathophysiology of CGD.
Duox
Since the discovery of the Dual oxidases in the thyroid gland, extensive research has focused on revealing their physiological role in human tissues. Duox2 is an essential hydrogen peroxide-generating partner of thyroperoxidase required for organification of iodide during thyroid hormone biosynthesis. Although the presence of Duox in the lung and other mammalian tissues was recognized early, the functional importance of Duox expression outside of the thyroid gland was not appreciated until Duox transcripts were detected in abundance in epithelial cells in exocrine glands and along mucosal surfaces [13]. An innate immune function of Duox was proposed based on detection of these oxidases at highest levels in tissues that also produce high LPO. It had been long recognized that many exocrine secretions (mucus, saliva, milk, tears) contain (LPO), but the source of hydrogen peroxide supporting LPO activity in these secretions was unknown. A few studies suggested that some microbial species could themselves produce sufficient hydrogen peroxide to be toxic in the presence of LPO alone, although this notion cannot account for the action of LPO on all organisms or in some sites that are relatively sterile. Thus the Duox enzymes were proposed as novel hydrogen peroxide sources that support LPO in a variety of tissues, including the oral cavity, airways, and mucosal surfaces of the gastrointestinal tract. Later work demonstrated that Duox could serve innate immune antimicrobial functions in the GI tract of Drosophila as well.
Oral cavity
The suggestion that Duox provides hydrogen peroxide to support salivary lactoperoxidase was based on the observation that Duox2 expression was detected specifically in epithelial cells of major (terminal) ducts of salivary glands [13]. In contrast, LPO is synthesized in early phases of saliva formation, within salivary acinar cells. Salivary thiocyanate (SCN-) reaches sub-millimolar concentrations, and is considered the primary LPO substrate; to account for this, the sodium iodide symporter was proposed as the major SCN-carrier and was detected within intercalated ducts. Thus the Duox/SCN-/LPO anti-microbial system is fully assembled only in the final stages of saliva formation, as Duox2 provides hydrogen peroxide, the most labile component of the system, just prior to delivery into the oral cavity.
Airways
There has been renewed interest in the last few years in the LPO/H2O2/thiocyanate system as an important innate immune component of the airways [21], since lactoperoxidase was detected at levels as high as 1 % of the total protein in airway secretions of sheep, and its inhibition led to decreased clearance of bacteria from the airways. Duox1 expression was detectable only within the surface epithelium of bronchial and tracheal sections, whereas LPO expression was detected primarily in tracheal and bronchial submucosal glands. Thus, Duox and LPO are expressed in distinct or segregated sites in these tissues, and the assembly of the complete functional system in major airways occurs only on the surface, where Duox1 provides the most labile and limiting component, hydrogen peroxide (Fig. 5.). Indeed, cultured primary human bronchial epithelial cells release extracellular hydrogen peroxide in response to different calcium-mobilizing stimuli (ionomycin, ATP, thapsigargin), which is inhibited by the oxidase flavoprotein inhibitor DPI or by Duox1-targeted antisense oligonucleotides. In airway epithelium Duox is localized to the apical (not basolateral) plasma membrane of the cells. Recently, it was shown that this system is toxic against bacterial species (S. aureus, P. aeruginosa) on the apical surface of cultured primary airway epithelial cells from several mammalian species [21].
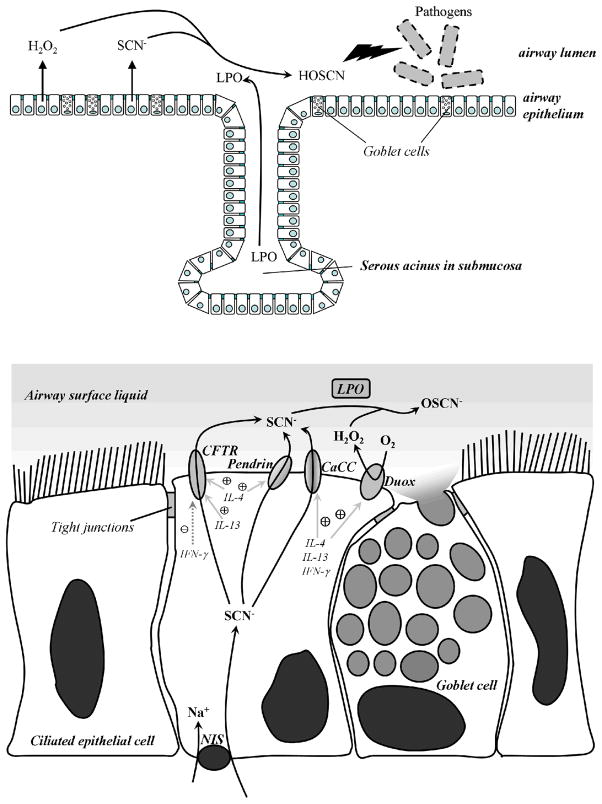
Figure 5. The Duox/Lactoperoxidase/Thiocyanate antimicrobial system in human airways.
Microbicidal hypothiocyanite anions (OSCN-) are formed in the airway surface liquid (ASL) by lactoperoxidase (LPO) through hydrogen peroxide-mediated oxidation of thiocyanate (SCN-). Lactoperoxidase is produced in serous acini of submucosal glands of the airways and transported into the ASL. Dual oxidases (Duox) are localized to the apical plasma membrane of airway epithelial cells, releasing hydrogen peroxide into the ASL, thereby providing the most labile component of the H2O2/LPO/SCN system. The sodium/iodide symporter (NIS) transports thiocyanate into the epithelial cells, while at least three different transporters can deliver thiocyanate into the ASL: calcium-dependent chloride channels (CaCC), pendrin, and the cystic fibrosis conductance regulator (CFTR). Under inflammatory conditions Th1 (IFN-γ) and Th2 (IL-4, IL-13) cytokines induce higher expression of the transporters and Duox. Goblet cells found in the airway epithelium produce and secrete mucin, the major component of mucus.
Three different mechanisms for transporting SCN- across the apical membrane of airway epithelial cells are known: calcium-activated chloride channels, pendrin (SLC26A4) and the cystic fibrosis transmembrane regulator (CFTR) (Fig. 5.). CFTR has been widely recognized as a Cl- transporter defective in cystic fibrosis (CF), although its ability to function also as a SCN- transporter was suggested to account for the enhanced susceptibility of cystic fibrosis patients to bacterial infections [13]. Later work showed that transcellular SCN- transport in CF airway epithelial cells is diminished, [SCN-] levels in CF airway liquid are lowered and killing of Pseudomonas aeruginosa, the most frequent pathogen in CF, is seriously impaired compared to normal controls. The impaired killing of CF airway cells was rescued by reintroducing a functional CFTR [21]. Thus, diminished availability of this component of the Duox/SCN-/LPO system leads to increased survival of pathogens in the airways. So far no mutations in Duox1 or LPO have been identified in man so that might contribute to chronic or acute airway infections of unknown origin.
Observations on the effects of different cytokines on airway epithelial cells suggest roles for both Duox isozymes in airway responses to proinflammatory signals. Duox1 expression is induced by the Th2 cytokines, IL-4 and IL-13, whereas Duox2 levels are increased by the Th1 cytokine, IFN-γ(Fig. 5.) [22]. Duox induction correlates with significantly enhanced apical H2O2 generation. IL-4 upregulates pendrin as well, which leads to increased transport of SCN- to the airway surface under inflammatory conditions [23]. Since IL-13 is a central cytokine in asthmatic disease, Duox-derived ROS may contribute to the pathogenesis of asthma. Duox2 expression is augmented by exposure to rhinovirus or the viral mimic, polyinosine:polycytidylic acid, suggesting Duox2 may have a role in IFN-γ-induced viral clearance. Children with biallelic Duox2 mutations suffer from severe hypothyroidism, although there are no reports to date of impaired innate immune response in these individuals [10].
Other functions for Duox in major airways have been proposed. Duox was suggested as a major source of H+ secretion into the airway surface liquid responsible for maintaining slightly acidic (pH 6.5–7.0) conditions optimal for LPO activity. Recently, a role for ROS produced by Duox1 in airway epithelial cells was suggested in mucus hypersecretion through a TNF-alpha-converting enzyme-EGF receptor pathway. Oxidative stress of various origins has been known to induce lung inflammation, and N-acetyl-cysteine, an efficient antioxidant, has been used therapeutically in a variety of lung inflammatory diseases. Duox proteins may participate in oxidative stress in the airways induced by a variety of environmental or pro-inflammatory agents. ATP released from injured epithelial cells stimulates purinergic P2 receptors on nearby intact cells, which were proposed to induce signaling cascades through Duox1 leading to cell migration and wound repair.
Gastrointestinal tract
Another site of high Duox2 and LPO expression is the lower GI tract, particularly in the rectum, where they can both work together to control proliferation of bacterial flora. Isolated rat rectal glands release H2O2 that is enhanced by the calcium ionophore, ionomycin, and inhibited by the flavoprotein inhibitor DPI. Duox2 mRNA and protein were both detected along the entire porcine digestive tract, particularly at high levels in the cecum and sigmoidal colon. Duox2 is mainly located at the apical plasma membrane of enterocytes, accumulating predominantly in terminally differentiated cells. A global gene expression profile study of non-inflamed colonic mucosal cells of Crohn’s patients and of healthy control individuals has shown that Duox2 expression is increased in the patients’ cells relative to the control group. This may relate to induction of Duox2 by IFN-γ, which has been implicated in Crohn’s disease. These preliminary data suggest innate immune defense roles of Duox in the lower gastrointestinal tract in maintaining normal gut flora and in the case of chronic inflammatory diseases.
An important role of ROS has been proposed in Drosophila, since infection of the insect gut induces immediate ROS production and flies missing some ROS-scavenging machinery show augmented mortality [24]. Membrane fractions of dissected intestines show basal hydrogen peroxide production that can be further increased by calcium and inhibited by DPI or by the extracellular calcium chelator, EGTA. ROS generation is decreased and mortality is significantly increased when Drosophila Duox (dDuox) RNAi constructs were expressed in flies infected with bacteria. The intestine of dDuox-RNAi flies infected with E. coli shows much higher bacterial numbers than that of normal flies. Reintroduction of either Drosophila or human Duox into the dDuox-RNAi-expressing mutants rescues ROS generation, limits bacterial proliferation in the gut, and decreases the mortality of these flies. Together these results suggest dDuox is the major source of antimicrobial ROS in Drosophila intestine and its activity is induced by microbial infection.
Nox1
The highest Nox1 expression is observed in the colon epithelium [2]. Nox1 mRNA was detected within the lower half of colon crypts, a site where cells divide and rapidly differentiate. However, given the short life span of these cells, Nox1 was proposed to function in differentiated epithelial cells. Later work detected Nox1 protein at highest levels on the luminal surfaces of epithelial cells within the upper portions of crypts, where the epithelial cells reach their terminally differentiated state. Initially, Nox1 was proposed to serve as a pro-mitogenic oxidase supporting cell proliferation, but this theory was later disfavored and an innate immune role of Nox1 is the presumed function in the colon [2,4]. Some of the earliest observations for Nox1 as a host defense enzyme showed direct induction of ROS production through TLR4 in guinea pig gastric pit cells by pathogenic Helicobacter pylori LPS. LPS induces Noxo1 simultaneously, consistent with the similar tissue-specific expression patterns of Nox1 and Noxo1. In colon epithelial cells, recombinant flagellin from S. enteriditis binds to TLR5, upregulates Nox1, and leads to higher ROS generation if both Nox1 and Noxo1 are present. Thus, gastric pit and colon epithelial cells detect virulence factors, which can in turn act through separated pathways to induce both transcriptional and post-translational events that enhance Nox1-mediated host defense responses. Nox1 is also detected at high levels in lymphoid cells of inflammatory lesions in the colon of Crohn’s disease and ulcerative colitis patients. Similar to Nox2 and Duox2, Nox1 expression is highly inducible by the inflammatory cytokine IFN-γ, further supporting its proposed function as a host defense oxidase. In inflammatory bowel disease imbalances of Th1 cytokine production (such as IFN-γ) and injury of colon epithelial cells caused by reactive oxygen species have been reported, consistent with roles for Nox1 in this disease process. The amount of Nox1 transcript increases from the ascending to descending colon segments, in parallel with the increased bacterial burden [3], further supporting a role of Nox1 in innate immunity.
Nox4
Early work on Nox4 suggested no innate immune functions for this oxidase, although recent observations suggest roles for Nox4 in LPS-induced proinflammatory responses in HEK293T, human aortic endothelial, and smooth muscle cells. Bacterial LPS induces production of ROS in HEK293T cells, which has been attributed to Nox4 [25]. Direct protein-protein interactions between TLR4 and Nox4 were shown to be necessary for LPS-induced ROS production and subsequent NF-kB activation. In aortic endothelial cells, LPS binds to Nox4, produces ROS, induces expression of intracellular adhesion molecule-1 (ICAM-1) and chemokines such as IL-8 and monocyte chemoattractant protein-1 (MCP-1). Migration and adhesion of monocytes to endothelial cells is increased because of enhanced chemokine production. All of these cellular responses are inhibited by Nox4-targeted siRNAs.
CXCL16 is a transmembrane chemokine expressed mainly on dendritic cells, macrophages, lymphocytes and smooth muscle cells. CXCL16 binds to and signals through CXCR6 receptors, whose expression is limited to certain types of T, B and NK cells and to aortic smooth muscle cells (ASMC). Binding of CXCL16 to CXCR6 is an important proinflammatory pathway that induces ASMC proliferation and contributes to the artherogenesis. Bacterial LPS upregulates CXCR6 expression through a signaling pathway of LPS-TLR4-Nox4-AP-1-CXCR6, thus Nox4-derived ROS produced in ASMCs may contribute to smooth muscle cell proliferation in artherosclerosis through this pathway. Taken together, these observations raised the interesting possibility that Nox4 participates in the function of the innate immune system by detecting and responding to microbial virulence factors.
Conclusions
Microbial killing through deliberate production of ROS by phagocytic cells has been appreciated for decades, as is evident in patients with CGD, who suffer from enhanced susceptibility to microbial pathogens and dysregulated inflammatory responses. Research in the last 20 years have revealed Nox2 system as a robust ROS-generating enzyme subject to elaborate controlling mechanisms that govern the sites and duration of deliberate ROS generation. Despite these advances, there is still debate on the mechanisms by which Nox2 mediates microbial killing within the confines of the phagosome and a new appreciation of novel roles served by Nox2 and the ROS it generates beyond the phagosome. With the recent expansion of genomic informatics, investigators have learned that the phagocytic oxidase is but one representative of a new Nox family of NADPH oxidases that serve a variety of essential signaling and biosynthetic functions. Several of the novel oxidases are also proposed to perform innate immune functions related to: 1) their predominant expression on epithelial (mucosal) surfaces, 2) their ability to deliver antimicrobial ROS on host interfaces with the external environment, and 3) their responsiveness to microbial pattern recognition or pro-inflammatory pathways. Together these observations suggest that several non-phagocytic members of the Nox family oxidases represent components of a “first line” of host defense that should be considered candidate mediators of inflammatory disease at these sites.
Acknowledgments
We thank Thomas E. DeCoursey and Erzsebet Ligeti for suggestions for improving the manuscript. This work was supported by the Division of Intramural Research of the NIAID, NIH.
Reference List
- 1.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 2.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 3.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 4.Leto TL, Geiszt M. Role of NADPH oxidases on host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 5.Segal BH, Leto TL, Malech HL, Gallin JI, Holland SM. Genetic, biochemical and clinical features of chronic granulomatous disease. Reviews in Molecular Medicine. 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 7.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 8.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 10.Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 11.Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, Abraham WM, Conner GE. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- 12.Klebanoff SJ. Basic principles and clinical correlates. 3. Lippincott Williams & Wilkins; 1999. Oxygen metabolites from Phagocytes; in Gallin JI and Synderman R (eds): Inflammation; pp. 721–769. [Google Scholar]
- 13.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 14.Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochimica et Biophysica Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–7. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi SD, Voyich JM, Braughton KR, Whitney AR, Nauseef WM, Malech HL, DeLeo FR. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol. 2004;172(1):636–43. doi: 10.4049/jimmunol.172.1.636. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 19.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126(1):205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol. 2003;29(2):206–12. doi: 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- 21.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(2):174–83. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579(21):4911–7. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178(8):5144–53. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 24.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]