Abstract
Background
Nearly half of all infiltrating leukocytes in rejecting human allografts are macrophages, yet, in comparison with T cells, much less is known about the contribution of this cell type to rejection. Our laboratory has previously described models of rejection of human skin or artery grafts in immunodeficient mouse hosts mediated by adoptively transferred allogeneic T cells. However, mature human monocyte/macrophages have consistently failed to engraft in these animals. Here, we describe the introduction of human CD68+ macrophages into irradiated immunodeficient mice by transplantation of enriched CD34+ hematopoietic stem-cells isolated from peripheral blood of G-colony-stimulating factor pretreated adults.
Methods
We investigated strains of immunodeficient mice bearing human tissue grafts (skin and artery) inoculated with 1×106 human CD34+ adult hematopoietic stem cells, peripheral blood monuclear cells autologous to the CD34 donor, or both for human cell engraftment.
Results
In the absence of T cells, CD68+ CD14+ macrophages infiltrate allogeneic human skin but produce little injury or thrombosis. Both responses are enhanced when combined with adoptive transfer of T cells autologous to the hematopoietic stem cells as exemplified by the induction of the macrophage activation marker CD163. CD68+ macrophages also infiltrate allogeneic arterial interposition grafts, producing intimal expansion and calcification in the absence of T cells.
Conclusions
These new models may be used to study the role of human macrophages in transplant rejection and other pathologies in vivo.
Keywords: Allograft rejection, Macrophages, Hematopoietic stem cells, Immunodeficient mice
Cell-mediated rejection responses of allogeneic organs often involve damage to graft blood vessels, especially of the endothelial cell (EC) lining (1, 2). The mechanisms underlying injury of allogeneic ECs in clinical transplantation are elusive for several reasons. First, commonly used rodent models may be misleading because human T-cell responses to allogeneic ECs differ in significant ways from those that occur in rodents. For example, rodent EC do not basally express major histocompatibility complex (MHC) class II molecules or the costimulator lymphocyte function-associated antigen-3, yet may express the costimulator CD86. In vitro, human EC can activate allogeneic CD4+ and CD8+ memory T cells through direct recognition of allogeneic MHC molecules whereas mouse EC cannot activate CD4+ effector cells (3). Second, observational and interventional studies of human transplant recipients are significantly limited by the inability to control experimental variables in clinical settings. Third, human cell culture experiments do not adequately approximate allograft rejection, an in vivo process. For these reasons, we have developed several models using C.B-17 severe combined immunodeficiency (SCID)/beige (bg) immunodeficient mouse hosts in which human memory T-cell responses to allogeneic skin or artery graft tissues can be studied in vivo (4–7). However, only T cells and, to a limited extent, B cells successfully engraft in these animals despite the presence of natural killer (NK) cells, monocytes, and dendritic cells in the initial inoculum (8). The models thus differ from rejecting clinical allografts in which macrophages, derived from blood monocytes, comprise as many as half of infiltrating leukocytes (9).
Since mature human blood monocytes are not readily transferable into mouse hosts, we turned to human hematopoietic stem-cell (HSC) transplantation based on the knowledge that transplantation of mouse HSC into an irradiated autologous host can fully regenerate the hematopoietic system, including macrophages. However, immunocompetent mice rapidly reject human hematopoietic cells, including HSCs by a combination of innate and adaptive immune effector mechanisms. Compromise of the adaptive immune system of mice first spontaneously arose in C.B-17 animals by mutation in the Prkdc gene whose protein product participates in immunoglobulin (Ig) and T-cell receptor rearrangements (10). Animals bearing this trait are referred to as having the SCID mutation. Despite an essentially absent adaptive immune system, C.B-17 SCID animals are still significantly resistant to adoptive transfer of human peripheral blood lymphocyte (PBL). Host NK cells were proposed to play a major role in this resistance because of their known capacity to reject allogeneic hematopoietic cells (11). Consistent with this interpretation, we found that resistance to adoptive transfer of human PBL was reduced in animals treated with antibody to asialo GM1, which depletes NK cells, or in C.B-17 SCID animals that also have the bg mutation that impairs the function of NK cells (as well as that of granulocytes and platelets) (4–6). The SCID mutation was bred into nonobese diabetic (NOD) mice by others to study the role of the adaptive immune system in the autoimmune phenotypes to which wild-type NOD mice are subject (12). In addition to their propensity to develop autoimmune disease, NOD mice are also deficient for certain class I MHC-related genes that play a role in the positive selection of NK cells from their progenitors (13). Consequently, compared with C.B-17 SCID mice, NOD SCID animals are additionally deficient in mature NK cells. For this reason, NOD SCID animals are more accepting of adoptively transferred human PBL than are C.B-17 SCID animals (14). (It was unclear if NOD SCID animals are also more accepting of adoptively transferred human T cells than are C.B-17 SCID/bg mice since these two strains had not previously been compared.) Despite their acceptance of mature T cells, NOD SCID animals still resist transplantation of human HSCs, even when irradiated to further impair immunity and to establish empty bone marrow niches. An additional mutation, namely knock out of the common gamma chain of the cytokine receptor family (γc), was introduced into NOD SCID animals to further compromise innate effector cells, many of which depend on γc signaling cytokines for their development (14). The resultant NOD SCID γc−/− strain has been reported to be an excellent host for human HSCs, allowing significant reconstitution of a human hematopoietic and immune system by HSCs after irradiating. There have not, to the best of our knowledge, been systematic comparisons between irradiated C.B-17 SCID/bg and NOD SCID γc−/− animal as recipients of human HSCs. There is also little known about how these animal strains compare with regard to acceptance of vascularized human tissues that may be recognized by elements of the innate immune system other than NK cells. Indeed many aspects of innate mechanisms of xenoreactivity are still unappreciated. For example, it has only recently been shown that NOD animals contain an allelic form of the signal regulatory protein (SIRP)-α inhibitory receptor on their macrophages that can interact with a human CD47 counter-receptor whereas non-obese diabetes-resistant (NOR) mice express an allele that cannot (15). It is not known which allelic forms SIPR-α are expressed in C.B-17 mice. The key points are that innate immune responses of different mouse strains to xenogeneic human grafts are not yet predictable and may vary with the type of graft.
In the present study, we directly compare the recently developed-NOD SCID γc−/− mouse strain(16), with SCID/bg animals as recipients of human skin, artery, or HSCs isolated from the peripheral blood of G-colony-stimulating factor (CSF)-mobilized adult donors. This source of HSCs allows us to combine stem-cell engraftment with adoptive transfer of alloreactive memory T cells that are important in allograft rejection (17). We find that SCID/bg adult animals offer major technical advantages over NOD SCID γc−/− as recipients of human skin and artery, whereas NOD SCID γc−/− mice much more readily accept human blood HSCs. However, human HSCs do give rise to mononuclear phagocytes in SCID/bg hosts and we use this model to show that human macrophages can actively contribute to experimental allograft rejection.
MATERIALS AND METHODS
Animals
All procedures and animal breeding was performed under protocols approved by the Yale Institutional Animal Care and Use Committee. SCID/bg mice were purchased from Taconic Farms, Germantown, NY and NOD/SCID γc−/− mice were generated from two breeding pairs of NOD/SCID γc−/− mice (a generous gift by Dr. Leonard Shultz, Jackson Laboratories, Bar Harbor, ME). SCID/bg animals were considered “leaky” at IgG levels more than 1 µg/mL measured by ELISA and excluded from experimental use (18). All animals were housed individually in microisolator cages and were fed autoclaved food and water. NOD/SCID γc−/− animals were routinely given Enrofloxacin (Baytril, 50 µg/kg, Bayer, Shawnee Mission, KS) in their drinking water.
Skin and Artery Transplantation
Human skin from deceased donors was obtained through the Yale University Skin Bank under a protocol approved by the Yale Human Investigation Committee and grafted to SCID/bg mice as previously described (5, 18). Distal branches of healthy appearing human epicardial coronary arteries were harvested from explanted recipient hearts during cardiac transplantation or from epigastric arterial segments obtained from living-related kidney donors under protocols approved by the Yale Human Investigation Committee and by the New England Organ Bank. Arterial grafts were implanted in immunodeficient mice by interposing size-matched (~1 mm diameter, 3–5 mm length) human artery segments into the infrarenal aorta of a 5- to 8-week-old recipient mouse using an end-to-end anastomotic technique as previously described (5).
Preparation and Characterization of Adult Human HSCs
Discarded anonymized human leukapheresis collections from adult blood enriched for CD34+ stem cells through mobilization by G-CSF were obtained from the Yale-New Haven Hospital Blood Bank under a protocol approved by the Yale Human Investigation Committee and peripheral blood mononuclear cells (PBMCs) were isolated using Lymphocyte Separation Medium (MP Biochemicals, Irvine, CA) according to the manufacturer’s instructions. CD34+ HSCs were further enriched using anti-human CD34 microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Populations enriched for CD34+ cells and residual CD34-depleted PBMCs were aliquotted and cryopreserved in 10% dimethyl sulfoxide/90% fetal bovine serum at −196°C as previously described (5). In some cases, lineage marker negative CD34+ HSCs were purified using a RoboSep automated cell separator (Stem Cell technologies, Vancouver, CA) with a positive selection kit (catalog number 18056) according to the manufacturer’s instructions. Cell preparations to be inoculated into animals were analyzed by two-color flow cytometry using a FACsort cytometer (BD Pharmingen, San Diego, CA) and CellQuest software. Approximately 1×105 cells were washed and incubated with a mouse anti-human CD34-fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (Miltenyi Biotech) as well as with one of the following phycoerythrin (PE)-labeled mouse anti-human antibody to human surface markers: CD3, CD11c, CD56, CD19 (all from Beckman Coulter Immunotech, Westbrook ME) or CD68 (BD Pharmingen).
HSC Engraftment
In pilot experiments, we found that HSC engraftment was not observed in the absence of γ-irradiation into SCID/bg or NOD SCID γc−/− mice, and we determined the optimal dose of γ-irradiation of SCID mouse recipients for HSC engraftment to be 350 cGy. A major effect of irradiation is believed to be opening up stem-cell niches within the bone marrow, but irradiation also further suppresses innate immunity. Although animals receiving only PBMC did not need to be irradiated to take up adoptively transferred T cells, irradiated animals receiving adoptively transferred PBMC reconstituted with much lower numbers of cells (1×107) compared with non-irradiated animals (3×108). Irradiation plus high numbers of PBMC (3×108) was lethal to the animals. Still, PBMC injection into irradiated hosts yielded only T cell (and B cell) reconstitution and did not allow for engraftment of human monocytes or other human immune cells accessory cells.
We also compared engraftment by lineage-depleted HSCs, partly enriched HSCs and HSC-depleted PBMCs and found that only partially enriched HSCs led to human monocyte/macrophage engraftment. In the optimized protocol for human skin grafts, immunodeficient mice, with or without healed human skin, were irradiated and injected with 1×106 enriched HSCs in a volume of 200 µL in sterile saline via lateral tail vein at 8 to 18 hr post-irradiation. Animals were provided with drinking water containing Enrofloxacin for the duration of the experiment. In experiments where T-cell adoptive transfer was performed, 3×108 CD34+-depleted PBMCs from the same donor as the CD34+-enriched HSCs were inoculated intraperitoneally into animals 2 weeks after CD34+ HSC injection. Control groups for T-cell experiments included nonirradiated animals not receiving human cells and nonirradiated animals inoculated with 3×108 CD34+-depleted PBMCs at the same time as the irradiated HSC recipients. In the artery model, animals were irradiated and given 1×106 enriched HSCs and were allowed to recover for up to 2 weeks postinjection. At this point, the animals seemed strong enough to survive the artery transplant surgeries. We chose to introduce HSC before surgery because the irradiation that was required to permit HSC engraftment produced injury and neointimal formation in artery grafts when the sequence was reversed in pilot experiments. In the case of skin grafts, we instead grafted skin first and then transferred HSCs. We used this sequence because previous experience with adoptive transfer of T cells had shown that human skin microvessels failed to inosculate with mouse microvessels in the wound bed of human T cells were already in the circulation. No evidence of injury was seen in human skin grafts after irradiation.
Flow Cytometric Analyses of Recipient Peripheral Blood and Bone Marrow
The presence of human cells in peripheral blood and bone marrow was evaluated by flow cytometry. In brief, at 6 weeks post-CD34+-enriched cell inoculation, peripheral blood was collected from heparinized retro-orbital venous samples. The animals were then euthanized and single-cell suspensions were prepared from bone marrow cells flushed from femurs and tibias and spleen cells expressed from the splenic capsule into approximately 1 mL phosphate-buffered saline/1% bovine serum albumin. Erythrocytes were lysed with ammonium chloride and the cells were washed and resuspended in staining buffer (phosphate-buffered saline, 0.1% bovine serum albumin). One cell sample was double stained with mouse anti-human CD45-FITC (Beckman Coulter Immunotech) and PE-conjugated rat anti-mouse CD45 (Sigma-Aldrich, St. Louis, MO) as described (11). Other samples of blood, bone marrow, and spleen cells were singly stained with PE-conjugated mouse anti-human CD11c, CD19, CD68, and CD56 (Beckman Coulter Immunotech). IgG-FITC and IgG-PE were used as negative control antibodies. Samples were analyzed using a FACsort and analyzed with Cell Quest software (BD Biosciences, Mountain View, CA).
Histology and Immunohistochemistry of Graft Tissues
Human skin grafts were harvested at indicated times and were formalin-fixed or paraffin-embedded or snap frozen in optimum cutting temperature (Satura Finetek USA, Torrance, CA) before sectioning. Immunostaining of slides was performed as described previously (18) using isotype-matched, nonbinding control Abs, or the following mouse anti-human monoclonal Abs: CD3 (UCHT1, IgG1) and CD56 from Dako (Carpinteria, CA); CD68, CD163, and CD20 from Vector Laboratories (Burlingame, CA); and CD11c and CD14 from BD Pharmingen. A biotinylated secondary antibody (Jackson Immunoresearch, Westgrove, PA) was used to detect the primary antibody. All anti-human mAbs used in this study are human specific and do not cross-react with mouse tissue. Mouse neutrophils were stained using rat anti-mouse Ly-6G and Ly6c antibodies and biotin-labeled mouse anti-rat IgG 2b secondary antibody (both from BD Pharmingen). In all cases, an avidin binding complex and AEC detection kit was used for color development (Vector Laboratories). The degree of skin graft microvascular damage was evaluated from hematoxylin-eosin-stained sections by a dermatopathologist (J.M.M.) blinded to the treatment groups using a previously described grading system (19). In brief, the percentage of dermal vessels showing injury, defined as EC loss or sloughing, was assessed from an average of three high-power (×200) fields using the following semiquantitative grading scale: grade 0, all vessels patent and uninvolved; grade 1, less than 25% of vessels show injury; grade 2, 50% of vessels show injury; and grade 3, more than 75% of vessels show injury. Artery grafts were harvested approximately 4 weeks after transplantation and analyzed with the same methods used for skin except the presence of vascular calcification was assessed using a Von Kossa stain.
Data Analysis
Specimens were pooled from several experiments for scoring. Results are expressed as the mean±SD. Statistical analysis was done using a one-way ANOVA followed by a Bonferroni correction. Differences between groups are considered as significant when P less than 0.05.
RESULTS
Human Skin and Artery Grafting in Immunodeficient Mice
We initially compared the ability of SCID/bg and NOD/SCID γc−/− animals to accept human skin grafts from the same donors. The majority (>95%) of skin grafts on SCID/bg animals seemed healthy with minimal injury or infiltration. In contrast, the majority of grafts (~75%) from the same skin sources in two independent experiments on NOD/SCID γc−/− animals showed extensive perivascular infiltrates and injury (Fig. 1). These infiltrating leukocytes stained positively for mouse neutrophil markers (Fig. 1C and D). This was an unexpected finding as human skin grafts from the same donor placed on C.B-17 SCID/bg animals are not infiltrated by mouse cells. (We did not test allogeneic mouse skin transplants on NOD SCID γc−/− animals because mouse skin grafts, unlike human skin grafts, are avascular and thus not a relevant comparator.) The presence of inflammation in control grafts precluded analysis of the human allogeneic response and no further experiments using human skin grafts were performed in NOD/SCID γc−/− recipients.
FIGURE 1.
Nonobese diabetic (NOD) severe combined immunodeficiency (SCID) γc−/− mice mount an innate immune response to human skin grafts. Human skin grafts harvested and stained with hematoxylin-eosin or for the presence of mouse neutrophils (Ly-6c, Gr-1) (n=4 per group). (A and B) hematoxylin-eosin of C.B.-17 SCID/bg mouse and NOD SCID γc−/−, respectively; (C and D) IHC for mouse neutrophils of C.B-17 SCID/bg mouse and NOD SCID γc−/−, respectively. 400× magnification of mouse neutrophils (D-inset).
We next compared the ability of those same strains of mice to accept human artery segments as infrarenal aortic interposition grafts again using the same donors (4). In contrast to a more than 95% success rate in SCID/bg recipients, NOD SCID γc−/− animals uniformly developed peripheral hind limb paralysis (requiring euthanasia) or died (by day 1 due to postsurgical vascular thrombosis). Two different skilled microsurgical operators (T.Y and Y.W.) experienced similar adverse outcomes in NOD SCID γc−/− animals over several operating sessions. Without further manipulations, such as antiplatelet or anticoagulation therapy, we find that this animal strain cannot be used for vascular graft surgery and no further experiments using artery grafts were performed in this study using NOD/SCID γc−/− recipients.
Human HSC Engraftment and Adoptive Transfer of Human PBMC
CD34+-enriched circulating mononuclear cells were isolated from leukapheresis products from G-CSF-mobilized adult donors as described in the Materials and Methods section. Approximately 70% of theses cells are lin− CD34+ HSCs (Table 1). Among CD45+ cells at least 50% were also positive for CD34, consistent with differentiating progenitors. These enriched HSCs were introduced into SCID/bg or NOD SCID-γc−/− irradiated adult recipients.
TABLE 1.
Flow cytometric data of CD34+ preparation before inoculation into animals
| Phenotype | Percentage±SD |
|---|---|
| CD34 (stem cell) | 41.80±0.97 |
| CD3 (T cell) | 18.13±3.63 |
| CD19 (B cell) | 5.53±2.33 |
| CD56 (NK cell) | 3.05±0.33 |
| CD11b (myeloid cells) | 40.80±3.45 |
| CD11c (dendritic cells and myeloid cells) | 44.33±2.82 |
| CD68 (macrophage) | 18.12±3.46 |
| Lin- | 67.49 |
Percentage±SD (standard deviation) of certain types of human cells contained in CD34+ isolation of G-CSF mobilized leukapheresis product injected into animals. Data pooled from 3 independent HSC donors.
HSC, hematopoietic stem cell; CSF, colony-stimulating factor.
Animals were sacrificed at 6 weeks postinoculation and mononuclear cells from peripheral blood, bone marrow, and spleen were collected and analyzed by flow cytometry. Peripheral blood from SCID/bg (n=14) contained low levels of human CD45+ cells. In contrast, NOD SCID γc−/− (n=5) animals demonstrated significantly larger populations of circulating CD45+ human cells, comprising up to 28% of the total PBMCs. In bone marrow, CD45+ cells were present in both strains of mice but the numbers in NOD SCID-γc−/− were again much greater. Bone marrow from NOD SCID γc−/− animals showed readily detectable populations of CD11b, CD11c, CD56, and CD19 positive cells (Table 2). We were unable to further phenotype the few human cells in the bone marrow of SCID/bg animals due to the low level of engraftment.
TABLE 2.
Flow cytometric analysis of cells isolated from peripheral blood and bone marrow in SCID/bg and NOD SCID-γc−/−mice
| Peripheral blood |
Bone narrow |
||||||
|---|---|---|---|---|---|---|---|
| Mouse strain | Treatment | CD45 | CD45 | CD11c | CD11b | CD56 | CD3 |
| SCID/Bg | no HSC | 0 | 0 | 0 | 0 | 0 | 0 |
| NOD/SCID-γc−/− | no HSC | 0 | 0 | 0 | 0 | 0 | 0 |
| SCID/Bg | HSC | 0.28±0.19 | 0.29±0.17 | 0 | 0.22±0.13 | 0 | 0 |
| NOD/SCID-γc−/− | HSC | 28.41±29.60 | 56.9±22.30 | 32.66±21.30 | 2.76±2.05 | 1.89±0.77 | 0 |
Percentage of human CD markers on cells isolated from peripheral blood and bone marrow. Data represents averages and standard deviations.
HSC, hematopoietic stem cell; SCID/bg, severe combined immunodeficiency/beige; NOD, nonobese diabetic.
Since the adoptive transfer of PBMC yields only T and B cell reconstitution and inoculations of CD34+ HSC allows for engraftment of human macrophages, we wished to see what human cell types would engraft in animals that received both types of inocula. To analyze the effects of combining HSC engraftment with PBMC adoptive transfer, SCID/bg animals were given the following treatments (a) irradiation plus CD34+-enriched HSCs (n = 10), (b) irradiation and HSCs followed by 3×108 CD34+-depleted PBMCs (n = 6), (c) CD34+-depleted PBMCs alone (n = 6), or (d) no cells (n = 6). Table 3 shows that the levels of human cells were found in blood, bone marrow, and spleen of treated animals 6 weeks post-HSC inoculation. The lowest frequency of human CD45+ cells in all three compartments were found in animals receiving only CD34+-enriched HSCs, intermediate levels in animals receiving only PBMCs, and the highest levels in animals receiving both HSCs and PBMCs.
TABLE 3.
Summary of flow cytometric data of human cells isolated from peripheral blood, bone marrow, and spleen
| Peripheral blood |
Bone marrow |
Spleen CD45 |
|||||
|---|---|---|---|---|---|---|---|
| Inoculum | CD45 | CD45 | CD11b | CD11c | CD19 | CD56 | |
| Control | 0 | 0 | 0.12±0.14 | 0.07±0.010 | 0.04±0.01 | 0 | |
| HSCs | 0.44±0.09 | 6.77±4.63 | 1.92±0.31 | 0.55±0.06 | 0 | 0.18±0.05 | 1.95±1.22 |
| PBMCs | 3.36±1.02 | 3.37±0.01 | 0.15±0.01 | 0.09±0.01 | 0 | 0.01±0.01 | 4.19±2.82 |
| CD34+ and PBMC | 9.99±4.06 | 8.36±4.63 | 0.84±0.28 | 2.19±0.59 | 0.13±0.32 | 0.36±0.35 | 9.47±2.82 |
Human CD markers on cells isolated from peripheral blood, bone marrow and spleen of SCID/bg mice inoculated with human HSCs and/or PBMCs as described in Methods. Data represent averages±SD.
HSCs, hematopoietic stem cells; SCID/bg, severe combined immunodeficiency/beige; PBMCs, peripheral blood mononuclear cells.
Bone marrow suspensions from mice inoculated with no cells, CD34+, CD34+, and PBMC or PBMCs alone were analyzed by flow cytometry for human-specific markers (Table 3). Animals that received only single sources of cells, that is, HSCs or PBMCs did not display CD19 (B cells) or CD56 (NK cells) positive staining. However, CD19 and CD56 positive cells were present in recipients of HSCs plus PBMCs. Animals receiving PBMCs showed a small number of CD11c+ dendritic cells which when combined with CD34+ HSC inoculates expanded significantly. Staining for CD11b+ (primarily macrophages) was present in all groups although the doubly inoculated group showed the highest number of positive cells. Taken together, our data indicate that transfers of human HSC and PBMC may be used in combination to reconstitute a human monocyte/macrophage and a human T-cell compartment in the same animal.
Combined Human Skin and HSC Engraftment With and Without PBMC Transfer
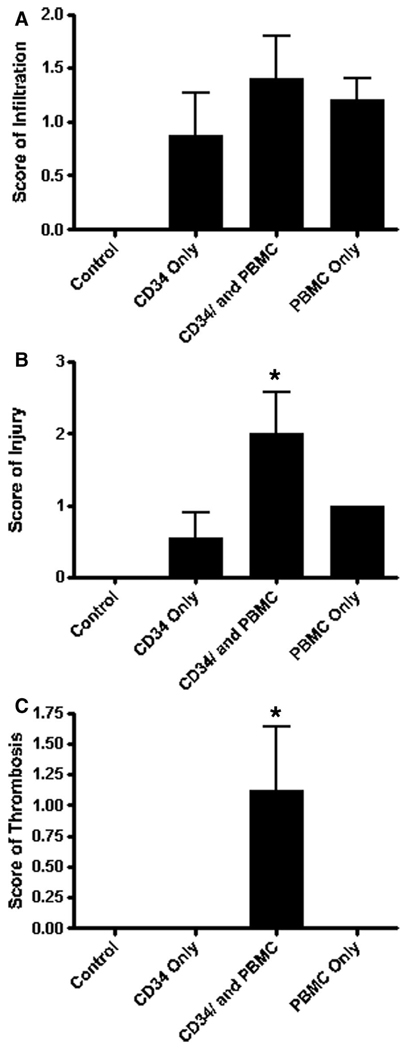
Based on our previous model of human T-cell transfer into SCID/bg mice bearing allogeneic human skin grafts, we wanted to see if human macrophages (produced by CD34+ HSC transfer), which have only limited capabilities to recognize allogeneic tissues, would also home to human skin grafts, and, if they did so, would they also produce damage to a human skin graft and its human endothelial-lined vascular supply. We analyzed the effects of HSC engraftment in SCID/bg animals bearing human skin from a donor allogeneic to the HSCs. Skin was placed first and allowed to heal for 5 weeks. Animals were irradiated and inoculated with human CD34+-enriched HSCs from a second donor. These results were compared with animals receiving skin and CD34+-depleted PBMC, skin, and HSCs plus CD34+-depleted PBMCs (the latter from the same donor as the HSCs) or skin only (control). Human skin grafts were harvested from animals at 6 weeks after HSC engraftment and were scored by a dermatopathologist, blinded to the treatment group, for the degree of infiltration, endothelial injury, and microvascular thrombosis (Fig. 2). All animals that received HSCs or PBMCs, or both showed some leukocytic infiltration, but these were not statistically different among the various groups of animals. Animals receiving HSCs plus PBMCs showed significantly stronger rejection responses, characterized by EC injury and thrombosis, compared with all other groups.
FIGURE 2.
Severe combined immunodeficiency/bg mice receiving both CD34+ hematopoietic stem cells and autologous peripheral blood mononuclear cells have significantly more injury and thrombosis than either cell type alone. Hematoxylineosin sections of human skin grafts were scored by a dermatopathologist (J.M.M.) blinded to treatment conditions for degree of (A) infiltration; (B) endothelial cell injury, and (C) microvascular thrombosis. Control animals bore human skin grafts with no human cell inocula.
Human cells infiltrating the human skin grafts were further characterized by immunohistochemistry (Fig. 3). Animals that received HSCs showed significant infiltrates composed almost entirely of human CD68+/CD14+ macrophages (Fig. 3F). Infiltrating cells lacked staining for human CD3, CD11c, CD56, CD15, or CD19 (data not shown). Animals that received only PBMC had infiltrates composed entirely of CD3+ T cells (Fig. 3A), consistent with previous experiments (4). Grafts from animals receiving both HSCs and PBMCs had mixed infiltrates of CD68+ and CD3+ cells (Fig. 3C and D). Skin grafts from animals receiving both HSCs and PBMCs also showed macrophage activation markers, not observed in the absence of T cells. These grafts demonstrated positive staining for both iNOS and CD163 (a macrophage activation marker). These data suggest that HSCs give rise to infiltrating human macrophages, whereas PBMCs give rise to T cells in SCID/bg mouse hosts, and that these cell populations collaborate to produce more EC damage and especially thrombosis in skin allografts than that produced by either cell type alone.
FIGURE 3.
Phenotype of human leukocytes infiltrating human skin graft infiltration is dependent on cell type inoculated. (I) Photomicrographs of hematoxylin-eosin sections of human skin harvested from severe combined immunodeficiency/bg mice (A) animals receiving 3×108 human peripheral blood mononuclear cells (PBMCs) alone and stained for CD3; (B) animals receiving 3×108 human PBMC alone and stained for CD68; (C) animals receiving 3×108 human PBMCs and 1×106 hematopoietic stem cells (HSCs) and stained for CD3; (D) animals receiving 3×108 human PBMCs and 1×106 human HSCs and stained for CD68; (E) animals receiving 1×106 HSCs and stained for CD3; and (F) animals receiving 1×106 HSCs and stained for CD68. (G) animals receiving 1×106 HSCs and stained for CD14 (inset is control animals bearing human skin grafts and no cells); (H) animals receiving 1×106 HSCs and stained for CD68; (I) animals receiving 3×108 human PBMCs and 1×106 HSCs and stained for iNOS; and (J) animals receiving 3×108 human PBMCs and 1×106 HSCs and stained for CD163 (a macrophage activation marker).
Combined Artery and HSC Engraftment
In a final series of experiments, we investigated the effects of allogeneic HSCs on artery grafts. Irradiated animals were reconstituted with 1×106 enriched HSCs and received human artery transplants within 2 weeks (precise time depending on tissue availability). Human artery grafts were harvested at 4 weeks postsurgery and analyzed by histology and immunohistochemistry. Artery grafts showed large neointimas in all animals that received HSCs compared with untreated paired control artery (Fig. 4A). Cells in the neointima of animals receiving HSCs stained positively for CD68+ macrophages (Fig. 4B and C). No human CD3 (Fig. 4D and E), CD11c, or CD19 cells could be found in these grafts (data not shown). Arteries of animals that received HSCs also showed amorphous hematoxylin staining deposits. Von Kossa stain (Fig. 4E and F) revealed that these deposits contain calcium salt. No calcium deposition was seen in control vessels. This extent of dystrophic calcification had not been previously observed in the arteries of animals receiving PBMC alone (20). Our new experiments suggest that CD68+ infiltrating macrophages can damage the human arterial grafts in the absence of detectable T cells and that one manifestation of injury is calcification. The extent of arterial injury produced by HSCs alone was too severe and rapid to allow subsequent adoptive transfer of PBMCs.
FIGURE 4.
Human artery segments engrafted into severe combined immunodeficiency/beige mice and inoculated with human hematopoietic stem cells (HSCs) develop CD68+ human macrophage infiltrates and dystrophic calcification. Photomicrographs of paired human coronary artery transplanted into SCID/beige mice. Animals received 1×106 adult HSCs within 2 weeks of artery transplantation. (A) Artery from animal receiving artery and no cells, stained for CD68; (B) artery from animal receiving HSCs and stained for CD68; (C) graph of counts of human CD68+ cells in human arterial grafts (n=10); (D) animals receiving artery and no cells and stained for CD3; (E) animals receiving HSCs and stained for CD3; (F) animals receiving artery and no cells and stained for Von Kossa; and (G) animals receiving HSCs and stained for Von Kossa.
DISCUSSION
We describe a new human-mouse chimeric model to study the role of human macrophages during allograft rejection in vivo. By inoculating irradiated adult SCID/bg mice with cells enriched for G-CSF mobilized HSCs from adult humans, we generated immunodeficient animals with human CD68+ CD14+ macrophages capable of infiltrating and injuring human tissues. In a human skin graft model, HSC inoculation led to infiltration by CD68+ macrophages into the allogeneic skin graft but little evidence of tissue injury. Subsequent adoptive transfer of CD34+ cell-depleted PBMCs, which give rise to circulating T cells, led to graft infiltration by both macrophages and T cells. This combination increased the extent of injury and caused much more prominent thrombosis than that caused either cell type alone. Injecting enriched populations of human CD34+ HCS into animals bearing allogeneic human artery grafts led to CD68+ infiltration, luminal occlusion by an expanded neointima, and dystrophic calcium deposition within the vessel wall. The cause of the neointimal growth may represent a response to injury as to macrophage-derived growth factor.
Attempts to develop models more closely reflecting human immune responses are continuing to be developed. Early models have been hampered by low levels of human cellular engraftment, relatively short life spans due to thymic lymphomas, and difficulty of the engrafted human cells to differentiate into fully functional T cells, B cells, myeloid cells, and dendritic cells in immunodeficient animals (12, 21, 22). Newly generated mouse strains that are even more profoundly immunodeficient, have enhanced human immune cell reconstitution [recently reviewed by Shultz et al. (14)]. A major advance has been the knock out of the interleukin-2 receptor (IL-2R) γ-chain locus that is required for high-affinity binding of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 and is essential for their signaling and function. This mutation impairs T- and B-cell development and completely prevents NK cell development and also prevents thymoma formation. Shultz et al. (16) developed NOD SCID γc−/− mice that have been found to be superior to other mouse strains for HSC reconstitution, giving rise to human CD3+ (CD4+ and CD8+), Ig B cells, myeloid cells, NK cells, and dendritic cells. Our experiments confirm the superiority of the NOD SCID γc−/− strain compared with SCID/bg recipients for HSC engraftment. However, in the present study, the NOD SCID γc−/− strain showed innate neutrophil responses to human skin grafts and thrombotic complications after human artery transplantation. We do not know precisely why this occurred, but the bg mutation, introduced to reduce NK cell function in C.B-17 SCID animals, does have effects on host neutrophils and platelet functions and these effects may contribute to observed differences in outcomes between the two strains tested. Another possible difference, especially relevant for neutrophil activation is a role for complement. The complement regulatory proteins of the mouse are different from those of human and may vary among different strains.
Previous models of allograft rejection developed in our laboratory have used SCID/bg mice bearing human skin or interpositional artery grafts followed by adoptive transfer of allogeneic human PBMC. The response to skin grafts is mediated by effector memory T cells (6, 17). Although a full complement of immune cells are present in the initial PBMC inoculate, there is no measurable engraftment of other leukocytes (e.g., NK cells, monocytes, or dendritic cells). We evaluated HSC transplantation as a complementary approach to T-cell adoptive transfer. Human CD34+ HSCs for reconstitution have been derived from a number of sources including cord blood (23, 24); fetal tissues (25) and G-CSF mobilized adult donors (16). We chose G-CSF mobilized adult donors since this is the only source that allows for the isolation of both enriched human CD34+ HSCs and memory T cells.
Evidence suggesting an active role for macrophages in allograft rejection has come from both clinical and animal studies (26). Clinical studies that deplete T cells from renal allograft recipients before transplantation have demonstrated the presence of monocytic infiltrates leading to graft rejection in the absence of T cells (27–29). Jose et al. (30), using a rat transplant model, demonstrated that specifically targeting the removal of macrophages by liposomal clodronate resulted in decreased graft damage. The animals developed a primary allograft response with subsequent lymphocyte accumulation. Tissue damage was attenuated due to a loss of macrophage-dependent delayed type hyper-sensitivity (DTH) effector mechanisms. This provided the first direct evidence of a role for macrophages in eliciting tissue damage during acute allograft rejection. To date there is no suitable small animal model to study the response of human macrophages to transplanted tissues, which has hindered fully elucidating their role in allograft rejection. The current report establishes a novel human allograft model whereby human macrophages reconstitute in immunodeficient mouse hosts and are themselves capable of causing damage to transplanted human skin or human artery grafts. We have concluded that damage in these models is caused by macrophages since no other human leukocytes can be identified in the tissues. However, attempts to rigorously test this conclusion by depletion of human macrophages with liposomal clodronate were technically unsuccessful due to death of animals that had been irradiated and injected with HSC (but not of control animals) after clodronate administration.
Human HSC transplantation into adult SCID/bg mice did not give rise to T or B cells. This raises the issue of how human monocyte/macrophages may recognize allogeneic tissue in the absence of lymphocytes. Human monocyte/macrophages may recognize nonclassical alloantigen via scavenger receptor (31). It is possible that the observed phenomena are not, in fact, alloreactions but rather involve innate monocyte reactions to tissues injured by surgical trauma or ischemia. The time course for such reactions is relatively prolonged, but much of this delay is likely due to the slow rate at which HSC engraft and give rise to macrophages. The recruitment of human macrophages from the circulation into these transplanted human tissues is unlikely to be due simply to the action of chemokine signaling since mouse macrophages, which are present in C.B-17 SCID/bg animals and respond to the same chemokines, are not recruited along with human macrophages to the grafts. If the response is nonspecific, accounting for the species selectivity, we hypothesize that human adhesion molecules expressed on ECs are also required.
Our new models can be used to study the role of macrophages in two distinct phenomena associated with tissue injury: thrombosis and calcification. In the skin graft model, an interaction between T cells and macrophages appear required for the development of thrombosis although the mechanism is unclear. A possible candidate is T-cell-mediated induction of tissue factor, the principle initiator of the coagulation system whose expression by macrophages is induced by engaged CD40 ligand expressed on activated T cells (32, 33). Although we did detect other signs of macrophage activation induced by T cells, namely iNOS and CD163, we did not find evidence of tissue factor in these specimens (unpublished data, NKS). Our artery grafts displayed prominent vascular calcification, a highly regulated process involving both inducers and inhibitors (34). That is a common response to soft tissue injury and systemic mineral imbalance (35). Vascular calcification is of clinical importance in many pathologic conditions including atherogenesis (36), ectopic calcification (37), or end-stage renal disease (38). The anatomical site of calcification within a vessel wall determines the nature and extent of the clinical manifestations (39, 40). Calcification of the intima is most often observed in atherosclerosis in association with lipids, macrophages, and smooth muscle cells. Medial calcification can exist independently of atherosclerosis and is associated with elastin and smooth muscle cells. The mechanisms whereby calcium is deposited may be similar in both vascular compartments, including the generation of matrix vesicles, apoptotic bodies, and the local expression of mineralization regulatory proteins (36). In our model, calcium deposits were observed in both intima and media. The development of an in vivo model to study the cell-mediated mechanisms regulating vascular calcification could lead to the development of novel therapeutics and strategies to address these problems.
In summary, we have developed novel in vivo small animal model systems in which to study the roles of macrophages in human allograft rejection. Our model may also have utility in the study of thrombosis and vascular calcification.
ACKNOWLEDGMENTS
The authors thank Bruce Fichandler for providing skin from deceased donors as well as Todd Jensen, Lisa Gras, and Louise Benson for their expert technical assistance. They also thank Dr. Jeffrey Platt for suggesting the possibility that differences in complement regulatory proteins may differentially influence neutrophil recruitment in different mouse strains.
This work was supported by NIH grants RO1-HL062188, P30-AR41942, PO1-HL070295, and RO1-HL051014.
REFERENCES
- 1.Gouldesbrough DR, Axelsen RA. Arterial endothelialitis in chronic renal allograft rejection: A histopathological and immunocytochemical study. Nephrol Dial Transplant. 1994;9:35. [PubMed] [Google Scholar]
- 2.Hruban RH, Beschorner WE, Baumgartner WA, et al. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Am J Pathol. 1990;137:871. [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes C, Wolos JA, Giannini EH, et al. Induction of T helper cell hyporesponsiveness in an experimental model of autoimmunity by using nonmitogenic anti-CD3 monoclonal antibody. J Immunol. 1994;153:3319. [PubMed] [Google Scholar]
- 4.Murray AG, Petzelbauer P, Hughes CC, et al. Human T-cell-mediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse. Proc Natl Acad Sci USA. 1994;91:9146. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellides GK, Kirkiles NC, Tereb DA, et al. Transplantation Models in human/mouse chimeras. In: Timmermann W, Gassel HJ, Ulrichs K, et al., editors. Organ transplantation in rats and mice: Microsurgical techniques and immunological principles. Berlin: Springer-Verlag; 1998. p. 65. [Google Scholar]
- 6.Pober JS, Bothwell AL, Lorber MI, et al. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003;25:167. doi: 10.1007/s00281-003-0135-1. [DOI] [PubMed] [Google Scholar]
- 7.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175:4886. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 8.Tereb DA, Kirkiles-Smith NC, Kim RW, et al. Human T cells infiltrate and injure pig coronary artery grafts with activated but not quiescent endothelium in immunodeficient mouse hosts. Transplantation. 2001;71:1622. doi: 10.1097/00007890-200106150-00023. [DOI] [PubMed] [Google Scholar]
- 9.Hancock WW, Zola H, Atkins RC. Antigenic heterogeneity of human mononuclear phagocytes: Immunohistologic analysis using monoclonal antibodies. Blood. 1983;62:1271. [PubMed] [Google Scholar]
- 10.Blunt T, Finnie NJ, Taccioli GE, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 11.Christianson SW, Greiner DL, Schweitzer IB, et al. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol. 1996;171:186. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 12.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180. [PubMed] [Google Scholar]
- 13.Christianson SW, Greiner DL, Hesselton RA, et al. Enhanced human CD4+ T cell engraftment in beta2-microglobulin-deficient NOD-scid mice. J Immunol. 1997;158:3578. [PubMed] [Google Scholar]
- 14.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 15.Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 16.Shultz LD, Lyons BL, Burzenski LM, et al. Humanlymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 17.Shiao SL, Kirkiles-Smith NC, Shepherd BR, et al. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179:4397. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 18.Kirkiles-Smith NC, Tereb DA, Kim RW, et al. Human TNF can induce nonspecific inflammatory and human immune-mediated microvascular injury of pig skin xenografts in immunodeficient mouse hosts. J Immunol. 2000;164:6601. doi: 10.4049/jimmunol.164.12.6601. [DOI] [PubMed] [Google Scholar]
- 19.Sultan P, Murray AG, McNiff JM, et al. Pig but not human interferon-gamma initiates human cell-mediated rejection of pig tissue in vivo. Proc Natl Acad Sci USA. 1997;94:8767. doi: 10.1073/pnas.94.16.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Ahmad U, Yi T, et al. Alloimmune-mediated vascular remodeling of human coronary artery grafts in immunodeficient mouse recipients is independent of preexisting atherosclerosis. Transplantation. 2007;83:1501. doi: 10.1097/01.tp.0000264560.51845.67. [DOI] [PubMed] [Google Scholar]
- 21.Mosier DE, Gulizia RJ, Baird SM, et al. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1998;335:256. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot T, Faktorowich Y, Lubin I, et al. Enhancement of T-cell-depleted bone marrow allografts in the absence of graft-versus-host disease is mediated by CD8+ CD4− and not by CD8− CD4+ thymocytes. Blood. 1992;80:2406. [PubMed] [Google Scholar]
- 23.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain (null) mice. Blood. 2005;106:1565. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traggiai E, Chicha L, Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 25.Gimeno R, Weijer K, Voordouw A, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104:3886. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 26.Matheson PJ, Dittmer ID, Beaumont BW, et al. The macrophage is the predominant inflammatory cell in renal allograft intimal arteritis. Transplantation. 2005;79:1658. doi: 10.1097/01.tp.0000167099.51275.ec. [DOI] [PubMed] [Google Scholar]
- 27.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2007;76:120. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 28.Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 29.Zhang PL, Malek SK, Prichard JW, et al. Monocyte-mediated acute renal rejection after combined treatment with preoperative Campath-1H (alemtuzumab) and postoperative immunosuppression. Ann Clin Lab Sci. 2004;34:209. [PubMed] [Google Scholar]
- 30.Jose MD, Ikezumi Y, van Rooijen N, et al. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Dhanireddy KK, Kirk AD. Human monocytes as intermediaries between allogeneic endothelial cells and allospecific T cells: A role for direct scavenger receptor-mediated endothelial membrane uptake in the initiation of alloimmunity. J Immunol. 2006;176:750. doi: 10.4049/jimmunol.176.2.750. [DOI] [PubMed] [Google Scholar]
- 32.Lindmark E, Tenno T, Siegbahn A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arterioscler Thromb Vasc Biol. 2000;20:2322. doi: 10.1161/01.atv.20.10.2322. [DOI] [PubMed] [Google Scholar]
- 33.Mach F, Schonbeck U, Libby P. CD40 signaling in vascular cells: A key role in atherosclerosis? Atherosclerosis. 1998;137 Suppl:S89. doi: 10.1016/s0021-9150(97)00309-2. [DOI] [PubMed] [Google Scholar]
- 34.Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63. doi: 10.1016/S1054-8807(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 35.Giachelli CM, Speer MY, Li X, et al. Regulation of vascular calcification: Roles of phosphate and osteopontin. Circ Res. 2005;96:717. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 36.Doherty TM, Asotra K, Fitzpatrick LA, et al. Calcification in atherosclerosis: Bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA. 2003;100:11201. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giachelli CM. Ectopic calcification: Gathering hard facts about soft tissue mineralization. Am J Pathol. 1999;154:671. doi: 10.1016/S0002-9440(10)65313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman WG, London G, Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 39.London GM, Marchais SJ, Guerin AP, et al. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14:525. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 40.Proudfoot D, Shanahan CM. Biology of calcification in vascular cells: Intima versus media. Herz. 2001;26:245. doi: 10.1007/pl00002027. [DOI] [PubMed] [Google Scholar]