Abstract
The dual oxidase-thiocyanate-lactoperoxidase (Duox/SCN−/LPO) system generates the microbicidal oxidant hypothiocyanite in the airway surface liquid by using LPO, thiocyanate, and Duox-derived hydrogen peroxide released from the apical surface of the airway epithelium. This system is effective against several microorganisms that infect airways of cystic fibrosis and other immunocompromised patients. We show here that exposure of airway epithelial cells to Pseudomonas aeruginosa obtained from long-term cultures inhibits Duox1-dependent hydrogen peroxide release, suggesting some microbial factor suppresses Duox activity. These inhibitory effects were not seen with the pyocyanin-deficient P. aeruginosa strain, PA14 Phz1/2. We showed that purified pyocyanin, a redox-active virulence factor produced by P. aeruginosa, inhibits human airway cell Duox activity by depleting intracellular stores of NADPH, as it generates intracellular superoxide. Long-term exposure of human airway (primary normal human bronchial and NCI-H292) cells to pyocyanin also blocks induction of Duox1 by Th2 cytokines (IL-4, IL-13), which was prevented by the anti-oxidants glutathione and N-acetylcysteine. Furthermore, we showed that low concentrations of pyocyanin blocked killing of wild-type P. aeruginosa by the Duox/SCN-/LPO system on primary normal human bronchial epithelial cells. Thus, pyocyanin can subvert Pseudomonas killing by the Duox-based system as it imposes oxidative stress on the host. We also show that lactoperoxidase can oxidize pyocyanin, thereby diminishing its cytotoxicity. These data establish a novel role for pyocyanin in the survival of Pseudomonas aeruginosa in human airways through competitive redox-based reactions between the pathogen and host.
Keywords: Human (Animals), Bacterial (Infections), Lung, Mucosa (Tissues), Cytotoxicity (Process), Cell surface molecules (Molecules)
Introduction
The respiratory system is a major exposure site for microbial entry into the body. As such, multiple mechanisms are responsible for preventing respiratory infections, including microbial detection systems, mucin and airway surface liquid (ASL)3 production, ciliary clearance, communication between epithelial cells and cells of innate and adaptive immunity, and production of antimicrobial compounds. One of the innate airway antimicrobial defense systems of renewed interest is the hydrogen peroxide-thiocyanate-lactoperoxidase (H2O2/SCN−/LPO)3 system, in which LPO uses H2O2 to catalyze oxidation of the pseudohalide SCN− into the microbicide hypothiocyanite (OSCN−): H2O2 + SCN− → OSCN− + H2O. The toxicity of this system has long been appreciated in other exocrine secretions, such as milk (1) and saliva (2). Targets of the H2O2/SCN−/LPO system include Streptococci, Staphylococci, Haemophilus influenzae (3), Pseudomonads, Escherichia coli (4), and viral and fungal pathogens (5, 6). LPO is found in sheep airways at concentrations as high as 1% of total soluble protein and its inhibition results in reduced microbial clearance (7). Significant levels of LPO are also detected in human airway secretions (3). The primary substrate of LPO, SCN−, is present in human airway secretions at an average concentration of 0.46 mM (3), which exceeds amounts needed to support LPO activity (8). Thiocyanate is transported into the ASL by epithelial cells by at least three apical plasma membrane transporters: the cystic fibrosis transmembrane conductance regulator (CFTR)3 (9), Ca2+-activated chloride channels, and pendrin (SLC26A4) (10). Comparatively low levels of H2O2 are detected in human airways, in the 1–10 μM range (11), which may be due to antioxidant properties of abundant LPO.
Although the H2O2/SCN−/LPO system of exocrine secretions has been studied for years, the source of H2O2 remained unclear until we reported high expression of NADPH oxidases (Nox)3, Dual oxidase 1 and Dual oxidase 2 (Duox)3, in epithelial cells of exocrine glands and along mucosal surfaces (12). The dual oxidases were first detected in the thyroid gland and were proposed to serve as H2O2 sources needed to support thyroperoxidase activity during thyroid hormone biosynthesis (13, 14). Duox expression was also detected in other non-thyroid tissues, including salivary glands, bronchial and tracheal surfaces, and the gastrointestinal tract (12, 15). Based on the parallel expression patterns of Duox and LPO in salivary glands, gastrointestinal tissues, and airways, we suggested a functional partnership of Duox in supporting antimicrobial activity of LPO in exocrine secretions (12). The Duox enzymes bind Ca2+ through their EF-hands and their activities are responsive to Ca2+-mobilizing agonists. These oxidases require maturation factors (Duox activators, Duoxa1 and Duoxa2) to be transported to their final destination, the plasma membrane (16). In human major airways Duox is found in the surface epithelium (12), where it is concentrated along the apical aspect (17, Ueyama, Lekstrom and Leto, unpublished). LPO is produced in acinar pockets of submucosal glands of human airways, but accumulates in the airway surface liquid layer (3, 12). Duox releases extracellular H2O2 from the apical surface of airway epithelial cells (17) where, together with SCN−, it would effectively support LPO-mediated killing of airway pathogens. Recently, primary airway epithelial cells and tracheal explants of different mammalian origin were shown to kill Pseudomonas aeruginosa and Staphylococcus aureus in vitro in a Duox-, LPO- and SCN−-dependent manner (18).
P. aeruginosa is an opportunistic pathogen of human airways that usually infects immunocompromised host (cystic fibrosis (CF)3, chronic obstructive pulmonary disease, pneumonia, burn, HIV, or cancer chemotherapy patients) (19). P. aeruginosa harbors a variety of cell-attached and extracellular virulence factors that are induced through quorum sensing signals as a consequence of bacterial overgrowth and biofilm formation in chronically infected individuals. One of the secreted factors is pyocyanin (Pyo)3, a blue heterocyclic metabolite of phenazine compounds that is redox-active (reviewed in (20)) and toxic against a range of host organisms. Many effects of Pyo on airway epithelial cells have been described; it causes cellular senescence and ciliary dyskinesia, induces IL-8 secretion, decreases glutathione levels and inhibits catalase activity (reviewed in (21)). Despite this spectrum of effects, its redox activity is considered the primary basis for its action. Pyo is a zwitterion that easily crosses cell membranes; in the cytosol it reacts with reduced NADH or NADPH, becomes reduced, and donates an electron to molecular oxygen, thereby producing intracellular superoxide (O2.-) anions (22).
Because Pyo and the Dual oxidases share some of the same substrates (molecular oxygen and NADPH), we investigated possible competitive interactions of Pyo and Duox enzymes in human airway epithelial cells. We show here that Duox1- and calcium-dependent H2O2 release by airway epithelial cells is abolished by prior exposure to P. aeruginosa obtained from long-term cultures. This was attributed to Pyo production by long-term cultures. Purified Pyo inhibits Duox activity and expression based on its ability to compete for intracellular NADPH and inflict oxidative stress on the host. Furthermore, we show that physiological levels of LPO can effectively detoxify Pyo. Thus, competitive redox reactions are involved in airway epithelial host defense against microbial infection, as well as in the microbe’s counter-offensive adaptation to the host environment.
Materials and Methods
Primary human cells and cell lines
The human pulmonary carcinoma cell line, NCI-H292, was purchased from ATCC (CRL-1848). Cells were grown in RPMI-1640 medium (Invitrogen) containing 10% FBS, 1% penicillin-streptomycin, 1% L-Glutamine, 1% sodium-pyruvate and 1% HEPES. The following cytokines were used: IL-4 (10 ng/mL), IL-13 (10 ng/mL) and IFN-γ (1 U/mL) (recombinant human, R&D Systems).
Primary normal human bronchial epithelial (NHBE)3 cells were isolated from normal tissue at Lonza (Walkersville, MD), cultured for one passage in 75 cm2 Falcon flasks in Bronchial/Tracheal Epithelial Cell Basal Medium (BEBMR) containing all SingleQuotR BEGM supplements, with the following modifications based on observations reported by Gray et al. (23): human epidermal growth factor (25 ng/ ml; Collaborative Research); all-trans retinoic acid (5 × 10−8 M; Sigma); bovine serum albumin (1.5 μg/ ml; Sigma); bovine pituitary extract (1% vol/ vol SingleQuotR Lonza); Gentamycin and Amphotericin B (50 μg/ ml). Upon reaching 80% confluence the cells were trypsinized and seeded onto 6- or 24-well polyester (0.4 micron pore) membrane transwells (Transwell-Col or Transwell Clear (Costar), precoated with rat tail collagen I; Collaborative Research) at a density of 20–50 × 103 cells/ cm2. When cells reached confluence, the upper chamber medium was removed and the lower medium was replaced with air-liquid interface (ALI)3 medium composed of: 50% BEGM/ 50% DMEM, supplemented as above (Lonza, Walkersville, MD). Cells were maintained in the air-liquid interface (ALI) format as long as 28 days by feeding daily with ALI medium. After one week of culture on ALI the cells formed a sealed monolayer that exhibited transepithelial resistances exceeding 1000 Ohm/cm2. Antibiotics were omitted from the culture medium two days before microbial killing experiments, typically on day 19–24.
K562 cells were purchased from ATCC (CCL-243), transduced retrovirally with NADPH components (p47phox, p67phox and gp91phox), and then clonally selected for high levels of the reconstituted Nox2-based NADPH oxidase (referred to as K562 +++ cells; (24)).
Bacterial strains
The following bacterial strains were used: P. aeruginosa ATCC 10145 (PA 10145; American Type Culture Collection); PAO1 wild-type (Pseudomonas Mutant Library, University of Washington, Seattle), PA14 wild-type and Pyo-deficient mutant PA14 PhzM (gift from Frederick M. Ausubel, Harvard Medical School, Boston (25)), and the PA14 phenazine-deficient mutant, Phz1/2 (provided by You-Hee Cho, Sogang University, South Korea (26)). Burkholderia cepacia was a gift from Dr. Steve Holland (NIAID, NIH, USA). Bacteria were grown in Luria-Bertani broth (KD Medical), and incubated for up to 3 days (shaking, 37°C). Where mentioned, densities of the cultures were determined from absorbance at 600 nm.
Western blotting
Airway cells were washed three times with cold calcium- and magnesium-free PBS and then lysed by NP-40 lysis buffer (Boston Biosciences) containing 150 μM PMSF (Fluka Biochemika) and 1% protease inhibitor cocktail (dissolved in DMSO; Sigma). Lysates were centrifuged and protein concentrations in supernatants were determined using the BCA assay (Pierce). Equal amounts of protein were loaded and electrophoresed on SDS-polyacrylamide gels (8%; Tris-Glycine Gel, Invitrogen). Gels were blotted on nitrocellulose membrane (Invitrogen) using the TransBlot SD semi-dry blotting cell (Bio-Rad). Blots were blocked overnight in TTBS (TBS-buffer containing 5% milk powder and 0.05% Tween-20). Blots were incubated with primary antibodies (RT, 1hr, TTBS), washed three times with TTBS and then probed with secondary HRP-linked antibodies (RT, 1hr, TTBS). After repeated washes, blots were developed by chemiluminescence using the Lumigen DS detection kit (GE Healthcare). The primary antibodies used in this study were: anti-Duox (rabbit, polyclonal; 1:2000) (15); anti-β-actin (rabbit, polyclonal, Sigma; 1:2000); anti-α-tubulin (mouse, monoclonal, Santa Cruz; 1:2000). Secondary antibodies used in this study were: HRP-linked anti-rabbit IgG from donkey (GE Healthcare; 1:1000); HRP-linked anti-mouse IgG from sheep (GE Healthcare; 1:1000).
RNA interference
To silence gene expression by RNA interference, 5 × 105 NCI-H292 cells per well were seeded onto 6-well plates (Beckton Dickinson) one day prior to transfection. Cells were transfected at 20–30 % confluence with either 50, or 100 nM Duox1-specific siRNAs (Applied Biosystems) for four hours in OPTI-MEM medium (Invitrogen) using Lipofectamine 2000 transfection agent (Invitrogen). Cytokine-treatment by IL-4 and IL-13 (each 10 ng/mL) was started immediately after transfection. Gene silencing efficiency was validated by Western blot analysis 72 hrs post-transfection. The following siRNAs were used (sequence of the sense strand):
Duox1 #1: 5′ GGACUUAUCCUGGCUAGAG 3′ (siRNA#: 24969);
Duox1 #2: 5′ GGAUAUGAUCUGUCCCUCU 3′ (siRNA#: 108942);
Duox1 #3: 5′ CCAUGUGUUGGUUGAAGAU 3′ (siRNA#: 117547);
Duox1 #4: 5′ GCUAUGCAGAUGGCGUGUA 3′ (siRNA#: 117546);
β-actin: 5′ GAUGAGAUUGGCAUGGCU 3′;
negative control: 5′ CCGUAUCGUAAGCAGUACU 3′.
Purification of pyocyanin
Pyocyanin was prepared from supernatants of wild-type PA10145 and PA14 P. aeruginosa strains cultured for 2.5 days in LB medium. The filtered, bacterium-free culture supernatants were subjected to repeated chloroform extraction cycles, as described (27). In the first step, Pyo was extracted by adding chloroform to the crude supernatant, whereas the second aqueous extraction of the chloroform phase used acidified distilled water (pH 1.0). Subsequent chloroform extractions (repeated 5 times) required neutralization of the aqueous extracts. Final Pyo extracts were concentrated to 1–3 mM in distilled water and stored at 4° C in the dark. Pyo concentrations were determined based on an absorption coefficient of 2460 mM−1 cm−1 at 520 nm (protonated form at pH 1.0). The identity and purity of Pyo preparations were confirmed by mass spectrometry, revealing one major protonated species with MW= 211.08 (Mass spectrometry Unit, Research Technologies Branch, NIAID, NIH).
Measurement of hydrogen peroxide release
Extracellular H2O2 release was measured by a Luminol/HRP-based chemiluminescence assay. Trypsinized NCI-H292 cells (1.5 × 106/mL, 50 μL) were preincubated with or without 10 μM diphenylene iodonium (DPI)3 (37°C, 10 min) and were stimulated by addition of an equal volume of HBSS containing 1 mM Luminol, 20 U/mL HRP and agonist. Luminescence was measured in luminescence 96-well plate reader (Luminoskan Ascent, Thermo). This assay reports real-time production of H2O2.as reflected in the decay of oxidized luminol. The results are shown either as kinetics of H2O2 production, expressed as relative luminescence units (RLU3) versus time, or as integrated luminescence (int. RLU), in absolute numbers or normalized.
Extracellular H2O2 generation was quantified by the Amplex Red/HRP assay (Molecular Probes), which detects the accumulation of a fluorescent oxidized product and can be used to calculate absolute H2O2 yield with appropriate standards. On top of washed NCI-H292 or NHBE cells on transwells, 50 μL HBSS were placed containing ionomycin (1 μM) or ATP (300 μM). Supernatants were collected at the end of 30 min incubation and incubated in dark with equal volume of HBSS containing 100 μM Amplex Red and 20 U/mL HRP. After 30 minutes, fluorescence was measured in a microplate reader (Fluoroskan Ascent, Thermo) using excitation at 530 nm and emission at 590 nm. H2O2 release was quantified (fmol H2O2/20min/mm2) using standard calibration curves. Both assays measured extracellular, not intracellular, hydrogen peroxide, since omitting HRP from the reaction mix completely abolished the signals.
Measurement of superoxide production
Intracellular superoxide production was measured spectrophotometrically by the quantitative nitroblue tetrazolium (NBT3) reduction assay. 106/mL cells were incubated in the presence of 1.3 mg/mL NBT for 30 min. Cells were centrifuged and washed twice in HBSS to remove extracellular formazan particles. Cells and intracellular formazan precipitates were dissolved in 2M NaOH and DMSO, and the absorbance was measured at 720 nm in a microplate reader (Versamax, Molecular Devices).
Extracellular superoxide production was measured by the Diogenes cellular luminescence enhancement system (National Diagnostics). Cells (106/mL) were preincubated (10 min, 37°C) with or without 10 μM DPI on 96-well (opaque white) plate (Thermo). An equal volume of Diogenes reagent containing stimuli was added, and luminescence was measured in a luminescent 96-well plate reader (Luminoskan Ascent, Thermo Labsystems). Data are expressed as integrated relative luminescence units (int. RLU).
Measurement of changes in intracellular calcium concentration
Attached NCI-H292 cells were loaded with 4 μM FURA2/AM (Sigma) for 60 minutes in the dark. Loaded cells were trypsinized, washed and placed into wells of black 96-well plates (Corning). After stimulation, changes in fluorescence were measured using 340 nm and 390 nm excitation wavelengths, and emission at 510 nm. After the subtraction of the background of unloaded cells, the 340/390 ratio was determined.
Bacterial killing
The upper surfaces of NHBE cells differentiated on ALI transwells were washed, and fresh culture medium was placed in the bottom chamber. The cells were preincubated for 10 minutes with 2 μL of HBSS containing DPI, LPO, SCN−, catalase, ascorbic acid, cysteine and Pyo, added to their exposed apical surface as indicated, before the addition of an additional volume of 3 μL of HBSS containing all the same components, plus the calcium mobilizing agonist (ATP or ionomycin) and 5000 or 10000 CFUs of P. aeruginosa or B. cepacia. Airway cells and bacteria were co-incubated for 3 hours (37° C) before lysing cells in 1 mL HBSS containing 1 mg/mL saponin. One to ten serial dilutions were spread on LB-agar plates in triplicate. Colonies were counted the next day to calculate bacterial killing efficiencies.
In vitro oxidation of pyocyanin by peroxidases
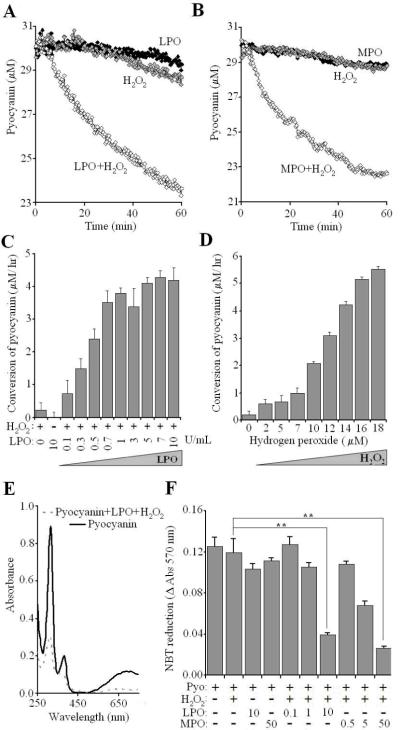
The in vitro oxidation of Pyo by peroxidases was monitored in a 96-well microplate spectrophotometer (Versamax, Molecular Devices) by measuring decreases in the concentration of the unoxidized, unprotonated form at its absorption maximum at 691 nm, based on a standard calibration curve (Fig. 7E). The data shown as “conversion of Pyo” in panels C and D of Fig. 7 reflects decreases in the concentration of the unprotonated, unoxidized form of pyoycanin with time. The reactions were conducted at 37° C in solutions supplemented with LPO, MPO, H2O2 and Pyo (at concentrations indicated in Fig. 7 legend), but lacking chloride. The pH was maintained above 6.0 to avoid absorbance changes (691 nm) related to Pyo acidification.
Fig. 7. Peroxidase-mediated detoxification of pyocyanin.
Changes in Pyo (initially 30 μM) were followed spectrophotometrically in the presence of 100 μM H2O2 and 10 U/mL (3.47 μM) LPO (A) or 50 μg/mL (335 nM) MPO (B). Shown is one representative result of three. (C) Pyo consumption (μM/hr) in the presence of 200 μM H2O2 and LPO (mean +/- S.E.M., n=4). (D) Pyo consumption (μM/hr) in the presence 5 U/mL (1.74 μM) LPO and H2O2 (mean +/- S.E.M., n=4). E) Absorption spectra of Pyo after overnight incubation in the presence or absence of 100 μM H2O2 and 20 U/mL LPO. (F) After overnight incubation (37° C) of solutions containing Pyo (30 μM), H2O2 (100 μM), LPO (0,1; 1.0; 10 U/mL) or MPO (05, 5.0, 50 μg/mL), 5 μLs were added to trypsinized and washed NCI-H292 cells (106/mL, 650 μg/mL NBT), incubated for 30min, and then intracellular O2.- production was determined (mean +/- S.E.M., n=3).
Statistical analysis
Data are represented either as mean +/- S.E.M. of at least three independent experiments or mean +/- S.D. of one representative experiment out of at least two similar independent ones. Significance levels were compared using t-test. *: p<0.05; **: p<0.01; ***: p<0.001. NS: not significant.
Results
Duox is induced in differentiated primary human bronchial epithelial cells cultured on air-liquid interface and in NCI-H292 cells treated with Th2 cytokines, IL-4 and IL-13
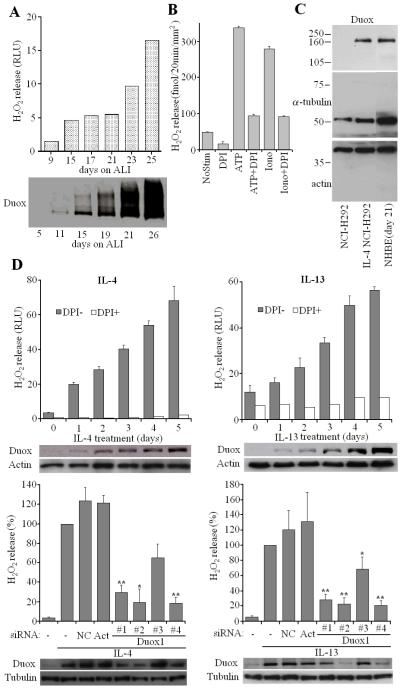
To study possible interactions of Duox and Pyo, we used two different airway epithelial models: primary normal human bronchial epithelial (NHBE) cells and a human mucoepidermoid pulmonary carcinoma cell line that also produces Duox, NCI-H292. Low passage NHBE cells were grown on transwells on air-liquid interface (ALI) to mimic the airway environment and promote a mature cellular phenotype (23). After seeding NHBE cells on ALI, they develop into polarized, terminally-differentiated cells over the course of 2–3 weeks, showing characteristic markers of mature airway epithelium (mucin expression, ciliogenesis). During this differentiation process, Duox expression and H2O2 release also increased gradually (Fig. 1A). H2O2 release was triggered by Ca2+-mobilizing agonists (ATP, ionomycin (iono)3) and was inhibited by the flavoenzyme inhibitor diphenylene iodonium (DPI) (Fig. 1B). Since Duox1 induction was demonstrated in NHBE cells by Th2 cytokines, interleukin-4 (IL-4) and interleukin-13 (IL-13) (28), we examined the effects of these cytokines on Duox levels in NCI-H292 cells. These cytokines also induce Duox in NCI-H292 cells after 3-day treatments at levels comparable with primary cells grown on ALI for 21 days (Fig. 1C), although the induced NCI H292 cells produced significantly lower amounts of H2O2. The IL-4-induced NCI-H292 cells produced 5.69 pmol H2O2/min/106 cells in response to ionomycin when measured on cell suspensions prepared at high cell concentrations (mean, n=2, Amplex Red/HRP). The same assay was not sensitive enough to detect H2O2 released from cells (IL-4 NCI-H292) when attached to transwells, because of the much lower amount of cells obtained under this condition. In contrast, ALI NHBE cells on transwells produced 247.3+/-5.45 fmol/20min/mm2 H2O2 (average+/- S.E.M., n=3; DPI-sensitive) in response to ATP (or similar amounts by ionomycin), indicating that the differentiated primary cells have a much higher H2O2 output than cytokine-induced NCI-H292 cells. This higher H2O2 output explains functional differences in microbial killing capabilities of the two cell types (see below).
Fig. 1. Duox-dependent hydrogen peroxide production by cytokine-induced NCI-H292 cells and normal human bronchial epithelial (NHBE) cells.
(A) Ionomycin-triggered H2O2 release (Luminol+HRP) and Duox protein levels were determined in NHBE cells cultured up to 26 days on air-liquid interface (ALI) transwells. (B) Quantified extracellular H2O2 release (Amplex Red+ HRP) by attached NHBE cells (ALI day 21) using ionomycin (1μM) or ATP (300 μM) as stimuli (mean +/- S.E.M., n=3). (C) Duox levels were compared by Western blotting in uninduced and cytokine-induced (3 days) NCI-H292 cells and NHBE cells (21 days on ALI; one representative exp, n=2). (D) Upper panels: NCI-H292 cells were treated with 10 ng/mL IL-4 or IL-13 for 0–5 days and were assayed for H2O2 release by luminescence in the presence or absence of DPI (10 μM). Shown are results (mean +/- S.D. of triplicates) from one representaive experiment of three. Inhibition of ionomycin-triggered H2O2 release by DPI (10 μM) in NCI-H292 cells after 3-day induction by cytokines was: 88.2 +/- 1.3% (IL-4, n=39) and 80.0 +/- 2.5% (IL-13, n=12) (mean +/- S.E.M.). Duox Western blotting during this time-course shown below. Bottom panels: NCI-H292 cells were treated with negative control, actin (Act) or different Duox1-targeted siRNAs (#1 - #4). Cells were induced by 10 ng/mL IL-4 or IL-13 for 3 days, Duox1 protein levels and ionomycin-triggered, DPI-sensitive H2O2 release (luminescence) was measured. Output is expressed as percentage of cytokine-induced H2O2 release detected in the absence of siRNAs (mean +/- S.E.M., n=4 for both cytokines). Relative inhibition of the H2O2 output by the Duox1 siRNAs: IL-4, #1: 70.5%, #2: 80.3%, #3: 35.1%, #4: 81.5% (mean, n=4); IL-13, #1: 71.9%, #2: 77.5%, #3: 31.1%, #4: 79.1% (mean, n=4). Significance levels are compared to NC.
IL-4 and IL-13 induce higher Duox levels steadily over the course of 5 days (Fig. 1D), whereas induction of Duox2 by the Th1 cytokine IFN-γ was less consistent (data not shown). Parallel with induction of Duox, DPI-sensitive H2O2 release by NCI-H292 cells increased following IL-4 or IL-13 treatments (Fig. 1D). Interestingly, both cytokines are detected at higher than normal levels in bronchial lavage from CF patients (29). Both, Duox protein levels and H2O2 release were diminished by Duox1-targeted siRNA-treatment. Three Duox1 siRNAs (#1, #2 and #4) out of four tested decreased DPI-sensitive H2O2 release by more than 70 percent (Fig. 1D lower panels). The results show that Duox1 is the principle source of Ca2+-dependent extracellular H2O2 release by NCI-H292 cells and that these cells are a suitable model to study expression and function of this airway oxidase.
Prolonged exposure to wild-type Pseudomonas aeruginosa PA10145 inhibits Duox-mediated hydrogen peroxide production
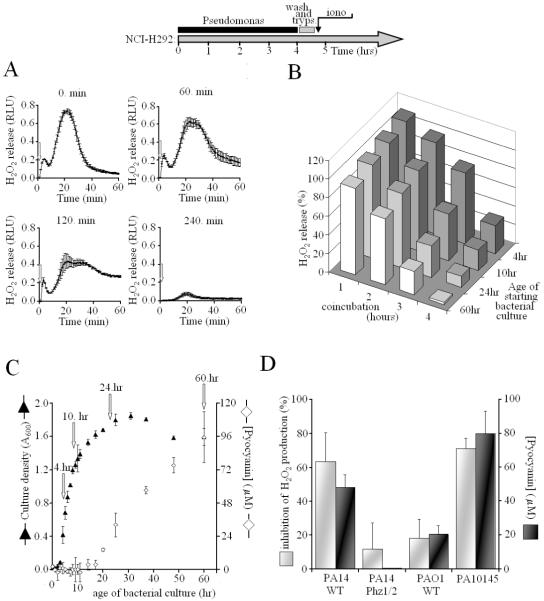
To study the influence of Pseudomonas on Duox-mediated H2O2 release, we incubated attached, cytokine-induced NCI-H292 cells with P. aeruginosa bacteria for up to four hours, then the bacteria were washed away and the NCI-H292 cells were stimulated by the Ca2+ ionophore ionomycin. We found that prolonged exposure of IL-4-induced NCI-H292 cells to WT PA10145 blocked Duox-mediated H2O2 release in response to ionomycin (Fig. 2A). H2O2 release decreased with increased bacterial exposure, and was entirely abolished after 240 min exposure to bacteria from overgrown (60 hr) cultures (Fig. 2A & B). During the 4-hour incubation, Duox protein levels do not change, nor was there evidence of degradation as judged by Western blotting (not shown). This inhibitory effect of co-incubation of bacteria with airway cells was diminished when exposed to fresh P. aeruginosa cultures, suggesting the bacteria produce some Duox inhibitory component in long-term culture (Fig. 2B). P. aeruginosa has a complex life cycle, starting with planktonic cells that seed microcolonies and then form macrocolonies, which leads to establishment of biofilms. This can be mimicked in overgrown suspension cultures. One indication of these changes is the production of a blue-green pigment, pyocyanin (Pyo), which appears as a consequence of quorum sensing in late phases of cultivation (Fig. 2C). Pyo is a good candidate for Duox inhibition, since it is secreted, cell permeable, and a redox-active compound capable of consuming intracellular NADPH.
Fig. 2. Exposure of airway cells cells to Pseudomonas aeruginosa inhibits Duox activation.
As shown on the scheme, attached, IL-4-induced NCI-H292 cells were coincubated with Pseudomonas aeruginosa for 0–4 hours. Bacteria grown for 4–60 hrs were washed and resuspended in RPMI and exposed to airway epithelial cells (MOI: 10:1). Afterthat bacteria were removed, NCI-H292 cells were washed (PBS), trypsinized and assayed for DPI-sensitive, ionomycin-triggered H2O2 release (Luminol+HRP). (A) Ionomycin-stimulated H2O2 release by IL-4-induced NCI-H292 cells is inhibited by exposure to P. aeruginosa (strain PA10145) for different times (0, 60, 120, 240 min). Kinetic data represent mean +/- S.D. of triplicates from one representative experiment out of three. (B) Ionomycin-stimulated, DPI-sensitive H2O2 release by IL-4-induced NCI-H292 cells was compared as a function of exposure time to PA10145 (bottom axis) and age of PA10145 culture added to cells (right axis). (C) Biosynthesis of Pyo in long-term cultures of PA10145 detected by O.D. of supernatants (A691, white diamonds). Bacterial culture densities were monitored by A600 (black triangles) and converted into concentration of CFUs (OD=1.0 corresponded to 109 CFUs/mL). Arrows denote times at different growth phases: 4hr (early); 10hr (late exponential); 24hr (early stationary); 60hr (late stationary). (D) Duox inhibition by P. aeruginosa strains (PA10145, PA14WT, PA14 Phz1/2 and PAO1) correlates with their Pyo production (mean +/- S.E.M., n=3). Bacteria were previously grown for 60 hrs, then added to NCI-H292 cells for 4 hrs. *: p<0.05, Phz1/2 compared to wild-type PA14.
Duox inhibition by Pseudomonas aeruginosa wild-type strains correlates with their pyocyanin-producing capabilities
In addition to the PA10145 WT strain, we compared two other wild-type strains that produce Pyo at different rates. Average Pyo concentrations in 2.5-day old cultures were: 55.5 μM (Pseudomonas aeruginosa 14 wild-type (PA14 WT)3), 21.2 μM (Pseudomonas aeruginosa PAO1 wild-type (PAO1 WT)3) and 92.3 μM (PA10145) (average, n=4) (Fig. 2D). Pyo appeared rapidly in the medium of PA14 WT and reached peak concentrations at 24–36 hours, whereas PA10145 was a slow producer but reached higher final concentrations. When 2.5-day old cultures of each wild-type strain were co-incubated with attached IL-4 NCI-H292 cells for 4 hours (MOI:10:1) and ionomycin-triggered H2O2 release was determined, the extent of Duox inhibition correlated with the Pyo producing capabilities of the wild-type strains (Fig. 2D). The fact that the pronounced inhibition of Duox activity by the wild-type PA14 strain disappeared when its phenazine-deficient mutant, Phz1/2, was used strongly suggested pyocyanin is the cause of Duox inhibition (Fig. 2D).
Duox activity is inhibited by pyocyanin
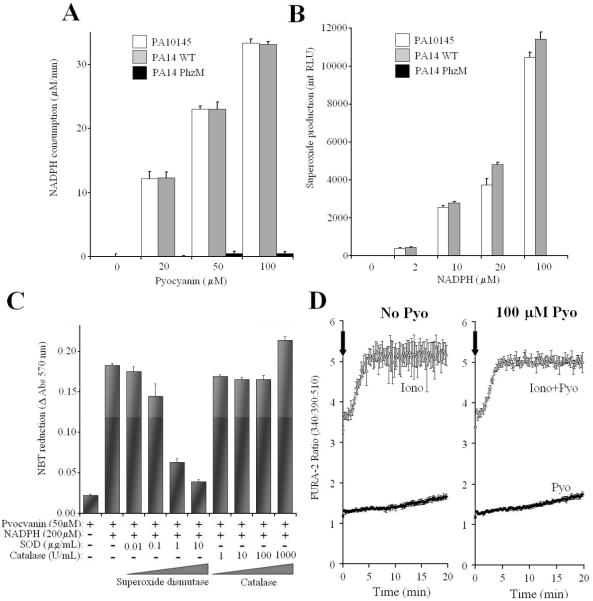
To confirm this relationship of Pyo production and Duox inhibition, we purified Pyo from supernatants of the two best wild-type producers (PA14 WT and PA10145). Pyo purified from both strains consumed NADPH (Fig. 3A) and produced O2.- (Fig. 3B and C) in vitro, while comparable extracts from the PA PhzM mutant had no activity. Fig. 3C shows that the primary product of pyocyanin-mediated reduction of oxygen is superoxide, not hydrogen peroxide. Purified pyocyanin by itself did not induce any calcium signals in FURA-2-loaded NCI-H292 (IL-4-induced) cells nor did it interfere with ionomycin-triggered increases in intracellular calcium concentrations (Fig. 3D).
Fig.3. Characterization of purified pyocyanin.
(A) Purified pyocyanin consumes NADPH in vitro. Supernatants of 2.5-day old cultures of P. aeruginosa wild-type (PA14 and PA10145) and Pyo-deficient mutant (PA14 PhzM) strains were processed in parallel to extract Pyo, as described in Materials and Methods. Purified Pyo, or equivalent volumes of the extracts from the mutant strain, were incubated in vitro with 1 mM NADPH for 20 min. NADPH consumption was measured by following the changes in absorbance at 340 nm and calibrated using samples with known NADPH concentrations. One representative result (mean +/- S.D. of trpilicates) of three independent experiments is shown. (B) Purified Pyo generates superoxide from NADPH. Pyo (50 μM), or equivalent mutant extracts, were incubated in vitro with NADPH in HBSS, and superoxide production was measured by Diogenes chemiluminescence (20 min). One representative result (mean integral luminescencence +/- S.D of triplicate assays) of three independent experiments is shown. (C) Pyocyanin reduces NBT in vitro through generation of superoxide. Pyo, NADPH, and SOD or catalase were incubated in vitro for 60 minutes in the presence of NBT, and absorbance changes at 570 nm were measured. Results are mean +/-S.D. of triplicates of one experiment out of two. (D) Pyocyanin does not affect calcium signaling in NCI H292 cells. FURA-2-loaded, IL-4-induced NCI-H292 cells were stimulated by ionomycin (1 μM), pyocyanin (100 μM), both or none of them. Changes in the ratio of FURA-2 fluorescence were followed for 20 min. Data are mean +/- S.D. of quadruplicates of one representative experiment. In four separate experiments the increases in FURA-2 ratios over the 20 min time were: Unstimulated: 0.49 +/- 0.057; Pyocyanin: 0.43 +/- 0.047; Ionomycin: 3.03 +/- 0.44; Ionomycin+pyocyanin: 3.16 +/- 0.52 (mean +/- S.E.M., n=4). Differences between the pyocyanin-free vs. pyocyanin-containing values were not significant.
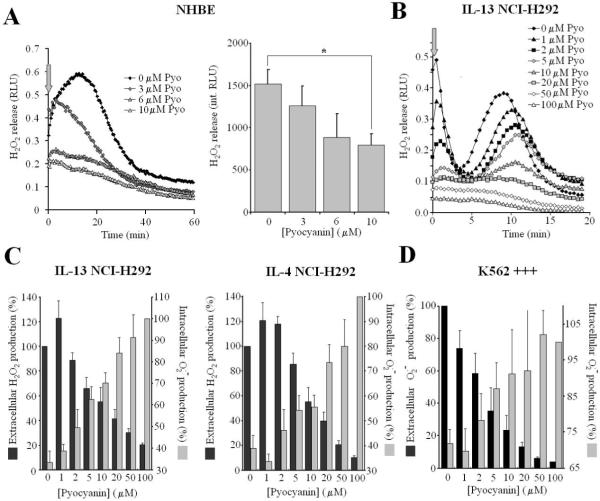
We then studied the effects of the purified toxin on Duox activity in different cell types. NHBE cells grown on transwell ALI cultures were washed, treated on their apical surface with Pyo (30 min; 3,6,10 μM) and washed again before measuring ionomycin-induced H2O2 release. Pre-treatment of NHBE cells with purified toxin inhibited Duox activity in a concentration-dependent manner, showing 50% inhibition at 10 μM Pyo (Fig. 4A).
Fig.4. Effects of purified pyocyanin on Duox activity in airway epithelial cells.
(A) Ionomycin-stimulated H2O2 release by adherent NHBE cells (ALI, day 21) is inhibited by Pyo (30 min pretreatments). Left panel: kinetics of one representative experiment (mean of duplicates) out of four. Right panel: averaged data (integrated RLUs over 60 min assays) of the four experiments each performed in triplicate (Luminol+HRP; mean +/- S.E.M.). (B) Representative kinetics of H2O2 release by IL-13-induced NCI-H292 cells when ionomycin (1 μM) and pyocyanin (0–100 μM) were added together. Each curve represents the mean of triplicate assays of one representative experiment; 5 others gave similar results (C) Pyo inhibits extracellular H2O2 release (black) while producing intracellular O2.- (grey). IL-4- and IL-13-induced NCI-H292 cells were stimulated by ionomycin (1 μM) and Pyo (0–100 μM) together, and both extracellular H2O2 release (Luminol+HRP; mean +/- S.E.M. of total integrated 20 min. output, normalized to pyo-free cells; IL-4: n=8, IL-13: n=6; all experiments performed in triplicate) and intracellular O2.- production (20 min. NBT reduction; mean +/- S.E.M. normalized to values obtained with 100 μM Pyo for both cytokines; n=3) were measured. D) K562+++ cells were stimulated by 100 nM PMA together with different concentrations of Pyo (0–100 μM). Shown are intracellular (NBT-reduction, mean +/- S.E.M. normalized to the Pyo-free cells, n=3) and extracellular (integrated Diogenes luminescence; mean +/- S.E.M normalized to 100 μM Pyo-treated cells, n=4) superoxide production over 20 min. time courses.
To test the possibility that Pyo entering cells and Duox compete for NADPH, we added Duox activator (Ca2+ ionophore) and the toxin together to cytokine-treated NCI-H292 cells and measured extracellular H2O2 release in parallel with intracellular O2.- production (Duox produces extracellular H2O2, whereas Pyo forms intracellular O2.- from the same intracellular pool of NADPH). Extracellular H2O2 release was inhibited in a dose-dependent manner by Pyo in both IL-13-induced (Fig. 4B and C) and IL-4-induced (Fig. 4C) NCI-H292 cells. Intracellular O2.- production was directly proportional to toxin concentrations and inversely proportional to extracellular H2O2 detected (Fig. 4C). The toxin-derived superoxide was detectable as extracellular hydrogen peroxide release as well in NCI-H292 cells not stimulated by ionomycin, but these levels were much lower than observed when Duox was activated (not shown). Thus, Pyo inhibits Duox activity as it ‘transforms’ extracellular H2O2 release aimed at destroying bacteria into intracellular O2.-, thereby exposing the cell interior to oxidative attack. We confirmed that NBT reduction by pyocyanin and NADPH occurs predominately through O2.- generation and not direct reduction under aerobic conditions, since it was inhibited by SOD in vitro (Fig. 3C). Furthermore, Pyo did not interfere with the luminol+HRP H2O2 detection system, as it was shown by using glucose+glucose-oxidase as alternative H2O2 source, which produced luminescence independent of Pyo concentrations (data not shown).
This inhibitory mechanism of Pyo could also apply to other Nox family members, since they all consume NADPH while reducing molecular oxygen into O2.- within an extracytoplasmic compartment. Pyo has been shown to inhibit the respiratory burst of PMA-stimulated human neutrophils (30). We confirmed these observations (data not shown). Furthermore, we used K562 cells reconstituted with the complete Nox2 system (K562 +++ cells) and showed that Pyo inhibited extracellular O2.- production in a dose-dependent manner, while producing intracellular O2.- (Fig. 4D).
Pyocyanin blocks cytokine-induced Duox up-regulation in airway epithelial cells
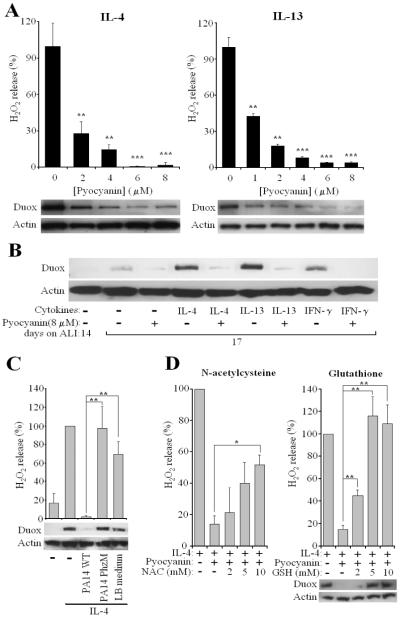
We tested whether Pyo also affects Duox protein levels following long-term treatment of airway epithelial cells by cytokines. When IL-4 or IL-13 was added together with Pyo to confluent NCI-H292 cells for 3 days, complete inhibition of Duox up-regulation was observed, which correlated with diminished H2O2 release (Fig. 5A) (inhibition by 8 μM pyocyanin was 85.0 +/- 2.9 % (mean+/- S.E.M., n=39). When the most potent inhibitory concentration of Pyo (8 μM) used on NCI-H292 cells was applied to primary NHBE cells, the toxin blocked Duox induction by all three cytokines, IL-4, IL-13 and IFN-γ (Fig. 5B). To confirm these observations, we subjected supernatants of PA14 wild-type, pyo-deficient PhzM and bacterium-free LB medium to the Pyo extraction and purification protocol and processed them parallel. When Pyo or the equivalent volume of the PhzM- or LB-extract were added together with IL-4 to NCI-H292 cells, only the wild-type extract containing Pyo had an inhibitory effect on H2O2 release and Duox protein levels, whereas the two other preparations were without effect (Fig. 5C). This confirms that Pyo and not some other residual bacterial components in the Pyo preparation are responsible for the observed inhibition. Since oxidative stress is considered to be the cause of most of the toxic effects of Pyo, we examined whether reactive oxygen species (ROS)3 scavengers had an effect on the Pyo-mediated inhibition of Duox up-regulation in IL-4-induced NCI-H292 cells. 10 mM concentrations of N-acetylcysteine (NAC)3 prevented partially, whereas 5 mM glutathione (GSH)3 prevented completely the inhibition of H2O2 release and corresponding decreases in Duox protein levels (Fig. 5D).
Fig. 5. Pyocyanin blocks up-regulation of Duox by cytokines.
(A) NCI-H292 cells were incubated 3 days with cytokines (IL-4 or IL-13) and Pyo (0–8 μM). Duox protein levels and ionomycin-triggered H2O2 release (DPI-sensitive) were measured relative to pyo-free cells (mean+/- S.E.M., n=4). Significance levels compared to cells not treated with pyo. (B) 14-day old NHBE cells were induced, or not, by three cytokines (IL-4, IL-13 and IFN-γ) +/- 8 μM Pyo. Cells were Western blotted 3 days later for Duox protein levels. Similar results were observed using cells from 3 different donors. (C) NCI-H292 cells were treated together with IL-4 and 8 μM Pyo prepared from the supernatant of PA14 wild-type strain or equivalent extract from PhzM mutant or LB medium by itself. After three days of induction, NCI-H292 cells were assayed for DPI-sensitive, iono-stimulated H2O2 release (Luminol+HRP) and Duox protein levels. Shown are the results (% of pyo-free cells) of three experiments each performed in triplicates (mean +/- S.E.M.). (D) ROS scavengers permit Duox induction in the presence of Pyo. NCI-H292 cells were treated with IL-4 and 8 μM Pyo +/- ROS scavangers (N-acetylcysteine (NAC) and glutathione (GSH)) for 3 days. Cells were assayed for ionomycin-stimulated H2O2 release and for Duox levels. Data are shown as % of values of cells treated only by IL-4 (mean +/- S.E.M., n=4 (GSH), n=3 (NAC)).
Pyocyanin inhibits killing of Pseudomonas aeruginosa by the Duox/LPO/SCN− system on airway cells
Next our aim was to test the presence of Pyo on the ultimate function of the H2O2/LPO/SCN− system, namely microbial killing. First, we confirmed that bacterial killing by primary NHBE cells is Duox dependent (18). Survival of Pseudomonas was unchanged when one or more components of the H2O2/LPO/SCN− system were omitted, and killing occurred only when the entire system was present (Fig. 6A). Inhibition of Duox by DPI, scavenging H2O2 by catalase or ascorbic acid, or omission of ATP as a stimulus of H2O2 release prevented Pseudomonas killing (Fig. 6A). We also showed that the system is lethal against another airway pathogen, Burkholderia cepacia (Fig. 6A); in this case H2O2 release was triggered by ionomycin. Addition of the ROS scavanger cysteine inhibited killing of B. cepacia (Fig. 6A). To investigate the effect of Pyo on killing, NHBE cells were preincubated with 2, 10, or 20 μM Pyo for 10 min, then 5000 c.f.u. of PA10145 were added together with LPO, SCN− and ATP. When Duox was activated by ATP, survival of PA10145 decreased dramatically showing a potent microbicidal effect of the Duox/LPO/SCN− system (Fig. 6B). Pretreatment of airway cells with Pyo blocked the killing of Pseudomonas (Fig. 6B). Pyo had no direct effect on the survival of the bacteria (Fig. 6B). These data show that Pyo is a potent virulence factor of P. aeruginosa capable of suppressing bacterial killing by the Duox/LPO/SCN− system.
Fig. 6. Pyocyanin inhibits microbicidal activity of the Duox/LPO/SCN− system on primary human airway cells.
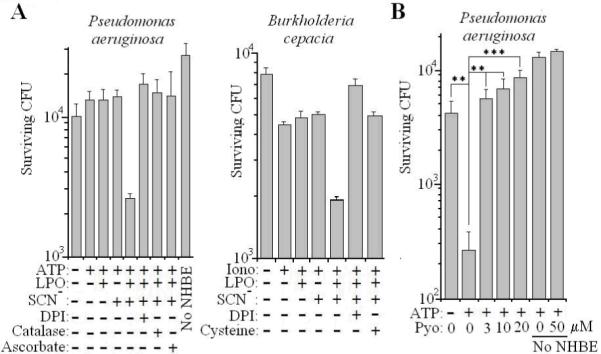
(A) 3-week old ALI cultures of NHBE cells were exposed for 3 hours to 10000 c.f.u. of P. aeruginosa PA10145 or 5000 c.f.u. of B. cepacia in the presence of different components or inhibitors of the Duox/LPO/SCN system, as indicated. Bacterial survival was measured by plating and colony counting. Data are mean +/- S.D. (quadruplicates) of one representative experiment out of two. (B) 3-week old NHBE cells were first incubated for 10 min with Pyo, then P. aeruginosa microbicidal assays were performed as in A. Data present mean +/- S.D. (quadruplicates) of one representative experiment out of two.
Lactoperoxidase and myeloperoxidase oxidize pyocyanin using hydrogen peroxide
In addition to LPO, appreciable levels of myeloperoxidase (MPO)3 are also found in the airway surface liquid. MPO is released from neutrophil granulocytes that migrate to the inner surface of airways. Both enzymes use H2O2 to oxidize a broad range of compounds and earlier studies have shown that MPO can oxidize several bacterial toxins: pneumolysin from Streptococcus pneumoniae, leukotoxin from Actinobacillus actinomycetemcomitans (31) and Clostridium difficile cytotoxin (32). Previous studies showed that Pyo is oxidized by hemin or microperoxidase, a degradation product of cytochrome c (21).
Here we show that both LPO and MPO can oxidize Pyo and convert it into a less toxic derivative. Neither LPO nor H2O2 alone decreased detectable Pyo levels, but when added together Pyo concentrations dropped (Fig. 7A). Similar results were obtained with MPO (Fig. 7B). Consumption of Pyo by LPO is dependent on the concentrations of LPO (Fig. 7C) and H2O2 (Fig. 7D). When Pyo is incubated with LPO and H2O2 together, the absorption spectrum of Pyo changes, with diminished absorbance peaks at 279 nm and 691 nm, reflecting the disappearance of the original form of the toxin (Fig. 7E). To show that conversion of Pyo by LPO and MPO renders it less toxic, we incubated 30 μM Pyo in the presence or absence of H2O2 and different concentrations of LPO and MPO. At the end of overnight incubations, each solution was added to NCI-H292 cells suspended in HBSS containing Nitro Blue Tetrazolium to measure intracellular O2.- production. The presence of H2O2, LPO or MPO alone did not decrease Pyo-mediated, intracellular O2.- production, but addition of the peroxidases together with H2O2 decreased the intracellular toxicity of Pyo (O2.- formation) in a dose-dependent manner (Fig. 7F). Thus, both LPO and MPO can protect human airway epithelial cells from the oxidative stress imposed by Pyo exposure.
Discussion
CF is an autosomal recessive genetic disorder that affects about 1 in 2500 individuals, mainly of Caucasian origin. The primary cause is mutations in the CFTR chloride channel, but how these genetic defects manifest in lung disease characterized by frequent and chronic bacterial infections remains a debated issue (33, 34). Models citing the reduced volume and increased viscosity (dehydration) of CF ASL that can impede ciliary clearance of airway pathogens are inadequate in explaining the unique susceptibility of CF patients to particular airway pathogens. Human mucosal surfaces are equipped with numerous antimicrobial systems. Among them, LPO and its substrate thiocyanate are well recognized as abundant, effective antimicrobial components of several exocrine secretions (milk, tears, saliva), although only recently has this system been appreciated within the ASL. Our laboratory suggested Duox1 and 2 serve as mucosal sources of H2O2 capable of supporting LPO activity in the generation of the microbicidal oxidant hypothiocyanite (12, 35). Later studies confirmed our observations detecting Duox1 as the predominant oxidase in human major airway epithelial cells, showed that Duox1 is induced by Th2 cytokines, IL-4 and IL-13 (28), and proposed other defense-related functions for Duox1, including acid (36) and mucin secretion (37). We suggested the immunocompromised phenotype in CF may reflect microbial killing defects by the airway Duox/LPO/SCN− system by noting that organisms typically infecting CF airways in early disease stages (i.e., Staphylococcus aureus, Burkholderia cepacia) also commonly infect chronic granulomatous disease patients, who suffer from defects in oxidative killing in phagocytes (12). Furthermore, we hypothesized that impaired performance of the Duox/LPO/SCN− antimicrobial system could stem from CFTR gene defects, since this channel is known to exhibit efficient SCN− transport activity (9). Two recent studies explored the model in more detail, showing the impaired SCN− transport activity of human CF airway epithelial cells is sufficient to compromise LPO- or ROS-dependent microbial killing in vitro (18, 38). Our current observations, together with these studies, indicate that the human airway Duox1/LPO/SCN− system is indeed capable of killing several pathogens that frequently infect the lungs of CF patients, including P. aeruginosa, S. aureus, B. cepacia, and H. influenzae. Further study is needed to confirm the consequences of CFTR mutations on the Duox/LPO/SCN− antimicrobial system in CF lungs and consider the effectiveness of supplemental SCN inhalation therapy in these patients.
Although the host factors that predispose CF patients to P. aeruginosa colonization are unclear, it is known that inducible microbial virulence factors have important roles in CF disease pathogenesis following the establishment of chronic infections. The importance of antimicrobial systems can become evident when targeted by microbial virulence factors. Production of Pyo and other virulence factors is turned on through microbial quorum sensing during advanced stages of chronic P. aeruginosa infection, usually coinciding with appearance of the mucoid phenotype and biofilm formation. Pyo is produced in the majority of P. aeruginosa clinical isolates from patients suffering from diverse lung diseases and is detected in high concentrations in PA-infected patients with bronchiestasis and CF (39). Studies on a variety of other P. aeruginosa-infected organisms showed Pyo-deficient mutant strains are markedly less capable of killing their hosts, including C. elegans, plants, Drosophila and mice (21). Our studies show that physiologically relevant Pyo concentrations interfere with the Duox/LPO/SCN− anti-microbial system on at least two levels: (1) Pyo enters airway cells, oxidizes NADPH, and produces O2.-, thereby inhibiting the extracellular production of H2O2 by Duox while imposing intracellular oxidative stress (Fig.8. process #1). (2) Long-term, Pyo also blocks cytokine induction of Duox1, such that the host is unable to respond to bacterial challenge with enhanced H2O2 release (Fig.8. process #2). Thus, this pathogen targets the Duox/LPO/SCN− system and by inhibiting it may improve survival of this and other bacterial species in the human respiratory tract. We show that the Duox/SCN−/LPO system is also able to kill B. cepacia. Although less common than P. aeruginosa infection, B. cepacia infection carries the worst prognosis for CF (40). In many cases, B. cepacia and P. aeruginosa coinfect CF patients (41); under these conditions, Pyo produced with chronic P. aeruginosa infection could compromise B. cepacia killing by the host Duox/SCN−/LPO system. In other cases, organisms may be directly susceptible to redox-based toxicity of Pyo, which may explain the predominance of P. aeruginosa in late stages of CF disease.
Fig. 8. Multiple redox-based interactions involving pyocyanin and the human airway Duox/LPO/SCN− system.
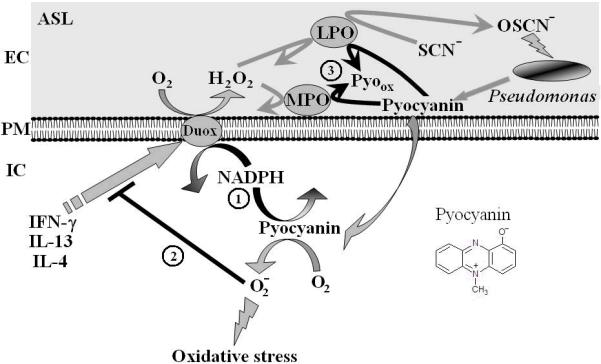
Duox-derived H2O2 supports LPO-mediated conversion of thiocyanate (SCN−) into hypothiocyanite (OSCN−), which is effective against oxidant-sensitive pathogens (several species that infect CF lungs). Pyo is a membrane-permeable microbial metabolite that enters cells and reacts with intracellular NAD(P)H (1). Diminished NADPH levels support lower Duox activity, thereby enhancing bacterial survival. Reduced Pyo reacts with oxygen and produces intracellular O2.- (oxidative stress), which inhibits cytokine induction of Duox (2) and limits H2O2 release. Duox-derived H2O2 and airway peroxidases can oxidize Pyo, thereby decreasing its cytotoxicity (3).
Airway oxidative stress is another feature of the enhanced inflammatory state of CF airways in advanced disease. Glutathione (GSH)3 levels are typically lower in CF ASL. We observed protective effects of GSH on Pyo-mediated inhibition of IL-4-induced Duox1 expression, raising the possibility that Duox protein levels in CF airway epithelium can be lower, leading to less efficient microbial killing by the H2O2/LPO/SCN− system. The protective effects of GSH and the GSH precursor NAC on Duox activity or expression may explain the improvement of lung function in CF following administration of either of the two drugs. How Pyo blocks Duox induction by these cytokines and how GSH and NAC prevent this inhibition will require further study, although it is known that Pyo can oxidize GSH directly (42). Pyo could alter transcription of Duox and other ROS responsive genes or interfere with Duox protein folding or transport.
Our observations on the effects of pyocyanin on airway Duox activity may be generalized to include NADPH oxidases in other cell types. Excessive neutrophil infiltration is another hallmark of chronically infected CF lungs. We confirmed earlier observations showing O2.- production by neutrophils is inhibited by Pyo (30). Our observations in Nox2-reconstituted cells suggest competition for common substrates (NADPH and oxygen) between Pyo and the Nox2-based phagocytic oxidase is the mechanism for inhibition in these cells as well.
In one final aspect of this redox-based interplay between airway epithelium and pathogen, we show that two airway peroxidases, LPO and MPO, are also capable of oxidizing Pyo into less toxic species no longer capable of exerting redox-based effects on the host (Fig.8. process #3). Here we showed that Pyo is detoxified by H2O2 and physiological levels of LPO or MPO and the products are less capable than untreated Pyo in inflicting intracellular oxidative stress on NCI-H292 airway cells. Similar observations were described in the detoxification of Pyo by microperoxidase 11, a peptide fragment of cytochrome c bound to heme, as reflected in diminished Pyo-induced IL-8 release by A549 cells (22). These findings suggest a new role for airway peroxidases and Duox in the ASL in detoxifying this virulence factor, but imply that Pyo can also prevent its own detoxification by inhibiting Duox when produced in excess by established biofilms. The results suggest augmentation of LPO-mediated Pyo oxidation in situ as a potential therapy for chronic P. aeruginosa infection.
In summary, our studies illustrate a complex network of redox-based interactions between human airway epithelial cells and P. aeruginosa, an opportunistic pathogen. Many of the reasons for its success in infecting immunocompromised individuals are not well understood, but the broad range of its virulence factors, its capability for biofilm formation, its resistance to many host defense mechanisms and its adaptability to changing environments certainly contribute. Our data show that the human airway epithelium is equipped with potent oxidant-based defense mechanisms that are effective against airway pathogens and that P. aeruginosa can adapt to this environment with redox-based counter-offensive mechanisms. The novel findings in the battle between P. aeruginosa and the airway epithelium presented here may help understand the pathogenesis of this microorganism.
Acknowledgements
This paper is dedicated to Dr. Bruno Reiter, a pioneer of the lactoperoxidase field who has provided invaluable advice and encouragement. The authors thank Carl Hammer (Mass spectrometry Unit, NIAID, NIH) for performing mass spectrometry analysis on pyocyanin preparations. We are also grateful for research materials from the following sources: The Pseudomonas Mutant Library (Manoil lab, Univ. of Washington, Seattle) for providing the PAO1 wild-type strain; Dr. Frederick M. Ausubel (Harvard Univ., Boston) for sending us the PA14 wild-type and PhzM strains; Dr. You-Hee Cho (Sogang Univ., South Korea) for providing the PA14 Phz1/2 strain and Dr. Steven M. Holland (LCID, NIAID, NIH) for the clinical isolate of B. cepacia. We thank Dr. Miklos Geiszt (Semmelweis Univ.) for many helpful discussions.
Footnotes
This work was supported by funding through the Intramural Research Program of the NIH, NIAID.
- ALI
- air-liquid interface
- ASL
- airway surface liquid
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- DPI
- diphenylene iodonium
- Duox
- Dual oxidase
- GSH
- glutathione
- H2O2
- hydrogen peroxide
- iono
- ionomycin
- LPO
- lactoperoxidase
- MPO
- myeloperoxidase
- NAC
- N-acetylcysteine
- NBT
- nitroblue tetrazolium
- NHBE
- normal human bronchial epithelial cells
- Nox
- NADPH oxidase
- NS
- not significant
- PA14
- Pseudomonas aeruginosa 14 strain
- PAO1
- Pseudomonas aeruginosa O1 strain
- Pyo
- pyocyanin
- RLU
- relative luminescence unit
- ROS
- reactive oxygen species.
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Reiter B, Perraudin JP. Lactoperoxidase: biological function. In: Everse J, Everse KE, Grisham MB, editors. Peroxidases in chemistry and biology. CRC Press; Boca Raton FL: 1991. pp. 143–180. [Google Scholar]
- 2.Pruitt KM. The salivary peroxidase system: thermodynamic, kinetic and antibacterial properties. J Oral Pathol. 1987;16:417–420. doi: 10.1111/j.1600-0714.1987.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 3.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol. 2003;29:206–212. doi: 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- 4.Reiter B, Marshall VM, Bjorck L, Rosen CG. Nonspecific bactericidal activity of the lactoperoxidases-thiocyanate-hydrogen peroxide system of milk against Escherichia coli and some gram-negative pathogens. Infect Immun. 1976;13:800–807. doi: 10.1128/iai.13.3.800-807.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtois P, van Beers D, de Foor M, Mandelbaum IM, Pourtois M. Abolition of herpes simplex cytopathic effect after treatment with peroxidase generated hypothiocyanite. J Biol Buccale. 1990;18:71–74. [PubMed] [Google Scholar]
- 6.Lenander-Lumikari M. Inhibition of Candida albicans by the Peroxidase/SCN-/H2O2 system. Oral Microbiol Immunol. 1992;7:315–320. doi: 10.1111/j.1399-302x.1992.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 7.Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, Abraham WM, Conner GE. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- 8.Pruitt KM, Mansson-Rahemtulla B, Tenovuo J. Detection of the hypothiocyanite (OSCN-) ion in human parotid saliva and the effect of pH on OSCN-generation in the salivary peroxidase antimicrobial system. Arch Oral Biol. 1983;28:517–525. doi: 10.1016/0003-9969(83)90184-x. [DOI] [PubMed] [Google Scholar]
- 9.Linsdell P, Hanrahan JW. Adenosine triphosphate-dependent asymmetry of anion permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. J Gen Physiol. 1998;111:601–614. doi: 10.1085/jgp.111.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. Am J Respir Crit Care Med. 2001;164:1012–1015. doi: 10.1164/ajrccm.164.6.2012139. [DOI] [PubMed] [Google Scholar]
- 12.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 14.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 15.El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M, Virion A, Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933–942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 16.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 17.Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 18.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr., Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez MI, Prince A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol. 2007;7:244–251. doi: 10.1016/j.coph.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Price-Whelan A, Dietrich LE, Newman DK. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol. 2006;2:71–78. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- 21.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Reszka KJ, O’Malley Y, McCormick ML, Denning GM, Britigan BE. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic Biol Med. 2004;36:1448–1459. doi: 10.1016/j.freeradbiomed.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 24.Leto TL, Lavigne MC, Homoyounpour N, Lekstrom K, Linton G, Malech HL, de Mendez I. The K-562 cell model for analysis of neutrophil NADPH oxidase function. Methods Mol Biol. 2007;412:365–383. doi: 10.1007/978-1-59745-467-4_24. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell. 1999;96:47–56. doi: 10.1016/s0092-8674(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 27.Cox CD. Role of pyocyanin in the acquisition of iron from transferrin. Infect Immun. 1986;52:263–270. doi: 10.1128/iai.52.1.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, Schendel DJ, Krauss-Etschmann S. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117:204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Muller M, Sorrell TC. Modulation of neutrophil superoxide response and intracellular diacylglyceride levels by the bacterial pigment pyocyanin. Infect Immun. 1997;65:2483–2487. doi: 10.1128/iai.65.6.2483-2487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark RA, Leidal KG, Taichman NS. Oxidative inactivation of Actinobacillus actinomycetemcomitans leukotoxin by the neutrophil myeloperoxidase system. Infect Immun. 1986;53:252–256. doi: 10.1128/iai.53.2.252-256.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ooi W, Levine HG, LaMont JT, Clark RA. Inactivation of Clostridium difficile cytotoxin by the neutrophil myeloperoxidase system. J Infect Dis. 1984;149:215–219. doi: 10.1093/infdis/149.2.215. [DOI] [PubMed] [Google Scholar]
- 33.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 34.Campodonico VL, Gadjeva M, Paradis-Bleau C, Uluer A, Pier GB. Airway epithelial control of Pseudomonas aeruginosa infection in cystic fibrosis. Trends Mol Med. 2008;14:120–133. doi: 10.1016/j.molmed.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 37.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581:271–278. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strausbaugh SD, Davis PB. Cystic fibrosis: a review of epidemiology and pathobiology. Clin Chest Med. 2007;28:279–288. doi: 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Thomassen MJ, Demko CA, Klinger JD, Stern RC. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am Rev Respir Dis. 1985;131:791–796. doi: 10.1164/arrd.1985.131.5.791. [DOI] [PubMed] [Google Scholar]
- 42.O’Malley YQ, Reszka KJ, Spitz DR, Denning GM, Britigan BE. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L94–103. doi: 10.1152/ajplung.00025.2004. [DOI] [PubMed] [Google Scholar]