Abstract
Background
There have been no studies that employ longitudinal data with more than two measurements and use methods of longitudinal data analysis to identify risk factors for incident albuminuria over time more effectively.
Study Design
Longitudinal study.
Settings & Participants
A subgroup of participants in the Strong Heart Study, a population-based sample of American Indians, in central Arizona, Oklahoma, and North and South Dakota. Diabetic participants without albuminuria were followed for a mean of four years.
Predictors
Age, sex, study center, high-density lipoprotein and low-density lipoprotein cholesterol, triglycerides, body mass index, systolic blood pressure, use of antihypertensive medication, smoking, hemoglobin A1c, fasting glucose, type of diabetes therapy, diabetes duration, plasma creatinine and urinary albumin/creatinine ratio (UACR).
Outcomes & Measurements
Albuminuria was defined as UACR ≥ 30 mg/g. Urine creatinine and albumin was measured by the picric acid method and a sensitive nephelometric technique, respectively.
Results
Among the 750 and 568 diabetic participants without albuminuria and with normal plasma creatinine at the 1st and 2nd examinations, 246 and 132 developed albuminuria by the 2nd and 3rd examinations, respectively. Incident albuminuria was predicted by baseline UACR, fasting glucose, systolic blood pressure, plasma creatinine, study center, current smoking, and use of angiotensin converting enzyme (ACE) inhibitors and antidiabetic medications. UACR of 10–30 mg/g increased the odds of developing albuminuria 2.7-fold compared with UACR < 5 mg/g.
Limitations
Single random morning urine specimen.
Conclusions
Many of risk factors identified for incident albuminuria can be modified. The control of blood pressure and glucose, smoking cessation, and use of ACE inhibitors may reduce the incidence of albuminuria.
INDEX WORDS: longitudinal analysis, risk factors, incidence, albuminuria, American Indians
INTRODUCTION
The presence of albuminuria, defined as urinary albumin to creatinine ratio (UACR) ≥ 30 mg/g (3.4 mg/mmol) in the urine of an individual with diabetes, predicts progression to diabetic nephropathy1. Albuminuria has been associated with increased risk of all-cause and cardiovascular disease (CVD) mortality in individuals with either type 1 or type 2 diabetes2–5, in hypertensive adults6, 7, and in the general population8, 9. Previous cross-sectional and cohort studies have shown that higher systolic blood pressure and cholesterol, worse glycemic control, and higher baseline albumin excretion rate predict albuminuria in type 2 diabetes10–14. To our knowledge no studies have employed data acquired at more than two time points or used methods of longitudinal data analysis to identify risk factors for albuminuria over time more effectively. The Strong Heart Study (SHS), a longitudinal population-based study of CVD and CVD risk factors in American Indians ages 45–74 years15, has shown high CVD mortality and high prevalence rates of diabetes and albuminuria in this population16–18. The purpose of this investigation was to use the three serial SHS examinations to evaluate risk factors for incident albuminuria over time.
METHODS
The design, survey methods and laboratory techniques of the SHS have been previously reported in detail15. The Indian Health Service, Institutional Review Boards, and participating tribes approved the study. Written informed consent was obtained from each participant. The SHS cohort comprises a population-based sample of 4,549 American Indians, aged 45 to 74 years at the 1st examination (1989–1991) who resided in central Arizona, Oklahoma, and North and South Dakota. Surviving cohort members were re-examined in the 2nd (1993–1995; 90%) and 3rd (1997–1999; 88%) examinations with identical laboratory and clinical examination methods. Each examination included a personal interview and a physical examination. Age, use of antihypertensive medication, type of diabetes therapy (use insulin alone, insulin with oral hypoglycemic agents, oral hypoglycemic agents, or lifestyle alone) and smoking status was ascertained at the interview. The physical examination included standardized blood pressure measurements, a 12-lead resting electrocardiogram, and a fasting blood sample for laboratory measurements, including plasma total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, glucose, and plasma creatinine.
Height and weight were measured with the participant in light clothing with shoes removed. Body mass index (BMI) was calculated as weight in kilograms/height in m2. Systolic and diastolic arterial blood pressures were measured three times while the participants were sitting; the mean of the last two measurements was used to estimate the blood pressure. All medications taken regularly by participants were presented at the examination and categorized according to the American Hospital Formulary Service Pharmacologic-Therapeutic Classification System. Use of classes of antihypertensive agents (angiotensin converting enzyme (ACE) inhibitors, β-blocking agents, calcium channel blocking agents and diuretics) have been previously summarized19. Use of statins was rare prior to their inclusion in the Indian Health Service formulary after completion of the 2nd SHS examination. Diabetes was defined by American Diabetes Association criteria20, i.e., taking antidiabetic medication or fasting glucose ≥126 mg/dl (7.0 mmol/l).
A random morning urine sample was collected to measure of creatinine and albumin. Urine creatinine and albumin were measured by the picric acid method21 and a sensitive nephelometric technique22, respectively. Nine baseline urine samples with albumin concentrations <0.20 g/dl (2 g/l), the lowest detection limit of the assay, were considered to have values of 0.19 g/dl (1.9 g/l) with corresponding UACRs <0.2 mg/g (0.02 mg/mmol).
The current analysis included diabetic participants without albuminuria (UACR<30 mg/g) and with normal plasma creatinine (≤1.5 mg/dl (132.6 µmol/l) for men and 1.3 mg/dl (114.9 µmodl/l) for women) at the 1st examination (n=750) or 2nd examination (n=568) (Figure 1). Measurements from each SHS examination of these participants were used. The generalized estimating equation (GEE) method23 of dichotomous outcome variable in longitudinal studies, a form of longitudinal logistic regression analysis, was used to identify risk factors for incident albuminuria. In the GEE model, an unstructured pairwise log odds ratio pattern was specified for the within-subject association between the two repeated binary responses (albuminuria, yes or no, at the 2nd and 3rd examinations) for each participant. Risk factor measurements for each participant without albuminuria at one examination were used to predict subsequent albuminuria at the next examination (mean of four years between examinations). Risk factors that were considered included age, sex, study center (Arizona, Oklahoma, North/South Dakota), HDL and LDL cholesterol, triglycerides, BMI, systolic blood pressure (SBP), use of antihypertensive medication (ACE inhibitors, other antihypertensive medications, and none), current smoking, HbA1c, fasting glucose, plasma creatinine, type of diabetes therapy (insulin including insulin with oral hypoglycemic agent, oral hypoglycemic agent alone or lifestyle alone), diabetes duration, and UACR within the normal range (<30 mg/g) at previous examination. We have previously shown in the SHS that albuminuria, even within the normal range (UACR<30 mg/g), predicts CVD events and CVD death24. Based on these results, for clinical utility we created categories of albuminuria within what was previously defined as “normal” range: <5, 5–10, and 10–30 mg/g (<0.56, 0.56–1.13, and 1.13–3.39) mg/mmol) and examined their odds ratios for developing frank albuminuria in the GEE model. Analyses were performed using SAS Version 9.0025. Interactions between study center and main effects were not statistically significant. Because the backward elimination is not included in the PROC GENMOD procedure in SAS software, variables were kept in the final GEE model by a manual backward elimination starting with all predictors in the model. The variable with the largest non-significant P value was removed and the model was refitted at each step to remove the least significant variable until all remaining variables had individual P values <0.05. An extension of the Hosmer-Lemeshow goodness-of-fit test for ordinary logistic regression to marginal regression models for repeated binary responses26 was used to assess the fit of the repeated binary response model. Statistical significance was defined as 2-tailed P<0.05 for all tests.
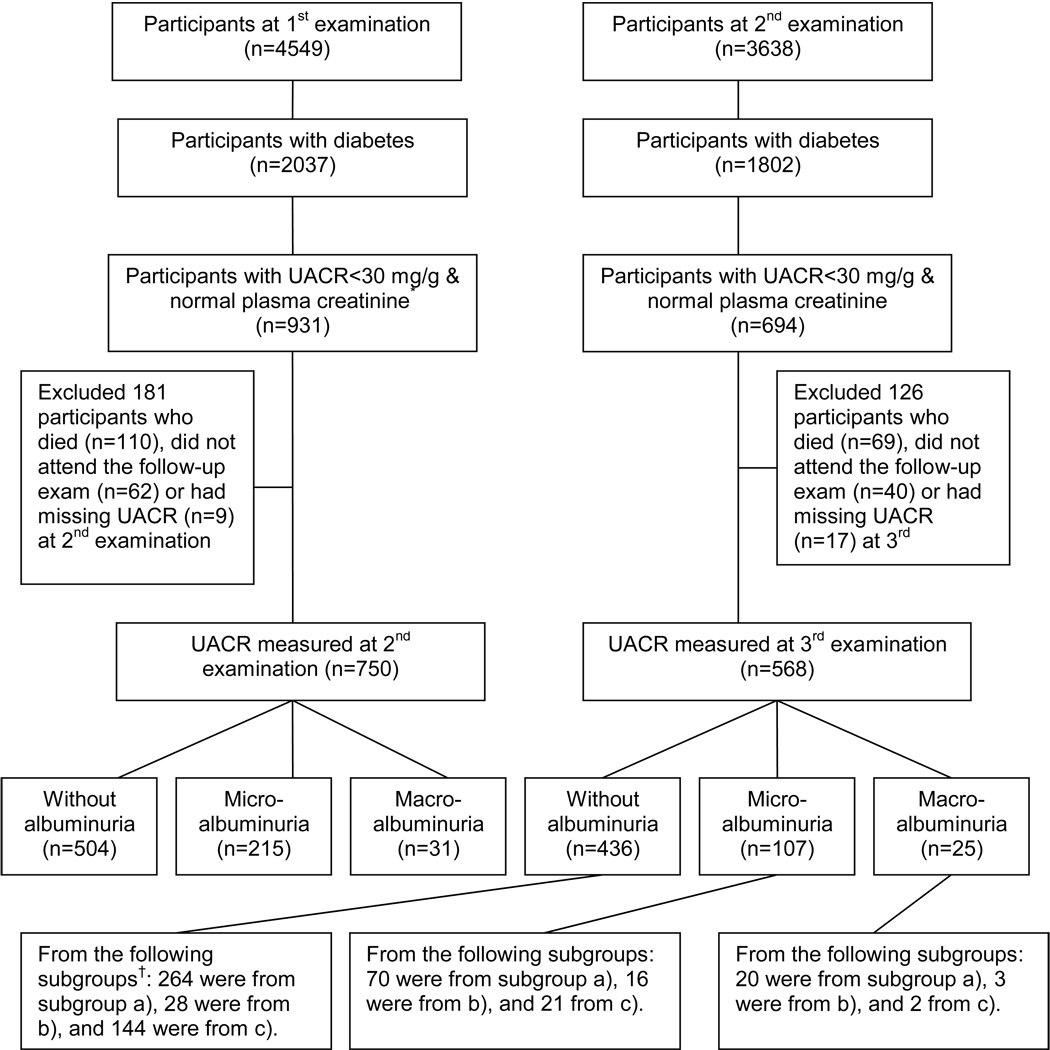
Figure 1.
Participant flow in the Strong Heart Study with development of albuminuria from the 1st examination to the 2nd examination and from the 2nd examination to the 3rd examination. * UACR: urinary albumin/creatinine ratio. Normal plasma creatinine: ≤1.5 mg/dl for men and 1.3 mg/dl for women. † Subgroups: a) the 504 diabetic participants without albuminuria at the 2nd examination; b) the 67 diabetic participants who had remission of albuminuria at the 2nd examination; and c) the 294 participants without diabetes or with missing diabetes status at the 1st examination who developed diabetes by the 2nd examination. To convert creatinine in mg/dL to µmol/L, multiply by 88.4.
RESULTS
The mean (SD) intervals from the 1st to 2nd SHS examination and from the 2nd to 3rd SHS examination were 3.92 (0.72) and 4.07 (0.65) years, respectively. Figure 1 shows participant flow with development of albuminuria from the 1st to 2nd SHS examination and from the 2nd to 3rd SHS examination. Among the 750 diabetic participants ages 45 to 74 years without albuminuria and with normal plasma creatinine at the 1st SHS examination, 504 (67%) remained free of albuminuria and 246 (33%) developed albuminuria (29% microalbuminuria and 4% macroalbuminuria) by the 2nd examination. Among the 568 diabetic participants without albuminuria and with normal plasma creatinine at the 2nd examination, 436 (77%) remained free of albuminuria and 132 (23%) developed albuminuria (19% microalbuminuria and 4% macroalbuminuria) by the 3rd examination. Sixty-seven of the 690 albuminuric diabetic participants at the 1st examination became free of albuminuria by the 2nd examination, and 294 of the 1953 participants without diabetes or with missing diabetes status at the 1st examination had developed diabetes by the 2nd examination. The 568 diabetic participants from the 2nd examination who also had UACR measured at the 3rd examination were from the following subgroups: a) 354 were from the 504 diabetic participants without albuminuria at the 2nd examination; b) 47 were from the 67 diabetic participants who had remission of abuminuria by the 2nd examination; and c) 167 were from the 294 participants without diabetes or with missing diabetes status at the 1st examination who developed diabetes by the 2nd examination. Thus, more diabetic SHS participants were free of albuminuria at the second examination than the number of diabetic participants who did not develop albuminuria between the 1st and 2nd SHS examination.
Table 1 presents baseline characteristics by the follow-up albuminuria status. Those who developed albuminuria between the 1st and 2nd SHS examination compared to those who did not were more likely to reside in Arizona, had higher fasting glucose, HbA1c, SBP and UACR, had longer diabetes duration, and were more likely to receive insulin or oral hypoglycemic agents at the 1st examination. Those who developed albuminuria between the 2nd and 3rd SHS examination compared to those who did not had higher triglycerides, fasting glucose, HbA1c, and UACR, and were more likely to smoke and receive antidiabetic medications at the 2nd examination.
Table 1.
Baseline characteristics by follow-up albuminuria status*
| 2nd examination | 3rd examination | |||||
|---|---|---|---|---|---|---|
| Risk factors† | Developed albuminuria | P- | Developed albuminuria | P- | ||
| Yes | No | value† | Yes | No | value | |
| (n=246) | (n=504) | (n=132) | (n=436) | |||
| Age (years) | 57.3 ± 7.6 | 56.5 ± 7.5 | 0.2 | 58.6 ± 7.3 | 59.0 ± 7.1 | 0.5 |
| Men | 85 (34.5) | 173 (34.3) | 0.9 | 136 (31.2) | 39 (29.5) | 0.7 |
| Study center | 0.01 | 0.9 | ||||
| Arizona | 105 (42.7) | 170 (33.7) | 43 (32.6) | 134 (30.7) | ||
| Oklahoma | 68 (27.6) | 193 (38.3) | 50 (37.9) | 168 (38.5) | ||
| North/South Dakota | 73 (29.7) | 141 (28.0) | 39 (29.5) | 134 (30.7) | ||
| Diabetes duration (years) | 6 (2–11) | 2 (0–8) | <0.001 | 6 (4–12) | 5 (2–10.5) | 0.5 |
| Body mass index (kg/m2) | 32.8 ± 6.2 | 33.0 ± 6.5 | 0.6 | 32.6 ± 6.5 | 33.4 ± 6.3 | 0.2 |
| LDL (mg/dl) | 111.1 ± 32.6 | 115.0 ± 33.2 | 0.1 | 112.8 ± 31.0 | 118.3 ± 32.9 | 0.1 |
| HDL (mg/dl) | 42.4 ± 10.0 | 43.6 ± 11.0 | 0.2 | 37.0 ± 11.4 | 38.6 ± 10.7 | 0.1 |
| Triglycerides (mg/dl) | 131 (95–188) | 124 (89–178) | 0.4 | 148 (108–233) | 135 (98–188) | 0.02 |
| Fasting glucose (mg/dl) | 202.9 ± 71.8 | 179.6 ± 64.6 | <0.001 | 208.5 ± 79.4 | 185.5 ± 72.7 | <0.01 |
| HbA1c (%) | 8.4 ± 2.2 | 7.6 ± 2.3 | <0.001 | 8.6 ± 2.4 | 7.9 ± 2.3 | <0.01 |
| Systolic blood pressure (mm Hg) | 128.7 ± 18.0 | 125.1 ± 16.4 | <0.01 | 128.1 ± 16.8 | 127.1 ± 16.4 | 0.5 |
| Current smoking | 74 (30.1) | 135 (26.8) | 0.3 | 46 (35.9) | 110 (26.0) | 0.03 |
| Use of Antihypertensive medication | 0.1 | 0.6 | ||||
| ACE inhibitors | 8 (3.2) | 26 (5.2) | 20 (15.1) | 82 (18.8) | ||
| Other antihypertensive medication | 65 (26.4) | 100 (19.8) | 27 (20.4) | 81 (18.6) | ||
| None | 173 (70.3) | 378 (75.0) | 85 (64.4) | 273 (62.6) | ||
| Type of diabetes therapy | <0.001 | <0.01 | ||||
| Lifestyle alone | 70 (28.5) | 258 (51.2) | 32 (24.2) | 166 (38.1) | ||
| Insulin‡ | 47 (19.1) | 61 (12.1) | 41 (31.1) | 88 (20.2) | ||
| Oral hypoglycemic agent alone | 129 (52.4) | 185 (36.7) | 59 (44.7) | 182 (41.7) | ||
| UACR (mg/g) | 12.7 (7.8–21.0) | 7.6 (4.2–12.2) | <0.001 | 14.6 (9.1–23.0) | 9.5 (6.0–14.5) | <0.001 |
| Plasma creatinine (mg/dl) | 0.85 ± 0.16 | 0.85 ± 0.15 | 0.9 | 0.84 ± 0.17 | 0.83 ± 0.17 | 0.4 |
The 1st examination was the baseline for outcome at the 2nd examination and the 2nd examination was the baseline for outcome at the 3rd examination. Data are presented as numbers (percentages) for categorical variables and means ± standard deviations or median (interquartile range) for continuous variables. To convert LDL cholesterol and HDL cholesterol in mg/dL to mmol/L, multiply by 0.02586; triglycerides in mg/dL to mmol/L, multiply by 0.01129; glucose in mg/dL to mmol/L, multiply by 0.05551; creatinine in mg/dL to µmol/L, multiply by 88.4.
Abbreviations: HDL, high-density lipoprotein; LDL low-density lipoprotein, ACE inhibitor, angiotensinconverting enzyme inhibitor; UACR, urinary albumin to creatinine ratio.
P value was based on Chi-square test for categorical variables and t-test for continuous variables (diabetes duration, total triglycerides, and UACR were normalized prior to the test) for comparing differences between albuminuria statuses.
Including insulin alone or insulin combined with oral hypoglycemic agents.
The estimated 4-year cumulative incidence rates of albuminuria and 95% confidence intervals after adjustment for baseline UACR are presented in Table 2 by sex, study center, and diabetes duration. There was no sex or study center difference in incidence of albuminuria. The incidence of albuminuria rose with longer diabetes duration (P for trend <0.001), controlled for baseline UACR, sex, and study center.
Table 2.
The generalized linear model and the model-based estimated 4-year cumulative albuminuria incidence (AI, %) and 95% confidence interval (CI)
| Diabetes duration (years)* | |||||||
|---|---|---|---|---|---|---|---|
| <5 | 5 to 10 | >10 | |||||
| Sex | Center† | AI | 95% CI | AI | 95% CI | AI | 95% CI |
| Men | AZ | 27.7 | 22.0–34.2 | 43.3 | 34.7–52.5 | 43.4 | 35.4–51.7 |
| OK | 18.1 | 13.9–23.1 | 30.1 | 23.0–38.3 | 36.3 | 28.8–44.6 | |
| ND/SD | 22.4 | 17.3–28.6 | 35.9 | 27.7–45.0 | 39.7 | 31.0–49.0 | |
| Women | AZ | 25.1 | 20.3–30.7 | 32.4 | 25.9–39.7 | 42.2 | 35.6–49.0 |
| OK | 17.9 | 14.1–22.5 | 29.2 | 22.9–36.5 | 31.4 | 25.2–38.5 | |
| ND/SD | 25.0 | 20.1–30.7 | 31.7 | 24.9–39.4 | 36.2 | 28.9–44.2 | |
AZ, Arizona; OK, Oklahoma; ND/SD, North/South Dakota.
Model used to estimate the above albuminuria incidence: Probability (an individual will develop albuminuria in 4-years)=exp(B)/[1+exp(B)], where B=2.6325+ 0.4776* log(UACR)+0.171*I(male)−0.1898*I(OK)+0.0343*I(ND/SD) +0.312*I(diabetes duration between 5 to 10 years)+0.5957*I(diabetes duration >10 years), and I(.) is the index function. The baseline UACR (urinary albumin to creatinine ratio) was set at its mean value when calculating the probabilities for each sex by center and by diabetes duration category. For the log(UACR) effect: odds ratio (OR)=1.61, P<0.001; sex effect: male vs female, OR=1.19, P=0.2; center effect: OK vs AZ, OR=0.83, P=0.2; ND/SD vs AZ, OR=1.03, P=0.5; ND/SD vs OK, P=0.8; diabetes duration effect: (5 to 10 years) vs (<5 years), OR=1.37, P=0.05; (>10 years) vs (<5 years), OR=1.81, P<0.001; (>10 years) vs (5 to 10 years), P=0.1.
Type 3 test P<0.01
Type 3 test P=0.3
The crude incidence of overt albuminuria in participants with baseline UACR <5, 5 to <10 and 10 to <30 mg/g was 16% (95% CI 12.2–20.8), 20.9% (CI 17.3–25.0) and 40.8% (CI 37.0–44.7), respectively. Compared to diabetic participants with baseline UACR <5 mg/g, albuminuria incidence was not significantly higher with baseline UACR of 5 to <10 mg/g (P=0.1), but was much higher with baseline UACR of 10 to <30 mg/g than with UACR in the other two categories (P<0.001).
The 4-year cumulative albuminuria incidence from the GEE model is presented in Table 3. In univariate analyses, UACR of 10–30 mg/g at previous examination, residence in Arizona compared with Oklahoma, higher LDL cholesterol, higher SBP, smoking, higher fasting glucose and HbA1c, diabetes duration ≥5 years, and use of anti-diabetic medications were significant predictors of incident albuminuria. In multivariate analyses, all of the above variables remained significant predictors of incident albuminuria except LDL cholesterol, HbA1c and diabetes duration. In addition, lack of ACE inhibitor use and higher baseline plasma creatinine predicted incident albuminuria. Diabetes duration and HbA1c did not remain in the final model, possibly because of high correlation between fasting glucose and HbA1c (Pearson r=0.74, P<0.001) and diabetes duration and type of diabetes therapy (Spearman ρ=0.48, P<0.001). Participants with UACR of 10–30 mg/g had 171% higher odds of incident albuminuria than those with UACR<5 mg/g at previous examination, independent of other significant risk factors. The extension of the Hosmer-Lemeshow goodness-of-fit test for marginal regression models for repeated binary responses provided no evidence for lack of fit in the final multivariate model (goodness-of-fit statistic χ2=11.74 with df=9, P=0.2).
Table 3.
Factors associated with 4-year cumulative albuminuria incidence
| Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|
| OR (95% CI) | Wald Chi-square |
P- value |
|||
| UACR (mg/g) | <0.001† | 43.9 | <0.001 | ||
| <5 | Referent | Referent | |||
| 5–10 | 1.38 (0.94–2.04) | 0.10 | 1.17 (0.79–1.71) | 0.4 | |
| ≥10 | 3.61 (2.53–5.14) | <0.001 | 2.71 (1.89–3.90) | <0.001 | |
| Female | 1.01 (0.78–1.31) | 0.9 | |||
| Age (per 5 years) | 1.01 (0.97–1.04) | 0.6 | |||
| Body mass index (per 1 kg/m2) | 0.99 (0.97–1.01) | 0.2 | |||
| Study center | 0.02 | 6.3 | 0.04 | ||
| Arizona | Referent | Referent | |||
| Oklahoma | 0.65 (0.49–0.87) | <0.01 | 0.65 (0.47–0.90) | 0.01 | |
| N/S Dakota | 0.82 (0.61–1.11) | 0.2 | 0.77 (0.55–1.09) | 0.1 | |
| HDL cholesterol (per 1 mg/dl) | 0.99 (0.98–1.00) | 0.2 | |||
| LDL cholesterol (per 1 mg/dl) | 0.99 (0.99–1.00) | 0.02 | |||
| Log (Triglycerides) (per 2.7 mg/dl) | 1.23 (0.99–1.53) | 0.07 | |||
| Systolic blood pressure (per 5 mm Hg) |
1.02 (1.00–1.03) | 0.01 | 1.02 (1.01–1.04) | 6.0 | 0.01 |
| Use of antihypertensive medication | 0.02 | 6.9 | 0.03 | ||
| None | Referent | Referent | |||
| ACE inhibitors | 0.68 (0.45–1.04) | 0.07 | 0.60 (0.38–0.95) | 0.03 | |
| Other antihypertensive medications | 1.28 (0.96–1.70) | 0.09 | 1.15 (0.83–1.59) | 0.4 | |
| Current smoking | 1.31 (1.01–1.70) | 0.04 | 1.49 (1.12–1.99) | 6.8 | <0.01 |
| Fasting glucose (per 10 mg/dl) | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.00–1.02) | 5.6 | 0.02 |
| HbA1c (per 1%) | 1.15 (1.09–1.21) | <0.001 | |||
| Plasma creatinine (per 1 mg/dl) | 1.39 (0.67–2.85) | 0.4 | 2.92 (1.27–6.69) | 5.9 | 0.01 |
| Diabetes duration (years) | <0.001 | ||||
| <5 | Referent | ||||
| 5–10 | 1.62 (1.21–2.17) | <0.01 | |||
| >10 | 2.11 (1.60–2.79) | <0.001 | |||
| Type of diabetes therapy | <0.001 | 23.4 | <0.001 | ||
| Lifestyle alone | Referent | Referent | |||
| Insulin‡ | 2.78 (1.99–3.89) | <0.001 | 2.35 (1.60–3.45) | <0.001 | |
| Oral hypoglycemic agent alone | 2.29 (1.75–3.00) | <0.001 | 2.02 (1.49–2.74) | <0.001 | |
UACR, urinary albumin to creatinine ratio; ACE, angiotensin converting enzyme. To convert LDL cholesterol and HDL cholesterol in mg/dL to mmol/L, multiply by 0.02586; triglycerides in mg/dL to mmol/L, multiply by 0.01129; glucose in mg/dL to mmol/L, multiply by 0.05551; creatinine in mg/dL to µmol/L, multiply by 88.4.
Adjusted odds ratios for variables included in the final generalized estimating equation model with categorical variable UACR, study center, and use of antihypertensive medication forced to the model and backward elimination for other variables with significant stay level 0.05.
P-value for type 3 test.
Including insulin alone or insulin combined with oral hypoglycemic agents.
In a sensitivity analysis, albuminuria was defined using sex-specific cutpoints (UACR ≥17 mg/g in men, ≥25 mg/g in women). UACR of 10–30 mg/g at previous examination, higher fasting glucose, higher SBP, residence in Arizona compared with Oklahoma, lack of ACE inhibitor use, and use of anti-diabetic medications were still significant, independent predictors of incident albuminuria. In addition, male sex (OR=1.55, 95% CI: 1.19–2.03) and lower HDL (OR=0.99, CI: 0.97–0.998) were also predictive of incident albuminuria, while plasma creatinine and smoking failed to reach statistical significance in this analysis.
To assess the period effect, an indicator variable for the period from the 2nd to 3rd SHS examinations was added to the GEE final model in Table 3. The results showed that odds of developing albuminuria were significantly lower between the 2nd and 3rd examinations than between the 1st and 2nd examinations after adjustment for all significant risk factors listed in Table 3 (odds ratio=0.44, 95% CI 0.32 to 0.60).
DISCUSSION
In this middle-aged to elderly diabetic population, UACR of 10–30 mg/g at previous examination, higher fasting glucose, residence in Arizona compared with Oklahoma, higher SBP, smoking, less use of ACE inhibitors, need for antidiabetic medications and higher plasma creatinine were significant, independent predictors of incident albuminuria. Diabetic participants with UACR of 10 to <30 mg/g at the previous examination had 2.7-fold odds of developing albuminuria compared with those with UACR<5 mg/g.
The odds of developing albuminuria between the 2nd and 3rd SHS examinations was significantly lower by 56% compared with that between the 1st and 2nd examinations after adjustment for other risk factors included in the multivariate model in Table 3. There are several possible explanations for the decline in odds for developing albuminuria over time. First is the possibility of misclassification (since the determination of albuminuria was based on a single random morning urine sample) at each evaluation; likewise, there were improvements in glycemic and blood pressure control which could have impacted the incidence of albuminuria27. In the SHS study, 9.7% (67 of 690) diabetic participants with albuminuria at the 1st examination became free of albuminuria at the 2nd examination while 19.0% (119 of 625) diabetic participants at the 2nd examination became free of albuminuria at the 3rd examination; there was no significant difference between the percentage of diabetic participants under good glycemic control (HbA1c<7%) at the 1st examination (41.5% (311 of 750)) and the 2nd examination (38.2% (217 of 568)) (P=0.2); however, the percentage of participants using ACE inhibitors among those using antihypertensive medications increased from 17% (34 of 199) at the 1st examination to 52% (102 of 197) at the 2nd examination (P<0.001), although the percentage of using ACE inhibitors at the 1st examination may be underestimated because 18% (36 of 199) of diabetic participants at the 1st examination only reported using non-specific hypotensive or antihypertensive medications. It is also known from long-term observational studies that less than half of patients develop nephropathy, irrespective of glycemic control28, 29. Thus, it seems most likely that the lower rate after the 2nd examination might reflect depletion of susceptible diabetic individuals actually at risk for diabetic nephropathy, given the relatively long diabetes duration in the cohort.
We found differences for odds of incident albuminuria by site. Participants from Oklahoma had 35% lower odds of developing albuminuria compared with those from Arizona or North/South Dakota. Reasons for the different odds of incident albuminuria among study sites may be explained by differences in access to health care, environmental factors, or by genetic variation. The Oklahoma site had the highest use of ACE inhibitors (12.9% in Oklahoma vs. 10.2% in Arizona and 7.2% in North/South Dakota) and other antihypertensive medications (25.0% in Oklahoma vs. 18.4% in Arizona and 18.1 in North/South Dakota) (p<0.001); likewise, glycemic control (HbA1c<7%) tended to be less tight in Arizona (28.9% in Arizona vs. 38.8% in Oklahoma and 39.3% in North/South Dakota) (p<0.01). Because at the 1st SHS examination of the SHS only a small number of participants at each site underwent dietary assessment, we can not evaluate if environmental factor such as intake of sodium or protein contributed to site differences for odds of incident albuminuria.
In our study, odds of incident albuminuria is elevated even in people with baseline UACR lower than traditional cutoff value (UACR<30 mg/g), which is consistent with findings from other studies12–14. Participants with UACR of 10–30 mg/g had 2.7-fold odds of developing albuminuria compared with those with UACR<5 mg/g. This increased odds of progression from “sub-threshold” levels of urinary albumin to frank albuminuria in our study adds to the current discussion of a possible new definition of albuminuria. Indeed, this observation is in accord with the results of several studies suggesting that either “high-normal levels of albuminuria” or “albuminuria within the normal range” are associated with increased risk of cardiovascular disease and death9, 24, 30–32.
This is the first study to report that type of diabetes therapy independently predicts subsequent albuminuria after adjustment for other risk factors. Diabetic men and women receiving insulin therapy (including insulin alone or with oral hypoglycemic agent) had 2.4 times odds of incident albuminuria, while those receiving only oral hypoglycemic agents had twice the odds of developing albuminuria compared with those whose diabetes was controlled with diet or exercise alone after adjustment for other significant risk factors listed in Table 3. We are aware of only one other study which addressed the association of antidiabetic therapy with incident albuminuria. Our findings are consistent with those reported in Pima Indians, i.e., incident albuminuria in subjects treated with either insulin or oral agents was 2.8 times that in those who had not received either drugs at the time of the initial examination when only controlled for age, sex, and diabetes duration10. The association of type of diabetes therapy with incident albuminuria may well reflect confounding by indication in the presence of more severe underlying diabetes; or unappreciated longer diabetes duration or impaired glucose tolerance because diabetes duration data were based only on self report and the need for antidiabetic medications may be a better indicator of duration and severity.
Our current longitudinal analysis confirms previous suggestions that systolic blood pressure, fasting glucose, and plasma creatinine are risk factors for incident albuminuria in type 2 diabetes as identified in previous prospective studies4, 10–12, 33, 34. In addition, we found that diabetic men and women who were current smokers had 49% higher odds of developing albuminuria after adjustment for other risk factors. This is consistent with findings from a prospective study in a population with older-onset type 2 diabetes35. In contrast, history of smoking, rather than current smoking, was found to be associated with microalbuminuria in one study of 108 patients with type 2 diabetes34 but not in another study of 191 patients with type 2 diabetes12.
Many other studies36–40 show that either ACE inhibitors or angiotensin II receptor blockers (ARBs) decrease both incident microalbuminuria and progression from microalbuminuria to macroalbuminuria (while also preserving renal function) in individuals with diabetes, irrespective of hypertension. We also found that participants using ACE inhibitors had 40% lower odds of developing albuminuria within four-year follow-up. None of our participants used ARBs at either the 1st or 2nd SHS examinations.
Age, male sex, cholesterol concentration, and plasma triglycerides have been reported as risk factors for incident albuminuria in some studies10, 12, 14 but not in others11, 13. The different results could be due to different definitions for albuminuria, sample size, and statistical models (logistic regression vs. Cox proportional hazards models). Although in our primary analysis, none of these variables listed above were significant risk factors, in an alternative analysis when albuminuria was defined using sex-specific cutpoints (UACR ≥17 mg/g in men, ≥25 mg/g in women), male sex and low HDL cholesterol were predictors of incident albuminuria. These sex-specific cutpoints may be warranted.
The strengths of the present study are its longitudinal design, the large sample size, and multivariable-adjusted analyses. A key limitation relates to outcome ascertainment: a single random morning urine specimen rather than multiple specimens or a timed specimen was collected to measure albumin and creatinine. A recent American Diabetes Association consensus guideline41 suggests that at least two urine collections be performed in a 3-to 6-month period to appropriately classify individual patients as normo-, micro- or macroalbuminuric. Because our study was not originally designed for clinical diagnosis of albuminuria in individuals, the collections were not performed accordingly. However, our methods are in accord with recent guidelines indicating that UACR calculated from a spot urine random sample (preferably a first morning specimen) correlates well with results of 24-hour urine collections42. This study was conducted in a single population, American Indians. However this population has been shown in many previous analyses to provide data that are relevant to all men and women with type 2 diabetes. Finally an intrinsic limitation of this and other studies focusing on incidence rates of an abnormality in a population initially free of that abnormality is that it will tend to overestimate the increase in its prevalence in the entire population because individuals who are reclassified from abnormal to normal (e.g., if UACR went from 31 to 29 mg/g) are not considered in this type of analysis.
In conclusion, in middle-aged to elderly diabetic men and women, higher UACR at previous examination, higher fasting glucose level, higher SBP, smoking, lack of ACE inhibitors use, place of residence, need for antidiabetic medications, and higher plasma creatinine were significant independent predictors of incident albuminuria. These data suggest that the odds for incident albuminuria may be substantially reduced by emphasis on blood pressure and glucose control, smoking cessation, and use of ACE inhibitors.
ACKNOWLEDGEMENTS
The authors acknowledge the assistance and cooperation of the Indian communities; without their support this study would not have been possible. The authors also wish to thank the Indian Health Service hospitals and clinics at each center, the directors of the Strong Heart Study clinics and their staff. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
Support: This study was supported by cooperative agreement grants U01-HL-41642, U01-HL-41652, and UL01-HL-41654 from the National Heart, Lung, and Blood Institute and, in part, by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
None.
REFERENCES
- 1.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. New Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 2.Arun CS, Stoddart J, Mackin P, MacLeod JM, New JP, Marshall SM. Significance of microalbuminuria in long-duration type 1 diabetes. Diabetes Care. 2003;26:2144–2149. doi: 10.2337/diacare.26.7.2144. [DOI] [PubMed] [Google Scholar]
- 3.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 4.Niskanen LK, Penttila I, Parviainen M, Uusitupa MI. Evolution, risk factors, and prognostic implications of albuminuria in NIDDM. Diabetes Care. 1996;19:486–493. doi: 10.2337/diacare.19.5.486. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Lee ET, Best LG, et al. Association of Albuminuria with All-cause and Cardiovascular Disease Mortality in Diabetes: The Strong Heart Study. The British Journal of Diabetes & Vascular Disease. 2005;5:334–340. [Google Scholar]
- 6.Jager A, Kostense PJ, Ruhe HG, et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:617–624. doi: 10.1161/01.atv.19.3.617. [DOI] [PubMed] [Google Scholar]
- 7.Romundstad S, Holmen J, Hallan H, Kvenild K, Ellekjaer H. Microalbuminuria and all-cause mortality in treated hypertensive individuals: does sex matter? The Nord-Trondelag Health Study (HUNT), Norway. Circulation. 2003;108:2783–2789. doi: 10.1161/01.CIR.0000103667.27493.32. [DOI] [PubMed] [Google Scholar]
- 8.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and non-cardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 9.Yuyun MF, Khaw KT, Luben R, et al. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: The European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol. 2004;33:189–198. doi: 10.1093/ije/dyh008. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RG, Knowler WC, Pettitt DJ, Hanson RL, Bennett PH. Incidence and determinants of elevated urinary albumin excretion in Pima Indians with NIDDM. Diabetes Care. 1995;18:182–187. doi: 10.2337/diacare.18.2.182. [DOI] [PubMed] [Google Scholar]
- 11.Park JY, Kim HK, Chung YE, Kim SW, Hong SK, Lee KU. Incidence and determinants of microalbuminuria in Koreans with type 2 diabetes. Diabetes Care. 1998;21:530–534. doi: 10.2337/diacare.21.4.530. [DOI] [PubMed] [Google Scholar]
- 12.Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314:783–788. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murussi M, Baglio P, Gross JL, Silveiro SP. Risk factors for microalbuminuria and macroalbuminuria in type 2 diabetic patients: a 9-year follow-up study. Diabetes Care. 2002;25:1101–1103. doi: 10.2337/diacare.25.6.1101. [DOI] [PubMed] [Google Scholar]
- 14.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 15.Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 16.Lee ET, Cowan LD, Welty TK, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988. The Strong Heart Study. Am J Epidemiol. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 17.Lee ET, Welty TK, Cowan LD, et al. Incidence of diabetes in American Indians of three geographic areas: The Strong Heart Study. Diabetes Care. 2002;25:49–54. doi: 10.2337/diacare.25.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Robbins DC, Knowler WC, Lee ET, et al. Regional differences in albuminuria among American Indians: an epidemic of renal disease. Kidney Int. 1996;49:557–563. doi: 10.1038/ki.1996.79. [DOI] [PubMed] [Google Scholar]
- 19.Hayslett JA, Eichner JE, Yeh JL, et al. Hypertension treatment patterns in American Indians: The Strong Heart Study. Am J Hypertens. 2001;14:950–956. doi: 10.1016/s0895-7061(01)02146-x. [DOI] [PubMed] [Google Scholar]
- 20.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 21.Chasson A, Grady H, Stanley M. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Tech. 1957;30:207–212. [PubMed] [Google Scholar]
- 22.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycaemia. Diabetologia. 1984;26:127–133. doi: 10.1007/BF00281119. [DOI] [PubMed] [Google Scholar]
- 23.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of longitudinal data. Oxford University Press; 2002. [Google Scholar]
- 24.Xu J, Knowler WC, Devereux RB, et al. Albuminuria within the "normal" range and risk of cardiovascular disease and death in American Indians: The Strong Heart Study. Am J Kidney Dis. 2007;49:208–216. doi: 10.1053/j.ajkd.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 25.SAS. SAS Release 9.00. SAS Institute: Cary, NC; 2002. [Google Scholar]
- 26.Horton NJ, Bebchuk JD, Jones CL, et al. Goodness-of-fit for GEE: an example with mental health service utilization. Statis Med. 1999;18:213–222. doi: 10.1002/(sici)1097-0258(19990130)18:2<213::aid-sim999>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Parving HH, Hommel E. Prognosis in diabetic nephropathy. BMJ. 1989;299:230–233. doi: 10.1136/bmj.299.6693.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25:496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- 29.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med. 1985;78:785–794. doi: 10.1016/0002-9343(85)90284-0. [DOI] [PubMed] [Google Scholar]
- 30.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and non-diabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 31.Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 32.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 33.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Internal Med. 1998;158:998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 34.Forsblom CM, Groop PH, Ekstrand A, et al. Predictors of progression from normoalbuminuria to microalbuminuria in NIDDM. Diabetes Care. 1998;21:1932–1938. doi: 10.2337/diacare.21.11.1932. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Klein BE, Moss SE. Incidence of gross proteinuria in older-onset diabetes. A population-based perspective. Diabetes. 1993;42:381–389. doi: 10.2337/diab.42.3.381. [DOI] [PubMed] [Google Scholar]
- 36.Chan JC, Ko GT, Leung DH, et al. Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int. 2000;57:590–600. doi: 10.1046/j.1523-1755.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 37.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 38.Agardh CD, Garcia-Puig J, Charbonnel B, Angelkort B, Barnett AH. Greater reduction of urinary albumin excretion in hypertensive type II diabetic patients with incipient nephropathy by lisinopril than by nifedipine. J Hum Hypertens. 1996;10:185–192. [PubMed] [Google Scholar]
- 39.Chan JC, Cockram CS, Nicholls MG, Cheung CK, Swaminathan R. Comparison of enalapril and nifedipine in treating non-insulin dependent diabetes associated with hypertension: one year analysis. BMJ. 1992;305:981–985. doi: 10.1136/bmj.305.6860.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trevisan R, Tiengo A North-East Italy Microalbuminuria Study Group. Effect of low-dose ramipril on microalbuminuria in normotensive or mild hypertensive non-insulin-dependent diabetic patients. Am J Hypertens. 1995;8:876–883. doi: 10.1016/0895-7061(95)00162-i. [DOI] [PubMed] [Google Scholar]
- 41.Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 42.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]