Abstract
We used fMRI to test the hypothesis that the nature of the neural response to taste varies as a function of the task the subject is asked to perform. Subjects received sweet, sour, salty and tasteless solutions passively and while evaluating stimulus presence, pleasantness and identity. Within the insula and overlying operculum the location of maximal response to taste vs. tasteless varied as a function of task; however, the primary taste cortex (anterior dorsal insula/ frontal operculum- AIFO), as well as a more ventral region of anterior insula, responded to taste vs. tasteless irrespective of task. Although response here did not depend upon task, preferential connectivity between AIFO and the amygdala (bilaterally) was observed when subjects tasted passively compared to when they performed a task. This suggests that information transfer between AIFO and the amygdala is maximal during implicit processing of taste. In contrast, a region of left lateral orbitofrontal cortex (OFC) responded preferentially to taste and to tasteless when subjects evaluated pleasantness and was preferentially connected to earlier gustatory relays (caudomedial OFC and AIFO) when a taste was present. This suggests that processing in the lateral OFC organizes the retrieval of gustatory information from earlier relays in the service of computing perceived pleasantness. These findings show that neural encoding of taste varies as a function of task beyond that of the initial cortical representation.
Keywords: insula, orbitofrontal cortex, amygdala, implicit processing, hedonic evaluation
Introduction
The taste signal is conveyed from the taste receptor cells and/or presynaptic cells (Chandrashekar et al., 2006; Tomchik et al., 2007) in the oral cavity via the cranial nerves VII (the chorda tympani branch of the facial nerve), IX (the glossopharyngeal nerve) and X (the vagus nerve) to the nucleus tractus solitarius (NTS), the first gustatory relay in the brain (Beckstead et al., 1980). Second-order gustatory fibres ascend ipsilaterally from the NTS to join the central tegmental tract and project to the parvicellular part of the ventroposteromedial nucleus of the thalamus (VPMpc) (Beckstead et al., 1980). The primary efferent projection from monkey VPMpc is located in ipsilateral insular/opercular cortex adjacent to the superior limiting sulcus (anterior insula and overlying frontal operculum - AIFO) and extends rostrally to the caudolateral orbitofrontal cortex (OFC) (Mufson & Mesulam, 1984; Ogawa et al., 1985; Pritchard et al., 1986). In humans, a cytoarchitectonically homologous region has been identified in AIFO (Petrides and Pandya, 2002). A second, less extensive, projection terminates in areas 3a, 3b, 2, and 1 along the lateral margin of the precentral gyrus (Pritchard et al., 1986). Thus, anatomical studies have identified two regions that receive afferent information from taste thalamus and thus it could be argued that there are two “primary” gustatory regions within the insula/operculum.
Neuroimaging studies of taste consistently report activation of the human homologues of the two primary areas identified in monkeys (Small et al., 1999; Verhagen & Engelen, 2006). However, activation often extends beyond these regions to include the ventral (Kinomura et al., 1994; Small et al., 2003) and posterior insula (Ogawa et al., 2005), as well as overlying Rolandic, frontal and parietal opercula (Kobayakawa et al., 1996; Faurion et al., 1998; Kobayakawa et al., 1999; Cerf-Ducastel et al., 2001). This suggests that taste information from the primary regions synapses with adjacent insular and opercular neurons.
One important question that remains to be answered is what factor or factors account for the variability in the location of maximal taste responses observed in neuroimaging studies? The insula is sensitive to a variety of sensations associated with eating including olfaction, oral somatosensation, swallowing, temperature and viscosity (Smith-Swintosky et al., 1991; Hamdy et al., 1999; Zald & Pardo, 1999; de Araujo & Rolls, 2004; Verhagen et al., 2004; Watanabe et al., 2004; Kadohisa et al., 2005a; Rolls, 2005). Thus, subtle differences in these parameters may affect taste related activity. Kobayakawa and colleagues have argued that the long stimulus presentation times traditionally employed by fMRI and PET studies precludes identification of activity in a quickly-adapting posterior parietal operculum (Kobayakawa et al., 1996; Kobayakawa et al., 1999; Ogawa et al., 2005). However, while they have shown that this posterior region shows the earliest response to taste (Kobayakawa et al., 1999; Ogawa et al., 2005), and can be identified using faster stimulus presentation with fMRI, there is no evidence for a direct gustatory projection to this region. Since this region is sensitive to attentional orienting to taste, we have argued that its recruitment during taste tasks reflects activation of the mouth area as a function of paying attention to taste in the mouth (Veldhuizen et al., 2007).
It is also possible that taste responses in the insula and overlying opercula are influenced by chemotopic or valence-specific organization. In an earlier study we reported different regions of insula and operculum respond to sweet compared to bitter solutions (Small et al., 2003), and Schoenfeld and colleagues (2004) report separate, as well as overlapping, insular activations to different tastes (Schoenfeld et al., 2004). However, there is no evidence for chemotopy in nonhuman primate studies (Scott & Plata-Salaman, 1999).
Finally, different paradigms have been employed across imaging experiments, with some requiring subjects to continuously rate intensity perception (Cerf et al., 1996; Faurion et al., 1998; Cerf-Ducastel et al., 2001), others requiring ratings of perceived pleasantness, quality and/or intensity preceding or following a functional run (Kobayakawa et al., 1996; Zald et al., 1998; Kobayakawa et al., 1999; Cerf-Ducastel et al., 2001; Ogawa et al., 2005), or following stimulus presentation (Zald et al., 1998; Mizoguchi et al., 2002; Zald et al., 2002; de Araujo et al., 2003b; Cerf-Ducastel & Murphy, 2004; Nitschke et al., 2006; Sarinopoulos et al., 2006), and yet others requiring subjects to report if they detect a stimulus (Small et al., 1997; Zald & Pardo, 2000; Veldhuizen et al., 2007). Others measure responses during passively tasting (Kinomura et al., 1994; Frey & Petrides, 1999; Barry et al., 2001; O’Doherty et al., 2001; O’Doherty et al., 2002; Frank et al., 2003; Small et al., 2003; Yamamoto et al., 2003; Schoenfeld et al., 2004). It is therefore possible that the different cognitive demands imposed by experimental paradigms may contribute to the variability of activations found. This possibility is consistent with recent work showing that trying to taste in the absence of taste results in enhanced responses in primary gustatory cortex in the insula and overlying operculum (Veldhuizen et al., 2007) and that neural response to the same taste differs depending upon whether subjects attend to its intensity or its pleasantness (Grabenhorst & Rolls, 2008).
The goal of the current study was to use fMRI to explore the role of task on neural encoding of taste. All subjects received sweet, sour, salty and tasteless solutions passively and while performing one of three tasks; taste detection, taste identification and evaluation of taste pleasantness. By collapsing across tastants and comparing response to all tastes vs. tasteless we could rule out differences in quality or perceived pleasantness contributing to differential activations. By employing brief stimulation periods in an event-related design we reduced the possibility that rapid adaptation would reduce responses. We predicted that the primary insular taste cortex would respond to taste compared to tasteless irrespective of the task but that beyond this initial cortical encoding there would be differential recruitment of regions, as well as in differential connectivity between regions, as a function of task, reflecting the influence of top-down influences on taste encoding.
Materials and Methods
Pilot study
Ten pilot subjects sampled and rated the intensity and pleasantness of our stimuli. A visual analog scale was used to collect pleasantness ratings (100mm scale anchored by 0 = least pleasant, 50 = neutral, 100 = most pleasant) and the general labeled magnitude scale was used to collect intensity ratings (anchored by 0 = barely detectable and 100 = most intense experience imaginable)(Green et al., 1996). Subjects verified that the stimuli used in our study were not highly aversive and were of moderate to weak intensity. The mean and standard deviation of pleasantness rating were 48.6 ± 0.49 for tasteless, 72.63 ± 3.15 for sucrose, 50.46 ± 3.55 for sour, and 46.30 ± 4.46 for salty. A within subjects ANOVA showed a main effect of taste {F(3,9) = 5.3; p = 0.05) with planned comparisons revealing that sweet is rated as more pleasant than all other stimuli (tasteless p = 0.0002; sour p = 0.002; salty p = 0.0003) tasteless as more pleasant than sour (p = 0.02) and no difference between sour and salty. Mean standard deviations for intensity ratings were 2.95 ± 1.01 for tasteless, 28.18 ± 5.14 for sucrose, 20.25 ± 3.29 for sour, 23.25 ± 4.14 for salty. A within subjects ANOVA showed a main effect of taste F 10.6(3,9) = 10.59; p 0.003) with tasteless being rated as less intense than all other stimuli (sweet p = 0.002, sour p = 0.001, salty = 0.002), sweet as more intense than sour (p = -.04) and no difference in intensity ratings between sour and salty.
Subjects
Nineteen healthy right-handed volunteers participated in the main study. Four subjects were excluded from the analysis due to technical difficulties during scanning. All subjects (9 women and 6 men; mean age: 25.4; range 22-31) participated with full informed consent and the study was approved by the Yale Human Investigation Committee. All subjects were classified as being right handed by the modified Edinburgh inventory (Oldfield, 1971). The average handedness score was 77 out of 100 (std err = ±10.13).
Experimental Paradigm
Subjects sampled one of four different solutions while they performed one of four different tasks (Figure 1). Gustatory stimuli consisted of a 0.5mL bolus of either sweet (5.6X10-1M sucrose), sour (1.0X10-2M citric acid), salty (1.8X10-1M NaCl) or tasteless (12.5mM KCL + 1.24mM NaHCO3) solutions. As is now standard practice (O’Doherty et al., 2001; O’Doherty et al., 2002; de Araujo et al., 2003a; Small et al., 2003; de Araujo & Rolls, 2004; Veldhuizen et al., 2007; Small et al., 2008), we employed the tasteless solution, which contains similar ionic components as saliva, rather than water as the baseline solution because water has been shown to activate the primary gustatory cortex (Frey & Petrides, 1999; Zald & Pardo, 2000). The tasks were to taste passively (P), detect the presence of a taste (D), identify the taste (ID) or indicate the perceived pleasantness (PP). During D subjects responded to the question “Is there a taste?” by pressing one of the buttons on either the left or right button boxes (representing ‘yes’ or ‘no’ responses, respectively). In ID they responded to the question “What is the taste?” with buttons representing ‘sweet, sour, salty, and tasteless’. In PP subjects responded to the question “How pleasant is the taste?” with buttons designated as ‘very pleasant, pleasant, unpleasant, and very unpleasant’. For the purposes of analysis “very pleasant” was assigned the numerical value of 4, “pleasant” a 3, “unpleasant” a 2 and “very unpleasant” a 1. The order the response buttons was counterbalanced so that some subject response options were ordered from left to right, and some were right to left. In P subjects were instructed to press one of the buttons at random. Subjects kept their eyes closed for the duration of the experiment.
Figure 1. Graphic depiction of long event-related design.
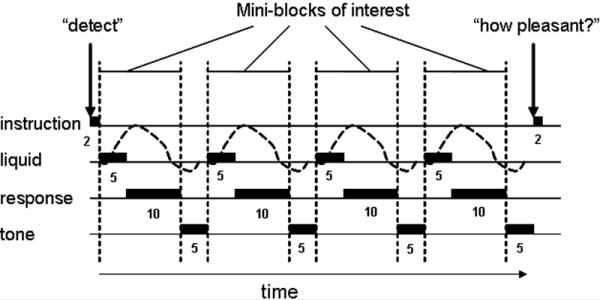
A long-event related design was used. Three different taste stimuli (sweet, sour, salty) and one tasteless stimulus, with similar ionic components to saliva (O’Doherty et al., 2001), were delivered (indicted by “liquid”. Every event began with the delivery of 0.5 cc of liquid over a 5 second period. The liquid was held in the mouth until a 400Hz tone played for 5 s signaling the window of the time during which subjects were allowed to swallow. Responses to instructions were made on manually held controllers after stimulus delivery and before the onset of the swallow tone (indicated by “response”. The dotted line indicates the predicted hemodynamic response function. Tasks were performed in blocks that began with a 2 second instruction indicating the task condition (“Is there a taste” D; “What is the taste” ID; “How pleasant is the taste” PP; “Randomly press” P). The subject was instructed to respond to the same task condition over 4 trials before the instruction changed. All 4 task conditions were presented during a run and counterbalanced to account for order effects.
A single “event” is depicted in figure 1 and consisted of an audio instruction, delivery of the liquid (taste or tasteless) over 5 seconds, a 10 second “hold” during which the subject was trained to hold the taste in their mouth and wait, and then an audio cue signaling them to swallow. We define the event of interest as the first 15 seconds of taste delivery (liquid + response, Figure 1) and model the swallowing as an event of no interest (i.e., a confound) (tone, Figure 1). Subjects were instructed to respond anytime after receipt of the taste and that the swallow audio cue also meant that only 5 seconds of response time remained before the commencement of the next trial.
Events were grouped into “instruction blocks” so that the same judgment was made for four events in a row. Subjects were never informed about the identity of the liquid they were about to receive and were equally likely to receive the tasteless, sweet, sour or salty stimulus. Each run comprised 4 instruction blocks for a total of 16 events per run. Each scanning session was comprised of 8 runs.
Every subject attended a training session approximately one week before scanning. During this session, they performed one mock run in an fMRI simulator to familiarize them subjects with the paradigm and button selections, to confirm that the subjects were comfortable with the confined conditions of the scanner and also served to identify any subject who found it difficult to swallow in a supine position.
Apparatus
Solutions were delivered via our custom-built gustometer (Veldhuizen et al., 2007), which consists of a laptop computer that controls independently programmable BS-8000 syringe pumps (Braintree Scientific, Braintree, MA). The pumps were set to deliver tastes at a flow rate of 6mL/min and controlled by a program written using Matlab 6.5.1 (MathWorks Inc., Sherborn, MA) and Cogent2000 v1.25 (Wellcome Department of Cognitive Neurology, London, UK). Each pump held a 60mL syringe connected to a 25-foot length of Tygon beverage tubing that terminated into a specially designed Teflon, fMRI-compatible gustatory manifold that was anchored to the MRI headcoil. The manifold was mounted by rigid tubing onto an anchoring block that clamped onto the bars of the headcoil. Tastant lines were arrayed around a tasteless line in a circular pattern and all tastants and rinses were delivered through a 1mm channel that passes through the entire manifold. These 1mm channels converged at a central point at the bottom end of the manifold. To prevent the subjects tongue from coming in contact with the 1mmchannels, a 7mm sphere was positioned directly under them and rested directly above the subject’s tongue. All subjects were instructed to allow the liquid to roll off of the sphere onto the tip of the tongue, but to refrain from swallowing until instructed. Use of the sphere allowed maintenance of constant tactile stimulation because it creates a constant focus of stimulation across all events.
fMRI Scanner
Images were acquired on a Siemens 3Tesla Trio scanner. Echoplanar imaging was used to measure the blood oxygenated level dependent (BOLD) signal as an indicator of cerebral activation. Subjects were given earplugs to reduce noise from the scanner and were instructed to keep their eyes closed and refrain from unnecessary movements for the duration of the scan. A vitamin E pill was taped to the subject’s left temple to indicate image laterality. A susceptibility weighted single-shot echoplanar sequence was used to image the regional distribution of the BOLD signal with TR = 2000 ms, TE = 20ms, flip angle = 90°, FOV = 220, matrix = 64 × 64, slice thickness = 3mm. Forty continuous slices were acquired in an interleaved mode to minimize signal contamination from adjacent slices. At the beginning of each functional run, the MR signal was allowed to equilibrate over 6 scans for a total of 12 sec, which were excluded from analysis. For anatomical localization, a T1 weighted 3D volume was acquired for each subject at the end of the scanning session (MP-RAGE with a TR/TE of 2530ms/3.66ms, flip angle of 15°, T1 of 1100ms, matrix size of 256 × 256, FOV of 22cm, slice thickness of 0.5mm). The 3D volume and functional slices contained identical orientation and were used in conjunction with the activation maps to localize the function and determine the anatomic regions for investigation of the time course data.
FMRI Data Analysis
Data were pre-processed on Linux workstations using SPM (Welcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/, London, UK)(Friston, 1994; Worsley & Friston, 1995) running in the Matlab software environment (MathWorks, Inc., Sherborn, MA). Functional images were time acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the scan immediately preceding the anatomical T1 image. The images, anatomical and functional, were then normalized to the Montreal Neurological Institute template (MNI-305), which approximates the anatomical space delineated by Talairach and Tournoux (Talairach & Tournoux, 1988). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. Functional images were smoothed with a 6mm FWHM isotropic Gaussian kernel. For time-series analysis on all subjects, a high-pass filter (128) was included in the filtering matrix in order to remove low-frequency noise and slow drifts in the signal, which could bias the estimates of the error. Condition-specific effects at each voxel were estimated using the general linear model. The response to events was modeled by a canonical hemodynamic response function (HRF), consisting of a mixture of 2 gamma functions that emulate the early peak at 5s and the subsequent undershoot. The temporal derivative of the hemodynamic function was also included as part of the basis set to account for small deviations in timing from the canonical HRF.
Parameter estimate images from designated contrasts were entered into a 2-way repeated measures ANOVA with stimulus (taste and tasteless) and task (I, D, PP, P) as within-subject factors. SPM assigns significance levels to the t-fields from all analyses using the theory of Gaussian random fields (2003). Individual SPMs were thresholded for display at a voxelwise p < 0.001 and cluster size of 3 (27 mm3). Unpredicted activations were considered significant at p < 0.05 FDR corrected across the whole brain for multiple comparisons. The insula, overlying operculum, and orbitofrontal cortex (OFC), and amygdala were designated as regions of interest based on prior results showing that these regions form the core of the gustatory network (Kinomura et al., 1994; Faurion et al., 1998; Zald et al., 1998; Frey & Petrides, 1999; Small et al., 1999; O’Doherty et al., 2001; O’Doherty et al., 2002; Small et al., 2003). WFU pick atlas (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003; Maldjian et al., 2004) was used to create a mask of these regions, which was then employed to perform the region of interest analyses for predicted regions. Activations isolated in masked SPMs were considered significant if their p-value was less than 0.05 FDR corrected for multiple comparisons across the entire mask. T-maps were thresholded at p<0.001 and k < 3 voxels.
Effective connectivity analyses were also planned and performed using the psychophysiological interaction toolbox in SPM2. Psychophysiological interaction (PPI) analyses allow the examination of the effective connectivity between brain regions (Friston et al., 1997) under different behavioral contexts (e.g. stimulus and/or task). The presence of a PPI can indicate that the behavioral context modulated either the output from the source region or the responsiveness of the target(s) (Friston et al., 1997). This type of model, therefore allows a restricted statement about the directionality of interregional influences. In the present study, individual PPI SPMs were entered into a random-effects group analysis and the t-maps were thresholded at P < 0.001 (uncorrected) with a cluster size of k<3 voxels. The SPM toolbox ensures correct derivation of the interaction term by deconvolving the source region signal, multiplying the deconvolved signal with the specified behavioral event vector (event onsets for tastes or tasks as detailed below) and reconvolving this interaction term with an HRF (Gitelman et al., 2003). Source region signals were calculated on a per subject basis as the first eigenvariate of the BOLD signals for all voxels within a 15mm radius sphere from the peak of the activation of interest. The ROIs were localized by finding for each subject their activation occurring within 15 mm of the group main effects (ANOVA) activation for each source region. New SPMs were computed for each subject, including as regressors, the interaction term, the source region signal, and the event onset variable.
Results
As stated in the methods section, four subjects were excluded from the analyses due to technical difficulties. These included evidence for scanner instability during the session or excess subject movement during scanning. Excessive movement was probed using the realignment parameters and was defined as movement greater than 1mm in any direction during any run. Thus analyses are based on 15 subjects.
Behavioral Data
The detection (D) and identification (ID) tasks were performed with high accuracy (average performance: D = 98%, ID = 98.25%). The tasteless solution was rated as pleasant (median = 3; mean = 3.03 standard deviation (SD) = 0.35); the sweet solution as very pleasant (median = 4; mean = 3.45; SD = 1.0), and the sour and salty solutions were rated as being equally unpleasant (sour: median=2, mean = 3.3; SD = 0.72 and salty: median = 2; mean = 0.49; SD = 0.48). A oneway ANOVA revealed a main effect of taste (F(3,42) = 15.365; p ≤0.05), with Tukey’s post-hoc test indicating that sour and salty stimuli were rated as more unpleasant than sweet and tasteless (sour vs. tasteless = p 0.05; sour vs. sweet p = 0.004; salty vs. tasteless p = 0.002; salty vs. sweet p = 0.0001). No other significant effects were observed and no stimulus was rated as very unpleasant. We note that the ratings collected in the scanner do not correspond exactly with the ratings collected in the pilot study. The lack of correspondence likely reflects use of different scales (categorical vs. VAS), especially since the categorical scale did not include a response option for neutral, rather than actual perceptual differences. We note that the primary objective of the in – scanner ratings was to focus the subject’s attention to a specific aspect of the stimulus, rather than to provide valid psychophysical ratings. Since subjects were instructed to keep their eyes shut, to avoid divided sensory attention, our method of collecting responses was limited to easily remembered assignment of categories to four buttons on the button box.
Neuroimaging Data
Main Effect of Stimulus
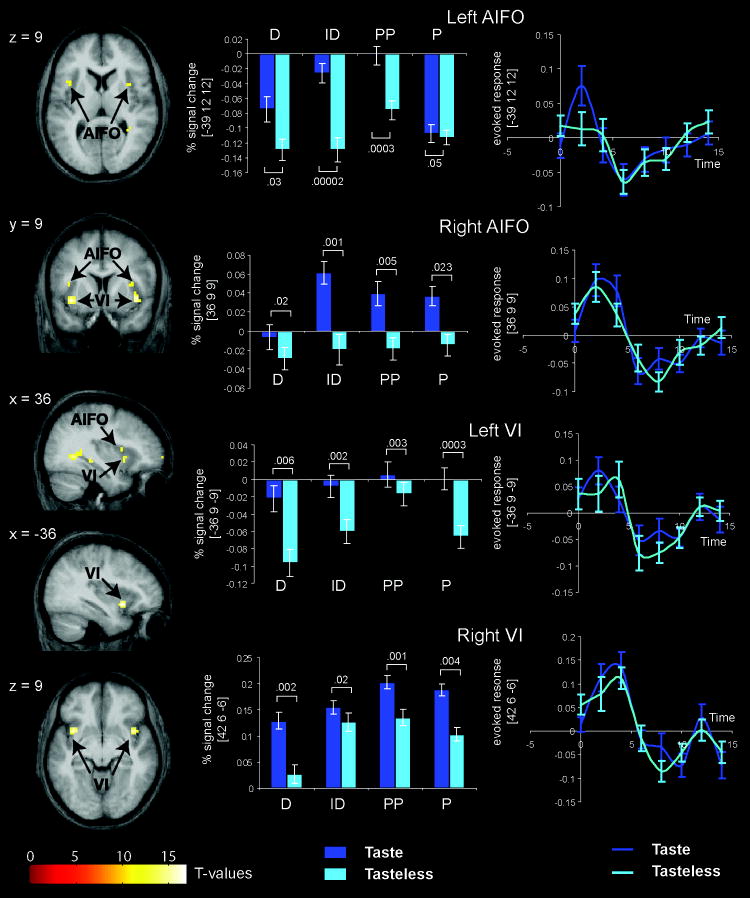
No significant effects were found in the whole brain analysis. Using the mask, a main effect of stimulus was observed bilaterally in the anterior dorsal insula and overlying operculum, as well as in the anterior ventral insula (Table 1). A t-test of taste – tasteless (Table 1) revealed that the main effect resulted from greater response to taste compared to tasteless and further examination of the individual t-tests for each of the simple contrasts showed that this difference was significant for P, ID, and PP but not D (Figure 2).
Table 1.
Main effect of stimulus
| Region | x, y, z | F | z | k | p |
|---|---|---|---|---|---|
| F-TEST (MAIN EFFECT STIMULUS) | |||||
| Left anterior ventral insula | -36, 9, -9 | 16.85 | 3.9 | 23 | 0.05 |
| Left anterior dorsal insula/frontal operculum | -39, 12, 12 | 12.65 | 3.2 | 12 | 0.05 |
| Right anterior ventral insula | 42, 6, -6 | 15.35 | 3.8 | 23 | 0.05 |
| Right anterior dorsal insula/frontal operculum | 36, 9, 9 | 12.00 | 3.2 | 3 | 0.05 |
| T-TEST (TASTE-TASTELESS) | x, y, z | T | z | k | p |
| Left anterior ventral insula | -36, 9,-9 | 4.1 | 3.9 | 23 | 0.04 |
| Left anterior dorsal insula/frontal operculum | -39, 12, 12 | 3.6 | 3.4 | 20 | 0.04 |
| Right anterior ventral insula | 42, 6, -6 | 3.9 | 3.8 | 73 | 0.04 |
| Right anterior dorsal insula/frontal operculum | 36, 9, 9 | 3.5 | 3.4 | 0.04 | |
| Right anterior dorsal insula/frontal operculum | 42, 15, 9 | 3.4 | 3.3 | 0.04 | |
Italics represent significant sub-peaks within a large cluster
Figure 2. Main effect of stimulus.
Brain sections (left column) show the location of the main effect of stimulus in the anterior insula/frontal operculum (AIFO) and ventral insula (VI) indicated with black arrows. The bar graphs (middle column) depict the average percent signal change over subjects (+/- s.e.m.) of the neural response at the peak voxel indicated by the arrows in the anatomical sections during perception of taste (light blue) and tasteless (dark blue) in each of the tasks (D = detection, ID = identification, PP = perceived pleasantness, and P = passive). Error bars represent standard error of the mean. The numbers represent uncorrected p-values extracted from contrasts of simple effects indicated by the lines. T-maps were thresholded at p < 0.001. Color bars represent t-values. Activation maps (and bar graphs) are based on the fit to the canonical HRF. The right column illustrates the time courses of the responses where response (in arbitrary units) to taste (dark blue) and to tasteless (light blue) are plotted as a function of time (in sec). Each time course represents the average response over subjects (+/- s.e.m.) extracted from the peak voxels. The hemodynamic response is estimated from the first eigenvariate at each TR (2 s.) in peristimulus time from the onset of the event until 7 scans after the event occurs. The responses are averaged across all occurrences of that event and averaged across tasks. Thus there is a data point at each 2 s interval. We note that the time course data may not correspond exactly with the bar graphs because the bar graphs reflected data fitted to the canonical HRF whereas the time courses are extracted using a finite impulse response model. These methods are standard within the spm_graph.m function.
Main effect of task
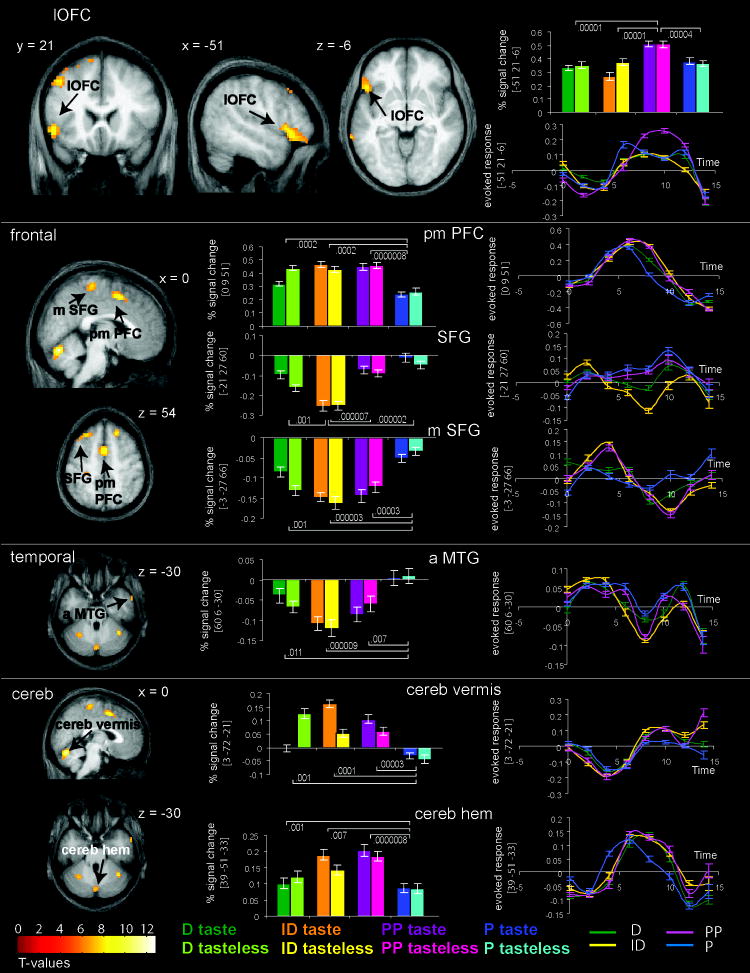
The whole brain analysis revealed many significant effects of task (Table 2 and Figure 3). These included responses in the left lateral OFC, cerebellar vermis, cerebellar hemispheres, superior frontal gyrus, lateral and medial prefrontal cortex, anterior and posterior middle temporal gyrus, and extrastriate cortex. The lateral OFC region was significant in the whole brain analysis and fell within the a priori region of interest (mask). There were no significant effects using the mask other than the left lateral OFC. Task effects were further examined in this region by comparing each task to all other tasks. As predicted the t-tests showed that the effect of task in the lateral OFC (extending into the inferior frontal gyrus) resulted from significantly greater response during the evaluation of perceived pleasantness compared to performance of any other task or passive receipt. Note that this was not an interaction with stimulus, and occurred equally for both taste and tasteless solutions.
Table 2.
Main effect of task
| Region | x, y, z | F | z | k | p |
|---|---|---|---|---|---|
| F-TEST (MAIN EFFECT TASK) | |||||
| Cerebellum (vermis) | 3, -72, -21 | 12.8 | 4.9 | 124 | 0.01 |
| Left Cerebellum (hemisphere) | -27, -57, -27 | 8.4 | 3.9 | 50 | 0.04 |
| Right Cerebellum (hemisphere) | 39, -51, -33 | 8.6 | 4.0 | 20 | 0.04 |
| Posterior medial prefrontal cortex | 0, 9, 51 | 10.5 | 4.3 | 79 | 0.02 |
| Left superior frontal gyrus (BA 8) | -21, 27, 60 | 9.8 | 4.3 | 99 | 0.02 |
| Left middle frontal gyrus (BA 8) | -42, 24, 45 | ||||
| Left medial superior frontal gyrus (BA 8) | -3, -27, 66 | 8.4 | 3.9 | 50 | 0.04 |
| Right superior frontal gyrus (BA 8) | 18, 33, 54 | 8.7 | 4.0 | 33 | 0.04 |
| Left lateral orbitofrontal cortex (BA 47) | -51, 21, -6 | 8.7 | 4.0 | 107 | 0.04 |
| Left lateral orbitofrontal cortex | -54, 33, -12 | 7.9 | 3.7 | 0.04 | |
| Left lateral orbitofrontal cortex | -48, 45, -12 | 6.8 | 3.5 | 0.06 | |
| Left posterior middle temporal gyrus (BA 21) | -69 -42 -6 | 7.8 | 3.7 | 10 | 0.04 |
| Right anterior middle temporal (BA 21) | 60, 6, -30 | 7.1 | 3.5 | 10 | 0.05 |
| Left extrastriate cortex (BA 19) | -18, -87, 33 | 7.6 | 3.7 | 24 | 0.04 |
Italics represent significant sub-peaks within a large cluster
Figure 3. Main effect of task.
Brain sections illustrate the location of brain regions in which a main of effect of task is present. Each panel (delineated with a white line) is devoted to a particular region. From the top this includes the lateral orbitofrontal cortex (LOFC); frontal cortex, including the posterior medial prefrontal cortex (pmPFC), superior frontal gyrus (SFG), and medial superior frontal gyrus (mPFC); anterior middle temporal guyrs (aMTG); and cerebellum (Cereb), including the vermis and hemisphere (Hem). Bar graphs depict the average percent signal change over subjects (+/- s.e.m.) of the neural response at the peak voxel indicated by the arrows in the anatomical sections during perception of taste and tasteless (D = detection, ID = identification, PP = perceived pleasantness, and P = passive). The color key for the bar graphs is located at the bottom of the figure. The numbers represent uncorrected p-values extracted from contrasts of simple effects indicated by the lines. The line graphs depict time course data of response to tasting during each condition in arbitrary units (+/- s.e.m.) extracted from the peak voxel (y-axis) and plotted against time in seconds (x-axis). See legend of figure 3 for further details. T-maps were thresholded at p < 0.001. Color bars represent t-values.
We also performed follow-up t-tests to determine the nature of the effects observed in the non-predicted but significant regions listed in Table 2. The cerebellar vermis, cerebellar hemispheres, and posterior medial prefrontal cortex responded preferentially to sapid stimulation when an evaluation was required compared to when subjects sampled passively. In contrast, the anterior middle temporal gyrus, medial and lateral aspects of the superior frontal gyrus, and extrastriate showed a significantly decreased response during the active compared to the passive tasks. No other significant effects were observed.
Stimulus by task interaction
No significant effects were observed for the whole brain or region of interest analysis when the t-map was thresholded at p < 0.001 and a cluster size of 3 voxels.
Simple Effects
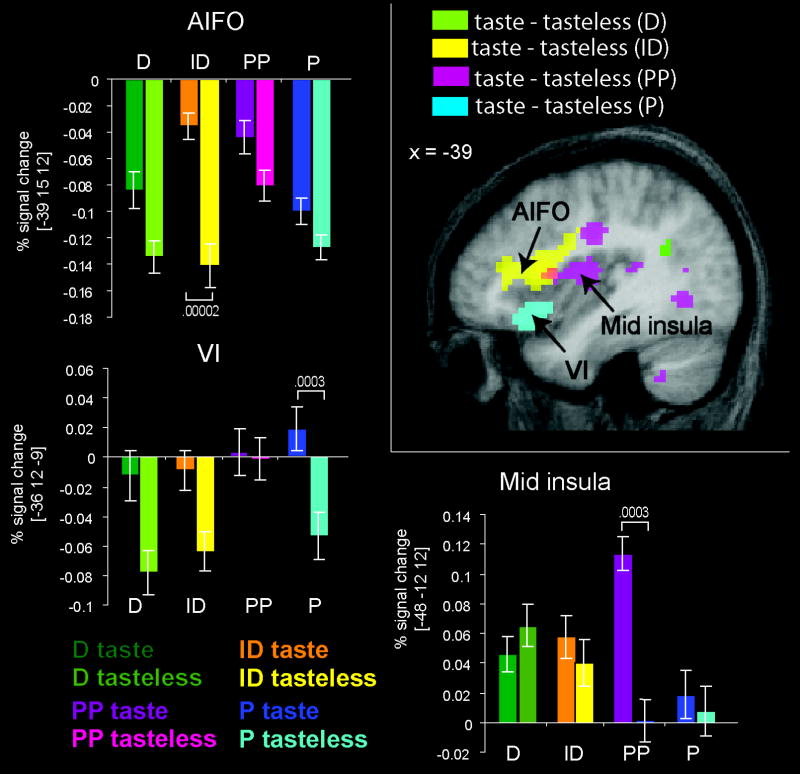
The results of the ANOVA show that sensing taste compared to tasteless produces bilateral responses in the anterior dorsal and ventral insula irrespective of task and that there is no significant task by stimulus interaction. This argues against functional specialization within the insula, at least based upon the divisions represented by our different tasks. However, there is considerable variability in the location of peak response to taste reported across taste studies (Small et al., 1999; Verhagen & Engelen, 2006). Since different studies use different tasks, we reasoned that some of the variability may arise as a function of task. We therefore examined the results from the simple contrasts of taste-tasteless under each of the four conditions to determine if there is anatomical variability in the location of the peak response under the four conditions. The results of these analyses are depicted in figure 4, where each contrast is represented by a different color. Attending to stimulus identity produced a large response in the left anterior dorsal insula at -39, 15, 12 (t = 4.3; z = 4.1; p = 0.02 and k = 59 – one tailed). Attending to pleasantness produced a response in the left mid and posterior insula at -48, -12, 12 (t = 4.2; z = 4.0; p = 0.04; k = 88 – one tailed) and -36, 6, 12 (t = 3.5; z = 3.4; p = 0.04; k = 10 – one tailed). Passively tasting produced a maximal response in the anterior ventral insula at -36, 12, -9 (t = 3.6; z = 3.5; p = 0.08; k = 16 – one tailed). No preferential response to taste vs. tasteless was observed when subjects performed the detection task. This latter observation is consistent with prior work showing that trying to detect a taste in the absence of taste activates the insular taste areas (Veldhuizen et al., 2007). We then contrasted response to taste in each condition vs. response to taste in all other conditions (eg. ID vs P, PP, and D). There was a trend for the left anterior insula to respond significantly more to taste when subjects attempted to identify the taste compared to the averaged activation observed when they were tasting and engaged in the three other tasks (-30, 24, 3; t = 3.5; z = 3.4; p = 0.06; corrected; k = 16). Simple contrasts between conditions showed that this trend arose because ID resulted in significantly greater response in the left anterior insula than D (detection task) (-33, 24, 3; t = 3.9; z = 3.7; k = 42) and trended towards a significantly greater response than during passive tasting (-36, 15, 9; t = 3.6; z = 3.5; p = 0.06; k = 11). There was no differential activation between task ID and task PP (perceived pleasantness). Thus, the left anterior insula tends to respond preferentially to taste when judging identity compared to passive tasting and compared to performing a detection task but not when compared to judging pleasantness. No other insular effects were observed.
Figure 4. Taste-tasteless as a function of task in the insula.
The sagittal brain section depicts the location of response isolated in the contrast of taste – tasteless for each of the four conditions (ID = identification; PP = perceived pleasantness; P = passive; D = detect), color-coded and superimposed upon the mean anatomical image. The SPM t-maps were thresholded at p < 0.005 for the purpose of illustration Activations appearing outside of the insula and overlying opeculum in this image are not significant. Bar graphs depict the average percent signal change over subjects (+/- s.e.m.) of the neural response at the peak voxel indicated by the black arrow (y – axis) for taste and tasteless across the four tasks. The color key is located at the bottom of the figure. T-maps were thresholded at p < 0.001.
Psychophysiological Interactions (PPIs)
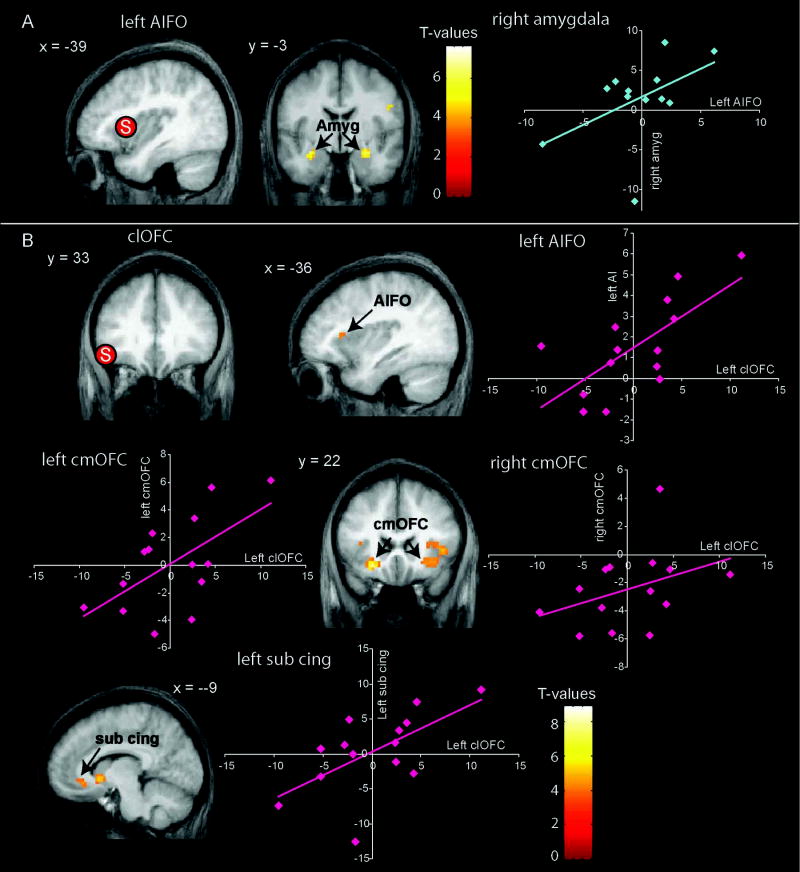
To determine whether the region of insula that responded to taste irrespective of task, and the region of lateral OFC that responded during hedonic judgments irrespective of stimulus, may differentially influence brain response elsewhere depending upon task (anterior insula) or stimulus (lateral OFC) we performed PPIs. Each of the four insular peaks was designated as a source region and 4 PPIs were performed for each (taste – tasteless for each task vs. all other tasks). The only significant finding was preferential connectivity between the left dorsal anterior insula and the amygdala bilaterally during passive sampling compared to active evaluation (-30, -6, -18; z = 4.4; k = 23; p < 0.0001 and 27, -3, -18; z = 4. 0; k = 18; p = 0.0001 (Figure 5). When the t-map threshold was dropped to p <0.005 a PPI was also observed between the right dorsal insula and the left amygdala (-27 -3 -24, k = 7, z = 3.08, p(uncorrected)= 0.001).
Figure 5. Connectivity analyses.
(A) Psychophysiological interactions (PPIs) with the left anterior insula/frontal operculum (AIFO) seed region. The sagittal image on the left shows the location (indicated with “S”) of the seed region in left AIFO from which data were extracted to perform the PPI. The coronal section depicts regions of the amygdala (Amyg) where a significant PPI is observed, reflecting greater connectivity between the amygdala and AIFO during passive tasting compared to tasting while subjects perform a task (D, ID, PP). The graphs show the parameter estimates from AIFO (x-axis) plotted against the parameter estimates from the right amygdala (y-axis) extracted for each subject in the contrast P vs. (D+ID+PP). Thus each dot represents a single subject. Note that a PPI between the right insula and the left amygdala was also observed at a reduced threshold but is not depicted here.
(B) PPIs with the left lateral orbitofrontal cortex (lOFC) seed region. The top left coronal section shows the location (indicated with “S”) of the seed region in lOFC from which data were extracted to perform a PPI. The remaining images depict regions where a significant PPI with the lOFC seed is observed, reflecting greater connectivity when subjects judge pleasantness of a taste compared to when they judge pleasantness of a tasteless solution. These regions include the left AIFO, left and right caudomedial OFC (cmOFC) and midline subcallosal cingulate (sub cing).. The graphs depict paremeter estimates extracted from the seed clOFC (x – axis) plotted against the parameter estimates from the peaks identified in the brain sections (y-axis). The color bars depict t-values. T-maps were thresholded at p < 0.001.
The left lateral OFC also served as the source region for the PPI of (taste vs. tasteless during hedonic evaluation). Greater connectivity between the lateral OFC and bilateral caudomedial OFC (cmOFC) ((-24 18 -12, z=5.0, p < 0.0001 and 33, 18, -9; z=3.7, p = 0.0002)), left ventral striatum (-9, 33, -9; z=3.4, p=0.0008), bilateral subcallosal cingulate (-18, 27, -15; z=3.4, p=0.0008 and 12, 36, -12; z=3.1, p=0.002), and bilateral anterior insula (45, 18, 3; z=3.6, p=0.0002; 45, 12, -9; z=3.4, p=0.006;-36, 24, 9; z=3.3, p=0.001) was observed when subjects received a taste compared to a tasteless solution (Figure 5). In contrast, no preferential connectivity was observed in tasteless compared to taste.
Discussion
We used fMRI to test the prediction that the nature of the neural response to taste varies as a function of the task the subject is asked to perform, reflecting the ability of top-down mechanisms to influence gustatory coding. More specifically, we found that task influences neural encoding of taste beyond the initial cortical representation in the anterior ventral and dorsal insula and overlying operculum. We suggest our findings are consistent with the existence of parallel pathways encoding taste beyond the initial representation in the anterior insula, with involvement of the amygdala during passive tasting and implicit encoding, and the lateral OFC during explicit hedonic evaluation.
Generalist responses in the anterior ventral and dorsal insula and overlying operculum
We identified bilateral responses in the anterior dorsal insula, extending into the frontal operculum and bilateral responses in the anterior ventral insula to taste-tasteless irrespective of task (Figure 2). Since our taste condition included sweet, sour and salty stimuli we conclude that these regions encode these taste qualities and that they are engaged in their representation irrespective of task demand. This finding is consistent with anatomical and physiological data in primates suggesting that the anterior dorsal insula represents primary taste cortex (Pritchard et al., 1986). The ventral insular region receives projections from both the anterior dorsal taste area, as well as from piriform cortex (Mufson & Mesulam, 1982). It also shows supra-additive responses to simultaneous perception of taste and odor (Small et al., 2004). It is therefore possible that the anterior ventral insula provides a second representation of basic taste information, which is then integrated with other oral sensations for the purpose of flavor and food representation.
Variation in the location of insular response to taste
Apart from the tendency for the left anterior dorsal insula to respond preferentially to taste when subjects judged quality, we did not find differential activation between tasks within the insula. However, a separate issue is whether task can lead to variability in the maximal location of the taste evoked activity. This question is of methodological interest because there has been considerable variability in the reported location of gustatory responses among studies that employ different tasks (Small et al., 1999; Verhagen & Engelen, 2006). Consistent with the possibility that the use of different tasks may contribute to the variation in the location of gustatory-evoked responses in the insula/opercular gustatory we found that the peak location for each of the simple contrasts of taste vs. tasteless differed (Figure 4).
Passive Tasting
Although we were unable to find evidence for functional specialization within the insula, we did observe that the effective connectivity between the anterior dorsal insula and the amygdala is greater during passive vs. evaluative tasting (Figure 5). In monkeys, there are direct projections from the anterior insula to the amygdala (Mufson et al., 1981; Mesulam & Mufson, 1985) and gustatory responses have been recorded and characterized in the primate amygdala (Scott et al., 1993; Yan & Scott, 1996; Kadohisa et al., 2005a; Kadohisa et al., 2005b) . Human neuroimaging studies also consistently show response here to taste (Zald et al., 1998; O’Doherty et al., 2001; O’Doherty et al., 2002; Small et al., 2003). Work in rodents indicate that there is an intimate and reciprocal relationship between the amygdala and insula in taste processing, in which stimulation of one region results in responses from the taste cells located in the other region (Grossman et al., 2008). The current finding adds to this literature by suggesting that whether information exchange between the amygdala and insula occurs depends upon attentional state, with preferential channeling of information during implicit processing. An alternative possibility is that during passive tasting the amygdala exerts an inhibitory influence on the anterior insula. This tonic inhibitory influence might then be disrupted during explicit evaluation of taste, resulting in decreased connectivity between the anterior insula and the amygdala. This latter hypothesis stems from our prior work suggesting that the amygdala might exert an inhibitory influence on taste coding. Specifically, resection of the amygdala for the treatment of pharmacologically intractable epilepsy results in increases in taste intensity perception (Small et al., 2001b; c). Since, the insula encodes concentration in rodents (Accolla et al., 2007) and monkeys (Scott et al., 1986; Yaxley et al., 1990; Scott & Plata-Salaman, 1999), and perceived intensity in humans (Small et al., 2003) , it stands to reason that the anterior insula might be the target region for the inhibitory influence from the amygdala.
Regardless of the nature of the mechanism, the proposal that the amygdala plays a preferential role in implicit encoding of taste is consistent with studies showing that the amygdala encodes salient but non conscious visual stimuli (Morris et al. 1998; Whalen et al., 1998), that it responds preferentially to implicit compared to explicit encoding of fearful images (Straube et al., 2004) and that cognitive evaluation may lead to inhibition of amygdaloid responses to visual stimulation (Pessoa et al, 2005). Considering the established role of the amygdala in implicit processing, together with the gustatory literature, our finding suggests that there may be a distinct pathway for implicit taste coding, which involves relay of taste information from dorsal insula to the amygdala, engagement of ventral insula and anterior middle temporal gyrus. We further suggest that since our passive tasting condition most closely approximates tasting under normal circumstances, it is possible it represents the default pathway, which is then disrupted when behavior demands explicit assessment.
Evaluative Tasting
A distinct subset of structures was preferentially engaged by evaluative vs. passive tasting (Figure 3). The only effect specified a priori, was that the OFC would be preferentially recruited during hedonic judgments. The OFC has been shown to play a key role in representing hedonic features of chemosensory stimuli (Rolls et al., 1989; Schoenbaum & Eichenbaum, 1995; Zald & Pardo, 1997; Zald et al., 1998; Tremblay & Schultz, 1999; O’Doherty et al., 2000; Royet et al., 2000; Tremblay & Schultz, 2000; Zatorre et al., 2000; O’Doherty et al., 2001; Royet et al., 2001; Small et al., 2001a; Gottfried et al., 2002; Zald et al., 2002; Anderson et al., 2003; de Araujo et al., 2003a; Kringelbach et al., 2003; Royet et al., 2003; Small et al., 2003; Haase et al., 2007; McCabe & Rolls, 2007). Consistent with the prediction, we found that the left lateral OFC, corresponding to area 47/12 according to the terminology of Petrides and Pandya (Petrides & Pandya, 1994), responded preferentially to taste and to tasteless during the hedonic evaluation. This same region of lateral OFC has been shown to respond preferentially when subjects are required to make hedonic compared to other types of judgments about odors (Royet et al., 2003). Considering our results with the work of Royet and colleagues it is tempting to speculate that this region plays a general role in hedonic evaluation in that it may be recruited irrespective of stimulus (e.g., taste and tasteless) or modality (e.g. odor and taste). Additionally, consistent with this interpretation, we found that connectivity with earlier gustatory relays, including the caudomedial OFC (Pritchard et al., 2005) and anterior dorsal and ventral insula, was greater when subjects judged taste compared to tasteless. This suggests that processing within the lateral OFC may actively organize the retrieval of sensory information from sensory-specific cortex in the service of computing and/or judging perceived pleasantness (Figure 3). However, we note that with PPI we are not able to distinguish whether the task modifies the output from the source or renders the target more sensitive to the source’s output, our interpretation remains speculative.
We also observed that the cerebellum was specifically engaged during evaluative tasting. This finding mirrors work in the olfactory system showing that the cerebellar vermis is recruited during evaluative compared to passive sensing of odors (Savic et al., 2000). The cerebellum is also sensitive to the intensity of chemosensory stimuli (Anderson et al., 2003; Small et al., 2003) and patients with cerebellar lesions have deficits in odor detection and identification (Mainland et al., 2005). Thus, the current finding adds to a growing body of literature implicating the cerebellum in chemosensation (Sobel et al., 1998).
Summary
In summary, we provide evidence for the existence of parallel pathways for encoding taste beyond the initial cortical representation in the anterior insula and overlying operculum. This is reflected both in differential recruitment of regions, well as in differential connectivity between regions as a function of task with involvement of the amygdala and anterior temporal neocortex during passive tasting and implicit encoding, and the cerebellum, anterior cingulate cortex, and lateral OFC during evaluative tasting and explicit encoding.
Acknowledgments
The authors would like to acknowledge Erica Mak for help with pilot testing, design, and data collection. Funding for this work was provided by National Institutes of Health (R01DC6706-02) awarded to DMS.
References
- Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JDE, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Barry MA, Gatenby JC, Zeiger JD, Gore JC. Hemispheric dominance of cortical activity evoked by focal electrogustatory stimuli. Chem Senses. 2001;26:471–482. doi: 10.1093/chemse/26.5.471. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Validation of a stimulation protocol suited to the investigation of odor-taste interactions with fMRI. Physiol & Behav. 2004;81:389–396. doi: 10.1016/j.physbeh.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Van de Moortele PF, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses. 2001;26:371–383. doi: 10.1093/chemse/26.4.371. [DOI] [PubMed] [Google Scholar]
- Cerf B, Faurion A, Mac Leod P, Van de Moortele PF, Lebihan D. Functional MRI study of human gustation. Neuroimage. 1996;3:s342. [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- de Araujo E, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo E, Rolls Et, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour in the human brain. Eur J Neurosci. 2003a;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003b;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Le Bihan D, Pillias AM. fMRI study of taste cortical areas in humans. Ann NY Acad Sci. 1998;855:535–545. doi: 10.1111/j.1749-6632.1998.tb10623.x. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Carter CS, Brooks S, May C, Fissell K, Stenger VA. The evaluation of brain activity in response to taste stimuli--a pilot study and method for central taste activation as assessed by event-related fMRI. J Neurosci Methods. 2003;131:99–105. doi: 10.1016/s0165-0270(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Re-examination of the human taste region: a positron emission tomography study. Eur J Neurosci. 1999;11:2985–2988. doi: 10.1046/j.1460-9568.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Selective attention to affective value alters how the brain processes taste stimuli. Eur J Neurosci. 2008;27:723–729. doi: 10.1111/j.1460-9568.2008.06033.x. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ’Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. J Neurosci Methods. 2007;159:98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol. 1999;81:1917–1926. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, Verhagen JV. Neuronal representations of stimuli in the mouth: the primate insular taste cortex, orbitofrontal cortex and amygdala. Chem Senses. 2005a;30:401–419. doi: 10.1093/chemse/bji036. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Verhagen JV, Rolls ET. The primate amygdala: Neuronal representations of the viscosity, fat texture, temperature, grittiness and taste of foods. Neuroscience. 2005b;132:33–48. doi: 10.1016/j.neuroscience.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, Yamaguchi T, Matsui H, Miyazawa H, Itoh H. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 1994;659:263–266. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, Takeda T, Saito S, Ogawa H. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett. 1996;212:155–158. doi: 10.1016/0304-3940(96)12798-1. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Ogawa H, Kaneda H, Ayabe-Kanamura S, Endo H, Saito S. Spatio-temporal analysis of cortical activity evoked by gustatory stimulation in humans. Chem Senses. 1999;24:201–209. doi: 10.1093/chemse/24.2.201. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Hardan AY, Yorbik O, Keshavan MS. Measurement of the orbitofrontal cortex: a validation study of a new method. Neuroimage. 2003;19:665–673. doi: 10.1016/s1053-8119(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N. Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci. 2005;25:6362–6371. doi: 10.1523/JNEUROSCI.0920-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCabe C, Rolls ET. Umami: a delicious flavor formed by convergence of taste and olfactory pathways in the human brain. Eur J Neurosci. 2007;25:1855–1864. doi: 10.1111/j.1460-9568.2007.05445.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. The Insula of Reil in man and monkey: Architectonics, connectivity and function. In: Peters A, Jones EG, editors. Cerebral Cortex. Plenum Publishing; 1985. pp. 221–222. [Google Scholar]
- Mizoguchi C, Kobayakawa T, Saito S, Ogawa H. Gustatory evoked cortical activity in humans studied by simultaneous EEG and MEG recording. Chem Senses. 2002;27:629–634. doi: 10.1093/chemse/27.7.629. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, Kosslyn SM, Rose RM, Davidson RJ. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:399–403. doi: 10.1097/00001756-200002070-00035. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ito S, Nomura T. Two distinct projection areas from tongue nerves in the frontal operculum of macaque monkeys as revealed with evoked potential mapping. Neurosci Res. 1985;2:447–459. doi: 10.1016/0168-0102(85)90017-3. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Wakita M, Hasegawa K, Kobayakawa T, Sakai N, Hirai T, Yamashita Y, Saito S. Functional MRI detection of activation in the primary gustatory cortices in humans. Chem Senses. 2005;30:583–592. doi: 10.1093/chemse/bji052. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Comparitive architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Elsevier; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- Pritchard TC, Edwards EM, Smith CA, Hilgert K, Gavlick A, Maryniak TD, Schwartz GJ, Scott TR. Gustatory neural responses in the medial orbitofrontal cortex of the old world monkey. J Neurosci. 2005;25:6047–6065. doi: 10.1523/JNEUROSCI.0430-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol & Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosci. 1989;1:53–70. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, Costes N, Holley A. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13:506–519. doi: 10.1006/nimg.2000.0704. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20:713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20:7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: implications for placebo. Brain Behav Immun. 2006;20:120–132. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf JM, Heinze HJ. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127:347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Scott TR, Karadi Z, Oomura Y, Nishino H, Plata-Salaman CR, Lenard L, Giza BK, Aou S. Gustatory neural coding in the amygdala of the alert macaque monkey. J Neurophysiol. 1993;69:1810–1820. doi: 10.1152/jn.1993.69.6.1810. [DOI] [PubMed] [Google Scholar]
- Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiol & Behav. 1999;67:489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol. 1986;56:876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman DR, Mesulam MM, Parrish TB. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. A role for the right anterior temporal lobe in taste quality recognition. J Neurosci. 1997;17:5136–5142. doi: 10.1523/JNEUROSCI.17-13-05136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;13:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish TB, Gitelman DR. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92:1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001a;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Jones-Gotman M. Changes in taste intensity perception following anterior temporal lobe removal in humans. Chem Senses. 2001b;26:425–432. doi: 10.1093/chemse/26.4.425. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Jones-Gotman M. Increased intensity perception of aversive taste following right anteromedial temporal lobe removal in humans. Brain. 2001c;124:1566–1575. doi: 10.1093/brain/124.8.1566. [DOI] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Plata-Salaman CR, Scott TR. Gustatory neural coding in the monkey cortex: stimulus quality. J Neurophysiol. 1991;66:1156–1165. doi: 10.1152/jn.1991.66.4.1156. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tomchik SM, Berg S, Woul Kim J, Chaudhar N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. J Neurophysiol. 2000;83:1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papthanassiou D, Crivellow F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Engelen L. The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci & Biobehav R. 2006;30:613–650. doi: 10.1016/j.neubiorev.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Kadohisa M, Rolls ET. Primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J Neurophysiol. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Abe S, Ishikawa T, Yamada Y, Yamane GY. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia. 2004;19:100–108. doi: 10.1007/s00455-003-0509-5. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yamamoto C, Takehara S, Morikawa K, Nakagawa S, Yamaguchi M, Iwaki S, Tonoike M, Yamamoto T. Magnetoencephalographic study of cortical activity evoked by electrogustatory stimuli. Chem Senses. 2003;28:245–251. doi: 10.1093/chemse/28.3.245. [DOI] [PubMed] [Google Scholar]
- Yan J, Scott TR. The effect of satiety on responses of gustatory neurons in the amygdala of alert cynomolgus macaques. Brain Res. 1996;740:193–200. doi: 10.1016/s0006-8993(96)00864-5. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- Zald D, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chem Senses. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 2002;87:1068–1075. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]
- Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121:1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281–286. [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Rouby C. Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport. 2000;11:2711–2716. doi: 10.1097/00001756-200008210-00021. [DOI] [PubMed] [Google Scholar]