Abstract
At hippocampal synapses, repetitive synaptic stimulation (RSS) in the theta frequency range (3–12 Hz) is associated with robust EPSP frequency facilitation (FF) and consequently, enhanced action potential (spike) generation and throughput. A complex, synaptically-induced hyperpolarization (SIHP) is also triggered by synaptic activation, and a Ca2+-dependent afterhyperpolarization (AHP) is triggered above spike threshold. With aging, the AHP is increased and impairs intracellular spike generation, at least in accommodation protocols. However, little is known about how these aging changes interact to affect spike generation at physiological frequencies of RSS, or if the SIHP also is modified in aging. Here we performed the first tests of the net impact of these excitatory and inhibitory aging changes on spike generation during RSS. We report that during RSS at spike threshold 1) spike throughput is well-sustained at theta frequencies in young and aged neurons; 2) an interposed AHP dampens spike generation, particularly in aged neurons and at higher frequencies; 3) compared to the AHP, the SIHP does not exert an equivalent inhibitory effect on spike throughput; and 4) in contrast to the AHP, the SIHP is reduced with aging. Together, these results are consistent with a model in which the source of the hyperpolarization is important in determining hippocampal spike throughput within the theta frequency range.
Keywords: synaptic hyperpolarization, aging, calcium, theta, fidelity, facilitation, hippocampus, AHP, frequency, accommodation
1. Introduction
Multiple excitatory and inhibitory potentials are known to influence synaptically-activated action potential generation, including the excitatory postsynaptic potential (EPSP), afterdepolarization (ADP), feed-forward and recurrent inhibitory postsynaptic potentials (IPSPs), a slow spike-induced, Ca2+-dependent afterhyperpolarization (AHP), and a complex synaptically-induced hyperpolarization (SIHP). Although the origin and functional impact of the AHP and SIHP have been analyzed in some detail (Alger, 1984; Madison and Nicoll, 1984; Newberry and Nicoll, 1984; Lacaille and Schwartzkroin, 1988; Storm, 1990; Sah and Bekkers, 1996; Saar et al., 2002; Otmakhova and Lisman, 2004; Wu et al., 2004; Metz et al., 2005), few studies have examined the interactive balance between concomitant excitatory and inhibitory processes on action potential throughput during physiological frequencies of repetitive synaptic activation. Further, this balance appears to shift in the hippocampus during aging, but it is not known how concurrent aging changes in the EPSP, SIHP and AHP interact to affect spike generation during repetitive synaptic stimulation (RSS).
RSS has been studied as a model of short-term synaptic plasticity across multiple frequencies (1–40 Hz), and is known to trigger powerful facilitation of EPSP amplitudes, which help maintain spike firing (frequency facilitation - FF) (Andersen and Lomo, 1967; Pitler and Landfield, 1987; Ouanounou et al., 1999; Thibault et al., 2001). Stimulation frequencies spanning the theta band (e.g., 3–12 Hz) have been found to be the most effective in inducing such short-term synaptic plasticity, and hippocampal theta rhythms also have been linked to memory consolidation (Landfield, 1977; Winson, 1978; Destrade, 1982; Jeantet and Jaffard, 1983; reviewed in Landfield and Thibault, 2001; Buzsaki, 2002; Hasselmo, 2005). Moreover, theta frequencies also preferentially induce long-term potentiation (LTP) (Larson and Lynch, 1986; Rose and Dunwiddie, 1986; Pavlides et al., 1988; Otto et al., 1991; Moore et al., 1993; Yeckel and Berger, 1998; Morgan and Teyler, 2001; Tombaugh et al., 2002).
With aging, decreases are seen in both hippocampal short-term synaptic plasticity (Landfield et al., 1986; Rosenzweig et al., 1997; Ouanounou et al., 1999; Thibault et al., 2001), and longer-term plasticity (Landfield et al., 1978; Deupree et al., 1993; Moore et al., 1993; Barnes et al., 1996; Norris et al., 1996; Rosenzweig et al., 1997; Bach et al., 1999; Rex et al., 2005; Lynch et al., 2006), as well as in single EPSP amplitude (Barnes and McNaughton, 1979; Landfield et al., 1986; Barnes et al., 1997; Thibault et al., 2001). In addition, one of the most consistently observed aging-dependent electrophysiological changes is an increase in amplitude and duration of AHPs evoked by intracellularly-activated action potentials (Landfield and Pitler, 1984; Kerr et al., 1989; Moyer et al., 1992; Potier et al., 1992; Kumar and Foster, 2002; Tombaugh et al., 2005; Disterhoft and Oh, 2006; Gant et al., 2006). AHP-mediated spike accommodation (reduction in spike frequency) during prolonged intracellular depolarization also is increased in aging, suggesting a role for the AHP in regulating action potential throughput (Moyer et al., 1992; Disterhoft et al., 1996; Gant et al., 2006). Furthermore, age-dependent increases in voltage-gated Ca2+ channels (VGCCs) that contribute to the AHP (i.e., L-type VGCCs) have been found (Thibault and Landfield, 1996), and have been suggested to underlie the increased AHP and accommodation (Landfield, 1987; Moyer et al., 1992; Disterhoft et al., 1996; Thibault et al., 2001; Disterhoft and Oh, 2007). In addition, activation-induced Ca2+ transients are increased in hippocampal neurons during aging (Thibault et al., 2001; Hemond and Jaffe, 2005; Gant et al., 2006), and recent findings indicate that altered Ca2+-induced Ca2+ release (CICR) may contribute to the increased AHP in aging (Kumar and Foster, 2005; Gant et al., 2006; Thibault et al., 2007), possibly in response to Ca2+ influx via L-VGCCs (Thibault et al., 2007). Importantly, some of these changes in Ca2+-mediated processes have been correlated with cognitive decline (Deyo et al., 1989; Disterhoft et al., 1996; Landfield, 1996; Thibault and Landfield, 1996; Tombaugh et al., 2005; Disterhoft and Oh, 2006) and synaptic plasticity (Landfield et al., 1986; Norris et al., 1996; Thibault et al., 2001).
However, it is still not clear how, or even if, larger AHPs seen in aging impair spike generation during RSS. To date, single neuron activity in the hippocampal formation, which can vary from 1 to 200 Hz in vivo (Buzsaki et al., 1992; Sik et al., 1995; Ylinen et al., 1995; Deadwyler et al., 1996; Traub et al., 1996), has not been found to differ clearly in aged animals. Some subfield specific changes have been reported (Mizumori et al., 1992; Shen et al., 1997; Asaka et al., 2005; Wilson et al., 2005), and a reduction in background firing rate has been seen during conditioning in aged non-learner rabbits (McEchron et al., 2001), consistent with the greater AHP in those animals (Deyo et al., 1989; Tombaugh et al., 2005; Disterhoft and Oh, 2006). However, failure of even a few spikes during a repetitive train may be important for memory function (e.g., Landfield and Thibault, 2001). Clearly, therefore, further analyses are required to elucidate the interactions of hyperpolarization and RSS-mediated spike generation.
Several ionic components have been identified that contribute to the SIHP, including IPSPs from activation of GABAb receptors, inactivation of Ih, intracellular Ca2+ and several K+ channels sensitive to β-adrenergic and cholinergic neuromodulation (Nicoll and Alger, 1981; Newberry and Nicoll, 1984; Lancaster et al., 2001; Otmakhova and Lisman, 2004; Wu et al., 2004). Moreover, the SIHP is activated synaptically even below spike threshold (Fig. 1 and Thibault et al., 2001; Otmakhova and Lisman, 2004; Wu et al., 2004). When triggered synaptically at or above action potential threshold, the SIHP and the AHP are activated in parallel and share common ionic mechanisms (Wu et al., 2004) that contribute to the net membrane hyperpolarization (Pitler and Landfield, 1987; Borde et al., 1999). However, although there have been some reports of smaller IPSPs with aging (Barnes and McNaughton, 1980; Billard et al., 1995; Potier et al., 2006), little is known about the effects of aging on the SIHP. Moreover, whereas the AHP has been studied for its role in modifying subthreshold synaptic signaling (Sah and Bekkers, 1996; Lancaster et al., 2001; Otmakhova and Lisman, 2004; Fernandez de Sevilla et al., 2007), its impact on spike throughput at action potential threshold has not been investigated in relation to aging. Here, we tested the effect of aging on several of these factors concurrently. The results provide the first evidence of aging changes in the total SIHP during RSS, in an unanticipated direction, and reveal a preferential impact of the AHP in regulating spike generation and throughput.
Figure 1. The AHP reliably shunts action potentials.
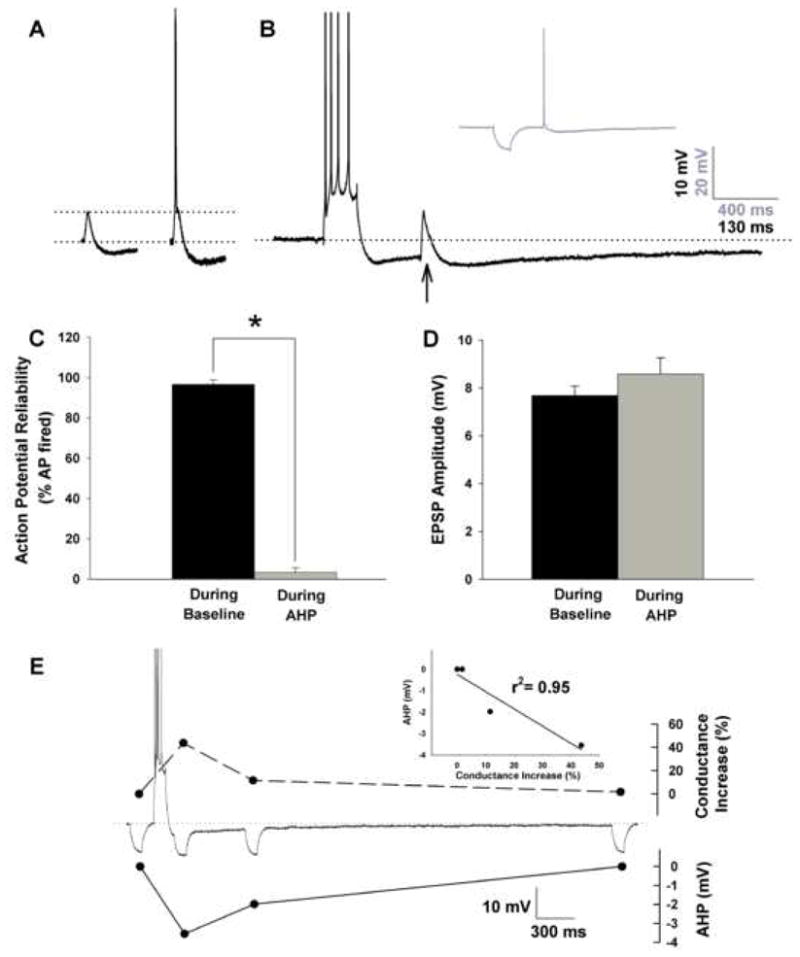
A, Examples of synaptically stimulated sub-threshold (left) and threshold EPSPs (right) recorded from −65 mV in a young-adult CA1 neuron. Note the presence of a substantial SIHP both below and at threshold for an action potential. B, The same synaptic stimulation intensity no longer elicits an action potential if placed near the peak of the AHP (arrow). Inset shows recovery of action potential in the same cells if the depolarizing current is replaced by a hyperpolarizing current of similar amplitude and duration. C, Percent failed action potentials (AP) during baseline measurements at 0.1 Hz and during the initial AHP. D, EPSP amplitudes measured during baseline or during the AHP are not significantly different. E, Changes in membrane K+ conductances (top) are correlated (inset) in time and amplitude with the AHP (bottom) in the same cell. Values represent means ± SEM. Asterisk indicates p < 0.05.
2. Materials and Methods
2.1. Slice preparations
All experiments were conducted in compliance with the institutional guidelines of the Animal Care and Use Committee at the University of Kentucky. Male F344 rats (4–6, and 21–22 months old) were anesthetized in a CO2 chamber prior to rapid decapitation. Brains were rapidly removed and transverse hippocampal slices (350 μm) were cut with a vibratome 3000 (TPI, Saint Louis, MO) into cold oxygenated artificial cerebrospinal fluid (ACF) of the following composition (in mM): 128 NaCl; 1.25 KH2PO4; 10 Glucose; 26 NaHCO3; 3 KCl; 0.1 CaCl2 and 2 MgCl2 (Thibault et al., 2001). Slices were placed in an interface-type chamber containing ACF with 2 mM CaCl2 (Ca-ACF) at 32° C and gassed with 95 % O2-5 % CO2. After at least 1 hour of recovery, individual slices were then transferred to a perfusion chamber (RC-22C, Warner Instruments, Co., Hamden, CT) equipped with a bottom net for Ca-ACF perfusion beneath the slice. The oxygenated Ca-ACF was delivered at 1.5–2 mL/min and was warmed to 32.0 ± 0.2° C using an inline heater (TC2Bip, Cell Micro Controls, Norfolk, VA) positioned one cm before the chamber inlet.
2.2. Electrophysiology
Data were acquired and analyzed using pCLAMP 8, a sharp-electrode amplifier (Axoclamp 2B), and a DigiData 1320 board (Axon Instruments, Union City, CA). Sharp intracellular electrodes were pulled from micro-hematocrit glass capillaries (Fisher Scientific, Pittsburgh, PA) on a Sutter Instruments (Novato, CA) P80 pipette puller, and had tip resistances of 80–120 MΩ when filled with 2 M KMeSO4 and 10 mM HEPES (pH 7.4). After impalement, neurons were held at −70 mV in current clamp mode with minimal current injection for approximately 5 min to allow for membrane stabilization. Input resistance measures were determined from the steady hyperpolarized potential reached in response to a 800 ms 200 pA hyperpolarizing current step. All experiments were conducted in current clamp mode with bridge balance compensation and capacitance neutralization. Voltage records were digitized at 2–20 kHz and low-pass-filtered at 1 kHz.
All intracellular AHPs were induced from a holding potential of −65 mV using a 100 ms current depolarization pulse delivered through the intracellular electrode, at sufficient intensity to generate four Na+ action potentials. This holding potential is more positive that that used previously (Thibault et al., 2001) and enhances measurement of hyperpolarizing potentials. In some case, a synaptic hyperpolarization was triggered from −65 mV by delivering four stimuli at 50 Hz to the Schaffer collaterals (see below), at intensities ensuring that four action potentials were elicited. AHP amplitudes were measured at the negative peak immediately following the depolarization pulse, during the medium AHP (mAHP), which typically lasts several hundred ms in hippocampal pyramidal neurons, and at 800 ms following the end of the step, during the slow AHP (sAHP), which has durations in the 1–3 sec range (Lancaster and Nicoll, 1987; Williamson and Alger, 1990; Sah and Faber, 2002; Stocker, 2004; Gu et al., 2005). AHP duration was measured from the end of the depolarizing step until return to baseline. For aging comparisons, AHPs were elicited every 30 s and averaged across a 3 min recording period.
Repetitive synaptic stimulation (RSS) was delivered through a twisted bipolar stimulation electrode (0.0045″ coated stainless steel, A-M Systems, Inc., Everett, WA) positioned in the Schaffer-collaterals/commissural fibers of the stratum radiatum. All synaptic activation protocols were delivered using a pair of SD9K stimulators (Astro Med Inc., Grass Instr., Warwick, RI), in combination with a S98 digital stimulator (Med Systems Corp., Greenvale, NY). Input-output (I/O) relationships were measured in every cell to determine action potential threshold. Synaptic stimulus intensity during RSS for all experiments was set to generate a single EPSP with amplitude just at Na+ action potential threshold. For all synaptic stimulation, pulse duration was 100 μs, and cells were held at −65 mV. The RSS train was delivered at 3, 7 or 15 Hz for 5 s and in some experiments, the 6th action potential was replaced with a burst of four intracellularly-induced action potentials (40–50 Hz), generating the AHP. The RSS train was then resumed at the same frequency. Because RSS delivered at several frequencies in the same cell might induce persistent changes in excitability or metaplasticity (Abraham and Bear, 1996; Mockett et al., 2002; Young and Nguyen, 2005), the order of frequency presentation was randomized for each cell and a 3 min rest period was imposed between each frequency tested. We monitored I/O functions between each 5 s frequency train, and overall, the RSS protocols did not affect spike threshold nor induce long-term plasticity changes.
Comparisons in synaptic EPSP amplitudes and action potential reliability were made in the presence or absence of a four-spike intracellularly triggered AHP (Fig. 1D) during baseline stimulation at −65 mV. The net effect of the AHP on EPSP amplitude (base to peak) depends on several factors, notably, an increase in EPSP driving force created by the membrane hyperpolarization, the increase in shunting membrane conductances which accelerates EPSP decay rate, and the amplitude of the AHP itself. As previously reported (Sah and Bekkers, 1996; Lancaster et al., 2001), single EPSP amplitudes were not significantly affected by the AHP. The presence of a synaptic action potential triggered at threshold and delivered at 0.1 Hz was monitored for three minutes either in the presence, or absence of an AHP. The percent action potentials missed was derived from the total number of synaptic stimuli applied during that period. Changes in membrane conductance (Fig. 1E) were derived from the inverse of membrane resistance, measured as the voltage deflection imposed during a 100 ms, 200 pA hyperpolarizing current injection delivered before the AHP, and at several time points during the AHP (at 75, 675, and 3675 ms).
EPSP amplitudes used to quantify frequency facilitation (% FF) during RSS were measured on the back side of the action potential (Mini Analysis, Synaptosoft, Inc., Fort Lee, NJ). The synaptic EPSPs measured at that point represent “mixed” EPSPs, which comprise currents from NMDA and AMPA receptors, but under some conditions, may also include currents from voltage-gated calcium channels and other depolarizing influences on the neuron, including the afterdepolarization (ADP) and persistent Na+ currents (Azouz et al., 1996; Staff et al., 2000; Wu et al., 2004; Metz et al., 2005; Fernandez de Sevilla et al., 2007; Metz et al., 2007). For example, about 20% of hippocampal neurons typically show little or no slow AHP, and upon activation, can develop a large ADP concomitant with action potential bursting (Azouz et al., 1996; Wu et al., 2004; Metz et al., 2007), which can overlap temporarily with our measures of the EPSP. Because of the goals of this study, we only included cells exhibiting an AHP. Therefore, although the ADP can influence EPSP measures and neuron excitability, it is unlikely to have had a major impact on our measures in this study. This conclusion is supported by observations that measures of FF taken on the left (front) side of the action potential, or on EPSPs missing an action potential (see Fig. 1A) were quantitatively very similar to those taken on the right side of the action potential. In addition, no cells showed signs of multiple spiking, a hallmark of ADP generating neurons (Azouz et al., 1996; Wu et al., 2004; Metz et al., 2005). Nevertheless, the caveat that these are “mixed” EPSP measures should be noted.
For % FF measures, mixed EPSP amplitudes were normalized to the first EPSP (E1) of the RSS train (% FF = (E2−E1)/E1*100; see Fig. 2A) where E2= the 5th or the average of the 6th–9th EPSPs. We measured the degree of EPSP facilitation at the 5th EPSP, and the average of the 6th to 9th EPSPs during baseline RSS (Fig. 2A) to confirm that by the 6th EPSP, % FF was steady. A similar analysis was conducted in RSS runs with interposed AHP (Fig. 3) to measure the impact of a 4-spike AHP on a steady baseline of EPSP facilitation and synaptic hyperpolarization (see below).
Figure 2. Mixed EPSP facilitation and synaptic hyperpolarization during RSS are greater in young-adult neurons compared to older cells.

A, Representative RSS at 7 Hz in a young-adult neuron. E1 (EPSP 1) and E2 (EPSP 2) describe the amplitude measures used to measure % FF of the EPSP. Horizontal bar indicates the time period during which FF was averaged for graphical representation in B. B, Quantitative group measures (means ± SEM) showing the effect of age on FF triggered at 3, 7 and 15 Hz. C, Quantitative data on SIHP measured across several frequencies. Synaptic hyperpolarization was measured during RSS at 3, 7 and 15, following a single action potential (AP), as well as following a burst of 4 action potentials stimulated synaptically at 50 Hz (inset; scale bar indicates 8 mV and 160 ms). Note that, unlike the AHP, the SIHP is smaller in aged compared to young-adult neurons. Values represent means ± SEM. Asterisk indicates p < 0.05.
Figure 3. Interposed AHP during RSS.

A, Examples of RSS with an interposed AHP in young-adult (left) and aged neurons (right). Several variables were measured during 1 s following the induction of an intracellularly-triggered AHP, including membrane hyperpolarization, percent FF and action potential generation. Cartoons of the stimulation protocols used are inset under each voltage trace and illustrate synaptic stimulation (upper trace) and intracellular current depolarization (lower trace). B, Percent action potentials missed during the AHP in young-adult and aged cells. Means ± SEM. Note that the high fidelity seen at 7 Hz in the young-adult group, is not present in the aged group. Values represent means ± SEM. Asterisk indicates p < 0.05.
Similar time points were used for measures of SIHP during RSS (Fig. 2, after the 5th EPSP and after individual EPSPs 6th – 9th), and during RSS with an interposed 4-spike AHP (Fig. 3). Maximal membrane hyperpolarization following a single synaptically activated action potential was determined from the first elicited action potential in the 3 Hz RSS train. These experiments confirmed that by the 5th EPSP, membrane hyperpolarization is steady, and also show that the synaptic hyperpolarization triggered by the 1st EPSP declines somewhat with subsequent repetitive firing during RSS.
2.3. Cell health and exclusion criteria
Neurons with input resistance below 35 MΩ, action potential amplitude less than 70 mV (measured from −65 mV), or holding current more than −200 pA at −65 mV were excluded from the study, as well as cells that did not exhibit a clear AHP. Overall yield of recorded neurons that met all criteria was approximately two cells per daily animal preparation (range: 1.6 to 2.4).
2.4. Statistical analyses
Variables were analyzed for main effects using 2-way ANOVA and repeated measures across frequencies and age groups. Fisher’s PLSD post-hoc comparisons were used (Statview, SAS Institute Inc., Cary, NC). P-values less than 0.05 were considered significant.
3. Results
Table 1 describes passive and active membrane properties obtained in 55 CA1 pyramidal neurons from 25 animals. Not all cells included in this study were subjected to every stimulation protocol. Resting membrane potential, input resistance, action potential height, electrode resistance, stimulation intensity at action potential threshold, and EPSP amplitude at threshold for an action potential did not change with aging. However, significant increases were observed in the aged group for measures of the medium AHP amplitude (mAHP; F(1,47) = 12.7; p < 0.001), the slow AHP amplitude (sAHP; F(1,47) = 19.2; p < 0.001) and AHP duration (F(1,47) = 15.6; p < 0.001).
Table 1.
Basic Neuronal Properties
| Young n = 31 | Aged n = 18 | p | |
|---|---|---|---|
| Input resistance | 50.3 ± 2.0 | 54.6 ± 2.5 | n.s. |
| Resting Potential (mV) | −64.1 ± 0.4 | −63.3 ± 1.1 | n.s. |
| AP height (mV) | 81.5 ± 1.3 | 78.5 ± 1.6 | n.s. |
| EPSP amplitude (mV) | 5.7 ± 0.6 | 6.0 ± 0.9 | n.s. |
| AP Threshold (μA) | 156.9 ± 13.7 | 141.7 ± 12.4 | n.s. |
| mAHP (mV) | 2.8 ± 0.2 | 4.0 ± 0.3 | p < 0.05 |
| sAHP (mV) | 1.4 ± 0.1 | 2.40 ± 0.3 | p < 0.05 |
| Duration (ms) | 2128.8 ± 142.8 | 3428.8 ± 357.2 | p < 0.05 |
Table 1 reports average measures and S.E.M. obtained in young-adult and aged CA1 pyramidal neurons, including properties of action potential (AP) height, excitatory post synaptic potential (EPSP) amplitude (average of 1st EPSP in all repetitive synaptic stimulation runs), threshold for synaptically eliciting an AP, amplitude of the medium and slow afterhyperpolarization (AHP) and duration of the AHP.
3.1. Single action potential reliability at −65 mV and during the AHP
In 12 young-adult cells, single action potentials were triggered with 0.1 Hz synaptic stimulation at threshold intensity either during baseline conditions at −65 mV (Fig. 1A), or near the peak of an intracellularly-induced AHP (Fig. 1B). Postsynaptic action potentials were elicited, on average, 97% of the time in all cells tested (Fig. 1C), however, the same stimulus intensity presented near the peak of the AHP only elicited an action potential 3% of the time (F(1,10) = 431.3; p < 0.0001). As noted, cellular mechanisms underlying the drop in action potentials during the AHP include triggering the EPSP from a more hyperpolarized potential as well as activating shunting membrane conductances correlated in time and amplitude with the AHP (Fig. 1E). These conductances are associated with the somatically-generated AHP and have been shown to reduce sub-threshold EPSP summation by decreasing EPSP half-width and decay time constant (Sah and Bekkers, 1996; Lancaster et al., 2001), in some cases, decreasing EPSP amplitudes (Saar et al., 2002; Fernandez de Sevilla et al., 2007). Here, and as previously reported (Sah and Bekkers, 1996; Borde et al., 1999; Lancaster et al., 2001), we show EPSP amplitudes were unaffected by the AHP (Fig. 1D).
3.2. EPSP frequency facilitation (FF) during repetitive synaptic stimulation (RSS) in young-adult and aged rat neurons
Growth of the EPSP during RSS is rapid and generally reaches maximal levels by the 6th synaptic event (Pitler and Landfield, 1987; Thibault et al., 2001). Examples of control RSS at 7 Hz and the ensuing FF are shown in Fig. 2A. As shown previously (Landfield et al., 1978; Ouanounou et al., 1999; Thibault et al., 2001), a main effect of aging was noted on EPSP facilitation during RSS, with greater FF seen in young-adult compared to aged animals (F(1,52) = 6.06; p <0.05, n=28 young-adult and n=26 aged neurons). Post-hoc analyses revealed that age differences were statistically significant and reliable primarily at 7Hz (p < 0.05; Fig. 2B). The weaker short-term 7 Hz EPSP facilitation seen in aged cells here likely contributes to decreases in longer forms of plasticity as seen in aging with theta-based LTP stimulation protocols (Larson and Lynch, 1986; Pavlides et al., 1988; Tombaugh et al., 2002; Rex et al., 2005), and also may alter encoding during information processing (Landfield et al., 1986; Ouanounou et al., 1999; Landfield and Thibault, 2001; Thibault et al., 2001).
3.3. Reliability of action potential throughput during control RSS
RSS delivered at 3, 7 and 15 Hz, was used to measure synaptic throughput by counting the number of action potentials elicited in each cell during the stimulation protocol. Young-adult neurons (n=13) did not drop a single action potentials at any frequency tested, firing 15, 35 and 75 action potentials during each 5 s protocol delivered at 3, 7 and 15 Hz, respectively. Further, aged neurons (n=12) responded equally well during these stimulation protocols, and only one aged neuron skipped several action potentials toward the end of the 15 Hz stimulation run. Thus, action potential throughput was robust and well-maintained, even in the presence of a large SIHP. Since the tendency for spike failure is dependent on the number of events (i.e., stimuli) delivered to the stimulating electrode, we normalized spike failure to the total number of events delivered. Even under these normalized conditions, input frequency did not significantly affect action potential throughput at either age during control RSS (data not shown).
3.4. Synaptically-induced hyperpolarization (SIHP) following single action potentials and during RSS
Surprisingly, and in contrast to the AHP, we found that the SIHP was greater in young-adult compared to aged rat neurons following a single action potential, a burst of action potentials, and during RSS at several frequencies (Fig. 2C). As reported for EPSP measures during RSS, SIHP during RSS also reached a steady level by the 6th action potential (Pitler and Landfield, 1987; Thibault et al., 2001). Greater SIHP was seen in young-adult neurons (n=13) compared to aged neurons (n=12) during 3 and 7 Hz RSS (F(1,23) = 5.23; p < 0.05; F(1,23) = 6.39; p < 0.0001 respectively; Fig. 2C). During 15 Hz stimulation protocols this difference did not reach significance. As previously reported, increasing stimulation frequency increases SIHP amplitude (Wu et al., 2004) as seen here in young-adult and aged animals (F(2,40) = 13.3; p <0.0001 and F(2,22) = 30.7; p < 0.0001, respectively). Post-hoc analyses revealed significant increases in SIHP from 3 to 7 Hz, and from 3 to 15 Hz, in both age groups (p < 0.0001). We also measured SIHP following a single action potential. The SIHP also was found to be larger in young-adult (n=13) compared to aged neurons (n=12) following a single spike (F(1,23) = 5.20; p < 0.05; Fig. 2C). Following a 50 Hz burst of synaptically-triggered action potentials (Fig. 2C inset), the SIHP was again greater in young-adult neurons (n=16) compared to aged neurons (n=19) (F(1,33) = 5.56; p < 0.05; Fig. 2C). Thus, during RSS at threshold for a single spike, the IPSP/hyperpolarization mechanisms (i.e., SIHP) are consistently larger in neurons from younger vs. older animals, even though a presumably larger single spike-induced AHP is embedded in the SIHP of older rats. These mechanisms however, are offset with facilitation of EPSP amplitudes during RSS, maintaining robust throughput irrespective of age at the frequencies tested here (see section 3.3).
3.5. Action potential throughput during RSS with an interposed AHP
We studied the inhibitory effects of a postsynaptic AHP by using an intracellularly applied current to elicit a 4-spike-burst after the 5th action potential during RSS. Action potential generation from synaptic stimulation was monitored during a 1 s window starting after the interposed burst (Fig. 3). A main effect of interposing an AHP during RSS triggered across the three frequencies was seen in 40 young-adult neurons (F(1,78) = 6.4, p < 0.05) and in 24 aged neurons (F(1,46) = 11.3, p < 0.01), with the number of action potentials elicited by RSS during the AHP decreasing when compared to the control stimulation condition (i.e., RSS without an interposed AHP), (Figs. 3A and B).
In addition, the effect of the AHP was considerably stronger in aged compared to young rat neurons (Fig. 3B) and a main effect of aging was seen in which spike generation was significantly less in older (n=24) compared to younger neurons (n=40) under conditions of an interposed AHP (F(1,62) = 6.96, p < 0.05). Post-hoc analyses revealed that aging differences were more prominent at 7 and 15 Hz (p < 0.05, for each). Interestingly, in young adult neurons, interposing an AHP immediately after the fifth action potential of the RSS significantly reduced synaptic throughput for a short period but only at 3 and 15 Hz (p < 0.05; p < 0.0001, respectively). At 7 Hz, in young-adult neurons, synaptic throughput was well-maintained during the AHP (Fig. 3B), and, in each cell, 7 action potentials were elicited during the 1 s window (n=13). In aged neurons however, the robust throughput seen at 7 Hz was not present. Instead, stimulation frequency was positively correlated with the percent action potential missed (Fig. 3B). Although the AHP can last several seconds, action potentials were dropped preferentially early during the AHP and for a short period, when AHP amplitude is greatest (see Fig. 3A).
3.6. Comparison of membrane hyperpolarization contributed by interposed AHPs and SIHPs
We quantified membrane hyperpolarization during the interposed AHP to determine whether the larger intracellularly-triggered AHP seen in aged neurons could account for the more extensive loss of action potentials seen during RSS in aged neurons. The degree of AHP-mediated membrane hyperpolarization within RSS was greater in aged (n=24) compared to young-adult (n=21) rat slices (F(1,43) = 6.50; p < 0.05). Consistent with the effects on spike failure (Fig. 3B), larger AHPs were induced in aged rat neurons at 7Hz (F(1,14) = 5.87; p < 0.05) and 15 Hz (F(1,14) = 6.52; p < 0.05) (Fig. 4). Thus, in aged neurons, increasing stimulation frequency increases both the AHP (Fig. 4) and the number of action potential missed (Fig. 3B), suggesting that the weaker synaptic reliability seen in aged slices reflects both the larger AHP as well as the weaker FF (Fig. 2B). The reason that the AHP-mediated hyperpolarization was greater with aging at higher frequencies may be that larger Ca2+ levels seen during FF and depolarization in these cells (Thibault et al., 2001; Hemond and Jaffe, 2005; Gant et al., 2006) primes the Ca2+-sensitive AHP mechanism.
Figure 4. Amplitude of the AHP component during RSS.

The amplitude of the AHP-mediated membrane hyperpolarization within RSS was measured by subtracting the membrane potential during the AHP from the membrane potential observed just prior to the AHP induction. Note that this is the AHP component which is added to the SIHP component, and that it is significantly smaller than the AHP triggered alone (Table 1). The degree of membrane hyperpolarization generated from the AHP was significantly greater in aged neurons compared to young-adult neurons. Values represent means ± SEM. Asterisks indicates p < 0.05.
Importantly, a similar relationship does not appear to hold between action potential throughput and the SIHP. The larger SIHP in young rat neurons is clearly not correlated with greater spike failure and cells with larger SIHP (younger rats) do not skip more action potential during RSS (see section 3.3). Furthermore, even when the sum of hyperpolarization from the SIHP and AHP is equivalent across aging (adding results of Fig. 2C to those of Fig. 4; see Fig. 5), there is less likelihood of spike failure in younger rat neurons (Fig. 3B). Thus, while both forms of hyperpolarization appear able to summate and contribute to total membrane hyperpolarization, the greater AHP in aged neurons appears to dampen spike throughput more effectively than the SIHP.
Figure 5. AHP and SIHP during aging.
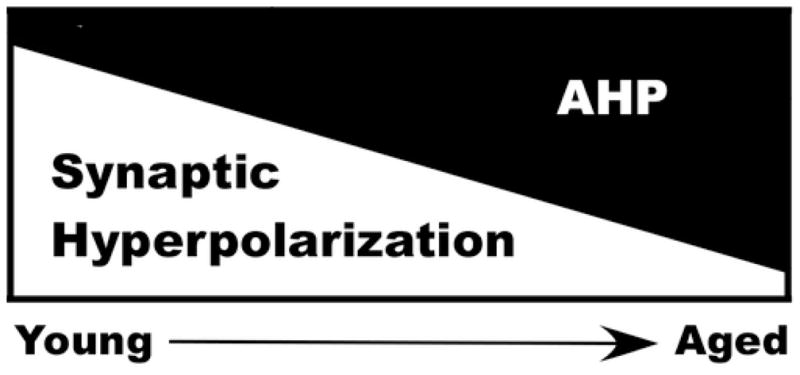
The SIHP is larger in young-adult animals and decreases with age, while the AHP is smaller in young-adult animals and increases with age, effectively shifting the two sources in opposite direction. Evidence reported here supports the model where, during aging, net membrane hyperpolarization is maintained, yet it is the AHP rather than the SIHP which governs action potential throughput.
4. Discussion
The present study provides the first integrative analysis of the effects of aging on parallel processes of synaptic excitability and multiple sources of postsynaptic hyperpolarization in controlling action potential generation during physiological frequencies of repetitive synaptic activation. The experiments were designed to address an unanswered question in the field of brain aging, namely: does the larger AHP affect synaptic throughput during repetitive activation? Several new observations on aging and excitability modulation emerge from these studies, including that 1) the SIHP, in sharp contrast to the intracellularly-induced AHP, is reduced with aging; 2) the SIHP, albeit being large and sustained, does not appear to regulate spike throughput as effectively as the AHP; and 3) with aging, the larger interposed AHP weakens spike throughput, and does so increasingly with higher frequencies of RSS.
4.1. Physiological implications
Because hippocampal cells firing in vivo exhibit bursts of activity on top of lower frequency, background firing (Muller and Kubie, 1989; Otto et al., 1991; Mizumori et al., 1992; Deadwyler et al., 1996), the effect of aging on the activity patterns generated here may have important implications for information processing. That is, we mimicked aspects of physiological activity patterns by interposing a four-spike AHP (40–50 Hz burst) within an RSS train of relatively slower frequencies (3–15 Hz), causing the AHP to summate with the activated SIHP. This paradigm resembles physiological conditions in which repetitive inputs through a set of synapses are modulated by a postsynaptic burst of activity (Borde et al., 1999), and also takes into account the dramatic increase in EPSP amplitudes seen during RSS. Under these conditions, our data show that the larger AHP associated with aging disrupts spike throughput at all frequencies of RSS, but with greater effects at higher theta frequencies and, therefore, potentially impairs summative processes critical for information storage (e.g., Landfield and Thibault, et al, 2001). Prior studies have shown that the AHP limits firing during accommodation protocols (Moyer et al., 1992; Thompson et al., 1996; Disterhoft and Oh, 2006; Gant et al., 2006), and the present studies extend these observations to conditions with physiological frequencies of background synaptic activity. These results are consistent with a prior study showing that FF of the EPSP is impaired with aging, but only at stimulation intensities above action potential threshold, when cellular communication is occurring (Thibault et al., 2001). Thus, our results lend further support to the view that the AHP may play a role in regulating plasticity (Disterhoft and Oh, 2007; Foster, 2007; Thibault et al., 2007).
4.2. Aging and the synaptically-induced hyperpolarization (SIHP)
During several seconds of RSS, and even in the presence of a sustained and large membrane hyperpolarization (~ 5 mV; Fig. 2), throughput was well-maintained in young-adult cells because of the robust FF seen in this group. Compared to younger cells, however, FF was reduced across RSS frequencies in aged neurons (Fig. 2B), but apparently was sufficient to maintain throughput in the presence of the SIHP (see section 3.3). Thus, during RSS, and irrespective of age or frequency, spike throughput was well maintained, balancing depolarizing and hyperpolarizing forces successfully.
Despite the spike maintenance in older neurons during control RSS, the hyperpolarization generated by the 4-spike AHP (beyond that provided by the SIHP), albeit modest, was more effective in dampening spike generation in cells from aged vs. young animals than was the larger SIHP in younger cells. This selective modulation may arise because the AHP is generated more proximally to the soma (Sah and Bekkers, 1996; Bekkers, 2000; Fernandez de Sevilla et al., 2007), and is mediated by greater or more topographically effective conductances, or is strongly Ca2+ dependent. That is, the aging difference in the AHP apparently depends upon both Ca2+ influx and Ca2+-induced Ca2+-release (Landfield and Pitler, 1984; Moyer et al., 1992; Disterhoft et al., 1993; Thibault and Landfield, 1996; Kumar and Foster, 2005; Gant et al., 2006), and Ca2+ may also induce increased shunting conductances to dampen spike initiation near the soma. In addition, multiple other somatic and dendritic K+ and Ca2+ conductances participate in shaping of synaptic inputs at CA1 pyramidal neurons (Sah and Bekkers, 1996; Magee, 1998; Bekkers, 2000; Johnston et al., 2003; Wu et al., 2004; Chen and Johnston, 2006; Metz et al., 2007), and their role in modulating spike throughput during RSS remain to be clarified.
We did not block IPSPs in the present study because prior work has shown that in the presence of GABAA receptor blockade, the remaining SIHP effectively regulates excitability during RSS (Pitler and Landfield, 1987). Nonetheless, a decrease in IPSPs and GABAergic neurotransmission has seen seen in the hippocampus with aging (Barnes and McNaughton, 1980; Billard et al., 1995; Potier et al., 2006), and it is not clear how much of the decline in SIHP is accounted for by this change. Clearly, therefore, further work will be needed to elucidate the underlying ionic mechanisms, physiological functions, and implications of aging changes in the SIHP.
4.3. Higher theta frequencies and aging
Prior subthreshold protocols have shown theta frequencies to be particularly efficient in inducing large membrane fluctuations (resonance), and improving membrane reliability during bouts of 7 Hz rhythms (Hu et al., 2002). Further, greater temporal coincidence through hippocampal networks also has been noted at these theta frequencies (Yeckel and Berger, 1998). Interestingly, hippocampal theta rhythms have long been associated with learning (Adey, 1970; Landfield, 1977) and memory storage processes (reviewed in Landfield and Thibault, 2001), and theta-patterned stimulation has been used extensively in LTP induction protocols, both in vitro and in vivo (Larson and Lynch, 1986; Pavlides et al., 1988; reviewed in Albensi et al., 2007). The present work provides the first quantitative analysis of the effect of the AHP on synaptic throughput during synaptic activation across several frequencies and shows that lower frequencies are less affected by aging. The decreased FF seen in aging, together with the greater number of skipped action potentials at higher theta frequencies, starting at 7 Hz, may well underlie the weaker theta-burst or primed-burst forms of LTP recorded in older animals (Moore et al., 1993; Rosenzweig et al., 1997; Tombaugh et al., 2002; Rex et al., 2005). Iterestingly, lower frequencies (3 Hz) appear to be more reliable in aging, and they are often associated with long-term depression (LTD) (Dudek and Bear, 1993; Norris et al., 1996; reviewed in Albensi et al., 2007), perhaps accounting in part for the weaker synaptic and cognitive phenotype seen in aging.
4.4. Summary
Our studies provide an initial analysis of the interplay between excitatory and inhibitory mechanisms in controlling spike generation during synaptic activation at multiple frequencies, and, in particular, of the relative influence of changes in these factors during aging. Notably, the results show that the balance between strength of EPSP facilitation on one hand, and degree, not of total hyperpolarization, but of selective components of hyperpolarization, on the other, modulates postsynaptic spike generation. Moreover, throughput at 7 Hz may have a small but significant advantage, possibly explaining in part, the prominence of this frequency range during exploration, learning and memory consolidation in rodents (Landfield, 1977; Winson, 1978; Destrade, 1982; Jeantet and Jaffard, 1983; reviewed in Landfield and Thibault, 2001; Buzsaki, 2002; Hasselmo, 2005).
Acknowledgments
We thank Dr. Philip Landfield for his discussions and comments on earlier versions of this manuscript. We also thank Drs. Blalock, Brewer and Norris for their critical reading of the paper. Supported by NIH grants AG04542, RR15592 and AG029268.
Footnotes
Disclosure Statement: The authors acknowledge that there are no actual or potential conflicts of interest that could inappropriately influence the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John C. Gant, Email: cgant@uky.edu.
Olivier Thibault, Email: othibau@uky.edu.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Adey WR. On-line computation in behavioral neurophysiology. Prog Brain Res. 1970;33:23–44. doi: 10.1016/s0079-6123(08)62441-3. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Oliver DR, Toupin J, Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp Neurol. 2007;204:1–13. doi: 10.1016/j.expneurol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Alger BE. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1984;52:892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Andersen P, Lomo T. Control of hippocampal output by afferent volley frequency. Prog Brain Res. 1967;27:400–412. doi: 10.1016/S0079-6123(08)63112-X. [DOI] [PubMed] [Google Scholar]
- Asaka Y, Mauldin KN, Griffin AL, Seager MA, Shurell E, Berry SD. Nonpharmacological amelioration of age-related learning deficits: the impact of hippocampal theta-triggered training. Proc Natl Acad Sci U S A. 2005;102:13284–13288. doi: 10.1073/pnas.0506515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492 (Pt 1):211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Neurophysiological comparison of dendritic cable properties in adolescent, middle-aged, and senescent rats. Exp Aging Res. 1979;5:195–206. doi: 10.1080/03610737908257198. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learn Mem. 1996;3:124–137. doi: 10.1101/lm.3.2-3.124. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–452. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Bekkers JM. Distribution of slow AHP channels on hippocampal CA1 pyramidal neurons. J Neurophysiol. 2000;83:1756–1759. doi: 10.1152/jn.2000.83.3.1756. [DOI] [PubMed] [Google Scholar]
- Billard JM, Lamour Y, Dutar P. Decreased monosynaptic GABAB-mediated inhibitory postsynaptic potentials in hippocampal CA1 pyramidal cells in the aged rat: pharmacological characterization and possible mechanisms. J Neurophysiol. 1995;74:539–546. doi: 10.1152/jn.1995.74.2.539. [DOI] [PubMed] [Google Scholar]
- Borde M, Bonansco C, Buno W. The activity-dependent potentiation of the slow Ca2+-activated K+ current regulates synaptic efficacy in rat CA1 pyramidal neurons. Pflugers Arch. 1999;437:261–266. doi: 10.1007/s004240050778. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Voltage-gated ion channels in dendrites of hippocampal pyramidal neurons. Pflugers Arch. 2006;453:397–401. doi: 10.1007/s00424-006-0097-y. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrade C. Two types of diencephalically driven RSA (theta) as a means of studying memory formation in mice. Brain Res. 1982;234:486–493. doi: 10.1016/0006-8993(82)90892-7. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Pharmacological and molecular enhancement of learning in aging and Alzheimer’s disease. J Physiol Paris. 2006;99:180–192. doi: 10.1016/j.jphysparis.2005.12.079. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6:327–336. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Moyer JR, Jr, Thompson LT, Kowalska M. Functional aspects of calcium-channel modulation. Clin Neuropharmacol. 1993;16(Suppl 1):S12–24. doi: 10.1097/00002826-199316001-00003. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez de Sevilla D, Fuenzalida M, Porto Pazos AB, Buno W. Selective shunting of the NMDA EPSP component by the slow afterhyperpolarization in rat CA1 pyramidal neurons. J Neurophysiol. 2007;97:3242–3255. doi: 10.1152/jn.00422.2006. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?--Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hemond P, Jaffe DB. Caloric restriction prevents aging-associated changes in spike-mediated Ca2+ accumulation and the slow afterhyperpolarization in hippocampal CA1 pyramidal neurons. Neuroscience. 2005;135:413–420. doi: 10.1016/j.neuroscience.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeantet Y, Jaffard R. Influence of the medial septal nucleus on the excitability of the commissural path-CA1 pyramidal cell synapse in the hippocampus of freely moving mice. Neuroscience. 1983;8:291–297. doi: 10.1016/0306-4522(83)90067-2. [DOI] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS, Campbell LW, Hao SY, Landfield PW. Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science. 1989;245:1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Intracellular calcium stores contribute to increased susceptibility to LTD induction during aging. Brain Res. 2005;1031:125–128. doi: 10.1016/j.brainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988;8:1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Hu H, Ramakers GM, Storm JF. Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. J Physiol. 2001;536:809–823. doi: 10.1111/j.1469-7793.2001.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW. Different effects of posttrial driving or blocking of the theta rhythm on avoidance learning in rats. Physiol and Behav. 1977;18:439–445. [Google Scholar]
- Landfield PW. ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol Aging. 1987;8:346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Aging-related increase in hippocampal calcium channels. Life Sci. 1996;59:399–404. doi: 10.1016/0024-3205(96)00318-9. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Thibault O. A neuroholographic model of memory: Theta rhythms, facilitation and Calcium channels. In: Gold PE, Greenough WT, editors. Memory consolidation: Essays in honor of James L. McGaugh. Washington, DC: American Psychological Association; 2001. pp. 295–320. [Google Scholar]
- Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150:85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J Neurophysiol. 1986;56:797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Res Rev. 2006;5:255–280. doi: 10.1016/j.arr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Weible AP, Disterhoft JF. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. J Neurophysiol. 2001;86:1839–1857. doi: 10.1152/jn.2001.86.4.1839. [DOI] [PubMed] [Google Scholar]
- Metz AE, Spruston N, Martina M. Dendritic D-type potassium currents inhibit the spike afterdepolarization in rat hippocampal CA1 pyramidal neurons. J Physiol. 2007;581:175–187. doi: 10.1113/jphysiol.2006.127068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz AE, Jarsky T, Martina M, Spruston N. R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:5763–5773. doi: 10.1523/JNEUROSCI.0624-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Barnes CA, McNaughton BL. Differential effects of age on subpopulations of hippocampal theta cells. Neurobiol Aging. 1992;13:673–679. doi: 10.1016/0197-4580(92)90089-g. [DOI] [PubMed] [Google Scholar]
- Mockett B, Coussens C, Abraham WC. NMDA receptor-mediated metaplasticity during the induction of long-term depression by low-frequency stimulation. Eur J Neurosci. 2002;15:1819–1826. doi: 10.1046/j.1460-9568.2002.02008.x. [DOI] [PubMed] [Google Scholar]
- Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3:57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- Morgan SL, Teyler TJ. Electrical stimuli patterned after the theta-rhythm induce multiple forms of LTP. J Neurophysiol. 2001;86:1289–1296. doi: 10.1152/jn.2001.86.3.1289. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The firing of hippocampal place cells predicts the future position of freely moving rats. J Neurosci. 1989;9:4101–4110. doi: 10.1523/JNEUROSCI.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981;212:957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol. 2004;92:2027–2039. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, Wible CG. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Ouanounou A, Zhang L, Charlton MP, Carlen PL. Differential modulation of synaptic transmission by calcium chelators in young and aged hippocampal CA1 neurons: evidence for altered calcium homeostasis in aging. J Neurosci. 1999;19:906–915. doi: 10.1523/JNEUROSCI.19-03-00906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Landfield PW. Postsynaptic membrane shifts during frequency potentiation of the hippocampal EPSP. J Neurophysiol. 1987;58:866–882. doi: 10.1152/jn.1987.58.4.866. [DOI] [PubMed] [Google Scholar]
- Potier B, Jouvenceau A, Epelbaum J, Dutar P. Age-related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience. 2006;142:187–201. doi: 10.1016/j.neuroscience.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Potier B, Rascol O, Jazat F, Lamour Y, Dutar P. Alterations in the properties of hippocampal pyramidal neurons in the aged rat. Neuroscience. 1992;48:793–806. doi: 10.1016/0306-4522(92)90267-6. [DOI] [PubMed] [Google Scholar]
- Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, Lynch G. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci Lett. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Learning-induced enhancement of postsynaptic potentials in pyramidal neurons. J Neurophysiol. 2002;87:2358–2363. doi: 10.1152/jn.2002.87.5.2358. [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, Skaggs WE, Weaver KL. The effect of aging on experience-dependent plasticity of hippocampal place cells. J Neurosci. 1997;17:6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff NP, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493 (Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Alger BE. Characterization of an early afterhyperpolarization after a brief train of action potentials in rat hippocampal neurons in vitro. J Neurophysiol. 1990;63:72–81. doi: 10.1152/jn.1990.63.1.72. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wu WW, Chan CS, Disterhoft JF. Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J Neurophysiol. 2004;92:2346–2356. doi: 10.1152/jn.00977.2003. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Spatial distribution of potentiated synapses in hippocampus: dependence on cellular mechanisms and network properties. J Neurosci. 1998;18:438–450. doi: 10.1523/JNEUROSCI.18-01-00438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, Nguyen PV. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. J Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


